Summary
Alzheimer's disease (AD) is the most common form of dementia worldwide. It is characterized by an imbalance between the production and clearance of amyloid β (Aβ) and tau proteins. In AD these normal proteins accumulate, leading to aggregation and a conformational change forming oligomeric and fibrillary species with a high β-sheet content. Active and passive immunotherapeutic approaches result in dramatic reduction of Aβ pathology in AD animal models. However, there is much more limited evidence in human studies of significant clinical benefits from these strategies and it is becoming apparent that they may only be effective very early in AD. Vaccination targeting only tau pathology has shown benefits in some mouse studies but human studies are limited. Greater therapeutic efficacy for the next generation of vaccine approaches will likely benefit from specifically targeting the most toxic species of Aβ and tau, ideally simultaneously.
Keywords: Amyloid-β, tau, immunotherapy, neurodegenerative disease, prion disease, oligomers, immunomodulation
Overview of the disease
Alzheimer's disease (AD) and related dementias currently affects more than 44 million people worldwide. If new therapeutics are not developed the number of people with AD and the associated costs are expected to escalate rapidly. Currently, the direct health-care costs in the USA alone is estimated to be $226 billion and is projected to reach $1.1 trillion in 2050[1]. The current global cost of care for AD is approximately $605 billion, or about 1% of the entire world's gross domestic product. AD is a neurodegenerative disease defined in the brain by pathological accumulation of amyloid β (Aβ) into extracellular plaques in the brain parenchyma and in the vasculature (known as congophilic amyloid angiopathy [CAA]), and abnormally phosphorylated tau that accumulates intraneuronally forming neurofibrillary tangles (NFTs) [2]. Pathological aggregation of Aβ and phosphorylated tau occurs in a sequential process; small numbers of monomers first aggregate into oligomers intraneuronally, which then continue to aggregate into the fibrils observed in amyloid plaques and NFTs. Both Aβ and tau oligomers have similar but not identical structural and biophysical properties including a high β-sheet content, some resistance to proteolytic degradation and neuronal toxicity. It is suggested that oligomers are the most neurotoxic species in AD as levels of these species correlate much better with cognitive symptoms than presence of plaques or NFTs. Recent studies have shown that oligomers of both Aβ and tau can be transmitted between neurons via “prion-like” mechanisms and can seed further pathology in the brain, and therefore could be responsible for the characteristic spread of AD pathology though specific brain regions [3,4]. Furthermore, there is also limited evidence to suggest that Aβ pathology can be transmitted from person to person[5].
AD is characterized as either early-onset (EOAD; <5% of all AD patients, with onset at <65yrs) or sporadic late-onset (LOAD; onset >65yrs). Autosomal dominant mutations in presenilin 1, presenilin 2 (PS1 and PS2) or the amyloid precursor protein (APP) account for only ~10% of all EOAD cases (~1% of all AD cases), leaving the cause of the majority of EOAD unexplained [6,7]. LOAD afflicts >95% of patients with AD and is related to both genetic and environmental factors [6,8-10]. Some of the known environmental risk factors for LOAD include level of physical activity, educational status, diabetes mellitus, hypertension and head injury [11,12]. Genome-wide association studies (GWAS) have identified over 20 loci that confer increased risk for LOAD, including genes involved in innate immunity, cholesterol metabolism and synaptic/neuronal membrane function [6,13]. The strongest identified genetic risk factor for LOAD is the inheritance of the apolipoprotein (apo) E4 allele, the protein product of which influences the aggregation and clearance of brain Aβ [14,15]. Rare variants of another gene that encodes the triggering receptor expressed on myeloid cells 2 (TREM2) have been reported as a significant risk factor for LOAD, with an odds ratio similar to apoE4 [16,17]. Additionally, impaired clearance of Aβ from the brain as a consequence of aging and/or inflow of serum Aβ into the CNS is thought to be an important factor in the development of LOAD [18]. Age associated degradation of the newly discovered brain glymphatic system has been recognized as a possible driver of Aβ accumulation in sporadic AD [18,19].
The direct link between APP, PS1 and PS2 genetic mutations and EOAD and evidence that triplication of APP gene in Down syndrome results in AD neuropathology resulted in the “amyloid hypothesis” of Alzheimer's disease [20,21]. The central idea proposed in this hypothesis is that Aβ aggregation, especially in its toxic oligomeric form, is the principal insult which produces neuronal toxicity and triggers downstream signaling events, which in turn lead to hyperphosphorylation of tau and development of NFTs. The amyloid hypothesis has been the subject of much debate over the years. The main criticisms of amyloid hypothesis are that people can have a high brain amyloid burden without any evidence of cognitive impairment, removal of brain Aβ doesn't prevent the progression of AD and there is a poor correlation of the anatomical distribution of plaques with neuronal loss and cognitive impairment[22]. However, supporters of the amyloid hypothesis suggest that cognitively normal individuals with a high amyloid burden have preclinical AD and that Aβ reducing therapies can prevent the progression of AD if given early enough in the disease[23]. Regardless, it is evident that despite the complexities of the role of Aβ in AD pathogenesis, it is still a central feature of AD neuropathology, therefore it is logical that therapeutic interventions designed to decrease Aβ followed. The most attractive approach to directly target Aβ was via immunotherapy. A similar immunotherapeutic approach has also been recently extended to targeting tau. In this manuscript, we will review both active and passive immunotherapeutic approaches along with preclinical and clinical data that has been used to target Aβ and pathological tau.
Active Vaccination Targeting Aβ in Humans
Preclinical studies showed that anti-Aβ targeting antibodies were able to prevent and disrupt Aβ fibrillization in vitro [24] and greatly reduced plaque burden and protected against cognitive deficits in vivo in transgenic mouse models of AD [25-31]. Further, immunohistochemistry also revealed that anti-Aβ antibodies generated in mice can label amyloid plaques on human AD brain sections, raising the possibility of such immune intervention being successful in humans. Importantly, these pilot preclinical trials revealed no evidence of toxicity in the immunized mice. These impressive results in preclinical studies prompted Elan/Wyeth's group to launch the first active immunization therapeutic approach for AD in a randomized, multiple-dose, dose-escalation, double-blind Phase I clinical trial (see Table 1). This trial, started in in April 2000 used the AN1792 vaccine, which was comprised of pre-aggregated Aβ1-42 and QS21 as an adjuvant. The vaccine was designed to generate a strong cell mediated immune response. In the initial Phase I trial, 80 people with mild to moderate AD were treated with AN1792 [32]. Multiple doses were tested and it was demonstrated that 56.9% of patients could mount an anti-Aβ humoral response. In the later segment of the phase I trial, polysorbate 80, which acts as an emulsifier, was added to increase the solubility of Aβ1-42. The increased emulsifier concentration caused a greater shift from a Th2 humoral response to a proinflammatory Th1 response [33]. A follow up phase IIa trial was conducted in October 2001 that involved 372 patients. This trial was terminated in January 2002 when 6% of immunized patients developed symptoms of aseptic meningoencephalitis [34,35], however follow-up assessment of treated patients continued. It was found that only 19.7% of the phase II patients were classed as responders, a rate much lower than in the Phase I trial, likely due to the fact that no patient received more than 3 immunizations, in comparison to the maximum 8 doses patients received in the Phase I trial. Post-mortem examination of patients who received AN1792 revealed a dramatic clearance of plaques in the brain parenchyma, thus validating the efficacy of this approach for amyloid fibril clearance in humans [35-40]. It was also shown that individual patients who had a comparatively high anti-Aβ titer had more reduced brain amyloid pathology at autopsy than those with a low anti-Aβ titer [37,38]. Remaining plaques had a “moth-eaten” appearance or appeared to have a “naked” dense core and were surrounded by microglia that were immunoreactive for Aβ, suggesting that microglia phagocytosis could be the mechanism of Aβ clearance. Important limitations of this approach was that treatment with AN1792 didn't clear NFTs, alter brain levels of Aβ oligomers or clear CAA [38-40]. A T-cell reaction was observed around some leptomeningeal vessels, suggesting that there was possibly an overstimulated immune response to the vaccine [35,41]. Neuroimaging revealed white matter lesions with or without evidence of brain edema, termed amyloid-related imaging abnormalities (ARIA). Most importantly, despite the clearance of amyloid pathology, treatment did not result in significantly improved cognitive function [42,43].
Table 1.
