Abstract
BACKGROUND
Convection-enhanced delivery of chemotherapeutics for the treatment of malignant glioma is a technique that delivers drugs directly into a tumor and the surrounding interstitium through continuous, low-grade positive-pressure infusion. This allows high local concentrations of drug while overcoming the limitations imposed by toxicity and the blood-brain barrier in systemic therapies that prevent the use of many potentially effective drugs.
OBJECTIVE
To examine the safety profile of a conventional chemotherapeutic agent, topotecan, via convection-enhanced delivery in the treatment of recurrent malignant gliomas and secondarily to assess radiographic response and survival.
METHODS
We performed a prospective, dose-escalation phase Ib study of the topoisomerase-I inhibitor topotecan given by convection-enhanced delivery in patients with recurrent malignant gliomas.
RESULTS
Significant antitumor activity as described by radiographic changes and prolonged overall survival with minimal drug-associated toxicity was demonstrated. A maximum tolerated dose was established for future phase II studies.
CONCLUSION
Topotecan by convection-enhanced delivery has significant antitumor activity at concentrations that are nontoxic to normal brain. The potential for use of this therapy as a generally effective treatment option for malignant gliomas will be tested in subsequent phase II and III trials.
Keywords: Clinical trial, Glioblastoma, Glioma, Topotecan
The inability to achieve tumoricidal concentrations of chemotherapeutic drugs in tumor cells is one of the primary causes of treatment failure in solid tumors. This is particularly pertinent in malignant gliomas, which are highly proliferative, invasive tumors that invariably recur after conventional treatment with radiation and chemotherapy. Better treatment strategies are needed, especially after recurrence when treatment options are limited and average survival is measured in weeks to months. Although chemotherapeutic drugs are available that demonstrate significant antitumor activity for gliomas in vitro and in animal models in which sufficient intratumoral cytotoxic concentrations can be attained, similar results are not achievable in humans because conventional systemic drug delivery methods result in limited efficacy owing to blood-brain barrier (BBB) restrictions and systemic toxicity. Malignant gliomas are locally invasive but rarely metastatic, as evidenced by predominately local recurrences, making them vulnerable to local regional delivery approaches such as convection-enhanced delivery (CED).1 The strategy of CED was developed by Bobo et al2 to deliver drugs directly into tumors and surrounding brain through the interstitial space. Stereotactically placed catheters connected to pumps provide a continuous, low-grade positive-pressure microinfusion that distribute drugs by bulk flow. The benefits include high local concentrations of drug while avoiding systemic toxicity. Mathematical and experimental models have demonstrated the advantages of bulk flow distribution with CED over simple diffusion methods such as systemic chemotherapy or passive local delivery methods, including controlled-release drug polymers and intrathecal administration.3–6
Previous clinical trials with CED for gliomas have been disappointing because of excessive toxicities or lack of tumor response, suggesting the need for safer and more efficacious drugs.7–10 Topotecan, a topoisomerase I inhibitor, is an ideal drug for CED of gliomas because (1) it is cytotoxic to glioma cells and nontoxic to normal brain; (2) topoisomerase I levels are higher in glioma cells and tumor tissue than in normal brain; and (3) it is a natural-product drug with high molecular weight and thus should minimally traverse the BBB from the brain to the systemic circulation.5,11–13 Previous clinical trials of topotecan delivered intravenously have shown minimal effects14; however, using CED in our rat in vivo models, we demonstrated significant antitumor efficacy and prolonged survival.5,11 We performed a prospective phase Ib dose-escalation study of topotecan by CED in patients with recurrent malignant gliomas to evaluate toxicity and quality of life (QoL) effects and to confirm antitumor activity radiographically.
METHODS
Patient Selection
All patients were treated at Columbia University Medical Center. The Institutional Review Board approved the study, and all patients gave informed consent. Eligibility criteria included (1) previously diagnosed supratentorial malignant glioma; (2) tumor progression evidenced by increasing contrast enhancement on magnetic resonance (MR) or computed tomography (CT) while on stable or increasing steroid dose; (3) prior treatment with external beam radiation; (4) MR imaging (MRI) demonstration of a stereotactically accessible enhancing mass of <65 cm3 without significant mass effect (eligible tumor size was limited to minimize the potential effects of mass and to focus simply on the drug concentration as a source of toxicity); (5) a Karnofsky Performance Score of at least 60; (6) a negative pregnancy test for women of childbearing potential; and (7) ability to give informed consent.
Patients were excluded if they had (1) diffuse subependymal or cerebrospinal fluid disease; (2) tumors involving the cerebellum or both hemispheres; (3) active infection requiring treatment or unexplained febrile illness; (4) HIV or hepatitis B or C positivity; (5) radiation treatment or chemotherapy within 4 weeks of enrollment; (6) systemic diseases associated with unacceptable anesthetic or operative risk; (7) prior treatment with topotecan; (8) age <1 year; (9) inability to undergo MR scanning; (10) white blood cell count ≤4.0 with an absolute neutrophil count ≤2000, hemoglobin <10.0, and platelets ≤100 000; and (11) abnormal liver, renal, and metabolic function panels >1.5 times the upper limits of normal.
