Abstract
Background and Purpose
There is evidence supporting a role for the nociceptin/orphanin FQ (N/OFQ; NOP) receptor and its endogenous ligand N/OFQ in the modulation of neurogenic inflammation, airway tone and calibre. We hypothesized that NOP receptor activation has beneficial effects upon asthma immunopathology and airway hyperresponsiveness. Therefore, the expression and function of N/OFQ and the NOP receptor were examined in healthy and asthmatic human airway tissues. The concept was further addressed in an animal model of allergic asthma.
Experimental Approach
NOP receptor expression was investigated by quantitative real‐time PCR. Sputum N/OFQ was determined by RIA. N/OFQ function was tested using several assays including proliferation, migration, collagen gel contraction and wound healing. The effects of N/OFQ administration in vivo were studied in ovalbumin (OVA)‐sensitized and challenged mice.
Key Results
NOP receptors were expressed on a wide range of human and mouse immune and airway cells. Eosinophils expressed N/OFQ‐precursor mRNA and their number correlated with N/OFQ concentration. N/OFQ was found in human sputum and increased in asthma. Additionally, in asthmatic human lungs N/OFQ immunoreactivity was elevated. NOP receptor activation inhibited migration of immunocytes and increased wound healing in airway structural cells. Furthermore, N/OFQ relaxed spasmogen‐stimulated gel contraction. Remarkably, these findings were mirrored in OVA‐mice where N/OFQ treatment before or during sensitization substantially reduced airway constriction and immunocyte trafficking to the lung, in particular eosinophils. N/OFQ also reduced inflammatory mediators and mucin production.
Conclusions and Implications
We demonstrated a novel dual airway immunomodulator/bronchodilator role for N/OFQ and suggest targeting this system as an innovative treatment for asthma.
Abbreviations
- AHR
airway hyperresponsiveness
- ECM
epithelial conditioned media
- EFS
electrical field stimulation
- EOL‐1
eosinophil‐like cell line
- GINA
Global Initiative for Asthma
- HASM
human airway smooth muscle
- HBEC
human bronchial epithelial cells
- HLMC
human lung mast cells
- HMC‐1
human mastocytoma cell line
- NOP receptor
N/OFQ peptide receptor
- N/OFQ
nociceptin/orphanin FQ
- OVA
ovalbumin
- PBEs
peripheral blood eosinophils
- ppN/OFQ
prepronociceptin
- SCF
stem cell factor
- TFA
trifluoroacetic acid
Tables of Links
| TARGETS |
| NOP receptor |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015).
Introduction
Nociceptin/orphanin FQ (N/OFQ) is the endogenous peptide activator of the N/OFQ receptor (NOP receptor), classified by The International Union of Basic and Clinical Pharmacology (IUPHAR) as a non‐opioid (or non‐classical) member of the opioid family. Since the discovery of the NOP receptor and its deorphanization, the N/OFQ‐NOP receptor system has revealed some intriguing pharmacology. Not least a dual action in pain processing with anti‐opioid actions supraspinally and antinociceptive actions in the spinal cord (Halford et al., 1995; Lambert, 2008). The vast range of additional peripheral actions for NOP receptor activation have also reinvigorated interest regarding its immunomodulatory actions and effects on isolated airway tissues.
The increasing prevalence of asthma is a major health problem affecting 235 million worldwide with an annual mortality of ~0.25 million (WHO, 2013). Asthma is a complex heterogeneous and devastating disease characterized by variable degree of airflow obstruction, airway hyperresponsiveness, chronic airway inflammation and airway remodelling (Brightling et al., 2012). These changes are the result of a crosstalk between resident structural airway smooth muscle and epithelial cells, progenitors including fibrocytes, infiltrating airway submucosal inflammatory cells (eosinophils and T‐cells) (Brightling et al., 2002a), localized mast cells within airway smooth muscle bundles (Brightling et al., 2002a) and Th2 cells (and their cytokines) (Brightling et al., 2002b).
With respect to airways, NOP receptor activation abolishes capsaicin‐ and electrical field stimulation (EFS)‐induced contraction in guinea pig airways (Shah et al., 1998; Corboz et al., 2000) and EFS‐induced contractions of an ex vivo human bronchial ring preparation (Basso et al., 2005). These effects have been attributed to inhibition of airway ACh and sensory neuropeptide release (Patel et al., 1997; Corboz et al., 2000). In ovalbumin (OVA)‐sensitized mice, capsaicin induces increased airway hyperresponsiveness (AHR) that may be partly mediated by reduced endogenous N/OFQ. More significantly, N/OFQ inhibits capsaicin‐induced bronchoconstriction in both naïve and OVA‐sensitized mice (D'Agostino et al., 2010). In addition, NOP receptor agonists are antitussive in preclinical models (McLeod et al., 2001).
In a clinical setting, combined anti‐inflammatory/bronchodilator therapy is effective in controlling asthma; however, ~10% of asthmatics display variable steroid‐resistant patterns of inflammation (Bousquet et al., 2009). The development of new therapies combining both bronchodilator and steroid‐free immunosuppressive profiles offer several advantages over the current treatments not least a simplified dosing regimen.
We hypothesized that the N/OFQ‐NOP receptor system plays a critical role in the pathogenesis of airway inflammation, airflow obstruction and hyperresponsiveness, the hallmarks of asthma. There are currently no data on N/OFQ‐NOP receptor expression in cells from human airways, and as such, its potential role in human asthma is unknown. We have addressed this hypothesis by investigating NOP receptor, prepronociceptin (ppN/OFQ) mRNA and N/OFQ peptide expression and function within airway tissue. We have used ex vivo human tissue from phenotyped asthmatic and non‐asthmatic patients and volunteers and compared data with an established in vivo OVA‐sensitized mouse model of asthma. We showed that N/OFQ is a candidate dual immunomodulator and bronchodilator.
Methods
Detailed methods are available in the Supporting Information.
Subjects
Asthmatic subjects and healthy controls were recruited in Leicester, UK, and their clinical characteristic are reported in Table 1 with the approval of the Leicestershire Ethics Committees. All patients gave written informed consent. Asthmatic subjects had a consistent history and objective evidence of asthma. Asthma severity was defined by Global Initiative for Asthma (GINA) treatment steps (mild–moderate GINA 1–3 and severe GINA 4–5). Subjects underwent extensive clinical characterization including sputum induction and video‐assisted fibreoptic bronchoscopic examination.
Table 1.
Clinical characteristics of healthy and asthmatic volunteers recruited for sputum analysis.
| Healthy (n = 29) | GINA 1–3 (n = 30) | GINA 4–5 (n = 55) | P value | |
|---|---|---|---|---|
| Age in years # | 50 ± 3 | 55 ± 2 | 56 ± 2 | 0.48 |
| Male, n (%) | 19 (66) | 20 (67) | 39 (58) | 0.85 |
| Smoking, n (%) | 9 (31) | 12 (40) | 16 (29) | 0.99 |
| Current | 2 (22) | 2 (17) | 3 (19) | |
| Ex | 7 (78) | 10 (83) | 13 (81) | |
| Smoking (pack years) # | 3.9 ± 1.5 | 6.3 ± 1.9 | 4.5 ± 1.2 | 0.60 |
| FEV1% predicted # | 105.3 ± 3.6 | 84.2 ± 4.1 | 69.3 ± 3.3 | <0.001 |
| FEV1/FVC% # | 78.5 ± 1.2 | 71.3 ± 2.1 | 34.5 ± 4.5 | <0.001 |
| Sputum neutrophils, % * | 50 (31–61.5) | 62.4 (55–78.5) | 56.3 (49–69) | 0.03 |
| Sputum macrophages, % * | 39.3(32.5–60.8) | 23.8(14.3–36.5) | 19.9 (14–28) | <0.001 |
| Sputum eosinophils, % * | 1.8 ± 0.6 (0–5) | 0.8 (0.5–1.8) | 2.9 (1.8–6.9) | <0.001 |
| Sputum epithelial cells, % * | 2.0 (1.0–3.3) | 1.0 (0.8–2.3) | 2.5 (1.8–4.0) | 0.03 |
| Sputum lymphocytes, % * | 0.3 (0–0.3) | 0.0 (0.0–0.3) | 0.3 (0.0–0.5) | 0.25 |
P value represents comparisons between healthy subjects and GINA 4–5 patients.
Mean ± SEM;
Median (interquartile range).
Cell isolation and culture
Pure human airway smooth muscle (HASM) bundles (Brightling et al., 2005), primary human bronchial epithelial cells (HBEC) (Martin et al., 2011) and human lung mast cells (HLMC) (Sanmugalingam et al., 2000) were isolated and cultured as described. Human mastocytoma (HMC‐1) and human eosinophil‐like (EOL‐1) cell lines were cultured, as described previously (Butterfield et al., 1990). Peripheral blood eosinophils (PBEs) were isolated from heparin‐treated peripheral venous blood from healthy control subjects and asthmatic volunteers using LS columns (Miltenyi Biotec, UK).
Membrane preparation and 125I radioisotope dilution assay
Membranes were prepared from freshly harvested cells, HASM (asthmatic and non‐asthmatic) and HMC‐1 cells at confluence. Cells were suspended in homogenizing buffer of Tris–HCl (50 mM), MgSO4 (5 mM) pH 7.4 with KOH and were homogenized followed by centrifugation at 4890 g for 10 min at 4°C. Membrane protein (20 μg of CHOhNOP, 200 μg of HASM and HMC‐1) was incubated in 0.5 mL of homogenization buffer containing 0.5% BSA, 10 μM peptidase inhibitors (amastatin, bestatin, captopril and phosphoramidon, Sigma‐Aldrich, Poole, UK) and various concentrations of [125I]‐N/OFQ (~10 pM–1 nM, Perkin Elmer, UK) for 1 h at room temperature. Non‐specific binding was defined in the presence of 1 μM unlabelled N/OFQ. Bound and free radioactivities were separated by vacuum filtration using a Brandel cell harvester onto Whatman GF/B filters (Fisher Scientific, Loughborough, UK). Filter‐bound radioactivity was assessed by a γ counter, and receptor density was calculated from dilution isotherms.
