Abstract
Background
Visceral leishmaniasis (VL), also known as kala-azar in the Indian sub-continent (ISC), is a major public health concern in Bangladesh, India, and Nepal, where it is caused by Leishmania donovani transmitted by the sand fly Phlebotomus argentipes. Various ecological parameters including air temperature, rainfall, wind speed, relative humidity, soil moisture, pH, and organic carbon are known to influence the oviposition of female sand flies, as well as the survival and development of larvae. However, more detailed knowledge on vector behavior, such as biting times, breeding places, and preferred hosts are needed to design optimal evidence-based vector control interventions.
Methods
In order to facilitate rational decisions regarding VL vector control, a systematic review was conducted to identify the prevailing practice and knowledge gaps in relation to vector bionomics and behavior. Search terms included ‘sand fly bionomics’, ‘habitat’, and ‘visceral leishmaniasis/kala-azar vector control’ using the Boolean operator AND to identify the country of interest, namely: Bangladesh, India, and Nepal. Both PubMed and Google search engines were used. Additional unpublished documents in the three countries were also analyzed.
Results
Information on the life cycle of VL vectors, their breeding behavior, infection rate with L. donovani, feeding behavior, and seasonal variation are useful for designing vector control operations. Unfortunately, none of the studies on the life cycle of P. argentipes was conducted in field settings of the ISC, so the publications from other locations had to be used for determining the duration of life cycle and development from egg to adult. However, information about breeding places, seasonal variation of vector densities, and 47 out of the selected 51 papers are available from the ISC and can be used for intelligent design of control operations.
Conclusion
Vector control services should undertake routine insecticide resistance monitoring and adapt indoor residual spraying rounds to the seasonality of vector densities. Further research is needed on potential animal reservoirs for L. donovani, on the breeding habitat, and life cycle of sand flies in the ISC.
Keywords: Kala-azar elimination, Vector characteristics, Sand fly, Phlebotomus argentipes
Introduction
Visceral leishmaniasis (VL), also known as kala-azar in the Indian sub-continent (ISC),1 is a parasitic disease that can be fatal if left untreated.2 It is estimated that globally, approximately 0.2–0.4 million VL cases occur each year. More than 90% of global VL cases occur in six countries: Bangladesh, India, Sudan, South Sudan, Ethiopia, and Brazil, and 20,000–40,000 estimated leishmaniasis deaths occur per year.3 India alone contributes 50% of global cases.4
Sand fly members of the subfamily Phlebotominae, within the Psychodidae family, are the primary vectors of VL, while Phlebotomus argentipes is the predominant vector in the ISC,5,6 Considering this, three countries (Bangladesh, India, and Nepal) have set a target for the elimination of VL by 2015, with the support of the WHO.7 This target was revised in 2014 by the Bangladesh declaration and now includes Bhutan and Thailand in the consortium to eliminate VL by 2017.8 One of the most important tools for VL elimination is integrated vector control,7 used to limit human–vector contact and thus to reduce parasite transmission. Planning effective national vector control programs requires a detailed understanding of the vector characteristics.
P. argentipes is the only vector of Leishmania donovani responsible for VL in the ISC. Various ecological parameters (i.e. air temperature, rainfall, wind speed, relative humidity, soil moisture, temperature, pH, and organic carbon)9 are known to influence the oviposition of gravid females sand flies, as well as the survival and development of larvae.10 Accordingly, sand flies seek microhabitats offering high humidity and favorable temperatures.11
Vector control activities in the three high burden countries (Bangladesh, India, and Nepal) focus on indoor residual spraying (IRS) twice per year, but this is hampered by many adverse factors such as flooding, insufficient allocation of funds, religious festivals, political events, and others. The quality of IRS suffers from a lack of high-quality spray pumps,12 shortage of spare parts, lack of proper supervision, and poor community acceptance,13 which often result in sub-standard insecticide concentrations on the wall.13,14 Use of insecticide-treated long lasting bed-nets would be an alternative option, as non-insecticidal bed-net usage is common and treatment with slow release insecticides would be feasible in a national program; in fact, it was observed in the three countries that 94% of the households were using the impregnated nets after community-based bed-net impregnation program.15 Nevertheless, whichever strategy is used, a thorough understanding of vector behavior and how habitats might change in relation to climate change is critical in designing and delivering effective interventions. Equally important is the change in human attitude toward VL control and the modification of vector control methods. A systematic review was conducted to address these factors and to identify any important knowledge gaps on VL vector bionomics.
Methods
Literature search
PubMed and Google search engines were used to identify relevant documents. The search terms ‘sand fly bionomics’, ‘sand fly habitat’, and ‘visceral leishmaniasis/kala-azar vector control’ were used in conjunction with the Boolean operator AND to identify the country of interest, namely: Bangladesh, India, or/and Nepal. Grey (unpublished) literature was also searched.
Inclusion criteria were studies on VL vector characteristics (globally but with an emphasis on Asia) and on VL vector control focusing at the three target countries (these documents frequently included information on insecticide resistance). Exclusion criteria were VL vector control studies beyond the target countries, publications and non-published documents with doubtful entomological assessments (i.e. studies where the study design, data collection procedure, and analysis were unclear), merely descriptive papers without analytical components, and opinion papers.
Results
Out of the 62 papers and documents identified by the literature search, 51 were included in the study.
Vector of VL (P. argentipes) and its taxonomic status
P. argentipes, the vector of VL in the northeast ISC, is also present in many Asian countries where leishmaniasis is absent. Indeed, it has been suggested that this species is composed of two or more populations, possibly sibling species, with different vectorial capacities.16
In southern Asia, the taxonomic status of the sand fly P. (Euphlebotomus) argentipes, which transmits L. donovani, was reassessed because the variation in morphology, behavior, and distribution suggested that this was a complex of sibling species.16 The putative complex is composed of the nomino-typical members P. argentipes sensu stricto, P. annandalei16 (status revived), and Phlebotomus glaucus16 (new status). An allolectotype (the type specimen of the opposite sex of the lectotype [syn. Lectoallotype]) is designated for the female of P. argentipes, as well as neotypes (a replacement syntype) of the males of P. annandalei and P. glaucus. Morphological descriptions, illustrations, and keys are available for the identification of adult males and females.17–19 Based on female morphological characteristics, P. argentipes, P. annandalei, and P. glaucus can be distinguished from each other using principal component analysis.