Active and passive immunization trials to treat AD (www.clinicaltrials.gov)
| ACTIVE | ||||
|---|---|---|---|---|
| Pharmaceutical | Trial | Stage | Status | Pathology being targeted |
| Aβ Target | ||||
| ELAN | AN1792 | Phase II | (2000-2002) halted, no clinical improvement, encephalitis in 6% of subjects | Aggregated Aβ1-42, QS21, Polysorbate80 |
| Novartis | CAD106 | Phase I Phase II |
Aβ titers, no change biomarkers Not reported |
Aβ1-6/Bacteriophage Qβ |
| Janssen/Pfizer | ACC-001 | Phase II | Not reported, finishing | Aβ1-6-QS21 |
| Affinis AG/GSK | AFFITOME AD02 | Phase II | Not reported, on going | Mimotope of unmodified Aβ N-terminus |
| Affinis AG | AFFITOME | Phase I | Ongoing | Mimotope of pyroglutamic-3 modified Aβ N-terminus |
| AC Immune | ACI-24 | Phase I/IIa | Not reported | β-sheet conformation of Aβ peptide |
| Tau Target | ||||
| Axon Neuroscience | AAD vac1 | Phase I | Ongoing continuation, tolerability, safety, antibody titers | Synthetic tau peptide residues 294-305 (involved in oligomerization of tau) coupled to KLH-ALUM |
| AC-Immune | ACI-35 | Phase I | Ongoing, tolerability, safety, antibody titers | Tau (pS396-pS404) in liposomes, with MPLA (TLR4 agonist) |
| PASSIVE | ||||
| Monoclonal antibody/Conformational state of Aβ target | ||||
| Janssen/Pfizer | Bapineuzumab AAB-001 | Phase II Phase III |
No clinical improvement, ARIA toxicity noted, trend to efficacy No improvement, halted |
Humanized 3D6, anti-Aβ1-5, binds all forms of Aβ (monomeric, oligomeric and fibrillar) |
| Eli Lilly | Solaneuzumab | Phase III(2) | No improvement overall, secondary analysis slight improvement in early AD; ongoing extension in early AD | Humanized mAb266 Anti-Aβ16-24, bindings monomeric Aβ |
| Janssen/Pfizer | Bapineuzumab AAB-003 | Phase I | Ongoing | Hu IgG4 3D6 to reduce ARIA, binds all forms of Aβ (monomeric, oligomeric and fibrillar) |
| Biogen | Aducanumab | Phase 1b | Concluded, Benefit in MMSE and CDR-SB at 3 and 10mg/kg doses, ARIA toxicity noted | Full human IgG1, to aggregated Aβ (fibrillar and oligomeric) |
| Biogen | Aducanumab | Phase III | Ongoing, ENGAGE and EMERGE trials will assess the efficacy and safety of aducanumab in approximately 2,700 people with early AD | Full human IgG1, to aggregated Aβ |
| Hoffman-La Roche | Gantenerumab DIAN |
Phase III | Ongoing, in autosomal dominant AD | Antibody to fibrillar form of Aβ Positions 3-12; 18-27 |
| Eli Lilly | Solaneuzumab A4 |
Phase III | Ongoing, asymptomatic AD with positive PET | Humanized mAb266 Anti-Aβ16-24, binds monomeric Aβ |
| AC Immune/Genentech | Crenezumab ABBY BLAZE API |
Phase II/III Phase II Phase II Phase III |
Failed to meet clinical co-primary endpoints No difference in biomarkers/ FDG-PET Ongoing in EOAD families with PS1 mut. E280A |
Hu IgG4 anti-multiple epitopes Aβ1-40, bindings all forms of Aβ (monomeric, oligomeric and fibrillar) |
Since this initial trial, five next generation active Aβ vaccination therapeutics have entered clinical trials (www.clinicaltrials.gov and see Table 1). Of these, two (ACC-001 from Janssen/Pfizer and Affitope AD02 from AFFiRiS AG/GlaxoSmithKline) were discontinued following Phase II trials. ACC-001 used the Aβ(1-6) fragment coupled to a carrier protein, and the surface-active saponin adjuvant QS-21 [44]. The shorter N-terminal fragment of Aβ was used in an attempt to avoid the safety complications associated with using full length Aβ1-42 in the AN1792 trial. ACC-001 was designed this way to include a minimal B-cell epitope from the Aβ amino terminus, while avoiding a T-cell mediated inflammatory response. The Phase II trial was halted for reasons that have not been reported.
Affitope AD02 consisted of synthetic antigenic peptides called mimotopes to target the unmodified Aβ N-terminus [45]. Similar to ACC-001, these fragments also included the B-cell epitope while lacking the most common T-cell epitope. In Phase I testing AD02 was given subcutaneously to patients with mild to moderate AD and it showed a favorable safety and tolerability profile at one year. Phase II testing consisted of a multicenter trial involving 332 patients with early AD. The limited data that was released from this trial suggested that AD02 did not reach either primary or secondary outcomes, and the follow-up study was discontinued in June 2015.
Three other active vacciniation therapeutics are currently in active clinical trials (CAD106 by GlaxoSmithKline/Novartis, ACI-24 by AC Immune and a secondary Affitome drug by Affiris AG). CAD106 was also designed to target only the B-cell epitope, using the small amino-terminal Aβ fragment (Aβ1-6), in this case along with an adjuvant carrier that was derived from multiple copies of the coat protein of bacteriophage Qβ [46,47]. It was specifically shown to produce a humoral Aβ specific response without activation of a T-cell response in a transgenic mouse model of AD [48]. Phase I testing concluded that patients with mild to moderate AD tolerated two doses (50μg or 150μg) of the treatment well and no cases of meningitis, meningoencephalitis or vasogenic edema were reported. Nine patients reported serious adverse reactions, but none were thought to be secondary to the immunogen. Phase IIa testing was then completed, which included 58 patients with mild AD who received 3 initial injections (either subcutaneous or intramuscular) of 150μg of CAD106 followed by 4 injections of 150μg of CAD106 in the open-label extension study[49]. Partial results have been reported that indicated antibody maturation and a favorable tolerability profile after 2.5 years [49,50]. A Phase II/III trial will begin in November 2015 administering CAD106 to 1,340 homozygous ApoE4 carriers as part of the Alzheimer Prevention Initiative study. All participants will be cognitively normal individuals who have a high risk of development of AD. Primary outcomes include time until diagnosis of MCI or AD and change in Alzheimer's Prevention Initiative composite cognitive score (www.clinicaltrials.gov).
AC Immune initiated Phase I/IIa trials in 2009 with their product, ACI-24, which works by generating a humoral immune response to Aβ1-15 in a primarily β-sheet conformation. The design is based on previous work by this group in an AD transgenic mouse model, where treatment with a tetra-palmitoylated Aβ(1-15) peptide that exists chiefly in a β-sheet conformation resulted in cognitive improvements that correlated with IgG anti-Aβ titers [51,52]. These initial trials are still ongoing and to date no results have been presented.
Finally, Affiris AG has also started another Phase I trial using the same technology as that used to develop AD02. This version specifically targets pyroglutamic-3 modified Aβ, a post-translational modified version of Aβ that renders it more prone to aggregation [53,54]. Pyroglutamic-3 modified Aβ is present in plaques and vascular amyloid deposits but is normally below the level of detection in CSF or plasma; however, it can be found in these biological fluids during therapeutic interventions where deposited Aβ has been mobilized [55].
Past Passive Immunization Approaches for AD
Multiple passive immunization strategies for AD have also been developed in the last 15 years. Passive immunization is generally considered a safer alternative to active vaccination and it allows very specific antigens or conformations to be directly targeted. Multiple transgenic mouse studies showed that passive immunization was a viable approach for the treatment of AD; treatment with anti-Aβ antibodies significantly reduced Aβ levels and resulted in cognitive benefits [56-58]. However, for passive immunization to be effective for AD there are various hurdles that need to be overcome: appropriate selection of antigen targets, the need for repeated injections in a chronic disease, blood–brain barrier penetration, potential to induce ARIA and the triggering of immune response to the antibodies that are injected. An additional consideration is that treatments with a monoclonal antibody are very expensive, typically in the range of >$150,000[59]. Multiple passive immunization approaches for AD have entered clinical trials and recent encouraging results from Biogen using their therapeutic aducanumab [60,61] have revitalized this field after earlier disappointing results using other therapeutics.