Study Design
This study was conducted as a prospective phase Ib open-label, nonrandomized, dose-escalation design. Patients received 40 mL infusion at 1 concentration of topotecan, depending on dose level assignment (GlaxoSmithKline; Philadelphia, Pennsylvania). Three new patients were initially considered at each dose level, and each patient received 1 treatment. The starting dose (level I) was 0.02 mg/mL, 1/10th the concentration that led to complete tumor regression without toxicity in a rat glioma model.5 Subsequent dose levels were 0.04, 0.0667, 0.1, and 0.133 mg/mL. If 2 patients at 1 dose level developed dose-limiting toxicity (DLT), the prior dose level would be considered the maximum tolerated dose (MTD).
Study Procedure
Steroids and antibiotics were administered throughout the infusion period. A standard MRI/CT-guided stereotactic biopsy was performed to confirm the presence of recurrent malignant glioma histologically. After confirmation, 2 Silastic infusion catheters (2.5-mm outer diameter, cerebrospinal fluid–peritoneal catheter; Integra; Plainsboro, New Jersey) were stereotactically placed directly into the enhancing tumor or adjacent brain at sites chosen to maximize coverage of the tumor and adjacent infiltrated brain on the basis of a presumed spherical distribution. Care was taken to avoid catheter placement closely adjacent to necrotic tumor, cystic regions, ventricles, or the cortical surface/subarachnoid space to prevent loss of drug to nonviable tissue or cerebrospinal fluid. Tumors with large postsurgical cavities or large cysts were excluded for CED. Postoperative CT was performed to assess catheter position. Two patients had postoperative imaging that suggested that the position could be optimized by pulling the catheter back slightly, which was performed without altering the rest of the treatment protocol. Infusion proceeded at 200 µL/h in each catheter for a continuous 100 hours via a Medfusion 2010 syringe pump (Medex, Inc, Carlsbad, California). After 40 mL topotecan infusion, the infusion was stopped and the catheters were removed at the bedside. Blood samples for routine studies and high-performance liquid chromatography analysis of active and inactive forms of topotecan15 were drawn at baseline, the end of the infusion, and 1, 2, and 4 weeks later.
Safety Assessment
Before treatment, patients underwent baseline physical and neurological examinations and neuropsychological assessment. Vital signs and neurologic status were monitored throughout the infusions. Patients returned as outpatients for follow-up evaluation every 4 weeks for 16 weeks and then every 8 weeks thereafter unless there were clinical or radiographic indications for more frequent monitoring. At those visits, patients underwent physical and neurological examinations, neuropsychological evaluations, and MRI with gadolinium to determine tumor response.
Neurocognitive status and QoL were evaluated serially with the Cognitive Stability Index (HeadMinder, Inc, New York, New York),16 which assesses attention, processing speed, visual memory, and reaction time, as well as the Short Form-36 questionnaire version 2.0,17 which measures overall physical and mental QoL. Summary scores were obtained as a baseline assessment before initiation of treatment on protocol and then after treatment at monthly intervals (for 4 months) and bimonthly thereafter.
Radiographic Assessment
Pretreatment MRI scans were obtained at Columbia University Medical Center an average of 4 days (range, 0–18 days) before topotecan infusion and at 4- and 8-week intervals after the infusion. All scans were performed with T2-weighted spin-echo, T2-weighted fluid-attenuated inversion recovery, and T1-weighted multiplanar scans before and after intravenous gadolinium contrast infusion. Over the course of the study, gadopentetate, gadodiamide, or gadobenate was used at 0.1 to 0.3 mmol/kg, depending on local availability, but the same agent and dose were used in serial scans on each subject at least for the first year after therapy.
Tumor volumes were calculated with a planometric segmented region of interest technique (GE Centricity PACS Version 2.1.1, Polygon Region of Interest Tool) with visual identification of the contrast enhancement margin (Robert L. DeLaPaz). Enhancement area was multiplied by image slice thickness to generate the volumes per slice, which were summed to generate total enhancement volume. Volumes of nonenhancing necrosis, cyst, or the surgical defect within the enhancing lesion area were subtracted from the total volume. Image slice thickness was 1, 1.5, or 2 mm in 13 cases and 5 mm in 3 cases. Fifteen pretreatment and all posttreatment lesion volumes were generated from gadolinium-enhanced T1-weighted MRI, and 1 pretreatment volume was calculated from contrast-enhanced CT with 1-mm slice thickness. All image sections were obtained contiguously with no intersection gap. Contrast enhancement signal intensity, above the minimum threshold for visual determination of the enhancing margin, was not used as a comparative criterion.
Tumor response was assessed by the change in contrast-enhancing volume of tumor on MRI, and each was classified as 1 of 3 response categories: Early response was defined as a decrease in contrast-enhancing volume of >50% through the first 3 to 6 months after therapy; progressive disease was defined as increasing contrast-enhancing volume (>25%) at ≥1 month after therapy until surgical resection or death; and pseudoprogression was defined as an increase in the contrast-enhancing volume of >50% followed by regression of enhancement and edema. To be considered an early response or pseudoprogression, the radiographic changes had to be sustained for at least 4 weeks with patients on a stable or decreasing dose of steroids. No patients received other antitumor therapy during the period of assessment of pseudoprogression.