P. glaucus is widespread in India, occurring sympatrically (biological species or speciation occurring in the same or overlapping geographical areas without interbreeding) with P. argentipes in L. donovani endemic foci, whereas P. annandeli is peripatric (formation of a new species through evolution) to the type species in Chennai, southern India.
‘In copula’ is a process of post-copulatory sexual selection occurring as correlated coevolution of male and female reproductive traits, which drives species isolation. This pattern has been implicated in reproductive isolation among the members of the P. argentipes complex.16
Life cycle of sand fly
The life cycle of the phlebotomine sand fly (P. argentipes) is presented in Fig. 1.18
Figure 1.
The life cycle of sand fly, Phlebotomus argentipes.
Eggs
The female sand fly (P. argentipes) requires blood meals to lay an average of 32.66 eggs.17 The time lag between the engorgement to oviposition is not less than six days.20 The eggs are elongated oval-shaped, pale at first and darkening following exposure to air with a single black ‘eye spot’. Within one to two weeks of oviposition (unless weather conditions become too cold), eggs hatch. The average incubation period for hatching lasts three–six days, with a mean of 3.96 days at a temperature of 25 ± 2 °C; the hatching rate under these conditions is 67.8%.17 If weather conditions become too cold, the eggs enter diapause during which they do not develop further. The development process restarts once temperature reaches sufficient levels (i.e. around 25 °C).17
Larvae
Originating from aquatic larvae, the larval stages have adapted to live in moist soil, where development takes on average 15.5 days (range 11–29 days).17 The larvae emerge through a J-shaped fissure and are legless, whitish, and with a dark head capsule.21 This usually occurs when humidity levels approach 100%, leading to soil moisture of ~12%.
The larvae possess a cylindrical, elongated, and segmented body. The first instar can be distinguished by the presence of two caudal bristles, while all subsequent instars have four caudal bristles. All larvae feed on dead organic matter and are often found in cracks of walls or rocks, animal burrows, caves, or below decaying leaves. The larvae cannot exist without water in the fluid form, but water may be bound by capillary action. The larvae, particularly younger instars, absorb water with their food and through the skin.17 In the urban areas, the larvae are found in the floors, loose bricks, and rat holes.17 Fourth instars can be distinguished by the presence of a prominent sclerite on the dorsum of the penultimate segment.
Pupae
Following the larval stage, the developing sand fly (P. argentipes) enters the pupal stage on floating debris or near the water’s edge. The pupae are golden brown and are affixed to the surface of the substrate on which they develop. Shortly before emergence, the wings and eyes turn black. The developmental period for pupal stage ranges from 6 to 10 days, with a mean of 7.65.17 Adults emerge just before dawn.22
Adults
Male sand flies emerge about 24 h before females, allowing time to rotate their external genitalia 180° to the correct position for mating.23 Although there have been no field studies of sand fly development time, the time from oviposition to adult emergence at ambient temperatures is around four–six weeks.21 Adult emergence takes place at a temperature of 25 ± 2 °C. During winter months (December–February), the development period decreases with the duration of each life cycle, from 25 to 40 days per generation.24
Only the adult female sand fly (P. argentipes) sucks vertebrate host’s blood, a requirement for egg production. Both males and females feed on plant sugars. The adults of P. argentipes are 2–3 mm long and because sand flies are so small, they have nicknames such as ‘no see ums’ and ‘punkies’. They cannot fly hence display a characteristic ‘hopping movement’ and consequently cannot move more than 106 meters from their breeding place,25 though they have been recorded from canopy of trees.26 Under favorable conditions, development from egg to adult takes approximately one month.18
Habitat and sampling
Research on vector breeding places is cumbersome and therefore scarce. Only 7.6% of the 144 soil samples collected from human dwellings, tree holes, cattle sheds, and mixed dwellings were positive for larvae of P. argentipes and other sand fly species.27 In another study in India, only 12 larvae (19.5% of soil samples) were found on earthen pots containing scrapings from cowshed floors and moist sand beds.28 In South India, extensive sampling was necessary to show that P. argentipes breeds in debris and at the base of shrubs.29 In Bihar, from a total of 204 soil samples collected from adult resting sites in human dwellings and cow sheds, only 5.4% were positive for immature P. argentipes and P. papatasi.30 Out of the 40 soil samples collected from various sites in cattle sheds, 13 larvae of P. argentipes were found in the four (10% of total samples) samples collected from the corners.31 In a VL endemic focus of Bihar, the highest number of positive soil samples was collected from cattle sheds, less in mixed dwellings, and none from human dwellings.32 In Nepal, the samples were collected from corners, feeding troughs, and near cattle tying poles of cattle sheds, but only 17.5, 25 and 30% of the samples, respectively, were positive for immature stages.33 A study in Bihar showed that intra-domestic soil was infested with two species of sand flies, P. argentipes (Annandale and Brunetti) and P. papatasi (Scopoli).