In 2014 the two most advanced phase III trials of passive immunization using Bapineuzumab and Solanezumab were reported as failing to demonstrate overall clinical improvement or any clear disease modifying results [62,63]. Bapineuzumab (an IgG1) is a humanized version of the mouse monoclonal antibody 3D6 (mouse IgG2b), which has an epitope of residues 1-5 of Aβ. Bapineuzumab binds to both monomeric and aggregated Aβ. In transgenic mouse studies, it was shown to cross the blood brain barrier and bind to plaques in the brain leading to Fc receptor mediated, microglial phagocytosis of Aβ plaques [56,64], a mechanism of action illustrated in Figure 1A. Phase II trials were then completed, testing multiple doses of bapineuzumab in patients with mild to moderate AD. Patients received six infusions that were given every 13 weeks at 4 different doses (0.15mg/kg, 0.5mg/kg, 1mg/kg and 2mg/kg) [65,66]. Overall, treatment with bapineuzumab did not result in a statistically significant improvement in cognitive testing. However, post-hoc analysis that only included subjects who received all infusions showed significant improvement in the pooled treated group compared to controls on the Disability Assessment of Dementia (DAD) and the Alzheimer's Disease Assessment Scale- Cognitive subscale (ADAS-Cog) [65]. In addition, ApoE4 patients showed significant (but small) cognitive improvement on the ADAS-cog, NTB, MMSE and CDR scales. 11C-PiB PET imaging in 28 patients showed that Bapineuzumab treatment resulted in a reduction of cortical fibrillar amyloid deposits compared to both baseline and control subjects over the 78 weeks of the trial [67]. One significant complication associated with Bapineuzumab treatment was the development of ARIA [68]. These abnormalities include increased FLAIR MRI signal due to parenchymal vasogenic edema and sulcal effusions (ARIA-E) and MRI abnormalities due to microhemorrhages and hemosiderosis as seen on the T2* weighted gradient echo (ARIA-H). 36 patients (17% of total patients) developed ARIA-E during treatment, however it was symptomatic only in 8 of these 36 patients (22% of patients with ARIA-E). ARIA-H occurred in 17 of the patients with ARIA-E and in 7 of 177 patients without ARIA-E [68]. The presence of ARIA were more common in ApoE4 carriers and patients who received the highest dose; of the 8 symptomatic patients 7 were apoE4 carriers and 6 were treated with the two highest doses of Bapineuzumab. It is likely that ApoE4 carriers showed more evidence of ARIA because of the increased levels of vascular amyloid in these patients, the removal of which causes increased vessel permeability [66,68]. This is a complication that any anti-Aβ antibody that binds to vascular amyloid will be prone to. Two phase III trials were initiated; one giving lower doses (0.5mg/kg limit) to 1,121 ApoE4 carriers and the other giving higher doses to 1,331 ApoE4 non-carriers (2mg/kg limit). Patients received an intravenous infusion of bapineuzumab every 13 weeks for 78 weeks[62]. C11-PiB-PET imaging showed that bapineuzumab treatment reduced fibrillar Aβ accumulation from baseline, and that this was most pronounced in ApoE4 carriers[69]. However, despite this target engagement, there was no clinical benefit observed in either ApoE4 carriers or non-carriers at any dose[62]. Furthermore, ARIA-E was observed in 15% of ApoE4 carriers who received bapineuzumab and in 4%, 9% and 14% of the ApoE4 non carriers that received 0.5mg/kg, 1mg/kg and 2mg/kg respectively. The explanations for the lack of cognitive improvement after treatment include treatment too late in the disease process or not targeting the most toxic Aβ species.
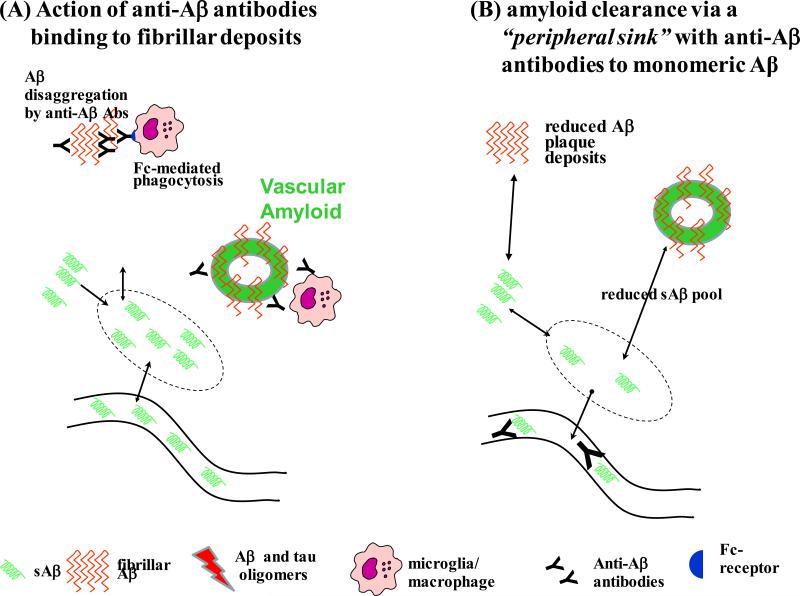
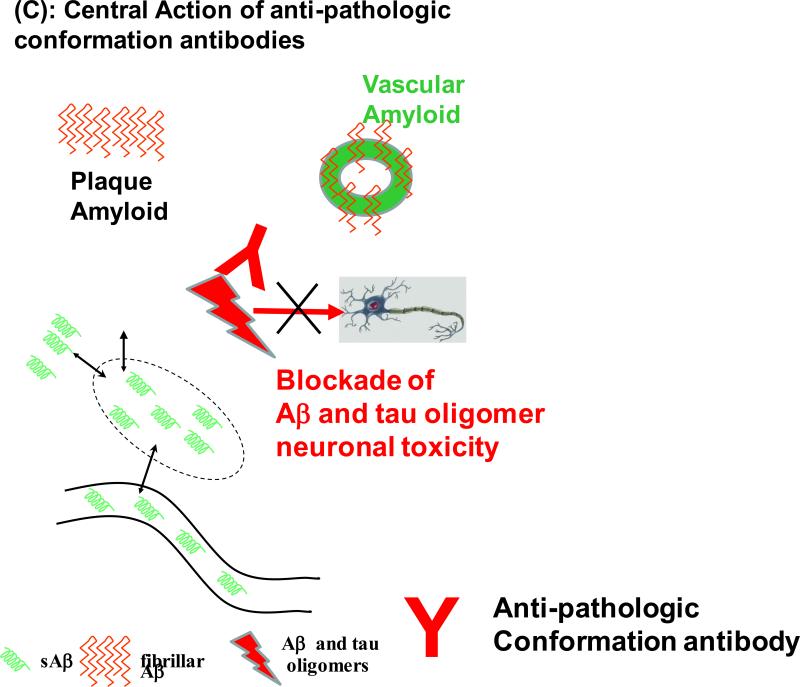
Figure 1. Different mechanisms by which immunotherapy can target AD related pathology.
A) Active immunization using Aβ peptides as an immunogen or passive immunization with antibodies that binding to fibrillar Aβ deposits will led to opsonization of plaques and vascular amyloid and resulting macrophage/microglia clearance of deposits. However, this may also lead to excessive inflammation and ARIA, in association with vessel amyloid being cleared.
B) Antibodies to Aβ that specifically bind monomeric forms (such as solanezumab), can sequester and clear sAβ in the peripheral circulation, forming a “peripheral sink”, whereby the brain Aβ peptide pool is reduced, gradually reducing deposited plaque and vessel amyloid. This method of AD pathology reduction, appears to be only potentially effective in very early stages of disease progress.
C) Active or passive immunization that specifically target oligomeric conformations of Aβ and/or tau have the advantage that these oligomeric species are thought to be the chief mediators of neurotoxicity. Such approaches have a much lower likelihood of inducing ARIA, as vessel amyloid is not directly targeted.
It is noteworthy that Pfizer and Janssen have also recently completed a Phase I clinical trial examining safety and tolerability of AAB-003, which is a derivative of bapineuzumab that differs in the Fc domain, designed to reduce effector function and hence possibly reduce ARIA (www.clinicaltrials.gov). Results from this study have not yet been released.
Solanezumab (an IgG1) is a humanized version of mAb 266 (mouse IgG2a) and has an epitope at residues 16-24 of Aβ. It recognizes soluble monomeric, not fibrillary Aβ and it is hypothesized that its mechanism of action is to act via a “peripheral sink” (see Figure 1B), whereby Aβ peptides are bound in the systemic circulation, acting to draw out the CNS pool of Aβ. Evidence for this mechanism include an immediate spike of systemic Aβ levels with Solunezumab application that correlates with the cerebral load of amyloid deposits, as well as the appearance of pyroglutamate modified Aβ species (a post-translational modification of Aβ that occurs after deposition) in the circulation with treatment[70-72]. The mechanistic strategy of using the peripheral sink, can also be achieved by other sAβ binding proteins. An example is the use of Affibodies that specifically bind monomeric Aβ, which are in preclinical development [73,74].
Two phase III Solanezumab trials have been completed (named EXPEDITION 1 and EXPEDITION 2) that included 1,012 and 1,040 patients with mild to moderate AD respectively. 400mg of solanezumab was administered intravenously every 4 weeks for 18 months. Cognitive testing performed at week 80 showed that solanezumab did not significantly improve cognitive function in the treatment group[63]. However, when patients with mild AD were studied separately, a small but statistically significant benefit was noted in the cognitive scores [63,66,75]. Plasma and CSF biomarker findings were consistent with target engagement[75]. Importantly, even though a much higher dose of Solanezumab was used in comparison to Bapineuzumab, ARIAs were not found as a complication despite the increased plasma Aβ [72,75]. Therefore, an additional Phase III trial (EXPEDITION 3) involving 2,100 patients with mild AD only was initiated in 2013 (www.clinicaltrials.gov). Results for this trial are expected in December 2016.
Inspired by Solanezumab's safety record and its cognitive benefits in mild AD, it has also been selected for two preventive or very early treatment trials; the Dominantly Inherited Alzheimer Network (DIAN) trial and the Anti-Amyloid Treatment for asymptomatic Alzheimer's disease (A4) trial. The DIAN trial is a five year Phase II/III trial that will target 210 asymptomatic/very mildly symptomatic adult children in families with known mutations and a diagnosis of familial AD. A4 will examine the effects of solanezumab in 1,150 individuals who are aged >65yrs and have biomarker evidence of brain amyloid deposition, thus meeting the criteria for preclinical AD. Results from both of these trials are expected in 2020.