RESULTS
Patient Characteristics
Sixteen patients with a median age of 50 years were enrolled. Ten patients had glioblastoma multiforme, and the other 6 had World Health Organization grade III glial tumors with an average enhancing volume of 16.1 cm3. Detailed demographic, tumor, and prior treatment data are presented in Table 1. One patient (patient 2) did not undergo surgical resection of the primary lesion owing to involvement of eloquent areas but instead underwent stereotactic biopsy. Because most patients were treated early in the study period before temozolomide became standard of care, 5 patients did not receive temozolomide during radiotherapy, 7 patients did not receive temozolomide after radiation, and 4 patients received no temozolomide at either point. Other chemotherapy regimens were given, including 1,3-bis (2-chloroethyl)-1-nitrosourea, thiotepa, and etoposide; bis-chloroethylnitrosourea alone; imatinib mesylate and hydroxyurea; and bevacizumab and irinotecan. The median and mean times from initial diagnosis to treatment with topotecan were 14.5 and 25.6 months, respectively (range, 4–100 months).
TABLE 1.
Patient Demographicsa
| Patient | Age, y/Sex | Pathology | Tumor Site | Tumor Volume, cm3 | Time From Diagnosis to Treatment, mo |
Previous Therapy |
|---|---|---|---|---|---|---|
| 1 | 54/M | AE | L P | 50.6 | 28 | Surg/RT/Chemo |
| 2 | 64/M | GBM | L F/P | 11.3 | 5 | RT/Chemo |
| 4 | 35/F | AE | L F/P | 17.8 | 100 | Surg/RT/Chemo |
| 5 | 50/M | AO | L F | 17.2 | 52 | Surg/RT |
| 6 | 67/M | AA | L P | 20.8 | 22 | Surg/RT |
| 7 | 47/F | GBM | L T | 20.5 | 59 | Surg/RT/Chemo |
| 8 | 46/M | GBM | R F | 30.8 | 4 | Surg/RT/Chemo |
| 9 | 59/F | GBM | L P | 7.6 | 5 | Surg/RT/Chemo |
| 10 | 48/M | GBM | R P/O | 21.6 | 10 | Surg/RT/Chemo |
| 11 | 46/M | AO | L F | 2.6 | 47 | Surg/RT |
| 12 | 50/M | GBM | L P | 6.5 | 26 | Surg/RT/Chemo |
| 13 | 66/M | GBM | R F | 11.7 | 11 | Surg/RT/Chemo |
| 14 | 71/F | AA | R F | 4.5 | 10.5 | Surg/RT/Chemo |
| 16 | 28/M | GBM | L F | 4.4 | 5.5 | Surg/RT/Chemo |
| 17 | 22/F | GBM | L P | 2.8 | 7 | Surg/RT/Chemo |
| 18 | 66/M | GBM | R P | 17.6 | 18 | Surg/RT/Chemo |
AA, anaplastic astrocytoma; AE, anaplastic ependymoma; AO, anaplastic oligodendroglioma; Chemo, chemotherapy; F, frontal; GBM, glioblastoma multiforme; O, occipital; P, parietal; RT, radiation therapy; Surg, surgery.
Toxicities and Pharmacokinetic Studies
The serum concentrations of active and inactive forms of topotecan were undetectable (<0.5 ng/mL) at all time points in all patients. At 1 week after infusion, 1 patient at level II had grade 1 leukopenia and 1 patient at level V had grade 2 thrombocytopenia, both of which resolved by week 2 with no adverse sequelae.
The first patient (patient 14) treated at the highest dose (level V, 0.133 mg/mL topotecan) experienced a DLT, manifested by a transient right parietal syndrome with dysmetria and hemineglect (Table 2). After delivery of 3.53 mg of the planned 5.32 mg of topotecan (66%), treatment was terminated and the symptoms improved, although some permanent deficits remained. The fourth patient (patient 18) treated at level V experienced a DLT consisting of left upper-extremity weakness. Although this resolved spontaneously, level IV (0.1 mg/mL) was determined to be the MTD, and recruitment was terminated. Other serious adverse events in the level V group, not attributable to the study treatment, included a deep venous thrombosis in the patient with the parietal syndrome and a seizure in another patient with preexisting seizures. No patients had hepatic, renal, or metabolic toxicities. See Table 3 for all identified toxicities.
TABLE 2.
Toxicity Results
| Patient | Treatment Level |
Pretreatment/ Posttreatment Karnofsky Performance Score |
Dose-Limiting Toxicity |
|---|---|---|---|
| 1 | I | 70/70 | |
| 2 | I | 70/60 | |
| 4 | I | 90/90 | |
| 5 | II | 80/80 | |
| 6 | II | 90/80 | |
| 7 | II | 90/90 | |
| 8 | III | 90/100 | |
| 9 | III | 80/90 | |
| 10 | III | 100/100 | |
| 11 | IV | 100/100 | |
| 12 | IV | 100/90 | |
| 13 | IV | 80/80 | |
| 14 | V | 60/50 | Parietal syndrome |
| 16 | V | 80/80 | |
| 17 | V | 80/80 | |
| 18 | V | 80/80 | Upper extremity weakness |
TABLE 3.