A recent longitudinal study in Bihar (unpublished data; Vijay Kumar) revealed that P. argentipes could only be detected in a small proportion of indoor samples, but none in outdoor samples. This was confirmed by another study from India with a few soil samples being positive in cattle sheds and human dwellings.34 In summary, on the basis of larval collections, it appears that P. argentipes prefers to breed in cattle sheds rather than in human houses. This preference appears to be associated with the alkaline soil of cattle sheds. In contrast, P. papatasi prefers breeding in the soil of human houses with neutral pH.35
Impact of microclimates on habitat
A cross-sectional survey was conducted to find the suitable locations of P. argentipes in relation to environmental characteristics between VL endemic and VL non-endemic districts in India. The above-noted study’s findings indicated that indoor temperature and relative humidity were the best predictors for P. argentipes distribution. The final model was highly significant (p value <0.0001). Factor analysis confirmed breeding preferences by P. argentipes. Soil covered by vegetation (expressed as Minimum Normalized Vegetation Index), marshy land, orchards, and settlements showed high sand fly breeding in an endemic region, whereas water bodies and dense forests were preferred sand fly breeding places in non-endemic areas (Bihar and Jharkhand). Soil examinations showed that soil pH and moisture were higher in VL endemic sites compared to non-endemic sites. The pH of the soil in breeding locations was alkaline (range 7.50–8.50, mean 7.84, SD 0.30) in the endemic site; however, in the non-endemic site, the pH was slightly acidic (range 6.05–6.18, mean 6.12 SD 0.06). The moisture content in the endemic site was much higher (12.2%) than in the non-endemic site, which symbolized suitable conditions for vector habitation.36 Temperature from 20 to 24.9° C had a highly significant effect on P. argentipes density in Nepal.37
VL infection rates of sand flies
In Nepal, PCR amplifications were made on 135 pools of P. argentipes and confirmed by sequencing. The infection rate of vectors ranged from 0.47 to 0.58% for Leishmania parasites.38
Feeding behavior
Most or all species of sand flies probably feed on plant sugars, while in general, female Phlebotomus feed on mammalian blood.17 In nature, most species of insects are gonotropically concordant, taking one blood meal for each batch of egg maturation. However, autogeny (the ability to lay eggs without blood meal) has been reported in other species of sand flies but not in P. argentipes.39 Regarding P. argentipes, the reports are conflicting; in some areas, more sand flies were caught biting cattle, while in others, humans were found to be the preferred host. In West Bengal, during the post-DDT era, species showed increased zoophily, which was attributed to diversion toward cattle by irritability to DDT. Furthermore, sampling either from cowsheds or human habitation gave variable results, indicating that host preference of P. argentipes varies widely in different biotopes.40 In Nepal, more P. argentipes were collected from cattle sheds than from human dwellings using three techniques, namely: morning indoor hand (aspirator) collection, pyrethrum spray sheet collections, and sticky trap collections. In cattle sheds, the mean density of P. argentipes was 10.21/man-hour, which was significantly higher than for other recorded species.37 Man-hour density of P. argentipes varied between 5.36–10.96 in North Bihar and 11.20–21.40 in Patna/Nalanda districts, where sand flies were collected using standard aspirators.41 In West Bengal, human blood was isolated from 21.5 and 69.6% of P. argentipes found in cowsheds and human dwellings, respectively.40 The same was true in case of the bovid blood index: 97.9% prevalence in cowsheds and 44.0% prevalence in human dwellings. The study highlighted that P. argentipes is an indiscriminate feeder and feeds on the host that is in the immediate vicinity. Another study in West Bengal showed that P. argentipes was mainly zoophilic and exophilic, and was found in the living rooms of houses both in urban and rural areas, but fed on humans in the absence of bovines.42 A similar type of observation was made in Bihar where P. argentipes preferred to feed on bovine hosts, secondly humans, and thirdly avian spp.43 This was also reported by another study in Bihar where P. argentipes was primarily zoophilic, preferring animal bait seven times more than the human bait.44 Blood meals of 304 P. argentipes and 206 P. papatasi, collected from different biotopes from two VL affected districts in West Bengal, were tested against seven different antisera by modified Ouchterlony gel diffusion techniques. It appeared that the host preference of P. argentipes varied widely in different biotopes, but was mainly zoophilic (62.80%), preferring to feed on man as the second choice (24.92%); however it is also a ‘chance feeder’ according to biotopes (a usually small or well-defined area that is uniform in environmental conditions and in its distribution of animal and plant life). Multiple blood meals are also prevalent in P. argentipes at a much higher rate than that of P. papatasi.45 However, blood meal analyses proved anthropophilic and zoophilic tendencies of P. argentipes.28 Another study from Bihar highlighted that sand flies mostly fed on humans, followed by cattle, buffalo, and goats;46 these findings conflicted with another study that was conducted in same state.43 P. argentipes from northeastern India was shown to be more exophilic and exophagic than previously reported.26 In Nepal, light traps were used on palm trees, banana plantations, bamboo groves, paddy fields, vegetable fields, and mixed vegetation across five different villages. No P. argentipes were collected from palm trees, but 1.7 and 0.33 P. argentipes per light trap per night were collected from banana plants in two villages (unpublished data, ML Das). This suggests that in India, P. argentipes has adapted to an exophilic and exophagic behavior probably due to DDT pressure. An entomological study in Eastern Nepal showed that night biting activity of P. argentipes was at its peak during midnight.47
Human risk factors for infection
Poor housing conditions, lack of waste management, and open sewerage at the household level may increase sand fly breeding and resting sites, as well as their access to humans. Sand flies are attracted to crowded housing due to a greater number of hosts.48
A study in Nepal showed that sleeping outside in warmer months increased the risk (CI: 1.0–4.1; p = 0.05) of infection.49 Sleeping outside was associated with increased risk (OR: 4.7, p = 0.004) of disease in Uttar Pradesh, India.50 Unfortunately, no information is available for sleeping practices in Bangladesh.
Seasonal variation of vector densities
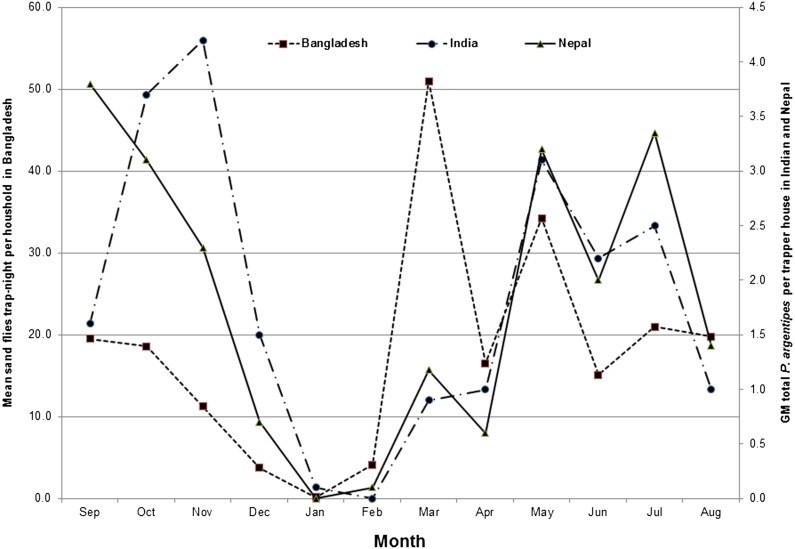
In Bihar, the maximum number of immature stage of sand flies in soil samples was found in January, and the minimum in September.32 The emergence of adult P. argentipes from soil samples was observed from April to October.35 A study in West Bengal highlighted that sand flies were present throughout the year with minimum abundance in winter and maximum during monsoon and post-monsoon months.51 In Bihar, seasonal fluctuations in sand fly density showed peaks in November and from May to August. The highest proportions of gravid female sand flies were captured during May. Of the sand flies trapped, the majority was P. argentipes (72.1% of 52,653 sand flies).52 In Bangladesh, the peak sand fly densities were found in March. From December to February, the density was low due to cool temperatures (Fig. 2).53 In Nepal, the density of P. argentipes showed two peaks, one during April and May and another in September and October.47 The seasonality of P. argentipes is similar in India and Nepal, with two annual density peaks around May and October (Fig. 2).54 Density of P. argentipes was significantly higher in summer (March to June) in comparison to monsoon months (July–October) and winter (November–February).37 Thus, the most intense transmission of L. donovani in the ISC occurs during two periods: a pre-winter peak in September–November and a post-winter peak in March–May.55
Figure 2.