The DIAN trial will also test a secondary passive immunization approach using Gantenerumab (Hoffmann-La Roche). Gantenerumab is a fully human IgG1 antibody that selectively binds to fibrillar/aggregated Aβ. Gantenerumab induces clearance of Aβ by activating microglial phagocytosis [76]. Target engagement in patients with mild to moderate AD was shown in a small study that observed fibrillar amyloid removal in the brains of 16 patients using 11C-PiB PET imaging, however ARIA was a concern[77]. Gantenerumab was unsuccessful in a phase II/III trial that was initiated in 2010 (called Scarlet RoAD), which examined the effect of monthly subcutaneous injections of Gantenerumab (105mg or 225mg) in patients with preclinical AD. This trial was discontinued in December 2014 because of lack of efficacy on primary and secondary outcomes. However, there is still hope that Gantenerumab may be successful in treating mild AD, which is currently being examined in a Phase III trial involving 1,000 patients.
Crenezumab (Genentech) is a relatively new antibody being tested in a passive immunization approach. It interacts with multiple species of Aβ [78]. The IgG4 backbone of Crenezumab reduces its effector function on microglia; hence reducing the likelihood of ARIA and other inflammatory complications. Two Phase I trials showed that ARIA was not a safety concern after treatment with Crenezumab. Phase II trials that included 450 people with mild to moderate AD receiving monthly subcutaneous injections of Crenezumab (15mg/kg) were completed in 2014 and are currently in an open label extension trial. Full results from this study have not yet been released, however, it was reported that it missed its primary endpoints of improved score on cognitive testing. However, there was a suggested treatment benefit in mild AD cases only, similar to that seen in solanezumab. It was announced in July 2015 that Crenezumab was moving into Phase III testing, initially examining individuals with prodromal AD. A secondary Phase I trial was also initiated in February 2015 examining intravenous administration in comparison to the subcutaneous route used previously. Crenezumab is also currently being tested in the Alzheimer's Prevention Initiative (API) phase II prevention trial, to be performed in ~ 300 people of a Colombian kindred with PS1 mutation (E280A). A very severe AD phenotype is seen in carrier of this mutation, characterized by Aβ deposition from ~ 25 years.
The most promising results to date for a passive immunotherapy approach for AD have come from Aducanumab (Biogen). Aducanumab is a fully human IgG1 that recognizes aggregated/fibrillar Aβ and was derived from healthy, aged donors who were cognitively normal, with the rationale that these donors’ immune system had successfully resisted AD and their antibodies could be turned into a therapeutic using an approach termed “reverse translational medicine”. In the summer of 2012 Biogen started “PRIME” a multiple-dose phase Ib study in 166 individuals with prodromal or mild AD. Subjects were randomized to placebo, 1mg, 3mg, 6mg or 10mg of Aducanumab with IV infusions given every 4 weeks over a 52 week period. Subjects were all amyloid positive on PET imaging at trial screening and were subject to repeat amyloid imaging at ~24 weeks and at the end of the trial. It was initially reported that statistically significant improvement on the MMSE was obtained at the 3 and 10mg/kg dosage[61](during the initial report data was not available on the 6mg/kg dose group). There was evidence of dosage dependent improvement on the clinical dementia rating scale sum of boxes (CDR-SB) as well as dose dependent reductions in brain amyloid. However the incidence of ARIA-E was relatively high at 5%, 43% and 55% in the 1-3, 6 and 10mg/kg, respectively in apoE4 carriers and 9%, 11% and 17% in the apoE4 non-carriers, respectively[61]. In a latter presentation of the trial findings at the 2015 AAIC meeting, it was reported that the 6mg/kg group failed to show significant cognitive improvement, questioning the argument that aducanumab was showing evidence of a dose-dependent response. Also in this later report, incidence of ARIA-E in the apoE4 non-carriers was updated to 22% from the initial rate of 11%[60]. Regardless, this trial is the first to show some clinical benefits on measures such as MMSE and CDR-SB after passive immunization. Hence, a Phase III trial has started, seeking to enroll ~1,350 patients, with the expectation of data being presented in ~2018.
An important limitation of the passive immunization approaches described above is the lack of ability to specifically target Aβ oligomers, widely thought to be the most deleterious forms of Aβ. The current approaches either target only the soluble Aβ (i.e. Solanezumab), recognize all forms of Aβ (i.e Bapineuzumab and Crenezumab) or bind to aggregated Aβ (i.e. Aducanumab and Gantenezumab) [58]. The targeting of soluble Aβ via a peripheral sink mechanism is likely only to work in very early states of the AD process, most likely just in the preclinical stages. Neuropathological studies have shown that patients even at the mild cognitive impairment stage of AD can have both significant amyloid and tau related pathology [2,79]. In addition, the therapeutic targeting of normal soluble Aβ in a chronic disease, over potentially a long period of time, has the risk of interfering with its physiological functions, proposed to include neuroprotection, modulation of long term potentiation and innate immunity [80-82]. On the other hand, the targeting of aggregated forms of Aβ has the possibility of being effective in clinical AD (as indicated by the preliminary results with aducanumab), but this approach carries the risk of inducing ARIA in a significant proportion of patients, as the mechanism is dependent on microglia mediated clearance of deposits of both plaques and vascular amyloid.
Immunological Targeting of Tau Related Pathology
Hyperphosphorylated tau, the main component of NFTs, is another attractive target for immunotherapy approaches for AD. This primarily because tau related pathology correlates better with the degree of dementia than amyloid plaque burden [83-90]. Furthermore, it has been shown that tau pathology actually precedes the formation of amyloid plaques in the development of AD [91-93]. There was initial concern about the mechanism of how tau immunotherapy could work in vivo because of the intracellular location of NFTs. However, a number of recent studies have clearly shown that pathological/aggregated tau can be both released and internalized by neurons [94-97]. Therefore, it is certainly plausible that tau immunotherapy directly targets extracellular pathological tau instead of the intraneuronal inclusions. In line with this hypothesis, the first preclinical studies describing the success of tau active and passive immunotherapeutic approaches were described in 2007 [98] and 2010 [99,100] respectively. These studies showed that both active and passive immunotherapeutic approaches were able to decrease NFT burden in vivo. Following on from these studies, there are now currently many tau immunotherapeutic approaches in preclinical development[101].
These preclinical results suggest that immunotherapy directed towards tau holds promise; as a result two active vaccines targeting either non-phosphorylated (AADVac1) and phosphorylated tau (ACI-35) have entered phase I testing [102,103] (www.clinicaltrials.gov). AADVac1 (Axon Neuroscience SE) consists of a synthetic peptide derived from residues 294-305 of tau, which are involved in the oligomerization of tau, coupled to KLH, with alum as an adjuvant. 30 individuals with mild to moderate AD will be given three monthly subcutaneous injections and will then be followed for 18 months. ACI-35 (AC-immune) integrates tau393-408 (pS396/ps404) into liposomes using technology similar to the Aβ vaccine ACI-24. The ACI-35 incorporates MPLA, which is a TLR4 agonist as an adjuvant. In the trial two doses will be compared in people with mild to moderate AD. It is a randomized, double-blind, placebo-controlled study of safety, tolerability, and immunogenicity. It also has secondary outcomes for an initial look at biomarkers, functional outcomes, and clinical outcomes.
Innate Immunity Stimulation to Reduce AD Pathology
Studies conducted over two decades ago suggested the potential role of microglia in the formation and clearance of amyloid lesions in AD [104-106]. More recently, multiple studies have confirmed the importance of microglia in AD pathogenesis by identifying that several factors that regulate glial clearance and inflammatory reactions significantly increase the risk for sporadic AD (TREM2, CD33, CR1) [8,107]. Furthermore, the immune/microglia system is the top network associated with the development of sporadic AD[108]. Microglia lose their Aβ clearing capabilities as AD progresses [109-111]. Senescence of microglia function has been suggested to play a fundamental role in both AD and other neurodegenerative diseases [112]. Neuroinflammation can contribute to cognitive impairment and play a significant role in AD progression [113,114]; however, it is increasingly recognized that tightly regulated stimulation of innate immunity processes and specific microglia activation can be neuroprotective depending on the stimulus and the environment [115].
One of the most potent ways to stimulate the innate immune system is via the Toll-like receptors (TLRs). Modulation of TLR2, 4 and 9 signaling pathways has previously been shown to be critical in modulating Aβ deposition. Diffuse and fibrillar Aβ deposits are increased in TLR4 deficient mice in comparison to control mice [116], suggesting that TLR4 signaling is involved in Aβ clearance [117]. Additionally, microglia deficient in TLR2, TLR4, or the co-receptor CD14 are not activated by Aβ and do not show a phagocytic response [118]. Conversely, treatment with TLR2-, TLR4-, or TLR9- specific agonists accelerates Aβ clearance both in vitro and in vivo [119]. Importantly, it has been shown that stimulation of TLR9 by treatment with CpG oligonucleotides (ODN) containing unmethylated CpG sequences can decrease cortical and vascular Aβ levels and tau related pathology simultaneously, as well as improving cognitive function in transgenic mouse models of AD [120,121]. Various CpG DNA drugs that are TLR9 agonists have also been shown to be safe for humans [122]. Studies testing CpG DNA TLR9 agonists in aged squirrel monkeys are on-going. These monkeys develop extensive Aβ pathology[123]. Together, these preclinical studies suggest that modification of microglial function in neurodegeneration is a viable therapeutic target to potentially ameliorate both Aβ and tau pathologies.
