Abstract
Background
Bone tissue engineering and the research surrounding peptides has expanded significantly over the last few decades. Several peptides have been shown to support and stimulate the bone healing response and have been proposed as therapeutic vehicles for clinical use. The aim of this comprehensive review is to present the clinical and experimental studies analysing the potential role of peptides for bone healing and bone regeneration.
Methods
A systematic review according to PRISMA guidelines was conducted. Articles presenting peptides capable of exerting an upregulatory effect on osteoprogenitor cells and bone healing were included in the study.
Results
Based on the available literature, a significant amount of experimental in vitro and in vivo evidence exists. Several peptides were found to upregulate the bone healing response in experimental models and could act as potential candidates for future clinical applications. However, from the available peptides that reached the level of clinical trials, the presented results are limited.
Conclusion
Further research is desirable to shed more light into the processes governing the osteoprogenitor cellular responses. With further advances in the field of biomimetic materials and scaffolds, new treatment modalities for bone repair will emerge.
Keywords: Peptides, Mesenchymal stem cells, Bone healing, Growth factors
Background
After a traumatic insult to the bone, the musculoskeletal system mounts both local and systemic reactions facilitating the prompt restoration of the continuity of bone and normal function. Unfortunately, this process is not always successful. Approximately 5 % to 10 % of the fractures occurring are associated with impaired healing, including delayed union or non-union [1–5]. Fracture non-union often results in devastating outcomes for the patient and the surgeon [2, 5, 6], requiring a complex, long-lasting and expensive treatment, and a variable degree of morbidity is often a common finding [2, 7, 8].
In established non-unions and bone defects, bone grafting is a common procedure. It is estimated that 1.5 million bone grafting procedures are performed annually in the USA and this figure is rapidly increasing due to population ageing [2, 7, 9–13]. The intense research in this field seen over the last few decades, has resulted in the discovery of several proteins that can upregulate the bone healing response [14, 15]. Bone morphogenetic proteins (BMPs) are the most representative example, which have been granted US Food and Drug Administration (FDA) approval for clinical applications in recalcitrant long bone non-unions, lumbar fusion and open tibial shaft fractures [16–18]. Several other proteins have shown to upregulate the osteogenic bone healing process [19–22]. However, the high cost derived from the purification techniques and the high doses required due to the instability of these molecules in vivo are the two most significant points of concern [23]. Recombinant DNA technologies have simplified the production of these molecules and the discovery of a variety of osteogenic peptides has emerged [24].
The terms protein, polypeptide, oligopeptide and peptide are rather ambiguous and overlapping in their meaning [25]. Proteins usually refer to denote an entire biological molecule in a stable conformation, while peptides refer to short amino acid oligomers most commonly lacking a stable 3-dimensional structure. In general, they exert their effect through binding to specific high-affinity receptors on the respective target cell receptors [25].
To date, a number of peptides have been engineered to upregulate the osteogenic response. Although BMP-derived peptides are the most studied, other peptides also exist. The aim of this study is to identify the currently existing osteogenic peptides, other than those derived from BMPs and to investigate their impact in the upregulation of bone healing and bone regeneration.
Methods
This review was conducted in accordance to the PRISMA guidelines [26]. Publications from January 1980 to date were included.
Eligibility and exclusion criteria
Studies selected were original articles publishing results on the effect of different peptides on osteoblasts and osteoprogenitor cells as well as in vivo studies on bone healing. All studies that did not fulfil all eligibility criteria were excluded from further analysis. Exclusion criteria included manuscripts in languages other than English and those with incomplete documentation. Also, peptides related to BMPs or those related to cells types or conditions distant to bone healing or bone cells were excluded from the selection process as these were out of the scope of the manuscript.
Information sources
Studies were identified by searching PubMed Medline, Ovid Medline, Embase, Scopus, Google Scholar, and the Cochrane Library to retrieve all available relevant articles. The terms used for the search included combinations of primary keywords including ‘peptide’, ‘sequence’, and ‘motif’ with secondary keywords including ‘bone’, ‘osteoblast’, ‘bone healing’, ‘mesenchymal’, ‘fracture’, ‘non-union’, ‘osteoprogenitor cells’, ‘stem cells, ‘growth factor’, and ‘extracellular matrix’. The identified articles and their bibliographies, including any relevant reviews, were manually searched for additional potential eligible studies.
Study selection
Two of the authors (IP, MP) performed the eligibility assessment in an independent, unblinded and standardised manner. Most citations were excluded on the basis of information provided by their respective title or abstract. In any other case, the complete manuscript was obtained, scrutinised by the two reviewers and included if fulfilling the eligibility criteria.
Results
Out of 6017 papers that were initially identified, 197 met the inclusion criteria (Fig. 1) [27–223]. These studies are presented below.
Fig. 1.

Flowchart of study selection process
Parathyroid hormone-related peptides
Parathyroid hormone 1–34 peptide (Teriparatide)
Parathyroid hormone (PTH) is an 84-amino acid, naturally occurring protein that plays a major regulatory role in mammalian mineral ion homeostasis. The peptide derived from its 34 amino acid domain has similar activity to the full length protein [224]. PTH1–34 is one of the earliest artificially synthesized amino acid fragments that was granted approval for the prevention and treatment of osteoporosis. Among its several functions, PTH1–34 stimulates osteoblast proliferation, differentiation and prevents their apoptosis (Fig. 2) [51].
Fig. 2.
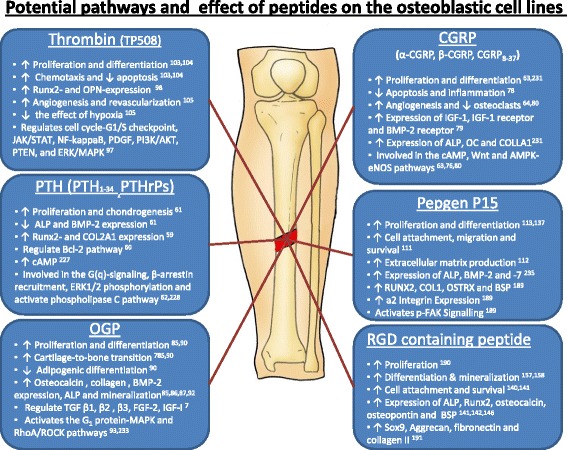
Potential pathways and effect of peptides on the osteoblastic cell lines
Synthetic matrix made of polyethylene-glycol containing PTH1–34 significantly stimulated in situ bone augmentation in rabbits [29]. Evidence from animal models shows that daily subcutaneous injections of PTH1–34 significantly increased the bone mineral content and density as well as the total osseous tissue volume, torsional strength and stiffness [27, 30]. Additionally, accelerated callus mineralization, increased bone density at the fracture site, and better mechanical properties of the united bone have been reported [27, 31, 32, 47–51].
To date, several case reports have indicated that teriparatide could facilitate the healing of sternal non-union [34], stress fractures [35], atrophic humeral shaft non-union [36], femoral non-union [37, 41, 42, 47, 225], hip fractures [40], delayed unions [38, 43, 44], periprosthetic fractures [45], and sacral and pubic insufficiency fractures [39].
In a prospective randomized double-blind study, Aspenberg et al. [28] analysed the effect of daily injections of 20 and 40 μg of recombinant teriparatide against placebo in post-menopausal women who sustained a distal radial fracture. Although they reported no significant difference between the teriparatide 40 μg versus placebo groups, they reported a shorter time to healing between the teriparatide 20 μg group and placebo (P = 0.006). In a post hoc subgroup analysis by the same group, blinded qualitative scoring of the calluses at 5 weeks suggested that patients who received PTH1–34 had a more ‘rich’ callus formation [33].
Parathyroid hormone-related protein
Human parathyroid hormone-related protein (PTHrP) consists of 139–175 amino acids and is a key regulator of cell growth, differentiation, and development of the foetal skeleton [59–62, 226–228]. Endogenous PTHrP plays an important role in fracture healing as demonstrated in an PTHrP haplo-insufficiency model where reduced cartilaginous and bony callus formation was noted together with reduced endochondral and osteoblastic bone formation [229]. However, a PTHrP1–34 maintains a less pronounced anabolic effect to the bone and osteoblasts possibly due its higher clearance rate [52, 53]. To overcome this weakness, several analogues have been developed to date [54–57].
PTHrP1–36 exerts an anabolic action to bone, including enhanced bone histological features and raised osteoblast differentiation markers in the long bones and plasma in mice [57]. Cavitary bone defects treated with PTHrP107–111 improve local bone induction in a rabbit femoral cavity defect model [58]. A C-terminally substituted analogue of PTHrP1–34, the RS-66271, was found to increase trabecular and cortical bone in ovarectomized osteopenic rats [54]. In an impaired bone healing animal model, daily injections of RS-66271 resulted in a larger callus area, greater stiffness and torque when compared with controls [56]. A similar analogue of PTHrP, the RS-50303, was found to enhance fracture healing in a rat femoral osteotomy model [55].