Seasonality and monthly variation of Phlebotomus sand fly density (Bangladesh) and P. argentipes (India and Nepal) observed per household trap during night by month: from September 2002 to August 2003 (Bangladesh) and 2006 to 2007 (India and Nepal).
Timing for vector control by IRS
Effective vector management programs are needed mostly when weather conditions favor increased sand fly abundance, which increases the likelihood of host–vector contact and the transmission of Leishmania parasites. IRS involves coating the walls and other surfaces of houses and animal dwellings. For several months, the insecticide will kill all susceptible insects that come in to contact with these surfaces. Accordingly, IRS reduces VL transmission by decreasing the sand fly survival.55
Timing of spraying is important. The residual activity of the insecticide must last through the periods of high vectorial density and hence intense VL transmission or else the spraying must be repeated. Maximum coverage of spraying is important to obtain a mass effect, i.e. also protecting persons in houses who were not sprayed in that area. According to operational research findings, the district program managers in India and Nepal reported that IRS should be carried out ideally before the peak vector season which is in May and November in India; April/May and September/October in Nepal; and March in Bangladesh. Considering this, the first cycle and second cycle of IRS should be carried out in February/March and May/June, respectively, in India, while in Nepal, the first cycle should be in March–May, and the second cycle in August–October.56 In Bangladesh, March/April and August/September serve as ideal seasons for treating the area with first and second cycles of IRS, respectively.57 In India, due to technical reasons viz., rainy season, festive seasons, flood, etc., the time gap between the execution of first and second cycles of IRS remained too short to cover the rest of the year, also inviting the situation of vector resurgence at the sprayed site. Hence, for maintaining the efficacy of IRS spraying, as well as controlling vector population, IRS should be carried out at regular intervals of six months57, depending on the residual decay rate of the insecticide and the peaks of vectorial capacity.
Insecticide resistance
Since 1990 and 1994, the use of DDT for VL vector control was completely banned in Nepal and Bangladesh, respectively, while it is still in use in India. The current synthetic pyrethroids used for VL vector control are: deltamethrin in Bangladesh, alpha cypermethrin in Nepal, and alpha cypermethrin on a pilot basis in India. A study showed that the vectors in Bangladesh were highly susceptible and the mortality at 24 h was 100% to deltamethrin 5WP.53 In Nepal, the susceptibility of vectors to deltamethrin 0.05% was between 96 and 100% and was much lower (mortality 62%) in bordering areas with India (of Bihar state).58 A literature survey covering the period 1978–2014 showed decreasing susceptibility level to DDT for P. argentipes in Bihar state and emerging decreased susceptibility to malathion (in one of the three studies), and deltamethrin (one in four studies).14
Discussion
The findings in our review contribute important information to design an effective vector control strategy in the three target countries (Bangladesh, India, and Nepal) (Table 1). Detailed knowledge of seasonal variation of vector densities, biting time and biting places, as well as host preferences can improve our understanding of prospects and limitations of certain interventions, and direct us to tailor effective control operations.
Table 1.
Summary of findings which are relevant for designing the vector control strategy in Bangladesh (BA), India (IN), and Nepal (NE)
| Topic area | Findings | Relevance for vector control |
|---|---|---|
| Seasonal variation of vector density |
|
IRS activities adapted to seasonal variation |
| Biting times |
|
Night biting increases effect of LNs |
| Biting places |
|
No effect of IRS and LN on outdoor biting; vector biting in cattle sheds reduces man–vector contact but also the effect of IRS and LNs |
| Host preference |
|
Preference for cattle reduces man biting: zoo-prophylaxis may be considered |
Lack of entomological information in the ISC
Within the Phlebotomine sub-family, two genera (genus Lutzomyia in New World and Phlebotomus in Old World) are responsible for transmitting different forms of leishmaniasis, contributing a huge burden to human public health. P. argentipes is the established vector of VL in Bangladesh, India, Nepal, and recently Bhutan.59–62 However, we identified an information gap regarding the VL vectors on the ISC. There is no study on the life cycle of P. argentipes in nature. The difficulty of identifying typical vector breeding sites is an important constraint on vector control measures.
Considering the above-mentioned larval studies, it seems that knowledge and understanding of the breeding behavior of the vector P. argentipes in the ISC is still incomplete55 and often the exact breeding habitats are unknown. However, it is likely that cattle sheds are important habitats for producing VL vectors. Therefore, larvae control activities can be focused on cattle sheds without ignoring other possible breeding habitats to optimize the effects of larviciding as part of integrated vector management.