Quantitative real‐time PCR
All airway and immune cells from volunteers and patients or cultured cells were prepared immediately or stored in RNAlater® (Ambion, Warrington, UK) before RNA extraction. Total RNA was extracted, and final RNA pellets (patient or cultured cells) were resuspended in PCR‐grade water. The mass of RNA was determined using an Eppendorf Biophotometer (Fisher Scientific, Loughborough, UK) and RNA purity crudely assessed from the 260/280 nm ratio, which was in the range of 1.9 to 2.1 for all samples using a nanodrop (Thermo Scientific, UK). Total RNA extracted was processed using Turbo DNA‐free® kit and a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Warrington, UK) according to the manufacturer's instructions. Quantitative RT‐PCR assessed mRNA quantity using commercially available TaqMan® gene expression assays from Applied Biosystems for the human NOP receptor (Hs00173471_m1), human ppN/OFQ or ppNOC (Hs00173823_m1), human CCL11 (eotaxin‐1; Hs00237013_m1), human CCL26 (eotaxin‐3; Hs00171146_m1) and GAPDH or β2 microglobulin. TaqMan probes for the genes under investigation and GAPDH contained different dyes and so were used in a duplex assay format. The thermal profile for quantitative real‐time PCR reactions in the StepOne instrument (Applied Biosystems) was 2 min at 50°C, 10 min at 95°C, 40 or 50 cycles of 15 s at 95°C and 1 min at 60°C. Non‐template controls were included for all samples. Results are expressed as ΔCt: the difference in cycle threshold of the gene of interest and the housekeeper gene, GAPDH or β2 microglobulin. Typical methodology is further described in Leonard et al. (2009).
Immunohistochemistry
Sequential 2 μm sections were cut from glycomethacrylate‐embedded asthmatic and healthy bronchial biopsies and stained using a polyclonal anti‐human N/OFQ antibody (1 in 200 dilution, Phoenix Europe GmbH, Germany) and appropriate isotype control rabbit IgG (Phoenix Europe GmbH). N/OFQ staining in airway smooth muscle was assessed using a semi‐quantitative score of no staining = 0, very low = 1, low = 2, moderate = 3, high = 4 and very high = 5.
Cell migration
The 24‐well or 96‐well Transwell migration assay was used to measure the migration of HMC‐1 cells, HLMCs, EOL‐1 cells and PBEs in response to chemoattractants. To investigate mast cell migration, 450 μL of stem cell factor (SCF) (10 ng·mL−1, R&D Systems, Abingdon, UK), CXCL10 (10 ng·mL−1, R&D Systems, Abingdon, UK) and HASM supernatants stimulated with recombinant TNF‐α for 24 h (10 ng·mL−1, R&D Systems, Abingdon, UK) were used as chemoattractants. We used epithelial conditioned media (ECM) and sputum from severe asthmatic volunteers (with very high levels of N/OFQ) as chemoattractants to study PBE and EOL‐1 cell migration. Chemoattractants were added to the bottom compartment of each well, with the exception of the negative controls. Cells were then added to the top chamber of each well (2.5 × 105 HMC‐1 cells; 1 × 105 HLMCs; 2.5 × 104 eosinophils and EOL‐1 cells). N/OFQ was added to the top compartment. To allow migration, mast cells (HMC‐1 and HLMCs) were incubated for 4 h at 37°C and EOL‐1 cells, eosinophils were incubated for 90 min at 37°C. Following this, cells present in the lower well were recovered and then counted on a flow cytometer or resuspended in trypan blue (0.4%) stain for counting with a haemocytometer by a blinded observer (Kaur et al., 2006).
Measurement of CCL11 and CCL26 by ELISA
HASM cells or undifferentiated HBECs (both 1 × 105 cells per well) were grown to confluency in a six‐well culture plate. Cells were then serum starved for 24 h and pre‐incubated with 300 nM of N/OFQ for 60 min at 37°C in the presence of peptidase inhibitors. Next, HASM cells were stimulated in the presence or absence of TNF‐α (10 ng·mL−1, R&D Systems, Abingdon, UK) and HBEC in the presence or absence of IL‐13 (10 ng·mL−1, R&D Systems, Abingdon, UK) for 24 h at 37°C. After 24 h, supernatants were collected, spun down to remove debris and stored at −80°C till further analysis. Colorimetric human CCL11 and CCL26 ELISAs (R&D Systems, Minneapolis, MN, USA) were performed according to the manufacturer's protocol.
Measurement of IL‐8 release from HMC‐1 cells
HMC‐1 cells were grown in six‐well culture plates and then pre‐incubated in the presence or absence of N/OFQ (300 nM) for 60 min at 37°C in the presence of peptidase inhibitors. Next, cells were stimulated in the presence or absence of SCF (10 ng·mL−1, R&D Systems, Abingdon, UK) for 24 h at 37°C. After 24 h, supernatants were collected, centrifuged at 2000 g for 5 min to remove debris and stored at −80°C till further analysis. IL‐8 concentrations were determined using a DuoSet ELISA Development Kit from R&D Systems.
Wound healing
Non‐asthmatic and asthmatic HBEC cells were seeded onto eight‐well culture plates, serum deprived for 24 h and then wounded using a sterile 200 μL pipette tip in a predetermined grid pattern. After being wounded, cells were washed and treated in the presence or absence of N/OFQ (3 and 300 nM). Non‐asthmatic and asthmatic HASM cells were seeded onto eight‐well fibronectin‐coated plates, serum deprived for 24 h in ITS medium, and then wounded using a sterile 200 μL pipette tip in a predetermined grid pattern. After being wounded, HASM cells were washed and treated in the presence or absence of N/OFQ (3, 30 and 300 nM). Wounds were then photographed at baseline, after 6 and 24 h. Wound areas were analysed by a blinded observer using imagej software, and the extent of repair was calculated and expressed as a percentage of wound healed area.
Sputum N/OFQ measurement by RIA
Acidified sputum samples were extracted using Strata C18‐E solid‐phase extraction cartridges with eluate. N/OFQ was measured by RIA as described previously (Williams et al., 2008).
Assessment of airway smooth muscle contraction by collagen gel analysis
Contractile properties of airway smooth muscle cells were assessed using a collagen gel contraction assay. Collagen gels were impregnated with HASM cells (2.5 × 105 cells) resuspended in DMEM with GlutaMAX‐1 supplemented with penicillin (100 U·mL−1), streptomycin (100 μg·mL−1), amphotericin (0.25 μg·mL−1), non‐essential amino acids (100 μM) (Invitrogen, Paisley, United Kingdom), sodium pyruvate (1 mM) and insulin‐transferrin‐selenium (1%) (Sigma‐Aldrich). Next, 450 μL of gel mixture was added to each well of a PBS 2% BSA pre‐coated 24‐well plate and allowed to polymerize at 37°C for 90 min. After polymerization, 500 μL DMEM with GlutaMAX‐1 (supplemented as above) was added to each well, and the gel was detached from the plastic surface to allow free contraction. Collagen gels were then incubated in the presence or absence of N/OFQ (3 and 300 nM) for 24 h at 37°C. Carbachol (100 μM) or bradykinin (10 nM) were then added to each well in an equal volume of the above media. The collagen gels were photographed at specified time points over a 3 h period by a blinded observer, and the gel size was calculated at specific time points, as a percentage of the well area, using imagej software (National Institutes of Health, USA). All gel conditions were performed in duplicate.
Animal studies
Animal studies reported are in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). The investigation was approved by the Veterinary Animal Care and Use Committee of the Second University of Naples (1966/7.17.2012) and conforms to the National Ethical Guidelines of the Italian Ministry of Health and the Guide for the Care and Use of Laboratory Animals (National Institute of Health, Bethesda, MD,USA, revised 1996). All experimental procedures were in accordance with Italian DLgs 26/2014, application of the EU Directive 2010/63/EU. BALB/c mice were obtained from Harlan Laboratory (Udine, Italy). Mice were housed in the animal facility of the Second University of Naples in standard conditions. Food and water were supplied ad libitum. Room temperature was 22°C–24°C, relative humidity was 40%–50%, and the day/night cycle was set at 12 h/12 h. Mice were acclimatized for 1 week before starting any procedures in 5 mice per cage.
Experimental protocol
Female BALB/c mice were used in this study. Animals were sensitized to OVA by s.c. injection with 0.4 mL of 10 μg OVA, absorbed to 3.3 mg of aluminium hydroxide gel in sterile saline at days 0 and 7. From day 21 to 23, all OVA‐sensitized mice were aerosol challenged (7‐min‐long daily sessions) with 1% OVA in PBS using an ultrasonic nebulizer (De Vilbiss Health Care, UK Ltd., Heston, Middlesex, UK). We used untreated animals (naïve mice) and OVA‐sensitized mice, treated with 100 μL of saline solution (vehicle) or 100 μL of N/OFQ (15 μg·kg−1). Two different experimental protocols were used for N/OFQ treatment. In the first protocol (post‐OVA sensitization), vehicle or N/OFQ were administered i.p. from day 21 to 23, 30 min before each OVA aerosol challenge (Figure 2A), while in the second protocol (pre‐OVA sensitization), vehicle or N/OFQ were administered i.p., at day 0 and 7, 30 min before each allergen injection (Figure 2B). Twenty‐four hours after the last aerosol challenge, animals were killed, and bronchopulmonary function, pulmonary tissue and bronchoalveolar lavage (BAL) fluid collection were performed.
Figure 2.
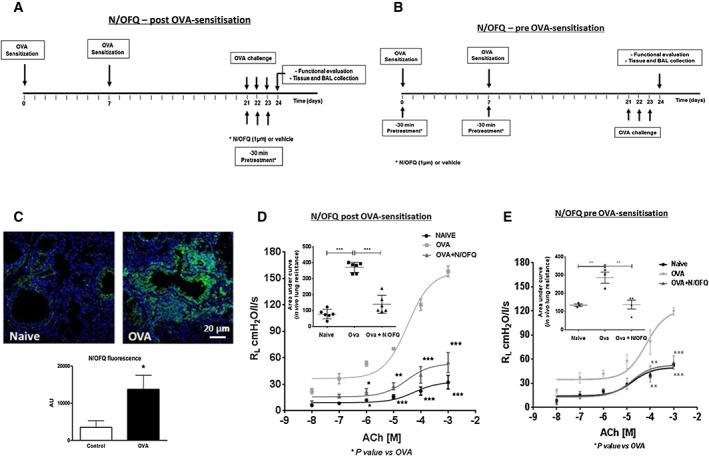
N/OFQ inhibits bronchial hyperreponsiveness. (A) In vivo experimental protocol: N/OFQ administered 30 min prior to OVA challenge (N/OFQ post‐OVA sensitization); (B) in vivo experimental protocol: N/OFQ administered 30 min prior to OVA‐sensitization (N/OFQ pre‐OVA sensitization); (C) representative image of N/OFQ (green) qualitative and quantitative expression in vivo by immunofluorescence, scale bars 20 μm; (D) measurement of ACh‐induced lung resistance in an in vivo model (N/OFQ post‐OVA sensitization) with AUC (inset, n = 6 mice); and (E) measurement of ACh‐induced lung resistance in an in vivo model (N/OFQ pre‐OVA sensitization) with AUC (inset), n = 4 mice. Data are expressed as mean ± SEM. Statistical comparisons by one‐way or two‐way anova followed by appropriate post hoc tests where relevant *P <0.05. N/OFQ administered at 1 μM is equivalent to 15 μg·kg−1.