Calcitonin gene-related peptide
Calcitonin gene-related peptides (CGRP) are found in two forms, α and β. α-CGRP derives from the Calca gene and consists of 37 amino acids [230]. It has a 20 % homology with calcitonin. In contrast, β-CGRP derives from a separate gene, termed Calcb, which is located in close proximity to Calca. In bone, CGRP is found in the sensory nerve endings in periosteum, bone marrow and metaphysis [66]. Among its several functions, CGRP has been found to stimulate the proliferation and differentiation, and to reduce the apoptosis of osteoprogenitor cells [63, 64, 75, 79, 231]. CGRP levels increase in patients with fractures, and it has been postulated that this plays an important role during the inflammatory stage of bone healing and overall during damaged tissue repair [72, 80]. Transgenic mice engineered to overexpress CGRP have been found to have high trabecular bone density and bone volume [65], also associated with an increase in bone formation rate. On the contrary, α-CGRP null mice developed osteopenia caused by a reduced bone formation rate [69]. CGRP was also found to stimulate the production of osteogenic molecules like IGF-I and BMP-2 [76–78].
Despite the abovementioned experimental evidence, limited in vivo studies have explored the potential supplementary effect of CGRP on bone healing. The literature suggests that, during fracture healing, the systemic levels of CGRP increase [72]. Fracture evokes an intense local in-growth of new nerve fibres containing CGRP thus possibly playing a role in the bone healing process [71]. When the bony innervation is disrupted locally, this results in an insufficient fracture callus [73]. Furthermore, Song et al. [70] speculated that the increased levels of CGRP secondary to traumatic brain injury may have led to the enhancement of fracture healing seen in this patient group. In an experimental model of induced fatigue bone damage, administration of CGRP or CGRP(8–37) increased reparative bone formation [74].
Osteogenic growth peptide (OGP)
OGP is a naturally occurring, highly conserved, 14-amino acid, H4 histone-related peptide [81], abundant in human and mammalian blood as well as in culture media of osteoblasts and fibroblasts [81, 82]. Following its dissociation from the a2-macroglobulin, the peptide is proteolytically cleaved to generate a C-terminal pentapeptide, which activates an intracellular Gi-protein-MAP kinase signalling pathway [232, 233].
OGP was found to exert an anabolic effect on bone cells, resulting in an increase of bone formation and overall bone mass [81, 83]. In vitro studies have shown that OGP can regulate osteoprogenitor cell proliferation, differentiation, alkaline phosphatase activity, osteocalcin secretion, collagen and matrix mineralization [85–87, 92]. In vivo OGP was found to regulate TGF-b1,b2,b3, FGF-2, IGF-I and aggrecan [85]. Further, transgenic mice overexpressing OGP have significantly increased peak bony mass [91].
Experimental fracture healing models have shown that OGP can serve as a potential candidate in enhancing the bone healing response (Table 1) [83–85, 88, 89]. Systemic administration of OGP accelerated bony union with enhanced bridging across the fracture gap, higher volume of callus and newly formed bone [85, 89]. Shuqiang et al. [84] treated 1.5-cm segmental defects in rabbits with an OGP incorporated in a PLGA scaffold. Their results showed a higher bony volume and acceleration of bone healing response.
Table 1.
In vivo animal studies presenting the effect of osteogenic growth peptide (OGP) on bone and bone healing
| Study/Year | Model | Mode of delivery | Results |
|---|---|---|---|
| Sun et al., 1998 [89] | Tibial fracture in rabbits | IV administration | • OGP treatment accelerated fracture union |
| Brager et al., 2000 [85] | Femoral fracture in rats | Systematic administration of OGP (25 ng/day) | • OGP enhances proliferation and differentiation of osteogenic cells possibly through the upregulation of TGF-beta |
| Gabet et al., 2004 [83] | Mid-femoral fracture in rats | Systematic administration of OGP | • OGP administration resulted in enhanced bridging across fracture gap, higher volume of callus and newly formed bone |
| Shuqiang et al., 2008 [84] | Radial 1.5 cm segmental defect in rabbits | OGP incorporated in PLGA | • The rate of bone formation and volume were statistically significantly upregulated in experimental group |
| Zhao et al., 2011 [88] | Distraction osteogenesis in rabbit tibia | Systematic administration of OGP (200 ng/kg/day) | • OGP treatments resulted in greater torsional stiffness, higher chondrocyte numbers and amount of newly formed bone |
Thrombin Peptide 508 (Chrysalin)
Thrombin peptide 508 (TP508), also known as Chrysalin, is a 23-amino acid synthetic peptide that represents the non-proteolytic receptor binding domain of thrombin. TP508 mimics some specific attributes of the thrombin, without the undesirable blood clotting effects. TP508 was found to enhance the proliferation and differentiation of and induces chemotaxis in human osteoblasts [103, 104]. It enhances VEGF-stimulated angiogenesis and attenuates effects of chronic hypoxia [105].
A number of in vivo animal models have all demonstrated that TP508 could have an upregulatory effect on bone healing (Table 2). Two animal studies, analysing the effect of TP508 loaded on PPF composite and microsphere scaffolds on segmental bone defects in rabbits, showed enhanced bone formation with a higher torsional stiffness of bone [99, 101]. TP508 injected into the fracture gap promotes fracture healing and increased blood vessel formation [95, 97, 102]. In animal models of distraction osteogenesis, injection of TP508 into the fracture gap resulted in enhanced bone formation and consolidation [94, 100]. In similar models, increased numbers of osteoblasts were apparent as well as the increased quality of bone [96, 98, 100].
Table 2.
In vivo animal studies presenting the effect of TP508 on bone and bone healing
| Study/Year | Model | Mode of delivery | Results |
|---|---|---|---|
| Hedberg et al., 2004 [101] | Segmental bone defect in rabbits | PPF composite scaffolds with 200 or 100 μg TP508 | • Enhance bone formation with 200 μg TP508 possibly due to the initial high burst of the molecule |
| Sheller et al., 2004 [99] | Segmental bone defect in rabbits | Microspheres with 100 μg or 200 μg TP508 | • Enhanced healing of the defects with higher torsional stiffness in the animals treated with TP508 |
| Li et al., 2005 [100] | Distraction osteogenesis in rabbits | 30 μg or 300 μg into the distraction gap | • Enhanced bone formation and consolidation, the 300 μg treatment group had the most advanced results |
| Wang et al., 2005 [102] | Femoral fracture in rats | 1 μg or 10 μg in the fracture gap | • TP508 found to promote fracture healing by inducing the levels of growth factors, inflammatory mediators and angiogenesis-related genes |
| Amir et al., 2007 [98] | Distraction osteogenesis in rabbits | 30 or 300 μg into the distraction gap | • Enhance bone regeneration with increased number of osteoblasts |
| Li et al., 2007 [97] | Rat femoral fracture model | 1 μg, 10 μg or 100 μg in the fracture gap | • TP508 accelerated fracture healing by upregulating the expression levels of molecules involved in cellular proliferation, cellular growth and apoptosis |
| Wang et al., 2008 [96] | Distraction osteogenesis in rabbits | Slow releasing TP508 preparation (300 μg in PPF/PLGA microparticles) | • Enhanced bone consolidation process with better quality bone |
| Hanratty et al., 2009 [95] | High energy femoral fracture in mice | 10 μg or 100 μg at fracture site, or 100 μg at muscles adjacent the fracture | • 100 μg in fracture gap significantly increased bone formation and fracture stiffness • Less scar tissue and increased blood vessel formation was noted when TP508 was injected in the adjacent to the fracture muscles |
| Cakarer et al., 2010 [94] | Distraction osteogenesis in rats | 10 μg and 100 μg percutaneously | • Significant larger area of consolidation in the animal receiving TP508; the higher dose was more effective |
In the clinical setting, TP508 has failed to display the same beneficial effects as in animal studies. A double-blinded, randomized, placebo controlled Phase III clinical trial has been conducted to analyse the effect of Chrysalin for the treatment of unstable displaced distal radial fractures [106]. The initial results demonstrated a statistically significant shorter time to the radiologic consolidation of the fractures but not differences in terms of the range of motion, grip strength and VAS or DASH scores [107]. Furthermore, the trial failed to show any statistically significant difference in the time of removal of the immobilization device, which was the primary end point of the study [108].