IRS: information needs
Very limited information is available on the impact of IRS on VL transmission in the three countries (Bangladesh, India, and Nepal). IRS is an expensive method compared to other vector control measures63 and requires intensive supervision. In the ISC, IRS has been shown to be very effective in reducing sand flies when conducted under strict supervision.64 However, it has also been shown that the outcome of IRS did not achieve optimum results when applied by national control programs in India and Nepal.56 Recent studies show the poor performance of IRS in national control programs on the ISC.13,14 Information on the seasonality of P. argentipes is important for timing the IRS program. As mentioned in our analysis, India and Nepal have two annual density peaks around May and October, while these peaks occur in March and September/October in Bangladesh53 and are positively associated with temperature and negatively associated with rainfall in both study sites.65 The multivariate climate model explained 57% of the monthly vector abundance. Considering the above-noted information and cross-border issues, IRS operations should be harmonized in the three countries (Bangladesh, India, and Nepal) to achieve the highest level of impact. It would be useful to conduct the national IRS first cycle between February and March (pre-monsoon) and the second cycle from July to September (post-monsoon). At the same time, national programs should reinforce the use of the monitoring and evaluation toolkits, developed for national IRS operation.57 These were tested in the field in the three countries and were found to be user friendly.13
Insecticide-treated bed-nets: information needs
The use of LLINs is another potential method of VL vector control, which has proven effective for reducing VL transmission in Bangladesh.66,67 A study in India and Nepal showed different results: random effects linear regression modeling showed that the cluster-wise distribution of LLINs significantly reduced the P. argentipes density/house by 24.9% (95% CI 1.80–42.5%) as measured by CDC light traps.68 There was however no significant difference in the risk of seroconversion over 24 months in the intervention compared with control clusters, and adjustment for covariates did not alter these conclusions.69 The use of untreated nets reduced the blood-feeding rate by 85% and human blood index by 42.2%, providing circumstantial evidence that untreated nets may provide some degree of personal protection against sand fly bites.70 In Nepal, a statistically significant correlation between antibodies to P. argentipes’ saliva and the average indoor density of female sand flies was observed, suggesting that vector densities are correlated with transmission risk. Additionally, changes in vector exposure were detected when sera from VL patients were assayed before, during, and after being protected from sand fly bites by untreated bed-nets.71 The efficacy of long lasting insecticide-treated nets (PermaNet 2.0, Vestergaard-Frandsen, Denmark) was tested in India and Nepal after two years of use against sand fly vectors. Between 63% (India) and 78% (Nepal) of the nets that had been distributed among the population developed holes over the course of two years.72 Deltamethrin residues fell from 55 mg/m2 to an average of 11.6 mg/m2 (India) and 27.9 mg/m2 (Nepal). However, on the basis of bioassay criteria, all LLINs tested met the WHO Pesticide Evaluation Scheme standard for LLIN effectiveness.72 After two washes during 18 months of use, the mean insecticide residues on PermaNet 2.0 and Olyset Net were 53.5 mg/m2 (97.3% of the target dose) of deltamethrin and 911.8 mg/m2 (91.2% of the target dose) of permethrin, respectively. These residues were close to the insecticide loads specified by the manufacturers of the two LLINs.73
Insecticide susceptibility: information needs
The success of any insecticide-based vector control program mostly depends on the response of the vector to particular insecticides. The current available information regarding insecticide susceptibility for controlling VL vectors in the three countries is incomplete and lacks updating which would guide vector control services to adjust the insecticides in use. Therefore, it is crucial to conduct insecticide-susceptibility assays.
In conclusion, insecticide resistance monitoring should be regularly undertaken by the vector control services and IRS spraying rounds should be better adapted to the seasonal variations of vector densities. The research community should be encouraged to collect evidence on the potential role of animal reservoirs for L. donovani and on the breeding habitat of sand flies. This will help better understand the transmission dynamics and improve vector control activities in the ISC.
Competing interests
The authors declare that they have no competing interests.
Notes on contributors
Rajib Chowdhury has made substantial contributions to conception and design; has collected information for review in this manuscript; has been involved in drafting the manuscript; and has given the final approval of the version to be published.
Vijay Kumar has been involved in drafting and revising the manuscript; has given the final approval of the version to be published.
Dinesh Mondal has been involved in drafting and revising the manuscript; has collected information for review in this manuscript; and has given the final approval of the version to be published.
Murari Lal Das has been involved in drafting and revising the manuscript; has given the final approval of the version to be published.
Pradeep Das has revised the manuscript critically for the content; has given the final approval of the version to be published.