Targeting Abnormal Protein Conformation of Both Aβ and Tau Related Pathologies
Oligomers are proposed to be the most toxic conformers of Aβ and aggregated tau. Both Aβ and tau oligomers have been demonstrated to spread extracellularly using prion like replicative mechanisms. Studies have shown that in the presence of Aβ pathology, therapeutic interventions that impede Aβ oligomer toxicity can reverse cognitive deficits with a remarkably short treatment duration, making these species an attractive therapeutic target [124,125]. Aβ and tau oligomers share structural and biophysical properties, such as a high β-sheet content, neuronal toxicity and partial resistance to proteolytic degradation. An important benefit of targeting only Aβ or tau oligomers is that the normal physiological function of the monomers of these proteins remains intact. An additional benefit of specifically targeting oligomers is that conformationally specific antibodies can be generated, which target the shared abnormal β-sheet confirmation of amyloid proteins, rather than a specific protein [126-128]. This approach has the benefit of simultaneously targeting both the Aβ and tau related pathologies. Our group has been engaged in this approach for the last several years, which has a mechanism of action illustrated in Figure 1C [58]. Conformational monoclonal antibodies were generated using a polymerized peptide derived from the carboxyl terminus of the British amyloidosis (ABri) peptide, oligomerised using glutaraldehyde as a cross linker that forms a stable population of oligomers, which we term pBri [129,130]. This peptide lacks any sequence homology to Aβ, tau or any other native human proteins [129,131,132]. As a result, this immunomodulatory approach has the decreased risk of causing auto-immune complications, as it is specific to pathological conformers and the immunogen does not have sequence homology to any mammalian peptide. We have shown that that pBri initiates a conformation selective immune response that is capable of targeting both phosphorylated tau and Aβ in AD transgenic mouse models, and results in improvement in cognitive deficits [130]. With this approach the reductions of deposited Aβ and tau are most likely related to interfering with the intermediate oligomeric forms of Aβ and tau before they fibrillize, rather than directly acting on the plaques and NFTs. Furthermore, using a passive approach, monoclonal antibodies derived by using pBri as an immunogen that recognize both Aβ and tau oligomers have shown therapeutic efficacy in AD model mice [133].
Expert Commentary
Currently there is no effective treatment for AD. However, there are many different active and passive immunization therapeutic approaches currently under development and in clinical trials. In addition pre-clinical studies are exploring innate immunity stimulation. Strategies that target monomeric Aβ peptides (with antibodies such as Solanezumab), leading to plaque reduction via a peripheral sink mechanism, could be effective if used very early in disease onset before the development of any clinical dysfunction. Antibodies that target aggregated Aβ, such as Biogen's Aducanumab, are showing some promise for symptomatic AD; however, this class of antibodies will likely have ARIA as a significant side effect, as part of the therapeutic targeting of deposited Aβ includes vessel amyloid. It also must be considered that the basis of these Aβ immunotherapy approaches, the amyloid hypothesis, cannot completely explain the complexity of AD. Therefore, the simplistic approach of a therapeutic that only decreases Aβ levels is unlikely to comprehensively treat AD. Indeed, post mortem analyses of the active vaccination trials in humans have revealed a significant decrease in plaque burden and strikingly reduced Aβ load relative to nonimmunized controls, but no cognitive improvement or long-term survival outcome [134]. Alternatively, it has been speculated that immunization was conducted in the late stage of the disease process, possibly outside of the clinically beneficial window of opportunity or that the individuals included in clinical trials had brain pathology other than that associated with AD that contributed to dementia [20,135]. Therefore, future trials must address these concerns.
Immunotherapy targeted towards tau pathology has also shown some promise, but there is limited data that can be effective in established disease and it bears the risk of toxicity. None of the existing trials specifically target oligomers of Aβ and/or tau. As these are the most toxic species such targeting is likely needed for greatest therapeutic efficacy. Furthermore a strategy that concurrently targets both Aβ and tau toxic oligomers might be most likely to be efficacious in symptomatic AD. It is known that Aβ and tau abnormal conformers interact; however, the relative importance of these abnormal conformers on driving AD pathology might differ from patient to patient. All individuals as they age develop some degree of tau pathology in the medial temporal lobes; however, Aβ pathology is a common but not a universal feature of aging even in the oldest old [136-138]. Hence tau and Aβ may be independent processes that show pathological synergy in the evolution of AD. AD patients at autopsy typically have some degree of concomitant pathology such as α-synuclein and/or TDP-43 accumulation [139,140]. Immunological targeting of the shared oligomeric structure of disease associated protein aggregates has the potential to also ameliorate this concomitant pathology. Such an approach has potential for multiple conformational neurodegenerative conditions.
Five-Year View
In the coming 5 years an anti-Aβ antibody targeting monomeric species will likely be approved for clinical use for pre-symptomatic disease. Over this period it is expected that PET imaging techniques, which are already available, for both amyloid and tau pathology will be in much wider use; readily identifying populations of patients who would most benefit from such a treatment. Approaches that target aggregated Aβ need to overcome the high prevalence of ARIA. This may be achieved by engineering the monoclonal antibodies to have reduced effector function and/or excluding subjects that are at greatest risk (ie. apoE4 carriers and those patients with significant vascular amyloid on imaging studies in development). We also expect more human trials of monoclonal antibodies targeting tau, given the importance of this pathology to symptomatic AD; however, this approach will likely have target engagement and toxicity issues. It is also likely that immunomodulatory approaches targeting abnormal protein conformation will go into clinical trial. We believe that ultimately this type of approach will have the greatest chance of efficacy in early and moderate stages of AD.
Key issues.
Immunotherapy is an attractive therapeutic strategy for AD; preclinical studies have shown both active and passive approaches can remove amyloid and tau pathology and, in many cases, improve cognitive function.
Numerous active and passive immunization strategies targeting Aβ have entered clinical trials. Aducanumab is the first mAb to show clinical benefits on widely used measures such as MMSE and CDR-SB.
A significant side effect of all passive immunization strategies targeting aggregated forms of Aβ are amyloid related imaging abnormalities (ARIA).
Two active vaccines targeting either non-phosphorylated (AADVac1) and phosphorylated tau (ACI-35) have entered phase I testing
Alternative approaches of active or passive immunization targeting abnormal protein conformation are promising future therapeutic strategies, since these are capable of targeting amyloid and tau pathology simultaneously.
Stimulation of the innate immune, which has been identified as a significant factor by GWAS in LOAD, is also showing promise as a therapeutic approach in preclinical studies.
Acknowledgments
This manuscript is supported by NIH grants NS073502, AG20245 and AG08051. It is also supported by the Seix Dow Foundation.