Toxicitiesa
| Toxicity | Patients Experiencing, n (%) |
Patients With Grade 3 or 4 Toxicity, n (%) |
|---|---|---|
| Seizure | 5 (31) | 1 (6) |
| Headache | 5 (31) | |
| Fatigue | 5 (31) | |
| Worsened hemiparesis | 5 (31) | |
| Gastrointestinal symptoms | 4 (25) | 2 (13) |
| Extremity/back pain | 4 (25) | |
| Deep vein thrombosis (with/ without pulmonary embolism) |
3 (19) | 3 (19) |
| Pneumonia | 3 (19) | 2 (13) |
| Word-finding difficulty | 3 (19) | |
| Memory impairment/confusion | 3 (19) | |
| Urinary symptoms | 3 (19) | |
| Right-hand dyscoordination | 2 (13) | |
| Poor wound healing | 2 (13) | |
| Thrombocytopenia/leukopenia | 2 (13) | |
| Dizziness | 2 (13) | |
| Anxiety/depression | 2 (13) | |
| Intracerebral hemorrhageb | 1 (6) | 1 (6) |
| Dry gangrene | 1 (6) | 1 (6) |
| Upper-extremity weakness | 1 (6) | 1 (6) |
| Right parietal syndrome | 1 (6) | 1 (6) |
| Tremor | 1 (6) | |
| Otherc | 3 (19) |
All symptoms experienced during the study period, whether attributable to the study treatment or not, are tabulated.
Intratumoral intracerebral hemorrhage occurred over a month after treatment and was not considered attributable to the study treatment.
Each of 3 patients experienced one of the following nonneurologic symptoms unrelated to the study treatment: rash, thrush, and sinusitis.
One patient (patient 1) with a recurrent parietal malignant ependymoma previously treated with high-dose chemotherapy and bone marrow transplant was admitted 7 weeks after treatment with worsening of his underlying seizure disorder, marked hyperglycemia, and fever. The patient died 4 days later after cardiopulmonary arrest. An autopsy revealed acute necrotizing pneumonitis with Aspergillosis, and cause of death was presumed metabolic and infectious complications caused by underlying disease, most likely not his topotecan treatment.
Response to Therapy
Radiographic responses followed 3 patterns: early response, progressive disease, or pseudoprogression. Early response occurred in 4 patients (25%). Of these, 1 patient showed decreased volume and died at 2 months; 2 showed decreased volume with later progression, 1 at 3 to 6 months and another at 1 year; and 1 showed stable disease with slight increase in volume (<25%) at 2 months, followed by decreased volume at 3 months and beyond (Figure 1). Progressive disease occurred in 5 patients (31%; Figure 2). Pseudoprogression occurred in 7 patients (44%). Among these, 5 patients exhibited progression and then regression within the first 3 months, followed by progression at 3 to 10 months. Two patients showed early regression to 1 and 6 months and progression to 6 and 8 months, respectively, followed by regression to 10 months (Table 2 and Figure 3). Thus, the early response rate was 25% and the late response rate (pseudoprogressors) was 44%. The total response rate to this single topotecan infusion was 69%.
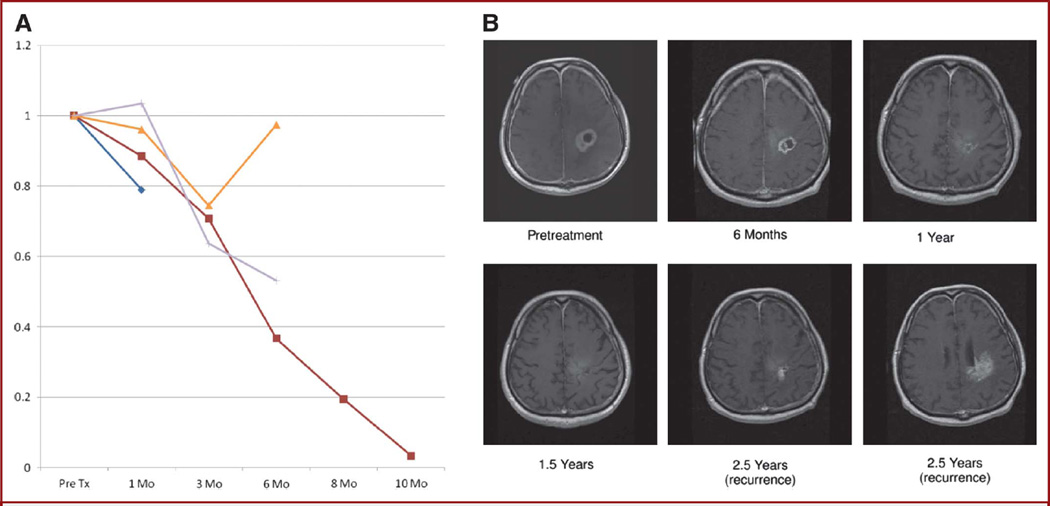
FIGURE 1.
Early response. A, each of the 4 early responding patients’ contrast-enhancing volume plotted over time. Early response refers to a decrease in contrast-enhancing volume through at least the first 3 months of therapy. B, serial contrast-enhanced T1-weighted MRI scans from the patient with the greatest response to 10 months in A showing complete resolution of contrast enhancement at 1.5 years (indistinct high signal was present on noncontrast scans) with tumor recurrence predominantly at the inferior treatment (Tx) margin at 2.5 years.