Airway hyperresponsiveness
AHR to ACh was assessed in an isolated and perfused mouse lung model as described in detail in Roviezzo et al. (2007). Mice were anaesthetized with ketamine HCl 40 mg kg‐1 i.p. and medetomidine hydrochloride 0.15 mg kg‐1 i.p and were exanguinated after incision of the renal vein as the method required. After 60 min, mean tidal volume was 0.21 ± 0.02 mL (n = 61), mean airway resistance 0.23 ± 0.08 cmH2Os·mL−1 and mean pulmonary artery pressure 2.9 ± 1.4 cmH2O. The airway resistance measured was corrected for the resistance of the pneumotachograph and the tracheal cannula of 0.6 cmH2Os·mL−1.
Bronchoalveolar lavage
Mice were anaesthetized with ketamine HCl 40 mg kg‐1 i.p. and medetomidine hydrochloride 0.15 mgkg‐1 i.p and then killed by cervical dislocation. Mouse BAL fluid was collected as follows: 1.5 mL of saline was instilled and withdrawn from the lungs via an intratracheal cannula; this lavage was performed three times, and different samples were collected. BAL fluid was centrifuged at 1000× g for 10 min at 4°C. The supernatant was transferred into tubes and stored at −70°C before use to analyse the cytokine production. Cell pellets were resuspended in PBS to a final volume of 0.5 mL for total and differential cell counting.
Total and differential cell count
Total cell count was performed using the Countess automated cell counter (Invitrogen), which evaluates cell number and viability using trypan blue stain according to the manufacturer's instructions. Differential counting was performed on Diff‐Quik (Reagena, Gentaur, Italy) stained cytospins. At least 200 cells were counted on each cytospin according to standard morphological criteria under light microscopy.
Cytokine assays
Measurement of cytokines in BAL fluid was performed taking advantage of the well‐established Luminex xMAP technology that allows measurement of a panel of analytes in a small sample volume (100 μL) simultaneously. The assays were performed using a Milliplex Cytokine Panel plate (Millipore‐Merck, Vimodrone‐Milan, Italy) according to the manufacturer's instructions on an automated immunoassay analyser (Luminex® 200™ System, Invitrogen, Milan, Italy) as detailed previously (Vignali, 2000). All samples were run in duplicate. Data were analysed using xponent software (1.9 version, Luminex® 200™ System, Invitrogen).
Histochemistry and immunofluorescence
Lungs were perfused and fixed in 10% phosphate‐buffered formalin. Tissue was embedded in paraffin and cut in 5 μm sections for histological analysis. For immunofluorescence, after deparaffinization and rehydration, tissue sections were treated with 10% normal donkey serum for 30 min at room temperature and then incubated with the primary antibodies diluted in PBS. After being washed several times with PBS, the sections were incubated with the FITC‐conjugated and tetramethyl rhodamine isothiocyanate‐conjugated secondary antibodies (Jackson ImmunoResearch). Nuclei were stained with DAPI. For the assessment of inflammation, sections were stained with haematoxylin–eosin (HE). To facilitate the recognition of eosinophils, a modified HE protocol was used (Meyerholz et al., 2009). The number of eosinophils mm‐2 of the peribronchial tissue was measured. The number of mast cells mm‐2 of the lung tissue was measured after staining with toluidine blue. Mucin production was assessed by immunolabelling with anti‐mucin 5 AC antibody (Abcam, Cambridge Science Park, UK). Mucin‐positive cells were quantified in the epithelial layer of the bronchi by counting labelled cells per total number of cells within the airway epithelium. N/OFQ expression within the mouse airways was investigated by immunolabelling with anti‐N/OFQ antibody (Novus Biologicals, Italy). All samples were analysed with a Leica fluorescence microscope (Leica Microsystems GmbH, Wetzlar, Germany) and a Zeiss LSM 700 confocal microscope (Carl Zeiss Microscopy GmbH, Jena, Germany). The values of corrected total fluorescence of N/OFQ per unit area of a peribronchial tissue from control (n = 3) and OVA mice (n = 3) were obtained using imagej software (imagej.nih.gov), adapted from Burgess et al. (2010).
Measurement of OVA‐specific IgE and IgG levels in BAL fluid by ELISA
Briefly, BAL fluid samples were mixed with an equal volume of trifluoroacetic acid (TFA: 1% v : v). Acidified samples were then loaded onto Strata C18‐E solid‐phase extraction cartridges and washed twice with 0.1% TFA. Samples were eluted with 0.1% TFA 3 mL 60% acetonitrile, lyophilized using a centrifugal evaporator and then freeze dried. Before assay, the sample was reconstituted in assay buffer. OVA‐specific IgE (Cambridge Bioscience, UK; assay range 20.7 pg·mL−1–20 ng·mL−1) and OVA‐specific IgG (2B Scientific, Oxford, UK, assay range 1.56–100 U·mL−1) ELISA was performed according to the manufacturer's protocol.
Statistical analysis
Analysis between groups was performed (PRISM Version 6 (GraphPad, CA, USA)) by paired or unpaired t‐tests and across groups by one‐way/two‐way anova with appropriate post hoc comparisons. Post hoc tests were only performed if F was significant. Due to the limitations in the availability of tissue material, some animal and human studies were performed with n < 5. However, no statistical analysis was performed for sample sizes less than 5. For sputum N/OFQ measurements, subjects were categorized into three groups: healthy, mild/moderate asthmatics (GINA 1–3) and severe asthmatics (GINA 4–5). Between group differences were analysed by unpaired t‐tests or Fisher's exact test. Correlations were assessed by Spearman rank (rs) coefficients. Values of P < 0.05 were considered significant.
Results
N/OFQ expression in asthmatic human airways and blood eosinophils
Severe asthmatics (GINA 4–5) had significantly higher levels of N/OFQ in sputum relative to healthy volunteers and GINA 1–3 subjects (Figure 1A). There was no statistical difference between levels of N/OFQ in the sputum from healthy and asthmatic subjects (mild–moderate). Although N/OFQ and FEV1/FVC did not correlate (Supporting Information Fig. SE1), we observed a weak correlation between the increased number of eosinophils in asthmatic patients (mild–moderate and severe) and sputum N/OFQ (Figure 1B, Table 1). In a search for the source of N/OFQ peptide, we assessed its expression in human airway tissue using IHC. N/OFQ staining appeared to be increased in asthma biopsies with a preferential sub‐epithelial and extracellular matrix location and a weak staining in HASM bundles (Figure 1C and D). These observations are consistent with sputum measurements and indicate that the eosinophils potentially release N/OFQ within the airways. In fact, ppN/OFQ mRNA transcripts were found in PBEs, but not in human airway structural cells, from healthy (expression observed in 5/9 independent samples; ΔCт = 21.73 ± 1.57) and asthmatic (expression observed in 5/7 independent samples; ΔCт = 18.31 ± 2.66) patients with no statistically significant differences (P < 0.05; Figure 1E).
Figure 1.
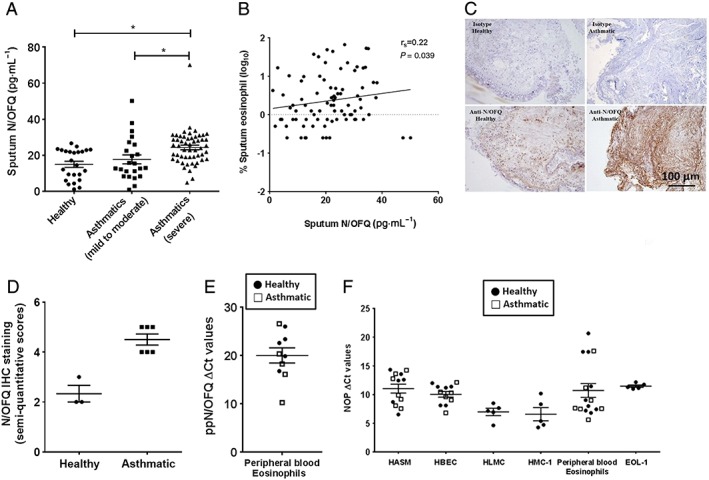
Endogenous N/OFQ expression is increased in human asthmatic airways; the NOP receptor is expressed in human airways. (A) Sputum N/OFQ levels from healthy (n = 29 subjects; n = 5 below detection limit), mild to moderate asthmatic (GINA 1–3; n = 30 patients; n = 7 below detection limit) and severe asthmatic (GINA 4–5; n = 55 patients; n = 2 below detection limit) donors; (B) correlation between sputum N/OFQ (pg·mL−1) and sputum eosinophils (%) in asthmatics (mild–moderate and severe); (C) IHC staining of human airway tissues for N/OFQ (representative image, n = 3 healthy donors and n = 6 asthmatic donors), scale bars 100 μm; (D) semi‐quantitative IHC staining score (n <5 for healthy human airway tissue); (E) quantitative real‐time PCR demonstrating ppN/OFQ mRNA expression on PBEs (n = 5 healthy and n = 5 asthmatic donors); and (F) quantitative real‐time PCR demonstrating NOP receptor mRNA expression in human airway structural and immune cells (n numbers represent cells from individual donors for HASM, HBEC, HLMC and PBEs and independent experiments for HMC‐1 and EOL‐1 cell lines). Data are expressed as mean ± SEM. Comparisons made by unpaired t‐test or one‐way anova followed by appropriate post hoc tests where relevant. *P <0.05.