Aditya Prasad Dash has revised the manuscript critically for important intellectual content; has given the final approval of the version to be published.
Axel Kroeger has made substantial contributions to the concept and design; has revised the manuscript at different stages, critically; and has given the final approval of the version to be published.
Acknowledgments
We wish to thank the Special Programme for Research and Training in Tropical Diseases (TDR-WHO) for the continued support.
References
- 1.Ahluwalia IB, Bern C, Costa C, Akter T, Chowdhury R, Ali M, et al. Visceral leishmaniasis: consequences of neglected disease in a Bangladeshi community. Am J Trop Med Hyg. 2003;69(6):624–8. [PubMed] [Google Scholar]
- 2.Bern C, Allen W, Chowdhury R, Ali M, Aman J, Wagatsuma Y, et al. Risk factors for kala-azar in Bangladesh. Emerg Infect Dis. 2005;11(5):655–62. 10.3201/eid1105.040718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvar A, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization-Regional Office for South-East Asia Regional technical advisory group for the kala-azar elimination programme. Report of the Fifth Meeting, Paro, Bhutan; 2013. New Delhi, India: (SEA-CD-280). [Google Scholar]
- 5.Sharma DA, Bern C, Varghese B, Chowdhury R, Haque R, Ali M, et al. The economic impact of visceral leishmaniasis on households in Bangladesh. Tropical Medicine and International Health. 2006;11(5):757–764. 10.1111/tmi.2006.11.issue-5 [DOI] [PubMed] [Google Scholar]
- 6.Rijal S, Koirala S, Van der Stuyft P, Boelaert M. The economic burden of visceral leishmaniasis for households in Nepal. Trans R Soc Trop Med Hyg. 2006;100(9):838–41. 10.1016/j.trstmh.2005.09.017 [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization Regional strategic framework for elimination of kala-azar from South East Asia region (2005–2015). SEA-VBC-85-REV-1 New Delhi, India; 2005. [Google Scholar]
- 8.WHO Regional Committee for South-East Asia Report of the sixty-seventh session, Dhaka, Bangladesh; 2014 Sept 9–12; New Delhi, India: World Health Organization, Regional Office for South-East Asia; 2014. p. 1–149. [Google Scholar]
- 9.Ghosh K, Mukhopadhyay J, Desai MM, Senroy S, Bhattacharya A. Population ecology of Phlebotomus argentipes (Diptera: Psychodidae) in West Bengal, India. J Med Entomol. 1999;36(5):588–94. 10.1093/jmedent/36.5.588 [DOI] [PubMed] [Google Scholar]
- 10.Sivagnaname N, Amalraj DD. Breeding habitats of vector sand flies and their control in India. J Commun Dis. 1997;29(2):153–9. [PubMed] [Google Scholar]
- 11.Kaul SM. Phlebotomine sand flies (Diptera: Psychodidae) from Khandwa and Hoshangabad Districts of Madhya Pradesh, India. J Commun Dis. 1991;23(4):257–62. [PubMed] [Google Scholar]
- 12.Kumar V, Kesari S, Chowdhury R, Kumar S, Sinha G, Hussain S, et al. User friendliness, efficiency and spray quality of stirrup pumps versus hand compression pumps for indoor residual spraying. Indian J Med Res. 2013;138:239–43. [PMC free article] [PubMed] [Google Scholar]
- 13.Huda MM, Mondal D, Kumar V, Das P, Sharma SN, Das ML, et al. Toolkit for monitoring and evaluation of indoor residual spraying for visceral leishmaniasis control in the Indian sub-continent: application and results. J Trop Med. 2011;2011:1– 11. doi: 10.1155/2011/876742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman M, Foster GM, Deb R, Singh RP, Ismail HM, Shivamb P, et al. DDT-based indoor residual spraying suboptimal for visceral leishmaniasis elimination in India. PNAS. 2015;112(28):8573–8. doi: 10.1073/pnas.1507782112 10.1073/pnas.1507782112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mondal D, Das ML, Kumar V, Huda MM, Das P, Ghosh D, et al. Estimating efficacy, cost, mass safety and acceptability of DWL, ITN and EM for controlling sand fly in the Indian subcontinent: a multi-country cluster randomized controlled trial (submitted for publication). [Google Scholar]
- 16.Ilango K. A taxonomic reassessment of the Phlebotomus argentipes species complex (Diptera: Psychodidae: Phlebotominae). J Med Entomol. 2010;47(1):1–15. 10.1603/033.047.0101 [DOI] [PubMed] [Google Scholar]
- 17.Kalra NL, Bang YH. 1988. Manual on entomology in visceral leishmaniasis. World Health Organization-Regional Office for South East Asia: New Delhi, India; SAE/VBC/35, p. 88. [Google Scholar]
- 18.Lewis DJ. The phlebotomine sand flies (Diptera: Psychodidae) of oriental region. Bull Br Mus (Nat Hist) Entomol. 1978;37(6):217–343. [Google Scholar]
- 19.Lewis DJ. A taxonomic review of the genus phlebotomus (Diptera: Psychodidae). Bull Br Mus (Nat Hist). 1982;45(2):121–209. [Google Scholar]
- 20.Killick–Kendrick R, Killick–Kendrick M. Biology of sand fly vectors of Mediterranean canine leishmaniasis. Proceedings of a canine leishmaniasis Forum; 1999 Jan 28–31; Barcelona (Sitges); 1999. p. 26–31. [Google Scholar]
- 21.Public Health Vectors and Pests (Sand fly) [cited 2016 Jan]. Available from: http://www.kznhealth.gov.za/environ/vector/sandfly.htm
- 22.IRAC: Resistance Management for Sustainable Agriculture and Improved Public Health [cited 2015 Feb]. Available from: http://www.irac-online.org/pests/sandfly-species/
- 23.Introduction to sand flies - life cycle [cited 2014 Jun]. Available from: http://pcwww.liv.ac.uk/leishmania/life_cycle__habitats.htm (accessed 2008 December 17)
- 24.Kumar V, Kesari S, Kumari K, Krishnakumari B, Venugopalan R, Das P. Comparison of in-vivo host animals as a blood-feeding source for laboratory rearing of the sand fly vector Phlebotomus argentipes (Diptera: Psychodidae). Ann Entomol Soc Am. 2011;104(3):429–33. doi: 10.1603/AN10045 [DOI] [Google Scholar]
- 25.Public Health Vectors and Pests (Sand fly) [cited 2013 Jun]. Available from: http://www.kznhealth.gov.za/environ/vector/sandfly.htm