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Association As. Alzheimer' Association Report. Alzheimer's and Dementia. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer's disease neuropathologic changes with cognitive status: a review of the literature. JNEN. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashe KH, Aguzzi A. Prions, prionoids and pathogenic proteins in Alzheimer disease. Prion. 2013;7(1):55–59. doi: 10.4161/pri.23061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jucker M, Walker LC. Pathogenic protein seeding in alzheimer disease and other neurodegenerative disorders. Ann. Neurol. 2011;70(4):532–540. doi: 10.1002/ana.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5•.Jaunmuktane Z, Mead S, Ellis M, et al. Evidence for human transmission of amyloid-beta pathology and cerebral amyloid angiopathy. Nature. 2015;525(7568):247–250. doi: 10.1038/nature15369. [Shows evidence for the first time that AD may be transmissible from person to person.] [DOI] [PubMed] [Google Scholar]
- 6.Guerreiro R, Hardy J. Genetics of Alzheimer's Disease. Neurotherapeutics. 2014 doi: 10.1007/s13311-014-0295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wingo TS, Lah JJ, Levey AI, Cutler DJ. Autosomal recessive causes likely in early-onset Alzheimer disease. Arch. Neurol. 2012;69(1):59–64. doi: 10.1001/archneurol.2011.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karch CM, Cruchaga C, Goate AM. Alzheimer's disease genetics: from the bench to the clinic. Neuron. 2014;83(1):11–26. doi: 10.1016/j.neuron.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertram L, Tanzi RE. The genetics of Alzheimer's disease. Prog. Mol. Biol. Transl. Sci. 2012;107:79–100. doi: 10.1016/B978-0-12-385883-2.00008-4. [DOI] [PubMed] [Google Scholar]
- 10.Kim DH, Yeo SH, Park JM, et al. Genetic markers for diagnosis and pathogenesis of Alzheimer's disease. Gene. 2014 doi: 10.1016/j.gene.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 11.Di Marco LY, Marzo A, Munoz-Ruiz M, et al. Modifiable Lifestyle Factors in Dementia: A Systematic Review of Longitudinal Observational Cohort Studies. J. Alzheimers. Dis. 2014 doi: 10.3233/JAD-132225. [DOI] [PubMed] [Google Scholar]
- 12.Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC. Public Health. 2014;14:643. doi: 10.1186/1471-2458-14-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karch CM, Goate AM. Alzheimer's disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry. 2015;77(1):43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanekiyo T, Xu H, Bu G. ApoE and Abeta in Alzheimer's disease: accidental encounters or partners? Neuron. 2014;81(4):740–754. doi: 10.1016/j.neuron.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potter H, Wisniewski T. Apolipoprotein E: essential catalyst of the Alzheimer amyloid cascade. Int. J. Alz. Dis. 2012;2012:489428. doi: 10.1155/2012/489428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hickman SE, El KJ. TREM2 and the neuroimmunology of Alzheimer's disease. Biochem. Pharmacol. 2014;88(4):495–498. doi: 10.1016/j.bcp.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boutajangout A, Wisniewski T. The innate immune system in Alzheimer's Disease. Int. J. Cell Biol. 2013;2013:e576383. doi: 10.1155/2013/576383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarasoff J, Carare R, Osorio R, et al. Clearance systems in the brain and Alzheimer's Disease. Nature Neurology Reviews. 2015;11(8):457–470. doi: 10.1038/nrneurol.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrone CD, Liu M, Black SE, McLaurin J. Interaction between therapeutic interventions for Alzheimer's disease and physiological Abeta clearance mechanisms. Front Aging Neurosci. 2015;7:64. doi: 10.3389/fnagi.2015.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holtzman DM, Mandelkow E, Selkoe DJ. Alzheimer disease in 2020. Cold Spring Harb. Perspect. Med. 2012;2(11) doi: 10.1101/cshperspect.a011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 22.Herrup K. The case for rejecting the amyloid cascade hypothesis. Nat Neurosci. 2015;18(6):794–799. doi: 10.1038/nn.4017. [DOI] [PubMed] [Google Scholar]
- 23.Musiek ES, Holtzman DM. Three dimensions of the amyloid hypothesis: time, space and 'wingmen'. Nat Neurosci. 2015;18(6):800–806. doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomon B, Koppel R, Frankel D, Hanan-Aharon E. Disaggregation of Alzheimer b-amyloid by site-directed mAb. Proc. Natl. Acad. Sci. USA. 1997;94(8):4109–4112. doi: 10.1073/pnas.94.8.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-b attenuates Alzheimer disease-like pathology in the PDAPP mice. Nature. 1999;400:173–177. doi: 10.1038/22124. [First to show active immunization targeting Aβ can clear amyloid deposits in vivo in an AD animal model.] [DOI] [PubMed] [Google Scholar]
- 26.Lemere CA. Immunotherapy for Alzheimer's disease: hoops and hurdles. Mol. Neurodegener. 2013;8(1):36. doi: 10.1186/1750-1326-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sigurdsson EM, Scholtzova H, Mehta P, Frangione B, Wisniewski T. Immunization with a non-toxic/non-fibrillar amyloid-b homologous peptide reduces Alzheimer's disease associated pathology in transgenic mice. Am. J. Pathol. 2001;159:439–447. doi: 10.1016/s0002-9440(10)61715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan D, Diamond DM, Gottschall PE, et al. Ab peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 29.Janus C, Pearson J, McLaurin J, et al. Ab peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 30.Sigurdsson EM, Knudsen EL, Asuni A, et al. An attenuated immune response is sufficient to enhance cognition in an Alzheimer's disease mouse model immunized with amyloid-b derivatives. J. Neurosci. 2004;24:6277–6282. doi: 10.1523/JNEUROSCI.1344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asuni A, Boutajangout A, Scholtzova H, et al. Ab derivative vaccination in alum adjuvant prevents amyloid deposition and does not cause brain microhemorrhages in Alzheimer's model mice. Eur. J Neurosci. 2006;24:2530–2542. doi: 10.1111/j.1460-9568.2006.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bayer AJ, Bullock R, Jones RW, et al. Evaluation of the safety and immunogenicity of synthetic Ab42 (AN1792) in patients with AD. Neurol. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- 33.Pride M, Seubert P, Grundman M, Hagen M, Eldridge J, Black RS. Progress in the active immunotherapeutic approach to Alzheimer's disease: clinical investigations into AN1792-associated meningoencephalitis. Neurodegener. Dis. 2008;5(3-4):194–196. doi: 10.1159/000113700. [DOI] [PubMed] [Google Scholar]
- 34.Wisniewski T, Frangione B. Immunological and anti-chaperone therapeutic approaches for Alzheimer's disease. Brain Pathol. 2005;15:72–77. doi: 10.1111/j.1750-3639.2005.tb00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boche D, Nicoll JA. The role of the immune system in clearance of Ab from the brain. Brain Pathol. 2008;18(2):267–278. doi: 10.1111/j.1750-3639.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bombois S, Maurage CA, Gompel M, et al. Absence of beta-amyloid deposits after immunization in Alzheimer disease with Lewy body dementia. Arch. Neurol. 2007;64(4):583–587. doi: 10.1001/archneur.64.4.583. [DOI] [PubMed] [Google Scholar]
- 37.Ferrer I, Boada RM, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer's disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masliah E, Hansen L, Adame A, et al. Ab vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurol. 2005;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- 39.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid- beta peptide: a case report. Nat. Med. 2005;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 40.Nicoll JA, Barton E, Boche D, et al. Abeta species removal after abeta42 immunization. J Neuropathol. Exp. Neurol. 2006;65(11):1040–1048. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- 41.Sadowski M, Wisniewski T. Disease modifying approaches for Alzheimer's pathology. Current Pharmaceutic Design. 2007;13(19):1943–1954. doi: 10.2174/138161207781039788. [DOI] [PubMed] [Google Scholar]
- 42.Gilman S, Koller M, Black RS, et al. Clinical effects of Ab immunization (AN1792) in patients with AD in an interupted trial. Neurol. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 43.Hock C, Konietzko U, Straffer JR, et al. Antibodies against b-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 44.Ryan JM, Grundman M. Anti-amyloid-beta immunotherapy in Alzheimer's disease: ACC-001 clinical trials are ongoing. J. Alzheimers. Dis. 2009;17(2):243. doi: 10.3233/JAD-2009-1118. [DOI] [PubMed] [Google Scholar]
- 45.Schneeberger A, Mandler M, Otawa O, Zauner W, Mattner F, Schmidt W. Development of AFFITOPE vaccines for Alzheimer's disease (AD)--from concept to clinical testing. J. Nutr. Health Aging. 2009;13(3):264–267. doi: 10.1007/s12603-009-0070-5. [DOI] [PubMed] [Google Scholar]
- 46.Winblad B, Andreasen N, Minthon L, et al. Safety, tolerability, and antibody response of active Abeta immunotherapy with CAD106 in patients with Alzheimer's disease: randomised, double-blind, placebo-controlled, first-in human study. Lancet Neurol. 2012;11(7):597–604. doi: 10.1016/S1474-4422(12)70140-0. [DOI] [PubMed] [Google Scholar]
- 47.Wisniewski T. Active immunotherapy for Alzheimer's disease. Lancet Neurol. 2012;11(7):571–572. doi: 10.1016/S1474-4422(12)70136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiessner C, Wiederhold KH, Tissot AC, et al. The second-generation active Abeta immunotherapy CAD106 reduces amyloid accumulation in APP transgenic mice while minimizing potential side effects. J Neurosci. 2011;31(25):9323–9331. doi: 10.1523/JNEUROSCI.0293-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farlow MR, Andreasen N, Riviere ME, et al. Long-term treatment with active Abeta immunotherapy with CAD106 in mild Alzheimer's disease. Alzheimers Res Ther. 2015;7(1):23. doi: 10.1186/s13195-015-0108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Graf A, Riviere ME, Caputo A, Farlow MR, Marotta G, Sanchez-Valle R. Active Abeta immunotherapy CAD106 pahse II dose-adjuvant finding study: safety and CNS biomarkers. Alz. Dementia. 2014;10:P274. [Google Scholar]