NOP receptor expression on HLMCs, HMC‐1 and airway structural cells
Interestingly, HLMCs and HMC‐1 cells expressed higher levels of NOP receptor mRNA transcript than HASM cells and HBECs. NOP receptor mRNA transcript was also detected in EOL‐1 cells and native eosinophils isolated from the peripheral blood of healthy and asthmatic volunteers. In contrast to N/OFQ, no differences were observed between healthy and asthmatic subjects documenting no disease signal (Figure 1F). Additionally, in a series of [125I]‐N/OFQ radioligand isotope dilution assays, cell membrane NOP receptor density was quantified on HASM (Bmax; 7.3 ± 1.1 fmol·mg−1 protein, n = 9) and HMC‐1 cells (Bmax; 17 ± 5.9 fmol·mg−1 protein, n = 3). NOP receptor density was significantly increased in HMC‐1 cells compared with HASM (P < 0.05; unpaired t‐test). A CHO cell line expressing recombinant human NOP receptors (positive control) expressed 1321 ± 60 fmol·mg−1 protein (n = 4) of NOP receptors. Moreover, N/OFQ treatment of activated HMC‐1 cells induced a small inhibition of the signalling messenger cAMP (19.3% at 300 nM N/OFQ; Supporting Information Fig. SE2).
Overall, these data demonstrate that the N/OFQ‐NOP receptor system is not only present in human airways but may also have a role in asthma as suggested by increased N/OFQ. The N/OFQ‐NOP receptor system may be positively involved in airway pathophysiology; we, therefore, considered whether the concentration was sufficiently increased to completely ameliorate inflammation in vivo and whether an additional exogenous N/OFQ supplement is required to induce beneficial effects.
Exogenous N/OFQ improves functional parameters and reduces inflammation in experimental allergic asthma
To investigate the impact of exogenous N/OFQ on allergic asthma, N/OFQ was administered in mice prior to allergen sensitization or during the challenge period to examine effects on established airway inflammation (Figure 2A and B). Airways of OVA‐challenged mice showed increased N/OFQ expression (Figure 2C), remarkably similar to that seen in human airways. N/OFQ treatment, either pre‐OVA or post‐OVA sensitization, reduced ACh‐induced bronchoconstriction. Importantly, N/OFQ given during OVA aerosol‐challenge (post OVA‐sensitization) appeared to induce a greater degree of inhibition in ACh‐induced AHR when compared with N/OFQ treatment during OVA sensitization (pre‐OVA sensitization) (62.5% vs. 51%; Figure 2D and E).
BAL analysis showed that N/OFQ administration significantly reduced total cell counts in OVA mice. Of note, OVA‐induced increase in eosinophils was markedly reduced by N/OFQ in both protocols, while the effect on lymphocytes was apparent only when N/OFQ was administered during OVA challenge (Figure 3A and B). There was no significant effect on neutrophils (Figure 3A and B) or alveolar macrophages (data not shown). Histological evaluation revealed an increase in peribronchial inflammatory infiltrates in the lungs of animals sensitized and challenged with OVA, and this was markedly reduced by N/OFQ (Figure 3C and D). Additionally, N/OFQ treatments appeared to reduce peribronchial eosinophil infiltration in OVA‐challenged mice (Figure 3E and F). Although bronchial and peribronchial mast cell accumulation was not observed, the total number of mast cells within the lung tissue of OVA‐sensitized and challenged mice increased. This was lower in N/OFQ‐treated groups (Figure 3G and H).
Figure 3.
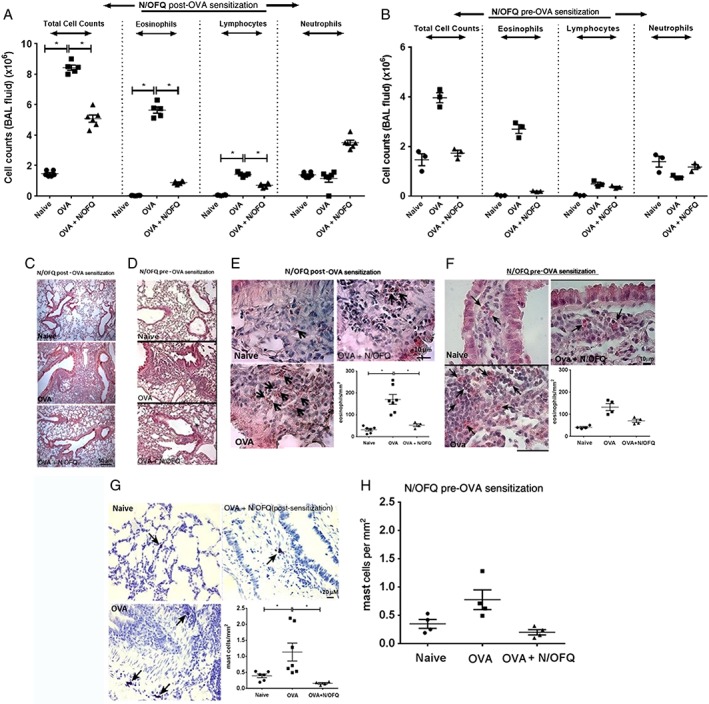
N/OFQ inhibits inflammatory cell infiltration in vivo and recruitment of inflammatory cells within mouse airway tissues. (A) Total and differential cell count in mouse BAL fluid in an in vivo model (N/OFQ post‐OVA sensitization, n = 6 mice), (B) total and differential cell count in mouse BAL fluid in an in vivo model (N/OFQ pre‐OVA sensitization, n = 3 mice). (C) Representative image of HE staining in a N/OFQ post‐OVA sensitized mouse airways (n = 6 mice). Scale bars 50 μm. (D) Representative image of HE staining in a N/OFQ pre‐OVA sensitization mouse airways (n = 3 mice). Scale bars, 50 μm. (E) Modified HE staining showing the kinetics of eosinophilia in a N/OFQ post‐OVA sensitization model (representative image, n = 7 mice) within peribronchial tissue. Scale bars, 10 μ. (F) Modified HE staining showing the kinetics of eosinophilia in a N/OFQ pre‐OVA sensitization model (representative image, n = 4 mice) within peribronchial tissue, scale bars, 10 μm. (G) Representative image of mast cells detected by toluidine blue staining, n = 7 mice. Scale bars, 20 μm. (H) Quantitative estimation of mast cells mm‐2 of mice airway tissue in a N/OFQ pre‐OVA sensitization mouse airways (n = 4 mice). Data expressed as mean ± SEM and analysed by one‐way anova followed by appropriate post hoc tests where relevant. *P <0.05.
Because Th1 and Th2 cytokines play an important role in allergic inflammation, to determine if N/OFQ affects inflammatory mediators in vivo, cytokine profile was analysed in BAL. When N/OFQ was administered during OVA sensitization and during the OVA challenge phase, the treatment significantly reduced allergen‐induced increases in IL‐4, IL‐5, IL‐12 and IL‐13 (Supporting Information Fig. SE3a–d; Figure 4A–D). Further administration of N/OFQ during OVA challenge significantly potentiated OVA‐stimulated increase in BAL IL‐10 levels and reduced OVA‐induced IL‐17 (Figure 4E and F). No effect was observed on IFN‐γ levels (not shown). However, it had no effect on IL‐10 and IL‐17 levels following administration during the OVA sensitization phase (Supporting Information Fig. SE3e and f).
Figure 4.
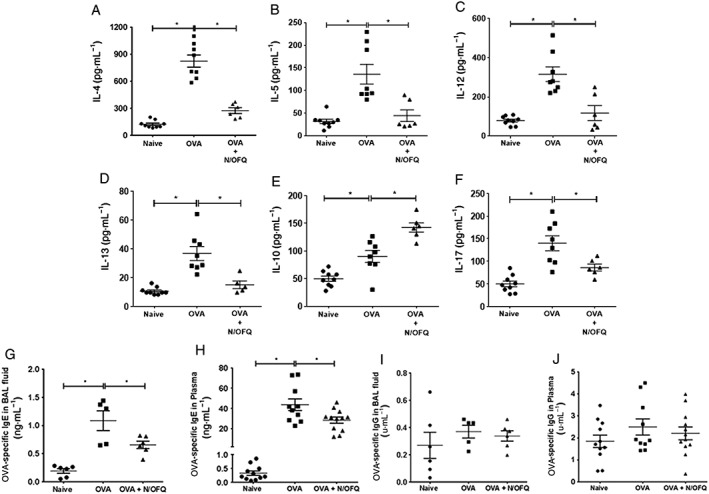
Administration of N/OFQ following sensitization and challenge with OVA regulates release of inflammatory mediators in vivo. (A) IL‐4, (B) IL‐5, (C) IL‐12, (D) IL‐13, (E) IL‐10 and (F) IL‐17 cytokine levels in mouse BAL fluid obtained from different treatment groups, n = 5–9 mice. Data are expressed as pg·mL−1 (mean ± SEM). (G) Measurement of OVA‐specific IgE in BAL fluid (n = 5 mice) and (H) in plasma (n = 10–12 mice). Data are expressed as ng·mL−1 (mean ± SEM). (I) Measurement of OVA‐specific IgG levels in mouse BAL fluid and (J) in mouse plasma. Data are expressed as U·mL−1 (mean ± SEM). Statistical comparisons by one‐way anova followed by appropriate post hoc tests where relevant. *P <0.05.
Finally, OVA‐specific IgE in BAL fluid and plasma samples were significantly reduced by N/OFQ (Figure 4G and H). N/OFQ treatment had no effect on OVA‐specific IgG levels in either BAL fluid or plasma (Figure 4I and J). These observations suggest a Th2‐selective immunomodulatory effect of N/OFQ in vivo.
N/OFQ inhibits agonist‐induced human airway smooth muscle cell contraction
Next, we determined whether the exposure to exogenous N/OFQ affects the functional properties of human airway structural cells. As an index of in vitro airway smooth muscle contractility, carbachol induced a significant time‐dependent collagen gel contraction initiating at 30 min post‐stimulation with a maximum effect after 180 min. There was a significant concentration and time‐dependent inhibition of carbachol‐induced contractility following 24 h pre‐treatment with 3 and 300 nM N/OFQ (P < 0.05 following repeated measures by two‐way anova). These inhibitory responses were significant from 60 to 180 min post‐agonist treatment (Figure 5A–C). We observed a similar effect of N/OFQ on bradykinin‐induced gel contraction (Supporting Information Fig. SE4). There was no significant difference between the effect of N/OFQ on agonist‐induced contraction of healthy and asthmatic HASMs.
Figure 5.