- 26.Poché RM, Garlapati R, Elnaiem DE, Perry D, Poché D. The role of Palmyra palm trees (Borassus flabellifer) and sand fly distribution in northeastern India. J Vector Ecol. 2012;37(1):148–53. doi: 10.1111/j.1948-7134.2012.00211.x [DOI] [PubMed] [Google Scholar]
- 27.Dhiman RC, Shetty PS, Dhanda V. Breeding habitats of phlebotomine sand flies in Bihar, India. Indian J Med Res. 1983;77:29–32. [PubMed] [Google Scholar]
- 28.Hati AK. Current status of leishmaniasis -vector biology. In: Mahajan RC, editor. Proceedings of the Indo-UK workshop on leishmaniasis; 1982 Dec 6–10; New Delhi: Indian Council of Medical Research; 1983. p. 84–91. [Google Scholar]
- 29.Rahman SJ, Menon PK, Rajagopal R, Mathur KK. Behaviour of Phlebotomus argentipes in the foothills of Nilgiris (Tamil Nadu), South India. J Commun Dis. 1986;18(1):35–44. [PubMed] [Google Scholar]
- 30.Kesari S, Palit A, Kishore K. Study of breeding habitats of sand flies–preliminary approach. J Commun Dis. 1992;24(1):62–3. [PubMed] [Google Scholar]
- 31.Kundu M, Basak B, Tandon N. A simple technique for detection and isolation of Phlebotomus argentipes larvae from soil samples. J Commun Dis. 1995;27(1):58–9. [PubMed] [Google Scholar]
- 32.Kesari S, Kishore K, Palit A, Kumar V, Roy MS, Sivakumar S, et al. An entomological field evaluation of larval biology of sand fly in Kala-azar endemic focus of Bihar–exploration of larval control tool. J Commun Dis. 2000;32(4):284–8. [PubMed] [Google Scholar]
- 33.Das ML, Roy L, Singh J. Preferential breeding sites of kala-azar vector, Phlebotomus argentipes in Nepal. Int J Trop Agric. 2008;26(3–4):323–7. [Google Scholar]
- 34.Sivagnaname N. Concern on vector control in kala-azar. Indian J Med Res. 2006;124:453. [PubMed] [Google Scholar]
- 35.Singh R, Lal S, Saxena VK. Breeding ecology of visceral leishmaniasis vector sand fly in Bihar state of India. Acta Trop. 2008;107(2):117–20. doi: 10.1016/j.actatropica.2008.04.025 (accessed 2008 August) [DOI] [PubMed] [Google Scholar]
- 36.Kesari S, Bhunia GS, Kumar V, Jeyaram A, Ranjan A, Das P. A comparative evaluation of endemic and non-endemic region of visceral leishmaniasis (kala-azar) in India with ground survey and space technology. MemInst Oswaldo Cruz. 2011;106(5):515–23. 10.1590/S0074-02762011000500001 [DOI] [PubMed] [Google Scholar]
- 37.Das ML. Studies on Phlebotomus argentipes Annandale & Brunetti (Diptera: Psychodidae). Vector of kala-azar in Eastern Part of Nepal. PhD thesis submitted in BHU; Varanasi, India; 2004. p. 1–205. [Google Scholar]
- 38.Bhattarai NR, Das ML, Rijal S, Auwera GVD, Picado A, Khanal B, et al. Natural infections of Phlebotomus argentipes with Leishmania and other trypanosomatids in a visceral leishmaniasis endemic region of Nepal. Trans R Soc Trop Med Hyg. 2009;103(11):1087–92. doi: 10.1016/j.trstmh.2009.03.008 [DOI] [PubMed] [Google Scholar]
- 39.Dinesh DS, Kumar V, Kesari S, Kumar AJ, Das P. Is Phlebotomus argentipes Annandale and Brunetti (Diptera: Psychodidae) autogenous? J Vector Borne Dis. 2008;45:174–5. [PubMed] [Google Scholar]
- 40.Addy M, Mitra AK, Ghosh KK, Hati AK. Host preference of Phlebotomus argentipes in different biotopes. Trop Geogr Med. 1983;35(4):343–5. [PubMed] [Google Scholar]
- 41.Kumar V, Kesari S, Kumar AJ, Dinesh DS, Ranjan A, Prasad M, et al. Vector density and the control of kala-azar in Bihar, India. Mem Inst Oswaldo Cruz. 2009;104(7):1019–22. [DOI] [PubMed] [Google Scholar]
- 42.Das S, Boreham PF, Bhattacharya NC, Sen Gupta PC. Prevalence and blood meal sources of Phlebotomus argentipes in West Bengal in 1972–73. Indian J Med Res. 1976;64(9):1307–13. [PubMed] [Google Scholar]
- 43.Mukhopadhyay AK, Chakravarty AK. Bloodmeal preference of Phlebotomus argentipes& Ph. papatasi of north Bihar, India. Indian J Med Res. 1987;86:475–80. [PubMed] [Google Scholar]
- 44.Dinesh DS, Ranjan A, Palit A, Kishore K, Kar SK. Seasonal and nocturnal landing/biting behaviour of Phlebotomus argentipes(Diptera: Psychodidae). Ann Trop Med Parasitol. 2001;95(2):197–202. [DOI] [PubMed] [Google Scholar]
- 45.Palit A, Bhattacharya SK, Kundu SN. Host preference of Phlebotomus argentipes and Phlebotomus papatasi in different biotopes of West Bengal, India. Int J Environ Health Res. 2005;15(6):449–54. 10.1080/09603120500392525 [DOI] [PubMed] [Google Scholar]
- 46.Garlapati RB, Abbasi I, Warburg A, Poché D, Poché R. Identification of blood meals in wild caught blood fed Phlebotomus argentipes (Diptera: Psychodidae) using cytochrome b PCR and reverse line blotting in Bihar, India. J Med Entomol. 2012;49(3):515–21. 10.1603/ME11115 [DOI] [PubMed] [Google Scholar]
- 47.Das ML, Karki P, Koirala S, Parija SC. Entomological study of sand fly vector of kala-azar in Eastern Nepal. Health Renaissance. 2007;1(4):23–8. [Google Scholar]
- 48.Leishmaniasis, World Health Organization [cited 2016 Jan]. Available from: http://www.who.int/mediacentre/factsheets/fs375/en/ (accessed 2016 March)
- 49.Bern C, Joshi AB, Jha SN, Das ML, Hightower A, Thakur GD, et al. Factors associated with visceral leishmaniasis in Nepal: bed-net use is strongly protective. Am J Trop Med Hyg. 2000;63(3, 4):184–8. [DOI] [PubMed] [Google Scholar]
- 50.Barnett PG, Singh SP, Bern C, Hightower AW, Sundar S. Virgin soil: the spread of visceral leishmaniasis into Uttar Pradesh, India. Am J Trop Med Hyg. 2005;73(4):720–5. [PubMed] [Google Scholar]
- 51.Ghosh K, Mukhopadhyay J, Desai MM, Senroy S, Bhattacharya A. Population ecology of Phlebotomus argentipes (Diptera: Psychodidae) in West Bengal, India. J Med Entomol. 1999;36(5):588–94. 10.1093/jmedent/36.5.588 [DOI] [PubMed] [Google Scholar]
- 52.Poché D, Garlapati R, Ingenloff K, Remmers J, Poché R. Bionomics of phlebotomine sand flies from three villages in Bihar, India. J Vector Ecol. 2011;36(S1):S106–17. 10.1111/j.1948-7134.2011.00119.x [DOI] [PubMed] [Google Scholar]