Cell-binding peptides
PepGen P-15
The P-15 peptide is a highly conserved peptide that consists of 15 amino acids identical to the cell-binding region of collagen type I [234]. P-15 enhances cell attachment to bone substitutes and upregulates extracellular matrix (ECM) production [112]. At the same time, it promotes cell survival and can be absorbed into a calcium phosphate substrate [111]. When P-15 is added in scaffold material, it results in a significantly higher gene expression of alkaline phosphatase (ALP), BMP-2 and BMP-7 [235]. This upregulated gene expression could suggest that P-15 promotes osteoblastic activity in human osteoblast cells. Indeed, P-15 was found to stimulate the proliferation and differentiation rate as well as the growth factor production of osteoblasts in vitro [113, 137, 189]. On the contrary, Vordemvenne et al. [104] reported that P-15 alone is not capable of upregulating the proliferation and calcifying potential of human osteoblasts in vitro. When combined with PDGF, a statistically significant increase in both proliferation and calcification was noted [104].
Preclinical results have shown that P-15-containing bone graft substitutes could facilitate bone healing and regeneration [118]. In bone defects, application of P-15-containing bone substitutes increased the rate of bone growth compared to the defects left empty or filled with bone substitute alone [120, 121, 123, 124, 132, 138]. In critical sized segmental defect in a rat radius, application of inorganic bone matrix together with P-15 resulted in positive effect on bone healing, without any immunogenic features and disease transmission risk [133]. The use of the same graft material was found as successful as autogenous bone graft in producing lumbar spinal fusion in an ovine model [119]. However, some controversial data exist, with some authors reporting less favourable results with P-15-containing graft substitutes [125, 126]. In addition, application of the P-15-containing graft substitutes was found to accelerate the process of early bone formation response but not the long-term effect [129, 131, 135].
The majority of clinical evidence derives from substitutes for the oral cavity [109, 110, 114–117, 122, 127, 128, 130, 134, 136, 139]. Periodontal osseous defects in 25 patients treated with combination of anorganic bovine-derived hydroxyapatite matrix and P-15 showed favourable clinical results [109]. In the treatment of non-unions limited evidence exists [236]. Gomar et al. [236] treated 22 patients with non-uniting fractures with P-15 containing bone graft substitutes. They reported a 90 % success rate and concluded that it could be an effective, safe and economical alternative to autologous bone grafting.
RGD containing peptide
Arginyl-glycyl-aspartic acid (RGD) sequence is found in several molecules and constitutes a system of cell surface signalling [237]. Evidence suggests that RGD enhances cell attachment and spreading of osteoblasts onto scaffolds and graft material [140, 141, 159, 188] whilst increasing cellular proliferation and the expression of ALP, Runx2, osteocalcin, osteopontin and bone sialoprotein [141, 142, 146, 190, 191]. Further, it promotes osteoblast differentiation and mineralization [143, 144, 146, 157, 158, 197].
Limited in vivo studies exist today analysing the effect of RGD on bone healing; however, several authors have investigated the effect of RGD peptides on implant surfaces. RGD coated implants were found to have an increased peri-implant bone formation and enhanced direct bone apposition even in areas of poor surrounding bone [148, 149, 151, 152, 155, 156]. This significantly increased the bone-to-implant contact [149]. When RGD-coated intramedullary nails were inserted into the tibia of male adult Wistar rats, the outcome was increased new bone formation [148]. Finally, it should be mentioned that RGD-containing scaffolds used to deliver growth factors, such as BMP-2 to promote bone regeneration in experimental fracture models, exist with favourable results [150]. In contrast to the abovementioned results, some fracture models have shown that RGD utilization could have detrimental effects. Hennessy et al. [153] showed that, when RGD was combined with adsorbed tibial proteins like fibronectin, vitronectin and fibrinogen, a markedly detrimental effect on mesenchymal stem cell (MSC) adhesion and survival was observed. No significant effects of an additional RGD coating on HA surfaces were detected in a rabbit model for cementless joint prostheses [154].
Other ECM-derived peptides
In addition to P-15 and RGD, other ECM-derived peptides are currently being developed for potential applications in amplifying the bone healing response. They represent signalling domains found along the ECM protein chains and are capable of interacting with receptors on the cellular membrane.
GFOGER (glycine-phenylalanine-hydroxyproline-glycine-glutamate-arginine) is a collagen-mimetic peptide. It selectively promotes α2β1 integrin binding, which is a crucial event for osteoblastic differentiation [162]. Implants coated with GFOGER were found to improve peri-implant bone regeneration and osseointegration [162, 164]. Results showed significantly accelerated and increased bone formation in non-healing femoral defects compared to uncoated scaffolds and empty defects. GFOGER could be utilized as a growth factor delivery vehicle, which can upregulate the fracture healing response [161].
The collagen-binding motif (CBM) is a cleavage product of osteopontin that can specifically bind to collagen [180]. The CBM was found to promote migration and osteogenic differentiation via the Ca2+/CaMKII/ERK/AP-1 signalling pathway [181]. In a rabbit calvarial defect model, application of an injectable gel containing synthetic CBM peptide resulted in increased cell adhesion and growth of osteoblasts followed by increased osteoblastic differentiation and marked bone formation [180, 238].
DGEA (Asp-Gly-Glu-Ala) is a recognition motif used by type I collagen to bind to α2β1 integrin [165]. This collagen peptide sequence has been shown to promote cell adhesion, spreading and osteogenic differentiation [163, 165, 166]. DGEA, engineered to express a heptaglutamate domain, was found to accumulate within bone tissue following intravenous injection [168], suggesting that such an approach could be used for drug to bone delivery. DGEA coupled with heptaglutamate-containing hydroxyapatite was found to enhance the adhesion and osteoblastic differentiation of MSCs as well as to increase new bone formation and bone-to-implant contact [167].
The SVVYGLR (Ser-Val-Val-Tyr-Gly-Leu-Arg) peptide sequence is found adjacent to the RGD sequence in osteopontin [239]. SVVYGLR peptide significantly enhanced the adhesion and proliferation of MSCs but also endothelial cell activity, resulting in an upregulation of neovascularization [169, 239, 240]. Experimental models of bone defects have shown that, when SVVYGLR was implanted together with a collagen sponge, an upregulation of osteogenesis and angiogenesis was observed [169, 239].
KRSR (lysine-arginine-serine-arginine) is a heparin-binding site found in fibronectin, vitronectin, bone sialoprotein, thrombospondin, and osteopontin [173]. KRSR increased osteoblast adhesion and osteogenic gene expression [171, 172, 177, 178]. Anodized nanotubular titanium coated with KRSR, RGDS (arginine-glycine-aspartic acid-serine) and molecular plasma deposition increased osteoblast density compared with uncoated substrates [174]. Likewise, KRSR and RGD coated on titanium promoted the greatest osteoblast densities relative to untreated titanium [175]. On the contrary, less favourable results in terms of stimulation of cell adhesion and spreading were reported by other studies [173, 179].
FHRRIKA (Phe-His-Arg-Arg-Ile-Lys-Ala) is a cell-binding and putative heparin-binding domain of bone sialoprotein. FHRRIKA could have a favourable effect on osteoblast adhesion, spreading and mineralization [183]. Osteoblast outgrowths from rat calvarial bone chips covered a significantly larger area on FHRRIKA surface [176]. Rat calvarial osteoblasts seeded into a scaffold containing the RGD and FHRRIKA sequences were found to remain viable and have higher proliferation kinetics compared to the controls in which no peptides were added [184].
Fibronectin (FN)-derived peptides have also shown to facilitate osteoblast adhesion, spreading and mineralization [185]. A fibrin-binding synthetic oligopeptide derived from FN was found to enhance new bone formation in rabbit calvarial defect model [187]. In addition, the multifunctional FN III9-10/12-14 greatly enhanced the regenerative effects of BMP-2 and PDGF-BB in a rat critical-size bone defect model [186].
NEMO-binding domain peptide (NBD)
The inhibitor of nuclear factor kappa-B kinase (IKK) is a high molecular weight complex consisting of two catalytic subunits (IKK-1 and IKK-2) and a non-catalytic regulatory subunit NF-kB Essential Modulator (NEMO or IKK-γ) [194]. NEMO interacts with both IKK subunits at the interacting region to amino acids 737–742, called the NEMO-binding domain (NBD) [194]. NBD peptide has shown to promote osteoblast differentiation and inhibit bone resorption [192, 193]. A protective role to the bone by blocking osteoclastogenesis and bone erosion in inflammatory arthritis was also noted [195]. In vivo evidence is limited to a murine tooth extraction model treated with lipopolysaccharide injection where TNF-a retarded bone regeneration [196].