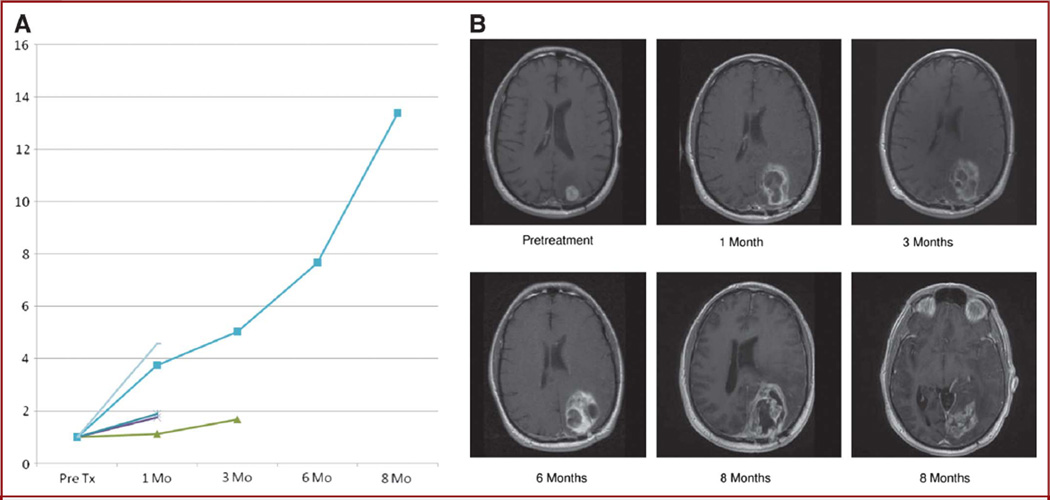
FIGURE 2.
Progressive disease. A, contrast-enhancing volume of each of the 5 patients with progressive disease after therapy plotted over time. Progressive disease refers to increasing contrast-enhancing volume at ≥1 month after therapy until surgical resection or death. B, serial contrast-enhanced T1-weighted MRI scans from the patient with the greatest progression at 8 months in A showing a rapid increase in contrast enhancement and necrosis at the treatment site and inferior to the treatment (Tx) margin (surgical resection followed the last scan).
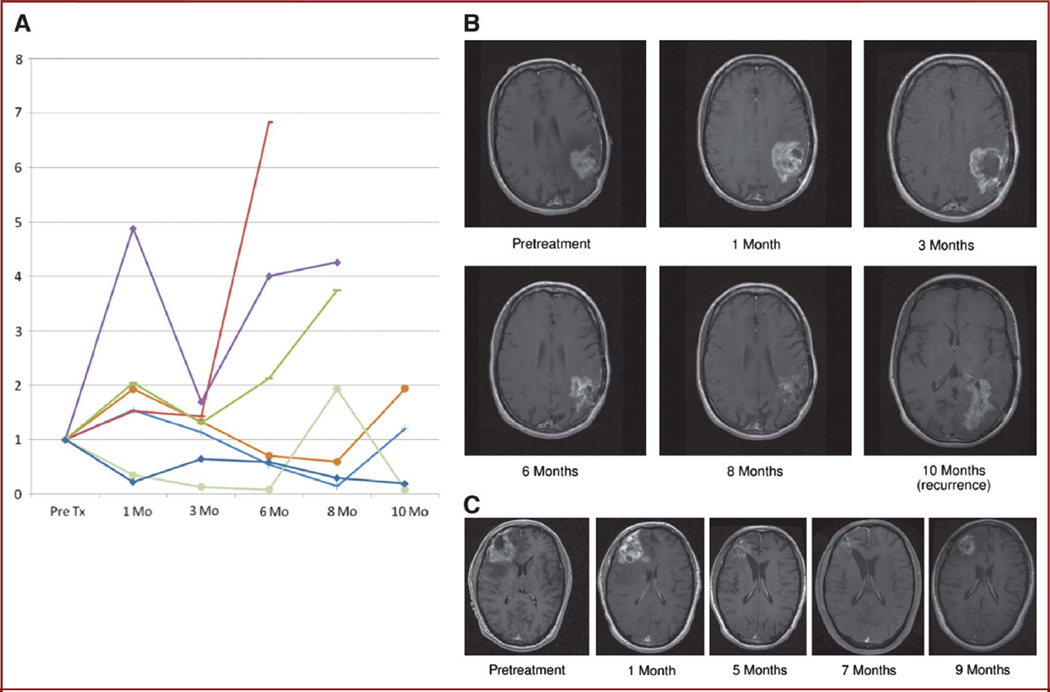
FIGURE 3.
Pseudoprogression. A, each of the 7 pseudoprogressors’ contrast-enhancing volume plotted over time. Pseudoprogression refers to an increase in the contrast-enhancing volume after therapy, followed by regression of enhancement and edema, especially within the first 3 months after therapy. B, serial contrast-enhanced T1-weighted MRI scans from a patient with pseudopregression at 1 to 3 months and response at 6 to 8 months without further therapy until surgical resection of tumor recurrence at the inferior treatment (Tx) margin at 10 months. C, serial contrast-enhanced T1-weighted MRI scans from a patient with pseudoprogression at 1 month and response at 5 to 7 months without further therapy until surgical resection of tumor recurrence at 9 months.