- 51.Muhs A, Hickman DT, Pihlgren M, et al. Liposomal vaccines with conformation-specific amyloid peptide antigens define immune response and efficacy in APP transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 2007;104(23):9810–9815. doi: 10.1073/pnas.0703137104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hickman DT, Lopez-Deber MP, Ndao DM, et al. Sequence-independent control of peptide conformation in liposomal vaccines for targeting protein misfolding diseases. J. Biol. Chem. 2011;286(16):13966–13976. doi: 10.1074/jbc.M110.186338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saido TC, Iwatsubo T, Mann DM, Shimada H, Ihara Y, Kawashima S. Dominant and differential deposition of distinct beta-amyloid peptide species, Ab N3(pE), in senile plaques. Neuron. 1995;14(2):457–466. doi: 10.1016/0896-6273(95)90301-1. [DOI] [PubMed] [Google Scholar]
- 54.Frost JL, Le KX, Cynis H, et al. Pyroglutamate-3 amyloid-beta deposition in the brains of humans, non-human primates, canines, and Alzheimer disease-like transgenic mouse models. Am. J. Pathol. 2013;183(2):369–381. doi: 10.1016/j.ajpath.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DeMattos RB, Lu J, Tang Y, et al. A plaque-specific antibody clears existing beta-amyloid plaques in Alzheimer's disease mice. Neuron. 2012;76(5):908–920. doi: 10.1016/j.neuron.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 56.Bard F, Cannon C, Barbour R, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of alzheimer disease. Nat Med. 2000;6(8):916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 57.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(15):8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wisniewski T, Goni F. Immunotherapeutic Approaches for Alzheimer's Disease. Neuron. 2015;85:1162–1176. doi: 10.1016/j.neuron.2014.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agadjanyan MG, Petrovsky N, Ghochikyan A. A fresh perspective from immunologists and vaccine researchers: Active vaccination strategies to prevent and reverse Alzheimer's disease. Alzheimers Dement. 2015 doi: 10.1016/j.jalz.2015.06.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sevigny J, Chiao P, Williams L, et al. Aducanumab (BIIB037), an anti-amyloid beta monoclonal antibody, in patients with prodromal or mild Alzheimer's disease: interim report of a randomized, double-blind, placebo-controlled, phase 1B study. Alz. Dementia. 2015;11:4484. [Google Scholar]
- 61•.Sevigny J, Chiao P, Williams L, Miao X, O'Gorman J. Randomized, double-blind, phase 1B study of BIIB037, an anti-amyloid beta monoclonal antibody, in patients with prodromal or mild Alzheimer's disease. Neurodegener. Dis. 2015;15(supplement 1):311. [Initial report of the clinical benefits of Aducanumab using measures such as MMSE and CDR-SB, in conjunction with amyloid reductions on PET brain imaging in trial subjects.] [Google Scholar]
- 62.Salloway S, Sperling R, Fox NC, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N. Engl. J. Med. 2014;370(4):322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doody RS, Thomas RG, Farlow M, et al. Phase 3 trials of solanezumab for mild- to-moderate Alzheimer's disease. N. Engl. J. Med. 2014;370(4):311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 64.Bard F, Barbour R, Cannon C, et al. Epitope and isotype specificities of antibodies to beta -amyloid peptide for protection against Alzheimer's disease- like neuropathology. Proc. Natl. Acad. Sci. U. S. A. 2003;100(4):2023–2028. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salloway S, Sperling R, Gilman S, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73(24):2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farlow MR, Brosch JR. Immunotherapy for Alzheimer's disease. Neurol. Clin. 2013;31(3):869–878. doi: 10.1016/j.ncl.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 67.Rinne JO, Brooks DJ, Rossor MN, et al. (11)C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9(4):363–372. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- 68.Sperling R, Salloway S, Brooks DJ, et al. Amyloid-related imaging abnormalities in patients with Alzheimer's disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11(3):241–249. doi: 10.1016/S1474-4422(12)70015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu E, Schmidt ME, Margolin R, et al. Amyloid-beta 11C-PiB-PET imaging results from 2 randomized bapineuzumab phase 3 AD trials. Neurology. 2015;85(8):692–700. doi: 10.1212/WNL.0000000000001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dodart JC, Bales KR, Gannon KS, et al. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model. Nat. Neurosci. 2002;5(5):452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- 71.DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer's disease. Science. 2002;295(5563):2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 72.Farlow M, Arnold SE, Van Dyck CH, et al. Safety and biomarker effects of solanezumab in patients with Alzheimer's disease. Alzheimers. Dement. 2012;8(4):261–271. doi: 10.1016/j.jalz.2011.09.224. [DOI] [PubMed] [Google Scholar]
- 73.Lindberg H, Hard T, Lofblom J, Stahl S. A truncated and dimeric format of an Affibody library on bacteria enables FACS-mediated isolation of amyloid-beta aggregation inhibitors with subnanomolar affinity. Biotechnol J. 2015 doi: 10.1002/biot.201500131. [DOI] [PubMed] [Google Scholar]
- 74.De Genst E, Muyldermans S. Development of a high affinity Affibody-derived protein against amyloid beta-peptide for future Alzheimer's disease therapy. Biotechnol J. 2015 doi: 10.1002/biot.201500405. [DOI] [PubMed] [Google Scholar]
- 75.Siemers ER, Sundell KL, Carlson C, et al. Phase 3 solanezumab trials: Secondary outcomes in mild Alzheimer's disease patients. Alzheimers Dement. 2015 doi: 10.1016/j.jalz.2015.06.1893. [DOI] [PubMed] [Google Scholar]
- 76.Bohrmann B, Baumann K, Benz J, et al. Gantenerumab: a novel human anti- Abeta antibody demonstrates sustained cerebral amyloid-beta binding and elicits cell-mediated removal of human amyloid-beta. J Alzheimers. Dis. 2012;28(1):49–69. doi: 10.3233/JAD-2011-110977. [DOI] [PubMed] [Google Scholar]
- 77.Ostrowitzki S, Deptula D, Thurfjell L, et al. Mechanism of Amyloid Removal in Patients With Alzheimer Disease Treated With Gantenerumab. Arch. Neurol. 2011;69:198–207. doi: 10.1001/archneurol.2011.1538. [DOI] [PubMed] [Google Scholar]
- 78.Adolfsson O, Pihlgren M, Toni N, et al. An effector-reduced anti-beta-amyloid (Abeta) antibody with unique abeta binding properties promotes neuroprotection and glial engulfment of Abeta. J Neurosci. 2012;32(28):9677–9689. doi: 10.1523/JNEUROSCI.4742-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morris JC. Mild cognitive impairment is early-stage Alzheimer disease: time to revise diagnostic criteria. Arch. Neurol. 2006;63(1):15–16. doi: 10.1001/archneur.63.1.15. [DOI] [PubMed] [Google Scholar]
- 80.Puzzo D, Privitera L, Leznik E, et al. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28(53):14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giuffrida ML, Caraci F, Pignataro B, et al. Beta-amyloid monomers are neuroprotective. J Neurosci. 2009;29(34):10582–10587. doi: 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soscia SJ, Kirby JE, Washicosky KJ, et al. The Alzheimer's Disease-Associated Amyloid beta-Protein Is an Antimicrobial Peptide. PLoS. ONE. 2010;5(3):e9505. doi: 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoshiyama Y, Lee VM, Trojanowski JQ. Therapeutic strategies for tau mediated neurodegeneration. J Neurol. Neurosurg. Psychiatry. 2013;84(7):784–795. doi: 10.1136/jnnp-2012-303144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sigurdsson EM. Immunotherapy targeting pathological tau protein in Alzheimer's disease and related tauopathies. J Alzheimers. Dis. 2008;15(2):157–168. doi: 10.3233/jad-2008-15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Noble W, Garwood CJ, Hanger DP. Minocycline as a potential therapeutic agent in neurodegenerative disorders characterised by protein misfolding. Prion. 2009;3(2) doi: 10.4161/pri.3.2.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kayed R, Jackson GR. Prefilament tau species as potential targets for immunotherapy for Alzheimer disease and related disorders. Curr. Opin. Immunol. 2009;21(3):359–363. doi: 10.1016/j.coi.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 87.Boutajangout A, Wisniewski T. Tau-based therapeutic approaches for Alzheimer's Disease - a mini-review. Gerontology. 2014;60:381–385. doi: 10.1159/000358875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42(3 Pt 1):631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 89.Bancher C, Braak H, Fischer P, Jellinger KA. Neuropathological staging of Alzheimer lesions and intellectual status in Alzheimer's and Parkinson's disease patients. Neurosci. Lett. 1993;162(1-2):179–182. doi: 10.1016/0304-3940(93)90590-h. [DOI] [PubMed] [Google Scholar]
- 90.Terry RD. The pathogenesis of Alzheimer disease: An alternative to the amyloid hypothesis. J. Neuropath. Exp. Neurol. 1996;55(10):1023–1025. [PubMed] [Google Scholar]