Cell penetrating peptides
Cell penetrating peptides (CPPs) are peptides that can transverse the cellular membrane and transport their ‘cargo’ into the cytoplasm [241]. Such cargos include proteins, siRNA, nanoparticles, oligonucleotides, and other peptides [242]. CPPs can derive from bacteria and viruses or synthesized in the laboratory [241, 242]. Jo et al. [199] demonstrated that the CPP-conjugated co-activator-associated arginine methyltransferase 1 (CARM1) protein can be delivered into human MSCs and change their global gene expression profile. Furthermore, upregulation of their differentiation capacity was noted [199]. In a rabbit calvarial defect model treated with CPP with a transcriptional factor fusion protein resulted in significantly increased bone formation [200]. Similarly, in a critical-size calvarial defect model, the inclusion of tetrameric CPPs in ex vivo transduction of recombinant adenovirus expressing BMP-2 into MSCs promoted highly mineralized bone formation [201].
Self-assembly peptides
Self-assembly peptides are another class of peptides, referred by some as ‘molecular Lego’, that are composed of alternating hydrophilic and hydrophobic amino acid residues [243]. These residues have the tendency to spontaneously adopt a β-sheet structure when exposed to monovalent cation solutions or placed under physiological conditions [203, 243]. The outcome of this process is the formation of self-assembled matrices with interwoven nanofibers.
RADA16-I (AcN-RADARADARADARADA-CONH2) is a synthetic commercially available peptide (PuraMatrix). MSCs exhibited higher levels of expression of ALP, osteocalcin and Runx2 genes in RADA16-I-containing demineralized bone matrix (DBM) compared to only DBM [203]. Cell adhesion, proliferation and differentiation of osteoblasts were found to be superior in the RADA16-I-containing scaffold [204]. In vivo data derived from a critically-sized femur defect in goats showed that the volume of newly formed bone from marrow-enriched RADA16-I/DBM was significantly higher compared to marrow-enriched DBM alone [203]. Other authors reported favourable outcomes with the utilization of RADA16-I self-assembly peptide [206–212]. The addition of BMP-2 in a hydrogel RADA16-I-containing scaffold significantly enhanced bone regeneration on the bone augmentation model in an animal bone defect model [205].
Peptide amphiphiles are another class of self-assembly peptides that can support osteoprogenitor cells and guide their differentiation [215, 216]. Mineralized matrices containing peptide amphiphiles were found to promote osteogenic differentiation of human MSCs [213]. The combination of peptide amphiphiles with MSCs and platelet-rich plasma was found to promote bone formation and enhance angiogenesis [214].
Other peptides
Numerous peptides have been isolated from the majority of the existing growth factors and bone-related proteins. Peptides derived from fibroblast growth factor were found to upregulate osteoblast differentiation [202, 244]. Similarly, peptides have been derived from molecules like BMPs, transforming growth factor-β, vascular endothelial growth factor, insulin derived growth factor, although their potential role in bone healing and regeneration remains obscure [217–219, 222]. Other peptides found to promote bone healing include the RANKL-binding peptide, AC-100, mechano growth factor E, and B2A2-K-NS (B2A) [223].
Discussion
Bone tissue engineering is a growing biomedical field. All recent advances in the field of growth factors, scaffolds and osteoprogenitor cells have boosted the application and further expansion of tissue engineering technologies. As far as growth factors are concerned, several drawbacks prohibit their widespread use. Difficulties arising from potential immunogenicity, large molecular weight, need for carriers for their delivery and instability in vivo are well recognized [188]. Moreover, concerns regarding their sterilization and their theoretical involvement in carcinogenesis also exist [23, 188, 245–247]. The discovery that small protein segments (peptides) have the capacity to exert a similar effect could overcome some of the abovementioned problems. Not only do they have low immunogenicity but they can also be easily synthesised and handled [188].
Chrysalin and teriparatide are two commercially available drugs that have been investigated as potential candidates in the upregulation of bone healing response in humans [28]. Their background in terms of pre-clinical and experimental evidence has been excellent. In humans, teriparatide resulted in a shorter time to healing with a ‘richer’ callus formation when used for the treatment of distal radial fractures [28]. These results, however, are rather weak and, according to the authors, they should be interpreted with caution and warrant further validation with more studies. Similarly, the use of Chrysalin for unstable displaced distal radial fractures demonstrated a shorter time to the radiologic consolidation of the fractures but no differences in terms of cast removal, range of motion, grip strength and VAS or DASH scores [107]. Therefore, one could hypothesise that the ‘exceptional’ results seen in the experimental animal models cannot be directly translated in clinical practice, at least as yet. It could be speculated that the differences in bone healing biology are responsible for these compelling results. In addition, differences in the study objectives in humans and animals are evident. The available in vitro and in vivo animal studies have limited their focus on the global osteogenic output. However, clinical studies are not limited to the radiologic appearance, but also several other parameters such as time for cast removal, range of motion, VAS or DASH scores, etc. It could be of speculation that a more radiologically rich callus formation is not necessarily associated with a better clinical outcome (Table 3). In addition, the potency of these peptides in humans and animals, as well as their stability and delivery challenges, are currently not fully understood.
Table 3.
Available clinical studies on the effect of peptides on bone healing
| Study, Year | Peptide used | Clinical application | Result |
|---|---|---|---|
| Yukna et al., 1998 [115] | P-15 | Periodontal osseous defects in 33 patients | • P-15 combined with anorganic bone matrix (ABM) yields better clinical results than freeze-dried bone allograft or open flap debridement |
| Yukna et al., 2000 [114] | P-15 | Periodontal osseous defects in 33 patients | • P-15 combined with ABM yields better clinical results than the ABM alone |
| Yukna et al., 2002 [122] | P-15 | Infra-bony periodontal defects in 25 patients | • Favourable 3-year results with P-15 combined with ABM suggest that it may have a beneficial effect long-term |
| Yukna et al., 2002 [109] | P-15 | Periodontal regeneration case report | • Uneventful results with no evidence of root resorption, ankylosis or untoward inflammation |
| Degidi et al., 2004 [128] | P-15 | Maxillary sinus augmentation in 7 patients | • Bone-replacement materials, without the addition of autologous bone, could be equally effective sinus augmentation |
| Gelbart et al., 2005 [110] | P-15 | Sinus floor augmentation in 12 patients | • New trabecular bone is formed after grafting P-15 combined with ABM in the sinus floor |
| Philippart et al., 2005 [130] | P-15 | Maxillary sinus floor grafting performed on 3 patients | • High degree of inorganic xenograft integration and natural bone regeneration |
| Gomar et al. 2007 [236] | P-15 | Non-unions and delayed union in 22 patients | • Full consolidation was achieved in 90 % of the cases • Safe, economical and clinically useful alternative to autograft in the repair of un-united fractures |
| Kasaj et al., 2008 [127] | P-15 | Infra-bony periodontal defects in 26 patients | • Significantly improved clinical outcomes compared to open flap debridement |
| Butz et al., 2011 [116] | P-15 | Sinus floor augmentation in 24 patients | • All implants placed in the augmented sites integrated and were restored prosthetically |
| Emam et al., 2011 [117] | P-15 | Sinus floor augmentation in 24 patients | • PepGen P-15 putty was found to be a promising osteoconductive graft for sinus augmentation, supporting immediate placement of implants |
| Aspenberg et al., 2010 [33] | Teriparatide | Distal radial fractures in 27 patients | • The results must be interpreted with caution • Radiographic quality at an early time point might be a sensitive variable, perhaps better than time to cortical continuity • Teriparatide appeared to improve early callus formation in distal radial fractures |
| Aspenberg et al., 2010 [28] | Teriparatide | Distal radial fractures in 102 patients | • Shortened time to healing for teriparatide group compared with placebo • These results should be interpreted with caution and warrant further study |
| Chintamaneni et al., 2010 [34] | Teriparatide | Sternal fracture non-union | • Consolidation of fracture |
| Oteo-Alvaro et al., 2010 [36] | Teriparatide | Humeral shaft non-union case report | • Consolidation of fracture |
| Chrysalin trial [106] | Chrysalin | Distal radial fractures in 274 patients | • Statistically significant shorter time to the radiologic consolidation of the fractures but no differences in terms of the range of motion, grip strength and VAS or DASH scores |
PepGen P-15 is another commercially available peptide that has been investigated in periodontal osseous defect models with favourable results. The vast majority of the available evidence comes from small osseous defects seen in dental and maxillofacial surgery (Table 3). There is limited evidence for long bone bony defects or non-unions. In the largest case series, PepGen P-15 containing bone graft substitutes were used in 22 patients with non-uniting fractures [236]. According to the authors, PepGen appeared to offer a safe, economical and clinically useful alternative to autologous grafting. However, additional randomized clinical studies are needed to define its effectiveness in this setting. In a similar note, the effectiveness of PuraMatrix warrants further clinical investigation as, although commercially available, its potential effectiveness for bone healing and regeneration is only limited to in vitro or animal studies.