Of the 16 patients studied, 4 remain alive at 310, 210, 132, and 105 weeks after treatment (Table 4). Survival ranged from 13 to >310 weeks with a median overall survival of 60 weeks. Progression-free survival ranged from 4 to 132 weeks with median progression-free survival of 23 weeks, including 2 patients who remain free of progression at 105 and 132 weeks. The 6-month progression-free survival and 6-month overall survival were 44% and 75%, respectively. Six-month progression-free survival in the group of patients with glioblastoma multiforme was 55%. Patient 1, because of his unrelated death not long after his treatment, was not evaluable for treatment response and was censored from future analyses.
TABLE 4.
Radiographic Resultsa
| Patient | Treatment Level |
Age, y/Sex | Pathologyb | Time to Progression, wk |
Responseb | Survival After Treatment, wk |
Time to MRI Progression, wk |
|---|---|---|---|---|---|---|---|
| 1 | I | 54/M | AE | c | ER | 8 | N/A |
| 2 | I | 64/M | GBM | 123 | ER | 152 | 128 |
| 4 | I | 35/F | AE | 18 | PP | 310d | 21 |
| 5 | II | 50/M | AO | 8 | PD | 13 | 4 |
| 6 | II | 67/M | AA | 8 | PD | 45 | 23 |
| 7 | II | 47/F | GBM | 4 | PP | 80 | 6 |
| 8 | III | 46/M | GBM | 39 | PP | 76 | N/A |
| 9 | III | 59/F | GBM | 27 | PD | 41 | 7 |
| 10 | III | 48/M | GBM | 29 | PP | 45 | 10 |
| 11 | IV | 46/M | AO | 74 | PP | 210d | 10 |
| 12 | IV | 50/M | GBM | 21 | PD | 60 | 5 |
| 13 | IV | 66/M | GBM | 15 | ER | 17 | N/A |
| 14 | V | 71/F | AA | 132d | PP | 132d | N/A |
| 16 | V | 28/M | GBM | 23 | PP | 57 | 6 |
| 17 | V | 22/F | GBM | 105d | ER | 105d | N/A |
| 18 | V | 66/M | GBM | 7 | PD | 25 | 6 |
AA, anaplastic astrocytoma; AE, anaplastic ependymoma; AO, anaplastic oligodendroglioma; GBM, glioblastoma multiforme; ER, early response; PD, progressive disease; PP, pseudoprogression.
Age, diagnosis, radiographic response, and survival data.
This patient died 8 weeks after treatment of disease complications unrelated to treatment but before progression.
Progression or death has not yet occurred in these patients.
QoL and Neurocognitive Functioning
Except for the 2 treatment level V patients with DLT, all patients remained ambulatory with no evidence of systemic toxicity. All QoL and neurocognitive measurements verified the safety and tolerability of the treatment. Average Karnofsky Performance Score before treatment was 84 and after treatment was 83. No significant change over time was found in the Cognitive Stability Index or the Physical or Mental Quality of Life, nor was a dose-dependent effect appreciated.
DISCUSSION
By demonstrating that topotecan delivered by CED can cause substantial tumor regression without significant toxicities in selected patients with recurrent malignant gliomas refractory to conventional therapy, we validate the concept that gliomas can be successfully treated with chemotherapy when delivery barriers are overcome. Supplementary studies examining QoL and neurocognitive functioning showed no significant treatment effects. The absence of detectable peripheral drug levels and the lack of significant bone marrow suppression demonstrate the advantage of topotecan by CED compared with intravenous delivery. Radiographic imaging documented standard regression of histologically verified tumors in 4 patients (25%) and late regression in the “pseudoprogression” group in 7 patients (44%), whereas only 5 patients (31%) showed no response. Additionally, 7 of 15 patients had >6 months of progression-free survival, including several patients with long-term sustained response. Toxicity was not seen at topotecan concentrations ≤0.10 mg/mL, which established the MTD. Neurologic DLTs occurred in 2 patients at the highest administered dose (level V, 0.133 mg/mL), including a parietal lobe syndrome and left upper-extremity weakness. Outcome data are difficult to interpret in phase I trials with selected populations; however, the median progression-free survival of 23 weeks and the median overall survival of 60 weeks compare favorably with historical control groups with recurrent malignant gliomas.10,18
An increasing volume of contrast enhancement after therapy is conventionally interpreted as evidence of tumor progression and treatment failure. However, a phenomenon called pseudoprogression was recognized in recent studies after observed transient increases in contrast enhancement and edema on MRI within the first 3 months after systemic temozolomide chemotherapy with radiation therapy, followed by radiographic stabilization or regression of disease.19–21 Although the mechanism of pseudoprogression is not clear, it has been suggested that the high-degree necrosis and apoptosis of tumor and endothelial cells caused by the treatment lead to edema, inflammation, and increased vascular permeability, which could mimic the edema and contrast enhancement associated with tumor progression.22 A comparable phenomenon associated with high degree of cell death may explain the observations in the present study.
A method of regional drug delivery, CED was pioneered by Bobo et al2 with demonstrated safety in several clinical trials.23–31 Bulk flow provides a relatively uniform distribution of drug within the treatment volume with a steep drop in drug concentrations outside the volume of distribution, beyond which further distribution ultimately occurs by diffusion. Our preclinical studies with CED of topotecan in rat glioma models found that topotecan perfused the entire cerebral hemisphere, providing therapeutic drug concentrations to tumor cells beyond the demarcations of visible tumor that are responsible for recurrence in most gliomas.5 For locally invasive tumors like glioblastomas, this treatment provides elevated concentrations of drug in the peritumoral region with undetectable serum drug concentrations, thereby avoiding systemic toxicity such as myelosuppression. Our previous studies showed that the BBB to natural-product drugs like topotecan was still partially intact in gliomas but not intact in metastatic brain lesions32; therefore, although this partially functioning BBB could impede the transit of systemically delivered topotecan into brain tumors, it is no longer an impediment with CED.