- 91.Braak H, Del TK. The pathological process underlying Alzheimer's disease in individuals under thirty. Acta Neuropathol. 2011;121(2):171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- 92.Elobeid A, Soininen H, Alafuzoff I. Hyperphosphorylated tau in young and middle-aged subjects. Acta Neuropathol. 2012;123(1):97–104. doi: 10.1007/s00401-011-0906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jack CR, Jr., Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet. Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clavaguera F, Bolmont T, Crowther RA, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 2009;11(7):909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clavaguera F, Grueninger F, Tolnay M. Intercellular transfer of tau aggregates and spreading of tau pathology: Implications for therapeutic strategies. Neuropharmacology. 2014;76(Pt A):9–15. doi: 10.1016/j.neuropharm.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 96.Liu L, Drouet V, Wu JW, et al. Trans-synaptic spread of tau pathology in vivo. PLoS ONE. 2012;7(2):e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dujardin S, Lecolle K, Caillierez R, et al. Neuron-to-neuron wild-type Tau protein transfer through a trans-synaptic mechanism: relevance to sporadic tauopathies. Acta Neuropathol Commun. 2014;2:14. doi: 10.1186/2051-5960-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98•.Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27(34):9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [Initial report that immunological targeting of tau pathology can be benefical in a tau mouse model.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J Neurochem. 2011;118(4):658–667. doi: 10.1111/j.1471-4159.2011.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chai X, Wu S, Murray TK, et al. Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. J Biol. Chem. 2011;286(39):34457–34467. doi: 10.1074/jbc.M111.229633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pedersen JT, Sigurdsson EM. Tau immunotherapy for Alzheimer's disease. Trends Mol Med. 2015;21(6):394–402. doi: 10.1016/j.molmed.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 102.Kontsekova E, Zilka N, Kovacech B, Novak P, Novak M. First-in-man tau vaccine targeting structural determinants essential for pathological tau-tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer's disease model. Alzheimers Res Ther. 2014;6(4):44. doi: 10.1186/alzrt278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Theunis C, Crespo-Biel N, Gafner V, et al. Efficacy and safety of a liposome- based vaccine against protein Tau, assessed in tau.P301L mice that model tauopathy. PLoS One. 2013;8(8):e72301. doi: 10.1371/journal.pone.0072301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wisniewski HM, Wegiel J, Wang KC, Lach B. Ultrastructural studies of the cells forming amyloid in the cortical vessel wall in Alzheimer's disease. Acta Neuropathol. 1992;84(2):117–127. doi: 10.1007/BF00311383. [DOI] [PubMed] [Google Scholar]
- 105.Frackowiak J, Wisniewski HM, Wegiel J, Merz GS, Iqbal K, Wang KC. Ultrastructure of the microglia that phagocytose amyloid and the microglia that produce beta-amyloid fibrils. Acta Neuropathol. 1992;84(3):225–233. doi: 10.1007/BF00227813. [DOI] [PubMed] [Google Scholar]
- 106.Wisniewski HM, Wegiel J. The role of microglia in amyloid fibril formation. Neuropathol. Appl. Neurobiol. 1994;20(2):192–194. [PubMed] [Google Scholar]
- 107.Yaghmoor F, Noorsaeed A, Alsaggaf S, et al. The role of TREM2 in Alzheimer's disease and other neurological disorders. J. Alz. Dis. Parkinsonism. 2014;4(5):160. doi: 10.4172/2161-0460.1000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang B, Gaiteri C, Bodea LG, et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer's disease. Cell. 2013;153(3):707–720. doi: 10.1016/j.cell.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fiala M, Lin J, Ringman J, et al. Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer's disease patients. J. Alzheimers. Dis. 2005;7(3):221–232. doi: 10.3233/jad-2005-7304. [DOI] [PubMed] [Google Scholar]
- 110.Majumdar A, Cruz D, Asamoah N, et al. Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol. Biol. Cell. 2007;18(4):1490–1496. doi: 10.1091/mbc.E06-10-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lai AY, McLaurin J. Clearance of amyloid-beta peptides by microglia and macrophages: the issue of what, when and where. Future. Neurol. 2012;7(2):165–176. doi: 10.2217/fnl.12.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Streit WJ, Xue QS. Human CNS immune senescence and neurodegeneration. Curr. Opin. Immunol. 2014;29C:93–96. doi: 10.1016/j.coi.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 113.Lee DC, Rizer J, Hunt JB, Selenica ML, Gordon MN, Morgan D. Review: experimental manipulations of microglia in mouse models of Alzheimer's pathology: activation reduces amyloid but hastens tau pathology. Neuropathol. Appl. Neurobiol. 2013;39(1):69–85. doi: 10.1111/nan.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lampron A, Elali A, Rivest S. Innate immunity in the CNS: redefining the relationship between the CNS and Its environment. Neuron. 2013;78(2):214–232. doi: 10.1016/j.neuron.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 115.Schwartz M, Kipnis J, Rivest S, Prat A. How do immune cells support and shape the brain in health, disease, and aging? J. Neurosci. 2013;33(45):17587–17596. doi: 10.1523/JNEUROSCI.3241-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain. 2006;129(Pt 11):3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jin JJ, Kim HD, Maxwell JA, Li L, Fukuchi K. Toll-like receptor 4-dependent upregulation of cytokines in a transgenic mouse model of Alzheimer's disease. J. Neuroinflammation. 2008;5:23. doi: 10.1186/1742-2094-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J. Neurosci. 2009;29(38):11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Michaud JP, Halle M, Lampron A, et al. Toll-like receptor 4 stimulation with the detoxified ligand monophosphoryl lipid A improves Alzheimer's disease-related pathology. Proc. Natl. Acad. Sci. U. S. A. 2013;110(5):1941–1946. doi: 10.1073/pnas.1215165110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Scholtzova H, Kascsak RJ, Bates KA, et al. Induction of Toll-like receptor 9 signaling as a method for ameliorating Alzheimer's disease related pathology. J. Neurosci. 2009;29(6):1846–1854. doi: 10.1523/JNEUROSCI.5715-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121•.Scholtzova H, Chianchiano P, Pan J, et al. Toll-like receptor 9 stimulation for reduction of amyloid b and tau Alzheimer's disease related pathology. Acta Neuropathol. Commun. 2014;2:101. doi: 10.1186/s40478-014-0101-2. [Initial report indicating that innate immunity stimulation can reduce both Aβ and tau related pathology in vivo.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv. Drug Deliv. Rev. 2009;61(3):195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 123.Scholtzova H, Williams L, Nehete P, Sabado R, Holmes A, Wisniewski T. Innate immunity stimulation via TLR9 in a non-human primate model of sporadic cerebral amyloid angiopathy. Alz. Dementia. 2013;9(4):p508. [Google Scholar]
- 124.Chung E, Ji Y, Sun Y, et al. Anti-PrPC monoclonal antibody infusion as a novel treatment for Ab oligomer cognitive cognitive deficits. BMC Neuroscience. 2010;11:130. doi: 10.1186/1471-2202-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Barry AE, Klyubin I, Mc Donald JM, et al. Alzheimer's disease brain-derived amyloid-beta-mediated inhibition of LTP in vivo is prevented by immunotargeting cellular prion protein. J. Neurosci. 2011;31(20):7259–7263. doi: 10.1523/JNEUROSCI.6500-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee EB, Leng LZ, Zhang B, et al. Targeting amyloid-beta peptide (Abeta) oligomers by passive immunization with a conformation-selective monoclonal antibody improves learning and memory in Abeta precursor protein (APP) transgenic mice. J Biol. Chem. 2006;281(7):4292–4299. doi: 10.1074/jbc.M511018200. [DOI] [PubMed] [Google Scholar]
- 127.Moretto N, Bolchi A, Rivetti C, et al. Conformation-sensitive antibodies against alzheimer amyloid-beta by immunization with a thioredoxin-constrained B-cell epitope peptide. J Biol. Chem. 2007;282(15):11436–11445. doi: 10.1074/jbc.M609690200. [DOI] [PubMed] [Google Scholar]
- 128.Wisniewski T, Prelli F, Scholtzova H, et al. Immunotherapy targeting abnormal protein conformation. Alz. Dementia. 2009;5(4, Suppl. 1):P113. [Google Scholar]
- 129.Goni F, Prelli F, Ji Y, et al. Immunomodulation targeting abnormal protein conformation reduces pathology in a mouse model of Alzheimer's disease. PLoS. ONE. 2010;5(10):e13391. doi: 10.1371/journal.pone.0013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130•.Goni F, Herline K, Peyser D, et al. Immunomodulation targeting both Ab and tau pathological conformers ameliorates Alzheimer's Disease pathology in TgSwDI and 3xTg mouse models. Journal of Neuroinflammation. 2013;10(1):150. doi: 10.1186/1742-2094-10-150. [Shows that targeting abnormal protein conformatoin can generate a humoral immune response to both Aβ and tau pathology concurrently.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vidal R, Frangione B, Rostagno A, et al. A stop-codon mutation in the BRI gene associated with familial British dementia. Nature. 1999;399(6738):776–781. doi: 10.1038/21637. [DOI] [PubMed] [Google Scholar]
- 132.Rostagno A, Tomidokoro Y, Lashley T, et al. Chromosome 13 dementias. Cell Mol. Life Sci. 2005;62(16):1814–1825. doi: 10.1007/s00018-005-5092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Herline K, Goni F, Drummond E, Marta-Ariza M, Prelli F, Wisniewski T. Characterization of a novel monoclonal antibody targeting pathological proteins in Alzheimer's Disease. JNEN. 2015;74(6):625–625. [Google Scholar]
- 134.Holmes C, Boche D, Wilkinson D, et al. Long term effects of Ab42 immunization in Alzheimer's disease: immune response, plaque removal and clinical function. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 135.Wisniewski T, Goni F. Immunotherapy for Alzheimer's disease. Biochemical Pharmacology. 2014;88:499–507. doi: 10.1016/j.bcp.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128(6):755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jellinger KA, Alafuzoff I, Attems J, et al. PART, a distinct tauopathy, different from classical sporadic Alzheimer disease. Acta Neuropathol. 2015;129:757–762. doi: 10.1007/s00401-015-1407-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jansen WJ, Ossenkoppele R, Visser PJ. Amyloid Pathology, Cognitive Impairment, and Alzheimer Disease Risk--Reply. JAMA. 2015;314(11):1177–1178. doi: 10.1001/jama.2015.9719. [DOI] [PubMed] [Google Scholar]
- 139.Walker L, McAleese KE, Thomas AJ, et al. Neuropathologically mixed Alzheimer's and Lewy body disease: burden of pathological protein aggregates differs between clinical phenotypes. Acta Neuropathol. 2015;129(5):729–748. doi: 10.1007/s00401-015-1406-3. [DOI] [PubMed] [Google Scholar]
- 140.Josephs KA, Whitwell JL, Weigand SD, et al. TDP-43 is a key player in the clinical features associated with Alzheimer's disease. Acta Neuropathol. 2014 doi: 10.1007/s00401-014-1269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]