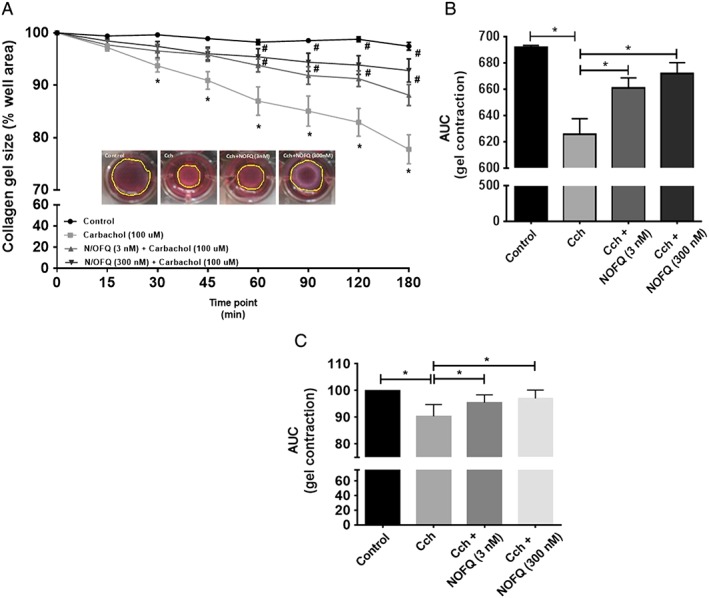
N/OFQ‐NOP activation modulates agonist‐induced HASM contraction. (A) Carbachol‐induced time‐dependent gel contraction (n = 7 HASM cells from seven independent donors); *statistical comparisons between carbachol and control; #statistical comparisons between N/OFQ and carbachol. (B) AUC‐carbachol response and (C) AUC‐carbachol response normalized to control (expressed as 100%). Data are expressed as mean ± SEM. Comparisons by one‐way anova followed by appropriate post hoc tests where relevant. *P <0.05; # P <0.05.
N/OFQ inhibits migration of human mast cells and eosinophils
Sputum from severe asthmatics containing 15–35 pg·mL−1 (8–19 pM) N/OFQ induced a significant increase in EOL‐1 and PBE migration. Addition of 30 or 300 nM exogenous N/OFQ significantly inhibited these effects (Figure 6A and B; Supporting Information Fig. SE5a and b).
Figure 6.
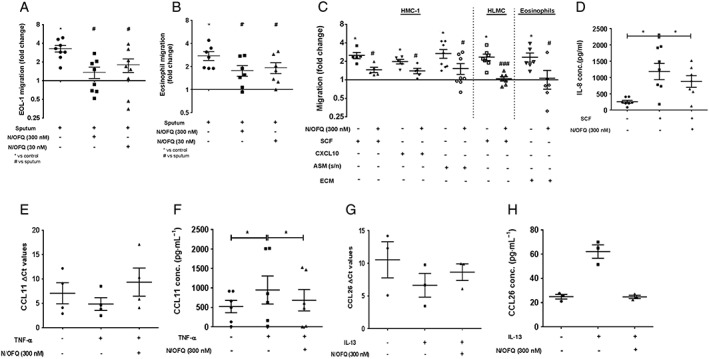
N/OFQ inhibits inflammatory cell migration by blocking mediator release in vitro. (A) Migration of EOL‐1 cells (n = 8 replicates) and (B) PBEs towards asthmatic sputum (n = PBEs from 7 independent donors). (C) Migration of mast cells and eosinophils through an 8 μm (pore‐size) transwell membrane (n = 5–7 independent donors). *Statistical comparisons with control, #statistical comparisons with relevant chemotactic stimuli. (D) SCF‐induced IL‐8 release from HMC‐1 cells (n = 7 replicates). (E) HASM CCL11 mRNA (n = 4 HASM cell samples from 4 independent donors) expression. (F) CCL11 levels in HASM supernatants (n = 6 HASM cell samples from 6 independent donors). (G) HBEC CCL26 mRNA (n = 3 HBEC samples from 3 independent donors) expression. (H) CCL26 levels in HBEC supernatants (n = 3 HBEC samples from 3 independent donors). Data expressed as mean ± SEM. Comparisons by one‐way anova followed by appropriate post hoc tests where relevant. *P <0.05; # P <0.05.
Chemoattractants including SCF, CXCL10 and supernatants from HASM stimulated with 10 ng·mL−1 of TNF‐α (HASM sn) induced an increase in HMC‐1 migration (SCF: 2.5‐fold over control; CXCL10: 1.98‐fold over control; HASM sn: 2.67‐fold over control). SCF‐induced HLMC migration (2.34‐fold over control) was inhibited by N/OFQ (Figure 6C; Supporting Information Fig. SE5c). ECM‐stimulated eosinophil migration (2.33‐fold over control) was also attenuated by 300 nM N/OFQ (Figure 6C; Supporting Information Fig. SE5d). Furthermore, N/OFQ (300 nM) significantly inhibited SCF, CXCL10 and HASM sn‐stimulated HMC‐1 migration (Figure 6C; Supporting Information Fig. SE5e and f).
N/OFQ inhibits the release of chemoattractants that mediate human mast cell and eosinophil migration
IL‐8 and TNF‐α play vital roles in the recruitment of mast cells to sites of inflammation (Nilsson et al., 1999; Olsson et al., 2004). SCF‐stimulated IL‐8 release from HMC‐1 cells was significantly inhibited by N/OFQ (Figure 6D). However, there was no effect on TNF‐α release (Supporting Information Fig. SE6).
CCL11 and CCL26 are involved in the activation and recruitment of PBEs (Garcia‐Zepeda et al., 1996; White et al., 1997). N/OFQ treatment demonstrated a trend towards reduced (increased ΔCt). TNF‐α‐stimulated CCL11 mRNA expression in HASM (Figure 6E) and TNF‐α‐stimulated CCL11 release appear to be inhibited by N/OFQ (Figure 6F). N/OFQ treatment appeared to reduce IL‐13‐stimulated CCL26 mRNA transcript and protein expression in HBEC (Figure 6G and h).
N/OFQ promotes wound repair of human airway structural cells but has no effect on cell proliferation
Features of airway remodelling include epithelial cell damage and mucus hypersecretion. Airway epithelial repair is regulated through the proliferation, migration and differentiation of cells adjoining the damaged area (Tam et al., 2011).
To test the effects of N/OFQ on the repair capacity of bronchial epithelium, confluent monolayers of undifferentiated healthy and asthmatic HBEC were scratch wounded in the presence or absence of the peptide. Although a complete wound closure (a combination of proliferation and migration or chemotaxis) was not achieved, N/OFQ appeared to promote wound repair of healthy HBECs and significantly induced wound closure of asthmatic HBECs (Figure 7A and B); the magnitude of the effect was larger in asthmatic HBECs. Similarly, N/OFQ also promoted wound closing in HASM cultures. Specifically, wound closure of non‐asthmatic HASM was promoted only by 300 nM N/OFQ, while asthmatic HASM wound closure was markedly increased with 3, 30 and 300 nM N/OFQ (Figure 7C and D). There was a significant time (P < 0.05) and dose‐dependent closure of HASM wound (P < 0.05; repeated measures by two‐way anova).
Figure 7.
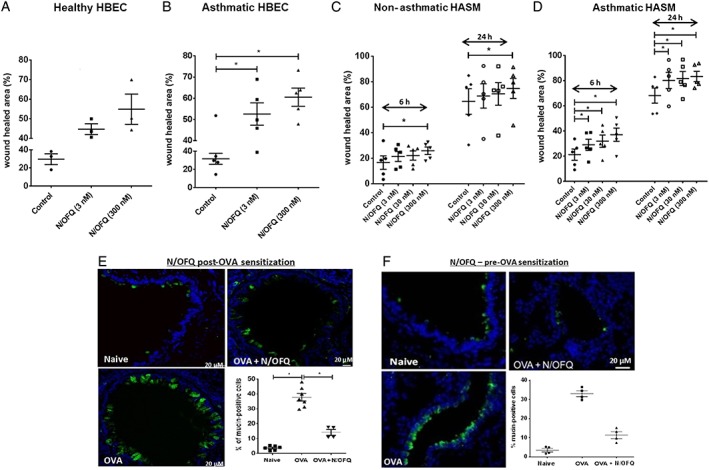
N/OFQ‐NOP activation promotes wound repair of human airway structural cells and inhibits recruitment of mucin‐labelled cells in vivo. (A) Wound repair of undifferentiated healthy (n = 3 HBEC samples from 3 independent donors) and (B) asthmatic HBEC (n = 5 HBEC samples from 5 independent donors). (C) Wound repair of non‐asthmatic (n = 5 HASM cell samples from 5 independent donors) and (D) asthmatic HASMs (n = 5 HASM cell samples from 5 independent donors). (E) Mucin‐labelled cells (green) in airway epithelium following administration of N/OFQ post‐OVA sensitization (n = airway tissue from 7 mice), scale bars 20 μm. (F) Mucin‐labelled cells (green) in airway epithelium following administration of N/OFQ prior to OVA sensitization (n = airway tissue from 4 mice). Data are expressed as mean ± SEM. Comparisons by one‐way anova followed by appropriate post hoc tests where relevant. *P <0.05; # P <0.05. In panels (C and D), there was a significant time (P <0.05) and dose‐dependent closure of HASM wound (P <0.05; repeated measures by two‐way anova).
Additionally, mitogen‐induced proliferation of HASMs, HMC‐1 cells alone (Supporting Information Fig. SE7) or when co‐cultured with HASMs was not influenced by N/OFQ (data not shown).
Finally, as the in vivo counterpart, the response of airway epithelium to the exogenous N/OFQ was evaluated. In animals sensitized and challenged with OVA, administration of N/OFQ reduced by ~50% the extent of epithelial damage (not shown) and decreased the fraction of mucin‐labelled epithelial cells (Figure 7E and F).
Discussion
Using a combination of complementary in vitro human and in vivo mouse studies, we showed that AHR, eosinophil and mast cell migration and inflammatory mediator release in the lungs were dramatically inhibited by N/OFQ. This is the first study to report a critical role for this system in asthma and describes a novel agent with combined anti‐hyperresponsiveness and immunomodulatory properties.
There is emerging evidence suggesting a generalized immunomodulatory role for the N/OFQ‐NOP receptor system (Miller and Fulford, 2007), and our data showed that asthmatic sputum had significantly elevated levels of N/OFQ. This may come from the increased eosinophil counts as there was a correlation between increased eosinophils and elevated N/OFQ. N/OFQ inhibited eosinophil and mast cell migration and attenuated the release of inflammatory mediators that play key roles in their recruitment. Interestingly, N/OFQ expression was found to be up‐regulated in the lung biopsies from asthmatic patients. In particular, N/OFQ was increased in the sub‐epithelial layer and extracellular matrix. We also demonstrated significant NOP receptor expression in human airway structural and inflammatory cells with ppN/OFQ expression only in eosinophils. However, we did not detect any significant increase in NOP receptor expression in asthma implying no disease signal. Functional in vitro studies showed that N/OFQ significantly inhibited agonist‐induced HASM‐embedded gel contraction, and we hypothesize that this could be an additional effect to its anti‐inflammatory role, a response that requires further investigation.