Given the benefits of CED over systemic delivery methods, many chemotherapeutic drugs are likely to be effective because much higher concentrations in the tumor and surrounding brain can be achieved. Previous clinical trials of CED with other agents showed only limited success, possibly because these trials mostly involved the use of toxin conjugates, which rely on highly specific binding to a single receptor on tumor cells.23,24,27,28,30 Such a target may be too specific for gliomas, which are known for their heterogeneity. A study with a more conventional chemotherapy drug, paclitaxel, was limited by excessive toxicity, possibly because its targets, microtubules, are necessary in the brain for transport of nutrients in all cell types of the brain.9 However, by targeting proliferative processes such as DNA repair, synthesis, and cell proliferation, topotecan provides a more clinically specific and effective mechanism of action. In our preclinical laboratory studies, topotecan was the most effective drug with a reasonable safety profile.5
Limitations of our study include the need to exclude patients with tumors >65 cm3 or whose tumors were adjacent to geometrically complex resection cavities. Other drawbacks include the need for a surgical procedure to implant catheters and infectious risks that limit the ability to permanently implant an external catheter or facilitate >1 treatment.
CONCLUSION
This phase Ib study establishes the MTD of topotecan by CED maintained with stable QoL and neurocognitive function. Results are encouraging; tumor regression was demonstrated even at drug concentrations lower than the MTD. The response rate of early and late responders (pseudoprogression) totaled 69%, which is significant for a group of relapsed patients who have failed previous treatments. The potential for use of this therapy as an effective treatment option for glial neoplasms will be tested in subsequent phase II and III trials.
Acknowledgments
We thank Venkat Seshan for assistance with statistical analysis; Michael Sisti, MD, and Guy McKhann, MD, for clinical support; May Huang for high-performance liquid chromatography analysis, and The Clinical Research Management Office of the Herbert Irving Comprehensive Cancer Center.
This study was supported by NIH grant 5RO1CA89395.
ABBREVIATIONS
- BBB
blood-brain barrier
- CED
convection-enhanced delivery
- DLT
dose-limiting toxicity
- MTD
maximum tolerated dose
- QoL
quality of life
Footnotes
Disclosures
The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
Informed consent was obtained from each patient in this study, and the protocols used were approved by the Institutional Review Board of Columbia University Medical Center. This study reports an unlabeled use of Topotecan.
REFERENCES
- 1.Barker FG, II, Chang SM, Gutin PH, et al. Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery. 1998;42(4):709–720. doi: 10.1097/00006123-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91(6):2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broaddus WC, Prabhu SS, Gillies GT, et al. Distribution and stability of antisense phosphorothioate oligonucleotides in rodent brain following direct intraparenchymal controlled-rate infusion. J Neurosurg. 1998;88(4):734–742. doi: 10.3171/jns.1998.88.4.0734. [DOI] [PubMed] [Google Scholar]
- 4.Groothuis DR, Benalcazar H, Allen CV, et al. Comparison of cytosine arabinoside delivery to rat brain by intravenous, intrathecal, intraventricular and intraparenchymal routes of administration. Brain Res. 2000;856(1–2):281–290. doi: 10.1016/s0006-8993(99)02089-2. [DOI] [PubMed] [Google Scholar]
- 5.Kaiser MG, Parsa AT, Fine RL, Hall JS, Chakrabarti I, Bruce JN. Tissue distribution and antitumor activity of topotecan delivered by intracerebral clysis in a rat glioma model. Neurosurgery. 2000;47(6):1391–1398. [PubMed] [Google Scholar]
- 6.Morrison PF, Chen MY, Chadwick RS, Lonser RR, Oldfield EH. Focal delivery during direct infusion to brain: role of flow rate, catheter diameter, and tissue mechanics. Am J Physiol. 1999;277(4 pt 2):R1218–R1229. doi: 10.1152/ajpregu.1999.277.4.R1218. [DOI] [PubMed] [Google Scholar]
- 7.Bidros DS, Liu JK, Vogelbaum MA. Future of convection-enhanced delivery in the treatment of brain tumors. Future Oncol. 2010;6(1):117–125. doi: 10.2217/fon.09.135. [DOI] [PubMed] [Google Scholar]
- 8.Rainov NG, Gorbatyuk K, Heidecke V. Clinical trials with intracerebral convection- enhanced delivery of targeted toxins in malignant glioma. Rev Recent Clin Trials. 2008;3(1):2–9. doi: 10.2174/157488708783330521. [DOI] [PubMed] [Google Scholar]
- 9.Lidar Z, Mardor Y, Jonas T, et al. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg. 2004;100(3):472–479. doi: 10.3171/jns.2004.100.3.0472. [DOI] [PubMed] [Google Scholar]