- 53.Chowdhury R, Dotson E, Blackstock AJ, McClintock S, Maheswary NP, Faria S, et al. Comparison of insecticide-treated nets and indoor residual spraying to control the vector of visceral leishmaniasis in Mymensingh District, Bangladesh. Am J Trop Med Hyg. 2011;84(5):662–7. doi: 10.4269/ajtmh.2011.10-0682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Picado A, Das ML, Kumar V, Dinesh DS, Rijal S, Singh SP, et al. Phlebotomus argentipes seasonal patterns in India and Nepal. J Med Entomol. 2010;47(2):283–6. doi: 10.1603/ME09175 [DOI] [PubMed] [Google Scholar]
- 55.Sharma U, Singh S. Insect vectors of Leishmania: distribution, physiology and their control. J Vector Borne Dis. 2008;45:255–72. [PubMed] [Google Scholar]
- 56.Chowdhury R, Huda MM, Kumar V, Das P, Joshi AB, Banjara MR, et al. The Indian and Nepalese programmes of indoor residual spraying for the elimination of visceral leishmaniasis: performance and effectiveness. Ann Trop Med Parasitol. 2010;105(1):31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Special Programme for Research and Training in Tropical Diseases (WHO/TDR) Monitoring and evaluation tool kit for indoor residual spraying: kala-azar elimination in Bangladesh, India and Nepal. Geneva, Switzerland: World Health Organization; 2010. Available from: http://apps.who.int/tdr/svc/publications/tdrresearchpublications/irstoolkit. [Google Scholar]
- 58.Dinesh DS, Das ML, Picado A, Roy L, Rijal S, Singh SP, et al. Insecticide susceptibility of Phlebotomus argentipes in visceral leishmaniasis endemic districts in India and Nepal. PLoS Neglected Trop Dis. 2010;4(10):e859(1–5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swaminath CS, Shortt HE, Anderson LAP. Transmission of Indian kala-azar to man by bites of Phlebotomus argentipes Annandale & Brunetti. Indian J Med Res. 1942;30:473–7. [PubMed] [Google Scholar]
- 60.Dinesh DS, Kar SK, Kishore K, Palit A, Verma N, Gupta AK, et al. Screening sand flies for the natural infection with Leishmania donovani, using a non-radioactive probe based on the total of the parasite. Ann Trop Med Parasitol. 2000;94:447–51. [DOI] [PubMed] [Google Scholar]
- 61.Bhattacharya SK, Rinzin N, Chusak P, Dash AP, Chowdhury R, Tobgay T, et al. Occurrence and significance of kala-azar in Bhutan. Indian J Med Res. 2010;132:337–8. [PubMed] [Google Scholar]
- 62.Yangzom T, Cruz I, Bern C, Argaw D, den Boer M, Velez ID, et al. Endemic transmission of visceral leishmaniasis in Bhutan. Am J Trop Med Hyg. 2012;87(6):1028–37. doi: 10.4269/ajtmh.2012.12-0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Das M, Banjara MR, Chowdhury R, Kumar V, Rijal S, Joshi AB, et al. Visceral leishmaniasis on the Indian sub-continent: a multi-center study of the cost of three interventions for the control of the sand fly vector, Phlebotomus argentepis. Ann Trop Med Parasitol. 2008;102(8):729–41. 10.1179/136485908X355274 [DOI] [PubMed] [Google Scholar]
- 64.Joshi AB, Das ML, Akhter S, Chowdhury R, Mondal D, Kumar V, et al. Chemical or non-chemical vector controls a contribution to the elimination of Visceral leishmaniasis on the Indian subcontinent: cluster randomized trials in Bangladesh, India and Nepal. BMC Med. 2009;7:54. doi: 10.1186/1741-7015-7-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Picado A, Das ML, Kumar V, Dinesh DS, Rijal S, Singh SP, et al. Phlebotomus argentipes seasonal patterns in India and Nepal. J Med Entomol. 2010;47(2):283–6. 10.1603/ME09175 [DOI] [PubMed] [Google Scholar]
- 66.Mondal D, Chowdhury R, Huda MM, Maheswary NP, Akther S, Petzold M, et al. Insecticide-treated bed nets in rural Bangladesh: their potential role in the visceral leishmaniasis elimination programme. Trop Med Int Health. 2010;15(11):1382–9. doi: 10.1111/j.1365-3156.2010.02635.x [DOI] [PubMed] [Google Scholar]
- 67.Mondal D, Huda MM, Karmoker MK, Ghosh D, Matlashewski G, Nabi SG, et al. Reducing visceral leishmaniasis by insecticide impregnation of bed-nets, Bangladesh. Emerg Infect Dis. 2013;19:1331–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Picado A, Das ML, Kumar V, Kesari S, Dinesh DS, Roy L, et al. Effect of village-wide use of long-lasting insecticidal nets on visceral leishmaniasis vectors in India and Nepal: a cluster randomized trial. PLoS Neglected Trop Dis. 2010;4(1):e587. 10.1371/journal.pntd.0000587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Picado A, Singh SP, Rijal S, Sundar S, Ostyn B, Chappuis F, et al. Longlasting insecticidal nets for prevention of leishmania donovani infection in India and Nepal: paired cluster randomised trial. BMJ. 2010;341:c6760. doi: 10.1136/bmj.c6760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Picado A, Kumar V, Das M, Burniston I, Roy L, Rijal S, et al. Effect of untreated bed nets on blood fed Phlebotomus argentipes in kala azar endemic foci in Nepal and India. MemInst Oswaldo Cruz. 2009;104(8):1183–6. [DOI] [PubMed] [Google Scholar]
- 71.Clements MF, Gidwani K, Kumar R, Hostomaska J, Dinesh DS, Kumar V, et al. Measurement of recent exposure to Phlebotomus argentipes the vector of Indian visceral leishmaniasis, by using human antibody responses to sand fly saliva. Am J Trop Med Hyg. 2010;82(5):801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Picado A, Singh SP, Vanlerberghe V, Uranw S, Ostyn B, Kaur H, et al. Residual activity and integrity of PermaNet(®) 2.0 after 24 months of household use in a community randomized trial of long lasting insecticidal nets against visceral leishmaniasis in India and Nepal. Trans R Soc Trop Med Hyg. 2011;106(3):150–9. doi: 10.1016/j.trstmh.2011.10.014 [DOI] [PubMed] [Google Scholar]
- 73.Das M, Roy L, Picado A, Kroeger A, Rijal S, Boelaert M. Deltamethrin and permethrin residue on long-lasting insecticidal nets after 18 months of use in a visceral leishmaniasis-endemic area in Nepal. Trans Roy Soc T Med Hyg. 2012;106(4):230–4. 10.1016/j.trstmh.2012.01.007 [DOI] [PubMed] [Google Scholar]