Asthmatic sputum contains several cytokines and chemokines that regulate eosinophil migration including IL‐5, IL‐8, RANTES, IgA and complexes of IL‐8–IgA (Louis et al., 1997). The increase in the levels of endogenous N/OFQ in sputum (~8–19 pM; 15–35 pg·mL−1) of asthmatic patients was several orders of magnitude lower than that required to exert beneficial effects in vitro. We therefore suggest that additional exogenous N/OFQ administration in the airways might present a new therapeutic strategy for asthma. This hypothesis is supported by our observation that spiking sputum from severe asthmatics (with reported high levels of endogenous N/OFQ) with additional N/OFQ (30 and 300 nM), which is over 2000‐fold higher than concentrations measured endogenously, significantly attenuated the migration of eosinophils towards asthmatic sputum.
N/OFQ is a naturally occurring peptide and does not cross the blood brain barrier (Lambert, 2008), and no significant adverse effects were reported in a clinical trial evaluating the urodynamic effects of intravesical administration of 1 μM N/OFQ in patients with neurogenic detrusor activity (Lazzeri et al., 2003). Therefore, any systemic/local administration of N/OFQ is unlikely to induce any unwanted central effects.
As a proof of concept, our in vivo experiments confirmed the hypothesis that exogenous N/OFQ can ameliorate the course of asthma. OVA sensitization followed by challenge has been widely used as a model of airway inflammation although this may not entirely reflect human asthma pathology. It does, however, retain many features of human allergic asthma including Th2 cytokine production, goblet cell hyperplasia, mast cell degranulation, IgE production, AHR and airway remodelling (Kumar and Foster, 2001; Gelfand, 2002; Kumar et al., 2008; Han et al., 2013). We found a significant reduction of allergen‐increased levels of IL‐4, IL‐5, IL‐12 and IL‐13, Th2 cytokines linked to inflammation (Brightling et al., 2002b). These observations were consistent with previous reports demonstrating inhibition of IL‐2 release and T‐cell proliferation by N/OFQ (Miller and Fulford, 2007; Easten et al., 2009). Surprisingly, we did not observe any significant effect of N/OFQ on IFN‐γ production, that is the principal effector of Th1‐mediated inflammation and has a protective effect against Th2‐driven immune responses (Teixeira et al., 2005). Additionally, N/OFQ was able to inhibit levels of OVA‐specific IgE in BAL fluid. However, it failed to modulate OVA‐specific IgG levels. These observations suggest a Th2 selective immunomodulatory effect of N/OFQ in vivo.
Of note, we administered N/OFQ either prior to or concurrent with OVA; this has important consequences for potential treatment paradigms. The observation that N/OFQ has efficacy in both models indicates that use in a clinical setting could involve both prophylaxis and control of acute symptoms. These data confirm and extend our recent study with the highly selective non‐native NOP agonist UFP‐112 (Sullo et al., 2013).
One of the characteristic features of airway remodelling is epithelial cell damage. Abnormal epithelial shedding in asthmatic patients with a variable degree of epithelial damage has been observed (Liu et al., 2013). Repair of airway epithelium is regulated through the proliferation, migration and differentiation of cells adjoining the damaged area (Tam et al., 2011). Glucocorticoids, one of the principal drug classes used in asthma, play a key immunomodulatory role in airway inflammation and provide a sustained repair potential for mechanically injured human airway epithelial cells (Barnes, 2002; Wadsworth et al., 2006). However, studies have also shown that steroids can adversely affect the repair process by suppressing the early‐stage migration and proliferation of airway epithelial cells (Wadsworth et al., 2006; Liu et al., 2013). Therefore, to identify a role for N/OFQ in repair, we explored the effect of this peptide on HBEC and HASM cell wound healing. We observed a significant up‐regulation in wound repair that relates more to lung cell homeostasis than immunomodulation. Importantly, this effect was predominant in cells from asthmatic airways. Additionally, N/OFQ reduced mucus production in vivo.
An important issue in chronic treatments is GPCR signalling desensitization due to persistent ligand binding at high doses. NOP receptor signalling is regulated by the process of homologous desensitization (Donica et al., 2013). Several factors are known to regulate this process including NOP receptor density, dose and duration of exposure, peptide and non‐peptide agonists. Acute exposure to its agonist N/OFQ does not induce NOP receptor down‐regulation (Dautzenberg et al., 2001). However, long‐term exposure to N/OFQ differentially induces down‐regulation in a time‐dependent manner (Hashimoto et al., 2002). Our in vitro assays demonstrated beneficial effects of N/OFQ at 3, 30 and 300 nM following administration of a single‐dose for either 4 or 24 h. It would therefore be interesting to investigate whether this N/OFQ administration regimen would result in desensitization of the NOP receptor. Another interesting observation would be to determine whether this would activate any compensatory mechanism(s) to maintain NOP receptor expression.
The main limitations of our study relate to lack of antagonist data and mechanistic information. Use of the antagonist UFP‐101 in several other studies is questionable as it may have partial agonist activity (Mahmoud et al., 2010) making interpretation problematic. Mechanistic details would be useful in particular the role of downstream signalling events in the N/OFQ‐NOP system including calcium channel modulation, activation of PKC, MAPK, extracellular signal‐regulated kinase 1/2 and Rho kinases; this would need further investigation (Baiula et al., 2013). Despite these limitations, key strengths of our findings include consistency of the observations between human data and an animal model and the magnitude of the effects observed. The anti‐inflammatory effects upon eosinophilic inflammation in the in vivo model are larger than typically observed with corticosteroids (Lee et al., 2008) and are similar to IL‐5 neutralization (Leckie et al., 2000). The magnitude of the effect upon cell migration was also larger than observed in response to corticosteroids or any anti‐inflammatory therapy we have investigated (Wardlaw et al., 2000). The effect upon airway hyperresponsiveness in vivo was similar to those described for anti‐IL‐13 and anti‐IL‐17 (Yang et al., 2004; Kinyanjui et al., 2013), but the effect upon inhibiting in vitro agonist‐induced airway smooth muscle contraction was the largest.
Clinical translation of this data set is a critical future development, but clearly, there are ‘non‐therapeutic’ issues with N/OFQ as it is a natural product already in the public domain. Supraspinal NOP receptor activation has the potential to produce hyperalgesic/antiopioid effects (Schröder et al., 2014), but our observations in human and mouse suggest the main effects on airways are purely peripheral. A simple clinical trial of nebulized N/OFQ both as a prophylactic and during an exacerbation of asthma is clearly warranted. The use of a nebulized formulation would reduce total body dosing, negate the likelihood of central spread and offer the advantage of a single entity combining anti‐hyperresponsiveness and immunomodulatory actions.
Our data suggest that endogenous N/OFQ is elevated in asthma, but its concentrations are too low to substantially modulate the immune system and AHR indicating that supplementation with exogenous N/OFQ is needed.
In conclusion, we have identified an important and an innovative role for N/OFQ in counteracting non‐neurogenic airway inflammatory responses and airway hyperresponsiveness. This combination of beneficial effects is rarely observed and supports our assertion that this opens a completely new potential target/strategy in the treatment of asthma.
Author contributions
S.R.S. is involved in the planning and design of the study, data collection and interpretation; performed and analysed quantitative PCR, migration experiments, in vitro ELISA measurements, collagen gel contraction assays, RIA, cAMP and [3H]‐thymidine incorporation assays; coordinated recruitment of healthy and asthmatic volunteers for sputum and eosinophil collection; analysed relevant clinical data, IL‐8 measurements in HMC‐1 cells, wound healing assays, IHC on airway tissues and contributed to writing of the manuscript. N.S. performed quantitative PCR, migration experiments, in vitro ELISA measurements and collagen gel contraction assays; involved in co‐ordinating animal experiments in Naples and contributed to writing of the manuscript. M.M. and G.S. performed animal experiments. J.M. performed IL‐8 measurements in HMC‐1 cells. R.S. and L.W. performed HASM and HBEC wound healing experiments respectively. K.U. and A.D.A. performed histochemistry and immunofluorescence analysis and contributed to writing of the manuscript. R.D.P. performed cytokine assays. R.B. coordinated recruitment of healthy and asthmatic volunteers for sputum and eosinophil collection and collected all relevant clinical patient data. M.P. performed sputum processing and sputum cell counts. V.M. performed immunohistochemistry on human airway tissues. F.R. made substantial contributions to the final version. G.C. and R.G. provided N/OFQ peptide and made an intellectual contribution to the pharmacology of the manuscript. B.D. coordinated animal experiments in Naples, made substantial contributions to the conception and design of the animal study and to drafting of the manuscript and supervised N.S.C.E.B. was involved in the planning and design of the study, data collection and interpretation; coordinated recruitment of healthy and asthmatic volunteers for sputum and eosinophil collection; analysed IHC on airway tissues; contributed to writing of the manuscript and supervised S.R.S., N.S., R.S., L.W., R.B., M.P. and V.M.D.G.L. was involved in the planning and design of the study, data collection and interpretation; established collaborative links with R.G., G.C. and B.D.; performed and analysed the cAMP and [3H]‐thymidine incorporation assays; contributed to the design of the animal study and drafting of the manuscript and supervised S.R.S., N.S. and J.M. All authors approved the final draft of the manuscript.
Conflict of interest
D.G.L. held a consultancy for work on Nociceptin, now lapsed, which was unlrelated to the research reported in this paper. The other authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organizations engaged with supporting research.
Supporting information
Figure S1 N/OFQ levels in asthmatic sputum do not correlate with FEV1/FVC ratio. Spearman correlation between sputum N/OFQ and FEV1/FVC ratio.
Figure S2 NOP receptors on HMC‐1 cells are coupled to inhibition of cAMP production.Measurement of cAMP formation in HMC‐1 cells (n = 7 independent experiments) following forskolin stimulation (1 M) significantly inhibited (P < 0.05) forskolin‐stimulated increase in cAMP formation. Data expressed as fold change over basal and was analysed by one‐way ANOVAwith Bonferroni's multiple comparison test. *P < 0.05.