- 10.Kunwar S, Chang S, Westphal M, et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12(8):871–881. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruce JN, Falavigna A, Johnson JP, et al. Intracerebral clysis in a rat glioma model. Neurosurgery. 2000;46(3):683–691. doi: 10.1097/00006123-200003000-00031. [DOI] [PubMed] [Google Scholar]
- 12.Burris HA., III Topotecan: incorporating It Into the treatment of solid tumors. Oncologist. 1998;3(1):1–3. [PubMed] [Google Scholar]
- 13.Matsumoto Y, Fujiwara T, Honjo Y, Sasaoka N, Tsuchida T, Nagao S. Quantitative analysis of DNA topoisomerase I activity in human and rat glioma: characterization and mechanism of resistance to antitopoisomerase chemical, camptothecin-11. J Surg Oncol. 1993;53(2):97–103. doi: 10.1002/jso.2930530210. [DOI] [PubMed] [Google Scholar]
- 14.Friedman HS, Kerby T, Fields S, et al. Topotecan treatment of adults with primary malignant glioma: the Brain Tumor Center at Duke. Cancer. 1999;85(5):1160–1165. [PubMed] [Google Scholar]
- 15.Rosing H, van Zomeren DM, Doyle E, et al. Quantification of topotecan and its metabolite N-desmethyltopotecan in human plasma, urine and faeces by high-performance liquid chromatographic methods. J Chromatogr B Biomed Sci Appl. 1999;727(1–2):191–203. doi: 10.1016/s0378-4347(99)00078-x. [DOI] [PubMed] [Google Scholar]
- 16.Erlanger DM, Kaushik T, Broshek D, Freeman J, Feldman D, Festa J. Development and validation of a Web-based screening tool for monitoring cognitive status. J Head Trauma Rehabil. 2002;17(5):458–476. doi: 10.1097/00001199-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36), I: conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 18.Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5(2):79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 20.de Wit MC, de Bruin HG, Eijkenboom W, Sillevis Smitt PA, van den Bent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63(3):535–537. doi: 10.1212/01.wnl.0000133398.11870.9a. [DOI] [PubMed] [Google Scholar]
- 21.McGirt MJ, Bulsara KR, Cummings TJ, et al. Prognostic value of magnetic resonance imaging-guided stereotactic biopsy in the evaluation of recurrent malignant astrocytoma compared with a lesion due to radiation effect. J Neurosurg. 2003;98(1):14–20. doi: 10.3171/jns.2003.98.1.0014. [DOI] [PubMed] [Google Scholar]
- 22.Roldan GB, Scott JN, McIntyre JB, et al. Population-based study of pseudoprogression after chemoradiotherapy in GBM. Can J Neurol Sci. 2009;36(5):617–622. doi: 10.1017/s0317167100008131. [DOI] [PubMed] [Google Scholar]
- 23.Kunwar S. Convection enhanced delivery of IL13-PE38QQR for treatment of recurrent malignant glioma: presentation of interim findings from ongoing phase 1 studies. Acta Neurochir Suppl. 2003;88:105–111. doi: 10.1007/978-3-7091-6090-9_16. [DOI] [PubMed] [Google Scholar]
- 24.Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3(12):1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 25.Patel SJ, Shapiro WR, Laske DW, et al. Safety and feasibility of convection-enhanced delivery of Cotara for the treatment of malignant glioma: initial experience in 51 patients. Neurosurgery. 2005;56(6):1243–1252. doi: 10.1227/01.neu.0000159649.71890.30. [DOI] [PubMed] [Google Scholar]
- 26.Popperl G, Goldbrunner R, Gildehaus FJ, et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET for monitoring the effects of convection-enhanced delivery of paclitaxel in patients with recurrent glioblastoma. Eur J Nucl Med Mol Imaging. 2005;32(9):1018–1025. doi: 10.1007/s00259-005-1819-7. [DOI] [PubMed] [Google Scholar]
- 27.Rand RW, Kreitman RJ, Patronas N, Varricchio F, Pastan I, Puri RK. Intratumoral administration of recombinant circularly permuted interleukin-4-Pseudomonas exotoxin in patients with high-grade glioma. Clin Cancer Res. 2000;6(6):2157–2165. [PubMed] [Google Scholar]
- 28.Sampson JH, Akabani G, Archer GE, et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol. 2003;65(1):27–35. doi: 10.1023/a:1026290315809. [DOI] [PubMed] [Google Scholar]
- 29.Voges J, Reszka R, Gossmann A, et al. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol. 2003;54(4):479–487. doi: 10.1002/ana.10688. [DOI] [PubMed] [Google Scholar]
- 30.Weber FW, Floeth F, Asher A, et al. Local convection enhanced delivery of IL4-Pseudomonas exotoxin (NBI-3001) for treatment of patients with recurrent malignant glioma. Acta Neurochir Suppl. 2003;88:93–103. doi: 10.1007/978-3-7091-6090-9_15. [DOI] [PubMed] [Google Scholar]
- 31.Yang W, Barth RF, Wu G, et al. Boronated epidermal growth factor as a delivery agent for neutron capture therapy of EGF receptor positive gliomas. Appl Radiat Isot. 2004;61(5):981–985. doi: 10.1016/j.apradiso.2004.05.071. [DOI] [PubMed] [Google Scholar]
- 32.Gerstner ER, Fine RL. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm. J Clin Oncol. 2007;25(16):2306–2312. doi: 10.1200/JCO.2006.10.0677. [DOI] [PubMed] [Google Scholar]