One avenue that warrants further investigation includes the combination of cell binding peptides with sub-functional doses of BMPs [197, 248]. As shown, for instance, by Visser et al. [248], when an absorbable collagen type I sponge functionalized with a synthetic collagen-targeted RGD containing low doses of BMP-2, ectopic bone formation was observed in rats. These low BMP-2 levels would have no significant effect if applied on their own.
Further research in the nanoscale phenomena governing biological materials and the heterojunction between cells and substrate could allow osteoinductive implants coupled with osteoconductive properties. Small molecules such as peptides could have a role to play in supporting and guiding the overall osteogenic response in such scenarios. Overcoming the peptide stability issues against proteolysis, which result in a short duration of activity and low bioavailability, is also crucial. In this context, expansion of our methodology for peptide designs with further research on ways to improve the incorporation of non-natural amino acids, cyclization and stable peptide bond engineering are crucial. The development of improved peptide motifs that could increase the osteogenic response in a compromised bone healing environment rather than cause an upregulation of the osteoblastic output, should further be explored. Another area of interest is the utilization of a ‘polytherapy’, i.e. the combination of several peptides targeting either a specific cell line or a specific phase of bone healing. Such an approach would, for instance, employ an osteoinductive peptide coupled by a peptide promoting the osteogenic or chondrogenic response. Scaffold technologies enabling a timed controlled release of such molecules could provide the right signals at the exact phase of the bone healing pathway. Therefore, further persistence in the design of peptide-scale molecules capable of targeting the upregulation of osteogenesis or form functional, structurally complex and well-defined scaffolds will lead to future clinical treatment modalities ranging from tissue replacement to tissue regeneration.
Conclusion
A significant number of peptides have been developed and investigated as potential candidates for the upregulation of bone healing response. In vitro and experimental animal models have been favourable, however, limited clinical evidence exists. Maturation of our knowledge in this field will give rise to novel biologically-derived molecules for applications in the clinical setting in cases where bone healing and bone regeneration are needed.
Acknowledgements
No acknowledgement to be made. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. No funds were received in support of this study.
Funding
No funds were received in support to this study. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Authors’ contributions
IP: Writing of the manuscript, study design, data collection and interpretation, preparation of revisions. MP: Data collection, assistance in study design and data interpretation. AL: Data collection, assistance in data interpretation. EJ: Generic input, study design. GMC: Contributed important intellectual content. PVG: Study senior author, study design, overview and final approval of the study. All authors read and approved the final manuscript.
Competing interest
The authors declare that they have no competing interests.
References
- 1.Hak DJ, Fitzpatrick D, Bishop JA, Marsh JL, Tilp S, Schnettler R, et al. Delayed union and nonunions: epidemiology, clinical issues, and financial aspects. Injury. 2014;45(Suppl 2):S3–7. doi: 10.1016/j.injury.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Hankenson KD, Zimmerman G, Marcucio R. Biological perspectives of delayed fracture healing. Injury. 2014;45(Suppl 2):S8–S15. doi: 10.1016/j.injury.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giorgio Calori M, Capanna R, Colombo M, De Biase P, O’Sullivan C, Cartareggia V, et al. Cost effectiveness of tibial nonunion treatment: a comparison between rhBMP-7 and autologous bone graft in two Italian centres. Injury. 2013;44(12):1871–9. doi: 10.1016/j.injury.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Panteli M, Pountos I, Jones E, Giannoudis PV. Biological and molecular profile of fracture non-union tissue: current insights. J Cell Mol Med. 2015;19(4):685–713. doi: 10.1111/jcmm.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannoudis PV, Panteli M, Calori GM. Bone healing: the diamond concept. In: Bentley G, editor. European Instructional Lectures. Volume 14th ed. Berlin Heidelberg: Springer; 2014. p. 3–16.
- 6.Giannoudis PV, Calori GM, Begue T, Schmidmaier G. Bone regeneration strategies: current trends but what the future holds? Injury. 2013;44(Suppl 1):S1–2. doi: 10.1016/S0020-1383(13)70002-0. [DOI] [PubMed] [Google Scholar]
- 7.Dahabreh Z, Panteli M, Pountos I, Howard M, Campbell P, Giannoudis PV. Ability of bone graft substitutes to support the osteoprogenitor cells: an in-vitro study. World J Stem Cells. 2014;6(4):497–504. doi: 10.4252/wjsc.v6.i4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zura R, Della Rocca GJ, Mehta S, Harrison A, Brodie C, Jones J, et al. Treatment of chronic (>1 year) fracture nonunion: heal rate in a cohort of 767 patients treated with low-intensity pulsed ultrasound (LIPUS) Injury. 2015;46(10):2036–41. doi: 10.1016/j.injury.2015.05.042. [DOI] [PubMed] [Google Scholar]
- 9.Ashman O, Phillips AM. Treatment of non-unions with bone defects: which option and why? Injury. 2013;44(Suppl 1):S43–45. doi: 10.1016/S0020-1383(13)70010-X. [DOI] [PubMed] [Google Scholar]
- 10.Goff T, Kanakaris NK, Giannoudis PV. Use of bone graft substitutes in the management of tibial plateau fractures. Injury. 2013;44(Suppl 1):S86–94. doi: 10.1016/S0020-1383(13)70019-6. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann G, Moghaddam A. Allograft bone matrix versus synthetic bone graft substitutes. Injury. 2011;42(Suppl 2):S16–21. doi: 10.1016/j.injury.2011.06.199. [DOI] [PubMed] [Google Scholar]
- 12.Calori GM, Colombo M, Mazza EL, Mazzola S, Malagoli E, Mineo GV. Incidence of donor site morbidity following harvesting from iliac crest or RIA graft. Injury. 2014;45(Suppl 6):S116–120. doi: 10.1016/j.injury.2014.10.034. [DOI] [PubMed] [Google Scholar]
- 13.Guimaraes JA, Duarte ME, Fernandes MB, Vianna VF, Rocha TH, Bonfim DC, et al. The effect of autologous concentrated bone-marrow grafting on the healing of femoral shaft non-unions after locked intramedullary nailing. Injury. 2014;45(Suppl 5):S7–S13. doi: 10.1016/S0020-1383(14)70013-0. [DOI] [PubMed] [Google Scholar]
- 14.Pountos I, Georgouli T, Kontakis G, Giannoudis PV. Efficacy of minimally invasive techniques for enhancement of fracture healing: evidence today. Int Orthop. 2010;34(1):3–12. doi: 10.1007/s00264-009-0892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Memeo A, Verdoni F, De Bartolomeo O, Albisetti W, Pedretti L. A new way to treat forearm post-traumatic non-union in young patients with intramedullary nailing and platelet-rich plasma. Injury. 2014;45(2):418–23. doi: 10.1016/j.injury.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 16.Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD. Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine (Phila Pa 1976) 2010;35(19):1794–800. doi: 10.1097/BRS.0b013e3181ecf6e4. [DOI] [PubMed] [Google Scholar]
- 17.Ronga M, Fagetti A, Canton G, Paiusco E, Surace MF, Cherubino P. Clinical applications of growth factors in bone injuries: experience with BMPs. Injury. 2013;44(Suppl 1):S34–39. doi: 10.1016/S0020-1383(13)70008-1. [DOI] [PubMed] [Google Scholar]