Figure S3 Administration of N/OFQ during OVA‐sensitisation regulates release of inflammatory mediators in vivo. (a) IL‐4, (b) IL‐5, (c) IL‐12, (d) IL‐13, (e) IL‐10 and (f) IL‐17 cytokine levels in mouse BAL fluid obtained from different treatment groups (N/OFQ pre and post OVA‐sensitization), n = 3. Data expressed as pg/ml (mean±SEM).
Figure S4 N/OFQ‐NOP activation modulates agonist‐induced HASM contraction. (a) Bradykinin‐induced time‐dependant gel contraction (n = HASM cells from 7 independent donors) and (b) AUC‐bradykinin response. Data expressed as mean±SEM. Comparisons made by two‐way ANOVA. Contraction data are a combination of cells harvested from healthy and asthmatic patients.
Figure S5 N/OFQ inhibits inflammatory cell migration in vitro. (a) Migration of EOL‐1 cells (n = 8 replicates) and (b) PBEs towards asthmatic sputum(n = PBEs from7 independant donors), (c) Migration of HLMC towards SCF (n = 7 independant donors), (d) Migration of PBEs (n = PBEs from 6 independent donors) to epithelial conditioned media, (e) Migration of HMC‐1 to SCF and CXCL10 (n = 5 replicates), (f) Migration of HMC‐1 to ASM supernatants (n = 8 replicates). Data expressed as mean ± SEM. Comparisons by one‐way ANOVA. *P < 0.05.
Figure S6 N/OFQ had no effect on TNF‐α release fromHMC‐1 cells. SCF‐induced TNF‐α release from HMC‐1 cells (n = 6 independent experiments) was not modulated by N/OFQ pre‐treatment. Data expressed as pg/ml (mean ± SEM) and analysed by paired t‐test.
Figure S7 N/OFQ does not modulate mitogen‐induced proliferation of HASMs and HMC‐1 cells. (a) [3H] thymidine incorporation was measured in HASM cultures stimulated with plateletderived growth factor (PDGF‐AB, 20 ng/ml) for 24 h in the presence or absence of N/OFQ expressed as fold change, (b) MTS colorimetric assay was performed to detect HMC‐1 cell viability and proliferation following stimulation with stem cell factor (SCF, 10 ng/ml) for 24h in the presence or absence of N/ OFQ expressed as fold change, (c) [3H] thymidine incorporation was measured in HASM cultures stimulated with platelet‐derived growth factor (PDGF‐AB, 20 ng/ml) for 24 h in the presence or absence of N/OFQ expressed as actual counts, (d) MTS colorimetric assay was performed to detect HMC‐1 cell viability and proliferation following stimulation with stem cell factor (SCF, 10 ng/ml) for 24 h in the presence or absence of N/OFQ expressed as actual counts. All data represent mean ± SEM (n = 6 independent experiments). Comparisons made by oneway ANOVA. *P < 0.05.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Acknowledgements
We would like to thank Professor Peter Bradding of the Institute for Lung Health, Department of Infection, Immunity and Inflammation, University of Leicester, Leicester, UK, for providing HMC‐1 and HLMC. This work was part funded by Asthma UK, Airway Disease Predicting Outcomes through Patient Specific Computational Modelling (AirPROM) project (funded through FP7 EU grant) and Leicester National Institute for Health Research (NIHR) Respiratory Biomedical Research Unit. This paper presents independent research funded by the NIHR. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. This work was supported (used for animal work) by PRIN 2010–2011 n. 2010Y4WMCR_005 from the Italian Ministry of Education, University and Research (MIUR).
Singh, S. R. , Sullo, N. , Matteis, M. , Spaziano, G. , McDonald, J. , Saunders, R. , Woodman, L. , Urbanek, K. , De Angelis, A. , De Palma, R. , Berair, R. , Pancholi, M. , Mistry, V. , Rossi, F. , Guerrini, R. , Calò, G. , D'Agostino, B. , Brightling, C. E. , and Lambert, D. G. (2016) Nociceptin/orphanin FQ (N/OFQ) modulates immunopathology and airway hyperresponsiveness representing a novel target for the treatment of asthma. British Journal of Pharmacology, 173: 1286–1301. doi: 10.1111/bph.13416.
References
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, et al. (2015). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiula M, Bedini A, Carbonari G (2013). Molecular mechanisms mediating nociceptin/orphanin FQ receptor signaling, desensitization and internalization. Curr Mol Pharmacol 5: 372–381. [PubMed] [Google Scholar]
- Barnes PJ (2002). Scientific rationale for inhaled combination therapy with long‐acting beta2‐agonists and corticosteroids. Eur Respir J 19: 182–191. [DOI] [PubMed] [Google Scholar]
- Basso M, Risse PA, Naline E, Calo G, Guerrini R, Regoli D, et al. (2005). Nociceptin/orphanin FQ inhibits electrically induced contractions of the human bronchus via NOP receptor activation. Peptides 26: 1492–1496. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Poli G, Acerbi D, Monno R, Ramael S, Nollevaux F (2009). Systemic exposure and implications for lung deposition with an extra‐fine hydrofluoroalkane beclometasone dipropionate/formoterol fixed combination. Clin Pharmacokinet 48: 347–358. [DOI] [PubMed] [Google Scholar]
- Brightling CE, Ammit AJ, Kaur D, Black JL, Wardlaw AJ, Hughes JM, et al. (2005). The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am J Respir Crit Care Med 171: 1103–1108. [DOI] [PubMed] [Google Scholar]
- Brightling C, Bradding P, Symon F, Holgate S, Wardlaw A, Pavord I (2002a). Mast‐cell infiltration of airway smooth muscle in asthma. N Engl J Med 346: 1699–1705. [DOI] [PubMed] [Google Scholar]
- Brightling CE, Symon FA, Birring SS, Bradding P, Pavord ID, Wardlaw AJ (2002b). TH2 cytokine expression in bronchoalveolar lavage fluid T lymphocytes and bronchial submucosa is a feature of asthma and eosinophilic bronchitis. J Allergy Clin Immunol 110: 899–905. [DOI] [PubMed] [Google Scholar]
- Brightling CE, Gupta S, Gonem S, Siddiqui S (2012). Lung damage and airway remodelling in severe asthma. Clin Exp Allergy 42: 638–649. [DOI] [PubMed] [Google Scholar]
- Burgess A, Vigneron S, Brioudes E, Labbé JC, Lorca T, Castro A (2010). Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B‐Cdc2/PP2A balance. Proc Natl Acad Sci 107: 12564–12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield JH, Weiler D, Peterson EA, Gleich GJ, Leiferman KM (1990). Sequestration of eosinophil major basic protein in human mast cells. Lab Invest 62: 77–86. [PubMed] [Google Scholar]
- Corboz MR, Rivelli MA, Egan RW, Tulshian D, Matasi J, Fawzi AB, et al. (2000). Nociceptin inhibits capsaicin‐induced bronchoconstriction in isolated guinea pig lung. Eur J Pharmacol 402: 171–179. [DOI] [PubMed] [Google Scholar]
- D'Agostino B, Orlotti D, Calò G, Sullo N, Russo M, Guerrini R, et al. (2010). Nociceptin modulates bronchoconstriction induced by sensory nerve activation in mouse lung. Am J Respir Cell Mol Biol 42: 250–254. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Wichmann J, Higelin J, Py‐Lang G, Kratzeisen C, Malherbe P, et al. (2001). Pharmacological characterization of the novel nonpeptide orphanin FQ/nociceptin receptor agonist Ro 64–6198: rapid and reversible desensitization of the ORL1 receptor in vitro and lack of tolerance in vivo . J Pharmacol Exp Ther 298: 812–819. [PubMed] [Google Scholar]
- Donica CL, Awwad HO, Thakker DR, Standifer KM (2013). Cellular mechanisms of nociceptin/orphanin FQ (N/OFQ) peptide (NOP) receptor regulation and heterologous regulation by N/OFQ. Mol Pharmacol 83: 907–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easten KH, Harry RA, Purcell WM, McLeod JD (2009). Nociceptin‐induced modulation of human T cell function. Peptides 30: 926–934. [DOI] [PubMed] [Google Scholar]
- Garcia‐Zepeda EA, Rothenberg ME, Ownbey RT, Celestin J, Leder P, Luster AD (1996). Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat Med 2: 449–456. [DOI] [PubMed] [Google Scholar]
- Gelfand EW (2002). Pro: mice are a good model of human airway disease. Am J Respir Crit Care Med 166: 5–6. [DOI] [PubMed] [Google Scholar]
- Halford WP, Gebhardt BM, Carr DJ (1995). Functional role and sequence analysis of a lymphocyte orphan opioid receptor. J Neuroimmunol 59: 91–101. [DOI] [PubMed] [Google Scholar]
- Han L, Sun YQ, Fu QL, Wen WP, Shi JB (2013). Development of allergic airway disease model in mice. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 48: 224–228. [PubMed] [Google Scholar]
- Hashimoto Y, Calo' G, Guerrini R, Smith G, Lambert DG (2002). Effects of chronic nociceptin/orphanin FQ exposure on cAMP accumulation and receptor density in Chinese hamster ovary cells expressing human nociceptin/orphanin FQ receptors. Eur J Pharmacol 449: 17–22. [DOI] [PubMed] [Google Scholar]
- Kaur D, Saunders R, Berger P, Siddiqui S, Woodman L, Wardlaw A, et al. (2006). Airway smooth muscle and mast cell‐derived CC chemokine ligand 19 mediate airway smooth muscle migration in asthma. Am J Respir Crit Care Med 174: 1179–1188. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). NC3Rs Reporting Guidelines Working Group. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinyanjui MW, Shan J, Nakada EM, Qureshi ST, Fixman ED (2013). Dose‐dependent effects of IL‐17 on IL‐13‐induced airway inflammatory responses and airway hyperresponsiveness. J Immunol 190: 3859–3868. [DOI] [PubMed] [Google Scholar]
- Kumar RK, Foster PS (2001). Murine model of chronic human asthma. Immunol Cell Biol 79: 141–144. [DOI] [PubMed] [Google Scholar]
- Kumar RK, Herbert C, Foster PS (2008). The ‘classical’ ovalbumin challenge model of asthma in mice. Curr Drug Targets 9: 503–510. [DOI] [PubMed] [Google Scholar]
- Lambert DG (2008). The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat Rev Drug Discov 7: 694–710. [DOI] [PubMed] [Google Scholar]