- 18.Schutzenberger S, Kaipel M, Schultz A, Nau T, Redl H, Hausner T. Non-union site debridement increased the efficacy of rhBMP-2 in a rodent model. Injury. 2014;45(8):1165–70. doi: 10.1016/j.injury.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Pountos I, Georgouli T, Henshaw K, Bird H, Giannoudis PV. Release of growth factors and the effect of age, sex, and severity of injury after long bone fracture. A preliminary report. Acta Orthop. 2013;84(1):65–70. doi: 10.3109/17453674.2013.765624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pountos I, Georgouli T, Henshaw K, Bird H, Jones E, Giannoudis PV. The effect of bone morphogenetic protein-2, bone morphogenetic protein-7, parathyroid hormone, and platelet-derived growth factor on the proliferation and osteogenic differentiation of mesenchymal stem cells derived from osteoporotic bone. J Orthop Trauma. 2010;24(9):552–6. doi: 10.1097/BOT.0b013e3181efa8fe. [DOI] [PubMed] [Google Scholar]
- 21.Pountos I, Panteli M, Panagiotopoulos E, Jones E, Giannoudis PV. Can we enhance fracture vascularity: what is the evidence? Injury. 2014;45(Suppl 2):S49–57. doi: 10.1016/j.injury.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Roberto-Rodrigues M, Fernandes RM, Senos R, Scoralick AC, Bastos AL, Santos TM, et al. Novel rat model of nonunion fracture with vascular deficit. Injury. 2015;46(4):649–54. doi: 10.1016/j.injury.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 23.Pountos I, Panteli M, Georgouli T, Giannoudis PV. Neoplasia following use of BMPs: is there an increased risk? Expert Opin Drug Saf. 2014;13(11):1525–34. doi: 10.1517/14740338.2014.968124. [DOI] [PubMed] [Google Scholar]
- 24.Massia SP, Hubbell JA. An RGD spacing of 440 nm is sufficient for integrin alpha V beta 3-mediated fibroblast spreading and 140 nm for focal contact and stress fiber formation. J Cell Biol. 1991;114(5):1089–100. doi: 10.1083/jcb.114.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dignass AU, Sturm A. Peptide growth factors in the intestine. Eur J Gastroenterol Hepatol. 2001;13(7):763–70. doi: 10.1097/00042737-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alkhiary YM, Gerstenfeld LC, Krall E, Westmore M, Sato M, Mitlak BH, et al. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34) J Bone Joint Surg Am. 2005;87(4):731–41. doi: 10.2106/JBJS.D.02115. [DOI] [PubMed] [Google Scholar]
- 28.Aspenberg P, Genant HK, Johansson T, Nino AJ, See K, Krohn K, et al. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res. 2010;25(2):404–14. doi: 10.1359/jbmr.090731. [DOI] [PubMed] [Google Scholar]
- 29.Jung RE, Hammerle CH, Kokovic V, Weber FE. Bone regeneration using a synthetic matrix containing a parathyroid hormone peptide combined with a grafting material. Int J Oral Maxillofac Implants. 2007;22(2):258–66. [PubMed] [Google Scholar]
- 30.Komrakova M, Stuermer EK, Werner C, Wicke M, Kolios L, Sehmisch S, et al. Effect of human parathyroid hormone hPTH (1-34) applied at different regimes on fracture healing and muscle in ovariectomized and healthy rats. Bone. 2010;47(3):480–92. doi: 10.1016/j.bone.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Mognetti B, Marino S, Barberis A, Martin AS, Bala Y, Di Carlo F, et al. Experimental stimulation of bone healing with teriparatide: histomorphometric and microhardness analysis in a mouse model of closed fracture. Calcif Tissue Int. 2011;89(2):163–71. doi: 10.1007/s00223-011-9503-3. [DOI] [PubMed] [Google Scholar]
- 32.Rowshan HH, Parham MA, Baur DA, McEntee RD, Cauley E, Carriere DT, et al. Effect of intermittent systemic administration of recombinant parathyroid hormone (1-34) on mandibular fracture healing in rats. J Oral Maxillofac Surg. 2010;68(2):260–7. doi: 10.1016/j.joms.2009.09.045. [DOI] [PubMed] [Google Scholar]
- 33.Aspenberg P, Johansson T. Teriparatide improves early callus formation in distal radial fractures. Acta Orthop. 2010;81(2):234–6. doi: 10.3109/17453671003761946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chintamaneni S, Finzel K, Gruber BL. Successful treatment of sternal fracture nonunion with teriparatide. Osteoporos Int. 2010;21(6):1059–63. doi: 10.1007/s00198-009-1061-4. [DOI] [PubMed] [Google Scholar]
- 35.Gomberg SJ, Wustrack RL, Napoli N, Arnaud CD, Black DM. Teriparatide, vitamin D, and calcium healed bilateral subtrochanteric stress fractures in a postmenopausal woman with a 13-year history of continuous alendronate therapy. J Clin Endocrinol Metab. 2011;96(6):1627–32. doi: 10.1210/jc.2010-2520. [DOI] [PubMed] [Google Scholar]
- 36.Oteo-Alvaro A, Moreno E. Atrophic humeral shaft nonunion treated with teriparatide (rh PTH 1-34): a case report. J Shoulder Elbow Surg. 2010;19(7):e22–28. doi: 10.1016/j.jse.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Paridis D, Karachalios T. Atrophic femoral bone nonunion treated with 1-84 PTH. J Musculoskelet Neuronal Interact. 2011;11(4):320–2. [PubMed] [Google Scholar]
- 38.Rubery PT, Bukata SV. Teriparatide may accelerate healing in delayed unions of type III odontoid fractures: a report of 3 cases. J Spinal Disord Tech. 2010;23(2):151–5. doi: 10.1097/BSD.0b013e31819a8b7a. [DOI] [PubMed] [Google Scholar]
- 39.Wu CC, Wei JC, Hsieh CP, Yu CT. Enhanced healing of sacral and pubic insufficiency fractures by teriparatide. J Rheumatol. 2012;39(6):1306–7. doi: 10.3899/jrheum.111458. [DOI] [PubMed] [Google Scholar]
- 40.Yu CT, Wu JK, Chang CC, Chen CL, Wei JC. Early callus formation in human hip fracture treated with internal fixation and teriparatide. J Rheumatol. 2008;35(10):2082–3. [PubMed] [Google Scholar]
- 41.Holm J, Eiken P, Hyldstrup L, Jensen JE. Atypical femoral fracture in an osteogenesis imperfecta patient successfully treated with teriparatide. Endocr Pract. 2014;20(10):e187–190. doi: 10.4158/EP14141.CR. [DOI] [PubMed] [Google Scholar]
- 42.Nozaka K, Shimada Y, Miyakoshi N, Yamada S, Hongo M, Kasukawa Y, et al. Combined effect of teriparatide and low-intensity pulsed ultrasound for nonunion: a case report. BMC Res Notes. 2014;7:317. doi: 10.1186/1756-0500-7-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tachiiri H, Okuda Y, Yamasaki T, Kusakabe T. Weekly teriparatide administration for the treatment of delayed union: a report of two cases. Arch Osteoporos. 2014;9:179. doi: 10.1007/s11657-014-0179-y. [DOI] [PubMed] [Google Scholar]
- 44.Fukuda F, Kurinomaru N, Hijioka A. Weekly teriparatide for delayed unions of atypical subtrochanteric femur fractures. Biol Ther. 2014;4(1–2):73–9. doi: 10.1007/s13554-014-0013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ochi K, Ikari K, Naomi A, Momohara S. Administration of teriparatide treatment for a challenging case of nonunion of periprosthetic fracture after total knee arthroplasty. Arch Osteoporos. 2013;8:159. doi: 10.1007/s11657-013-0159-7. [DOI] [PubMed] [Google Scholar]
- 46.Bashutski JD, Eber RM, Kinney JS, Benavides E, Maitra S, Braun TM, et al. Teriparatide and osseous regeneration in the oral cavity. N Engl J Med. 2010;363(25):2396–405. doi: 10.1056/NEJMoa1005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pietrogrande L, Raimondo E. Teriparatide in the treatment of non-unions: scientific and clinical evidences. Injury. 2013;44(Suppl 1):S54–57. doi: 10.1016/S0020-1383(13)70013-5. [DOI] [PubMed] [Google Scholar]
- 48.Morgan EF, Mason ZD, Bishop G, Davis AD, Wigner NA, Gerstenfeld LC, et al. Combined effects of recombinant human BMP-7 (rhBMP-7) and parathyroid hormone (1-34) in metaphyseal bone healing. Bone. 2008;43(6):1031–8. doi: 10.1016/j.bone.2008.07.251. [DOI] [PubMed] [Google Scholar]