- Lazzeri M, Calò G, Spinelli M, Guerrini R, Salvadori S, Beneforti P, et al. (2003). Urodynamic effects of intravesical nociceptin/orphanin FQ in neurogenic detrusor overactivity: a randomized, placebo‐controlled, double‐blind study. Urology 61: 946–950. [DOI] [PubMed] [Google Scholar]
- Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, et al. (2000). Effects of an interleukin‐5 blocking monoclonal antibody on eosinophils, airway hyper‐responsiveness, and the late asthmatic response. Lancet 356: 2144–2148. [DOI] [PubMed] [Google Scholar]
- Lee SY, Kim JS, Lee JM, Kwon SS, Kim KH, Moon HS, et al. (2008). Inhaled corticosteroid prevents the thickening of airway smooth muscle in murine model of chronic asthma. Pulm Pharmacol Ther 21: 14–19 Epub 2006 Oct 20. [DOI] [PubMed] [Google Scholar]
- Leonard AD, Thompson JP, Hutchinson EL, Young SP, McDonald J, Swanevelder J, et al. (2009). Urotensin II receptor expression in human right atrium and aorta: effects of ischaemic heart disease. Br J Anaesth 102: 477–484. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang M, Niu C, Luo Z, Dai J, Wang L, et al. (2013). Dexamethasone inhibits repair of human airway epithelial cells mediated by glucocorticoid‐induced leucine zipper (GILZ). PLoS One 8 e60705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis R, Shute J, Biagi S, Stanciu L, Marrelli F, Tenor H, et al. (1997). Cell infiltration, ICAM‐1 expression, and eosinophil chemotactic activity in asthmatic sputum. Am J Respir Crit Care Med 155: 466–472. [DOI] [PubMed] [Google Scholar]
- Mahmoud S, Margas W, Trapella C, Caló G, Ruiz‐Velasco V (2010). Modulation of silent and constitutively active nociceptin/orphanin FQ receptors by potent receptor antagonists and Na+ ions in rat sympathetic neurons. Mol Pharmacol 77: 804–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Thévenot G, Danel S, Chapron J, Tazi A, Macey J, et al. (2011). Pseudomonas aeruginosa induces vascular endothelial growth factor synthesis in airway epithelium in vitro and in vivo . Eur Respir J 38: 939–946. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod RL, Bolser DC, Jia Y, Parra LE, Mutter JC, Wang X, et al. (2001). Antitussive effect of nociceptin/orphanin FQ in experimental cough models. Pulm Pharmacol Ther 15: 213–216. [DOI] [PubMed] [Google Scholar]
- Meyerholz DK, Griffin MA, Castilow EM, Varga SM (2009). Comparison of histochemical methods for murine eosinophil detection in an RSV vaccine‐enhanced inflammation model. Toxicol Pathol 37: 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TR, Fulford AJ (2007). Regulation of nociceptin/orphaninFQ secretion by immune cells and functional modulation of interleukin‐2. Peptides 28: 2243–2252. [DOI] [PubMed] [Google Scholar]
- Nilsson G, Mikovits JA, Metcalfe DD, Taub DD (1999). Mast cell migratory response to interleukin‐8 is mediated through interaction with chemokine receptor CXCR2/Interleukin‐8 RB. Blood 93: 2791–2797. [PubMed] [Google Scholar]
- Olsson N, Taub DD, Nilsson G (2004). Regulation of mast cell migration by T cytokines: identification of tumour necrosis factor‐alpha and interleukin‐4 as mast cell chemotaxins. Scand J Immunol 59: 267–272. [DOI] [PubMed] [Google Scholar]
- Patel HJ, Giembycz MA, Spicuzza L, Barnes PJ, Belvisi MG (1997). Naloxone‐insensitive inhibition of acetylcholine release from parasympathetic nerves innervating guinea‐pig trachea by the novel opioid, nociceptin. Br J Pharmacol 120: 735–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. (2014). The IUPHAR/BPS guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucleic Acids Res 42: D1098–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roviezzo F, Di Lorenzo A, Bucci M, Brancaleone V, Vellecco V, De Nardo M, et al. (2007). Sphingosine‐1‐phosphate/sphingosine kinase pathway is involved in mouse airway hyperresponsiveness. Am J Respir Cell Mol Biol 36: 757–762. [DOI] [PubMed] [Google Scholar]
- Sanmugalingam D, Wardlaw AJ, Bradding P (2000). Adhesion of human lung mast cells to bronchial epithelium: evidence for a novel carbohydrate‐mediated mechanism. J Leukoc Biol 68: 38–46. [PubMed] [Google Scholar]
- Schröder W, Lambert DG, Ko MC, Koch T (2014). Functional plasticity of the N/OFQ‐NOP receptor system determines analgesic properties of NOP receptor agonists. Br J Pharmacol 171: 3777–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Page CP, Spina D (1998). Nociceptin inhibits non‐adrenergic non‐cholinergic contraction in guinea‐pig airway. Br J Pharmacol 125: 510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullo N, Roviezzo F, Matteis M, Ianaro A, Calò G, Guerrini R, et al. (2013). Nociceptin/orphanin FQ receptor activation decreases the airway hyper responsiveness induced by allergen in sensitized mice. Am J Physiol Lung Cell Mol Physiol 304: L657–L664. [DOI] [PubMed] [Google Scholar]
- Tam A, Tsang DP, Chan MY, Zhu N, Yam VW (2011). The airway epithelium: more than just a structural barrier. Ther Adv Respir Dis 5: 255–273. [DOI] [PubMed] [Google Scholar]
- Teixeira LK, Fonseca BP, Barboza BA, Viola JP (2005). The role of interferon‐gamma on immune and allergic responses. Mem Inst Oswaldo Cruz 100: 137–144. [DOI] [PubMed] [Google Scholar]
- Vignali DA (2000). Multiplexed particle‐based flow cytometric assays. J Immunol Methods 243: 243–255. [DOI] [PubMed] [Google Scholar]
- Wadsworth SJ, Nijmeh HS, Hall IP (2006). Glucocorticoids increase repair potential in a novel in vitro human airway epithelial wounding model. J Clin Immunol 26: 376–387. [DOI] [PubMed] [Google Scholar]
- Wardlaw AJ, Brightling C, Green R, Woltmann G, Pavord I (2000). Eosinophils in asthma and other allergic diseases. Br Med Bull 56: 985–100. [DOI] [PubMed] [Google Scholar]
- White JR, Imburgia C, Dul E, Appelbaum E, O'Donnell K, O'Shannessy DJ, et al. (1997). Cloning and functional characterization of a novel human CC chemokine that binds to the CCR3 receptor and activates human eosinophils. J Leukoc Biol 62: 667–675. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Asthma Update November. (2013). Available at http://www.who.int/respiratory/asthma/en/ (accessed: March 10 2015)
- Williams JP, Thompson JP, Young SP, Gold SJ, McDonald J, Rowbotham DJ, et al. (2008). Nociceptin and urotensin‐II concentrations in critically ill patients with sepsis. Br J Anaesth 100: 810–814. [DOI] [PubMed] [Google Scholar]
- Yang G, Volk A, Petley T, Emmell E, Giles‐Komar J, Shang X, et al. (2004). Anti‐IL‐13 monoclonal antibody inhibits airway hyperresponsiveness, inflammation and airway remodeling. Cytokine 28: 224–232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 N/OFQ levels in asthmatic sputum do not correlate with FEV1/FVC ratio. Spearman correlation between sputum N/OFQ and FEV1/FVC ratio.
Figure S2 NOP receptors on HMC‐1 cells are coupled to inhibition of cAMP production.Measurement of cAMP formation in HMC‐1 cells (n = 7 independent experiments) following forskolin stimulation (1 M) significantly inhibited (P < 0.05) forskolin‐stimulated increase in cAMP formation. Data expressed as fold change over basal and was analysed by one‐way ANOVAwith Bonferroni's multiple comparison test. *P < 0.05.
Figure S3 Administration of N/OFQ during OVA‐sensitisation regulates release of inflammatory mediators in vivo. (a) IL‐4, (b) IL‐5, (c) IL‐12, (d) IL‐13, (e) IL‐10 and (f) IL‐17 cytokine levels in mouse BAL fluid obtained from different treatment groups (N/OFQ pre and post OVA‐sensitization), n = 3. Data expressed as pg/ml (mean±SEM).
Figure S4 N/OFQ‐NOP activation modulates agonist‐induced HASM contraction. (a) Bradykinin‐induced time‐dependant gel contraction (n = HASM cells from 7 independent donors) and (b) AUC‐bradykinin response. Data expressed as mean±SEM. Comparisons made by two‐way ANOVA. Contraction data are a combination of cells harvested from healthy and asthmatic patients.
Figure S5 N/OFQ inhibits inflammatory cell migration in vitro. (a) Migration of EOL‐1 cells (n = 8 replicates) and (b) PBEs towards asthmatic sputum(n = PBEs from7 independant donors), (c) Migration of HLMC towards SCF (n = 7 independant donors), (d) Migration of PBEs (n = PBEs from 6 independent donors) to epithelial conditioned media, (e) Migration of HMC‐1 to SCF and CXCL10 (n = 5 replicates), (f) Migration of HMC‐1 to ASM supernatants (n = 8 replicates). Data expressed as mean ± SEM. Comparisons by one‐way ANOVA. *P < 0.05.
Figure S6 N/OFQ had no effect on TNF‐α release fromHMC‐1 cells. SCF‐induced TNF‐α release from HMC‐1 cells (n = 6 independent experiments) was not modulated by N/OFQ pre‐treatment. Data expressed as pg/ml (mean ± SEM) and analysed by paired t‐test.
Figure S7 N/OFQ does not modulate mitogen‐induced proliferation of HASMs and HMC‐1 cells. (a) [3H] thymidine incorporation was measured in HASM cultures stimulated with plateletderived growth factor (PDGF‐AB, 20 ng/ml) for 24 h in the presence or absence of N/OFQ expressed as fold change, (b) MTS colorimetric assay was performed to detect HMC‐1 cell viability and proliferation following stimulation with stem cell factor (SCF, 10 ng/ml) for 24h in the presence or absence of N/ OFQ expressed as fold change, (c) [3H] thymidine incorporation was measured in HASM cultures stimulated with platelet‐derived growth factor (PDGF‐AB, 20 ng/ml) for 24 h in the presence or absence of N/OFQ expressed as actual counts, (d) MTS colorimetric assay was performed to detect HMC‐1 cell viability and proliferation following stimulation with stem cell factor (SCF, 10 ng/ml) for 24 h in the presence or absence of N/OFQ expressed as actual counts. All data represent mean ± SEM (n = 6 independent experiments). Comparisons made by oneway ANOVA. *P < 0.05.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item