- 49.Manabe T, Mori S, Mashiba T, Kaji Y, Iwata K, Komatsubara S, et al. Human parathyroid hormone (1-34) accelerates natural fracture healing process in the femoral osteotomy model of cynomolgus monkeys. Bone. 2007;40(6):1475–82. doi: 10.1016/j.bone.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 50.Komatsubara S, Mori S, Mashiba T, Nonaka K, Seki A, Akiyama T, et al. Human parathyroid hormone (1-34) accelerates the fracture healing process of woven to lamellar bone replacement and new cortical shell formation in rat femora. Bone. 2005;36(4):678–87. doi: 10.1016/j.bone.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Whitfield JF, Morley P, Willick GE. Parathyroid hormone, its fragments and their analogs for the treatment of osteoporosis. Treat Endocrinol. 2002;1(3):175–90. doi: 10.2165/00024677-200201030-00005. [DOI] [PubMed] [Google Scholar]
- 52.Peggion E, Mammi S, Schievano E, Silvestri L, Schiebler L, Bisello A, et al. Structure-function studies of analogues of parathyroid hormone (PTH)-1-34 containing beta-amino acid residues in positions 11-13. Biochemistry. 2002;41(25):8162–75. doi: 10.1021/bi0200155. [DOI] [PubMed] [Google Scholar]
- 53.Karaplis AC, Deckelbaum RA. Role of PTHrP and PTH-1 receptor in endochondral bone development. Front Biosci. 1998;3:d795–803. doi: 10.2741/A322. [DOI] [PubMed] [Google Scholar]
- 54.Vickery BH, Avnur Z, Cheng Y, Chiou SS, Leaffer D, Caulfield JP, et al. RS-66271, a C-terminally substituted analog of human parathyroid hormone-related protein (1-34), increases trabecular and cortical bone in ovariectomized, osteopenic rats. J Bone Miner Res. 1996;11(12):1943–51. doi: 10.1002/jbmr.5650111216. [DOI] [PubMed] [Google Scholar]
- 55.Bostrom MP, Asnis P, Toledano T, Hill EL, Avnur Z, Vickery B. The enhancement of fracture healing in the rat using a PTHrP analog. J Orthop Trauma. 1999;13(4):294–5. doi: 10.1097/00005131-199905000-00041. [DOI] [Google Scholar]
- 56.Bostrom MP, Gamradt SC, Asnis P, Vickery BH, Hill E, Avnur Z, et al. Parathyroid hormone-related protein analog RS-66271 is an effective therapy for impaired bone healing in rabbits on corticosteroid therapy. Bone. 2000;26(5):437–42. doi: 10.1016/S8756-3282(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 57.de Castro LF, Lozano D, Portal-Nunez S, Maycas M, De la Fuente M, Caeiro JR, et al. Comparison of the skeletal effects induced by daily administration of PTHrP (1-36) and PTHrP (107-139) to ovariectomized mice. J Cell Physiol. 2012;227(4):1752–60. doi: 10.1002/jcp.22902. [DOI] [PubMed] [Google Scholar]
- 58.Trejo CG, Lozano D, Manzano M, Doadrio JC, Salinas AJ, Dapia S, et al. The osteoinductive properties of mesoporous silicate coated with osteostatin in a rabbit femur cavity defect model. Biomaterials. 2010;31(33):8564–73. doi: 10.1016/j.biomaterials.2010.07.103. [DOI] [PubMed] [Google Scholar]
- 59.Kim YJ, Kim HJ, Im GI. PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochem Biophys Res Commun. 2008;373(1):104–8. doi: 10.1016/j.bbrc.2008.05.183. [DOI] [PubMed] [Google Scholar]
- 60.Amling M, Neff L, Tanaka S, Inoue D, Kuida K, Weir E, et al. Bcl-2 lies downstream of parathyroid hormone-related peptide in a signaling pathway that regulates chondrocyte maturation during skeletal development. J Cell Biol. 1997;136(1):205–13. doi: 10.1083/jcb.136.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du P, Ye Y, Seitz PK, Bi LG, Li H, Wang C, et al. Endogenous parathyroid hormone-related peptide enhances proliferation and inhibits differentiation in the osteoblast-like cell line ROS 17/2.8. Bone. 2000;26(5):429–36. doi: 10.1016/S8756-3282(00)00264-7. [DOI] [PubMed] [Google Scholar]
- 62.Guo J, Liu M, Yang D, Bouxsein ML, Thomas CC, Schipani E, et al. Phospholipase C signaling via the parathyroid hormone (PTH)/PTH-related peptide receptor is essential for normal bone responses to PTH. Endocrinology. 2010;151(8):3502–13. doi: 10.1210/en.2009-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mrak E, Guidobono F, Moro G, Fraschini G, Rubinacci A, Villa I. Calcitonin gene-related peptide (CGRP) inhibits apoptosis in human osteoblasts by beta-catenin stabilization. J Cell Physiol. 2010;225(3):701–8. doi: 10.1002/jcp.22266. [DOI] [PubMed] [Google Scholar]
- 64.Villa I, Melzi R, Pagani F, Ravasi F, Rubinacci A, Guidobono F. Effects of calcitonin gene-related peptide and amylin on human osteoblast-like cells proliferation. Eur J Pharmacol. 2000;409(3):273–8. doi: 10.1016/S0014-2999(00)00872-4. [DOI] [PubMed] [Google Scholar]
- 65.Ballica R, Valentijn K, Khachatryan A, Guerder S, Kapadia S, Gundberg C, et al. Targeted expression of calcitonin gene-related peptide to osteoblasts increases bone density in mice. J Bone Miner Res. 1999;14(7):1067–74. doi: 10.1359/jbmr.1999.14.7.1067. [DOI] [PubMed] [Google Scholar]
- 66.Kruger L, Silverman JD, Mantyh PW, Sternini C, Brecha NC. Peripheral patterns of calcitonin-gene-related peptide general somatic sensory innervation: cutaneous and deep terminations. J Comp Neurol. 1989;280(2):291–302. doi: 10.1002/cne.902800210. [DOI] [PubMed] [Google Scholar]
- 67.Drissi H, Hott M, Marie PJ, Lasmoles F. Expression of the CT/CGRP gene and its regulation by dibutyryl cyclic adenosine monophosphate in human osteoblastic cells. J Bone Miner Res. 1997;12(11):1805–14. doi: 10.1359/jbmr.1997.12.11.1805. [DOI] [PubMed] [Google Scholar]
- 68.Drissi H, Lieberherr M, Hott M, Marie PJ, Lasmoles F. Calcitonin gene-related peptide (CGRP) increases intracellular free Ca2+ concentrations but not cyclic AMP formation in CGRP receptor-positive osteosarcoma cells (OHS-4) Cytokine. 1999;11(3):200–7. doi: 10.1006/cyto.1998.0415. [DOI] [PubMed] [Google Scholar]
- 69.Schinke T, Liese S, Priemel M, Haberland M, Schilling AF, Catala-Lehnen P, et al. Decreased bone formation and osteopenia in mice lacking alpha-calcitonin gene-related peptide. J Bone Miner Res. 2004;19(12):2049–56. doi: 10.1359/jbmr.040915. [DOI] [PubMed] [Google Scholar]
- 70.Song Y, Bi L, Zhang Z, Huang Z, Hou W, Lu X, et al. Increased levels of calcitonin gene-related peptide in serum accelerate fracture healing following traumatic brain injury. Mol Med Rep. 2012;5(2):432–8. doi: 10.3892/mmr.2011.645. [DOI] [PubMed] [Google Scholar]
- 71.Li J, Kreicbergs A, Bergstrom J, Stark A, Ahmed M. Site-specific CGRP innervation coincides with bone formation during fracture healing and modeling: a study in rat angulated tibia. J Orthop Res. 2007;25(9):1204–12. doi: 10.1002/jor.20406. [DOI] [PubMed] [Google Scholar]
- 72.Onuoha GN. Circulating sensory peptide levels within 24 h of human bone fracture. Peptides. 2001;22(7):1107–10. doi: 10.1016/S0196-9781(01)00434-X. [DOI] [PubMed] [Google Scholar]
- 73.Madsen JE, Hukkanen M, Aune AK, Basran I, Moller JF, Polak JM, et al. Fracture healing and callus innervation after peripheral nerve resection in rats. Clin Orthop Relat Res. 1998;351:230–40. doi: 10.1097/00003086-199806000-00028. [DOI] [PubMed] [Google Scholar]
- 74.Sample SJ, Hao Z, Wilson AP, Muir P. Role of calcitonin gene-related peptide in bone repair after cyclic fatigue loading. PLoS One. 2011;6(6):e20386. doi: 10.1371/journal.pone.0020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang L, Shi X, Zhao R, Halloran BP, Clark DJ, Jacobs CR, et al. Calcitonin-gene-related peptide stimulates stromal cell osteogenic differentiation and inhibits RANKL induced NF-kappaB activation, osteoclastogenesis and bone resorption. Bone. 2010;46(5):1369–79. doi: 10.1016/j.bone.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Calland JW, Harris SE, Carnes DL., Jr Human pulp cells respond to calcitonin gene-related peptide in vitro. J Endod. 1997;23(8):485–9. doi: 10.1016/S0099-2399(97)80306-8. [DOI] [PubMed] [Google Scholar]
- 77.Vignery A, McCarthy TL. The neuropeptide calcitonin gene-related peptide stimulates insulin-like growth factor I production by primary fetal rat osteoblasts. Bone. 1996;18(4):331–5. doi: 10.1016/8756-3282(96)00017-8. [DOI] [PubMed] [Google Scholar]
- 78.Millet I, Vignery A. The neuropeptide calcitonin gene-related peptide inhibits TNF-alpha but poorly induces IL-6 production by fetal rat osteoblasts. Cytokine. 1997;9(12):999–1007. doi: 10.1006/cyto.1997.0245. [DOI] [PubMed] [Google Scholar]
- 79.Xu G, Jiang D. The role and mechanism of exogenous calcitonin gene-related peptide on mesenchymal stem cell proliferation and osteogenetic formation. Cell Biochem Biophys. 2014;69(2):369–78. doi: 10.1007/s12013-013-9809-z. [DOI] [PubMed] [Google Scholar]
- 80.Zheng S, Li W, Xu M, Bai X, Zhou Z, Han J, et al. Calcitonin gene-related peptide promotes angiogenesis via AMP-activated protein kinase. Am J Physiol Cell Physiol. 2010;299(6):C1485–92. doi: 10.1152/ajpcell.00173.2010. [DOI] [PubMed] [Google Scholar]
- 81.Bab I, Gazit D, Chorev M, Muhlrad A, Shteyer A, Greenberg Z, et al. Histone H4-related osteogenic growth peptide (OGP): a novel circulating stimulator of osteoblastic activity. EMBO J. 1992;11(5):1867–73. doi: 10.1002/j.1460-2075.1992.tb05238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Greenberg Z, Gavish H, Muhlrad A, Chorev M, Shteyer A, Attar-Namdar M, et al. Isolation of osteogenic growth peptide from osteoblastic MC3T3 E1 cell cultures and demonstration of osteogenic growth peptide binding proteins. J Cell Biochem. 1997;65(3):359–67. doi: 10.1002/(SICI)1097-4644(19970601)65:3<359::AID-JCB6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 83.Gabet Y, Muller R, Regev E, Sela J, Shteyer A, Salisbury K, et al. Osteogenic growth peptide modulates fracture callus structural and mechanical properties. Bone. 2004;35(1):65–73. doi: 10.1016/j.bone.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 84.Shuqiang M, Kunzheng W, Xiaoqiang D, Wei W, Mingyu Z, Daocheng W. Osteogenic growth peptide incorporated into PLGA scaffolds accelerates healing of segmental long bone defects in rabbits. J Plast Reconstr Aesthet Surg. 2008;61(12):1558–60. doi: 10.1016/j.bjps.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 85.Brager MA, Patterson MJ, Connolly JF, Nevo Z. Osteogenic growth peptide normally stimulated by blood loss and marrow ablation has local and systemic effects on fracture healing in rats. J Orthop Res. 2000;18(1):133–9. doi: 10.1002/jor.1100180119. [DOI] [PubMed] [Google Scholar]
- 86.Fei Q, Guo C, Xu X, Gao J, Zhang J, Chen T, et al. Osteogenic growth peptide enhances the proliferation of bone marrow mesenchymal stem cells from osteoprotegerin-deficient mice by CDK2/cyclin A. Acta Biochim Biophys Sin Shanghai. 2010;42(11):801–6. doi: 10.1093/abbs/gmq086. [DOI] [PubMed] [Google Scholar]
- 87.Spreafico A, Frediani B, Capperucci C, Leonini A, Gambera D, Ferrata P, et al. Osteogenic growth peptide effects on primary human osteoblast cultures: potential relevance for the treatment of glucocorticoid-induced osteoporosis. J Cell Biochem. 2006;98(4):1007–20. doi: 10.1002/jcb.20836. [DOI] [PubMed] [Google Scholar]
- 88.Zhao ZY, Shao L, Zhao HM, Zhong ZH, Liu JY, Hao CG. Osteogenic growth peptide accelerates bone healing during distraction osteogenesis in rabbit tibia. J Int Med Res. 2011;39(2):456–63. doi: 10.1177/147323001103900213. [DOI] [PubMed] [Google Scholar]
- 89.Sun YQ, Ashhurst DE. Osteogenic growth peptide enhances the rate of fracture healing in rabbits. Cell Biol Int. 1998;22(4):313–9. doi: 10.1006/cbir.1998.0253. [DOI] [PubMed] [Google Scholar]
- 90.Chen ZX, Chang M, Peng YL, Zhao L, Zhan YR, Wang LJ, et al. Osteogenic growth peptide C-terminal pentapeptide [OGP(10-14)] acts on rat bone marrow mesenchymal stem cells to promote differentiation to osteoblasts and to inhibit differentiation to adipocytes. Regul Pept. 2007;142(1–2):16–23. doi: 10.1016/j.regpep.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 91.Smith E, Meyerrose TE, Kohler T, Namdar-Attar M, Bab N, Lahat O, et al. Leaky ribosomal scanning in mammalian genomes: significance of histone H4 alternative translation in vivo. Nucleic Acids Res. 2005;33(4):1298–308. doi: 10.1093/nar/gki248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.An G, Xue Z, Zhang B, Deng QK, Wang YS, Lv SC. Expressing osteogenic growth peptide in the rabbit bone mesenchymal stem cells increased alkaline phosphatase activity and enhanced the collagen accumulation. Eur Rev Med Pharmacol Sci. 2014;18(11):1618–24. [PubMed] [Google Scholar]
- 93.Chen Z, Wang X, Shao Y, Shi D, Chen T, Cui D, et al. Synthetic osteogenic growth peptide promotes differentiation of human bone marrow mesenchymal stem cells to osteoblasts via RhoA/ROCK pathway. Mol Cell Biochem. 2011;358(1–2):221–7. doi: 10.1007/s11010-011-0938-7. [DOI] [PubMed] [Google Scholar]
- 94.Cakarer S, Olgac V, Aksakalli N, Tang A, Keskin C. Acceleration of consolidation period by thrombin peptide 508 in tibial distraction osteogenesis in rats. Br J Oral Maxillofac Surg. 2010;48(8):633–6. doi: 10.1016/j.bjoms.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 95.Hanratty BM, Ryaby JT, Pan XH, Li G. Thrombin related peptide TP508 promoted fracture repair in a mouse high energy fracture model. J Orthop Surg Res. 2009;4:1. doi: 10.1186/1749-799X-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y, Wan C, Szoke G, Ryaby JT, Li G. Local injection of thrombin-related peptide (TP508) in PPF/PLGA microparticles-enhanced bone formation during distraction osteogenesis. J Orthop Res. 2008;26(4):539–46. doi: 10.1002/jor.20495. [DOI] [PubMed] [Google Scholar]
- 97.Li X, Wang H, Touma E, Qi Y, Rousseau E, Quigg RJ, et al. TP508 accelerates fracture repair by promoting cell growth over cell death. Biochem Biophys Res Commun. 2007;364(1):187–93. doi: 10.1016/j.bbrc.2007.07.202. [DOI] [PubMed] [Google Scholar]
- 98.Amir LR, Li G, Schoenmaker T, Everts V, Bronckers AL. Effect of thrombin peptide 508 (TP508) on bone healing during distraction osteogenesis in rabbit tibia. Cell Tissue Res. 2007;330(1):35–44. doi: 10.1007/s00441-007-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sheller MR, Crowther RS, Kinney JH, Yang J, Di Jorio S, Breunig T, et al. Repair of rabbit segmental defects with the thrombin peptide, TP508. J Orthop Res. 2004;22(5):1094–9. doi: 10.1016/j.orthres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 100.Li G, Ryaby JT, Carney DH, Wang H. Bone formation is enhanced by thrombin-related peptide TP508 during distraction osteogenesis. J Orthop Res. 2005;23(1):196–202. doi: 10.1016/j.orthres.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 101.Hedberg EL, Kroese-Deutman HC, Shih CK, Crowther RS, Carney DH, Mikos AG, et al. Effect of varied release kinetics of the osteogenic thrombin peptide TP508 from biodegradable, polymeric scaffolds on bone formation in vivo. J Biomed Mater Res A. 2005;72(4):343–53. doi: 10.1002/jbm.a.30265. [DOI] [PubMed] [Google Scholar]
- 102.Wang H, Li X, Tomin E, Doty SB, Lane JM, Carney DH, et al. Thrombin peptide (TP508) promotes fracture repair by up-regulating inflammatory mediators, early growth factors, and increasing angiogenesis. J Orthop Res. 2005;23(3):671–9. doi: 10.1016/j.orthres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 103.Li G, Cui Y, McIlmurray L, Allen WE, Wang H. rhBMP-2, rhVEGF(165), rhPTN and thrombin-related peptide, TP508 induce chemotaxis of human osteoblasts and microvascular endothelial cells. J Orthop Res. 2005;23(3):680–5. doi: 10.1016/j.orthres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 104.Vordemvenne T, Paletta JR, Hartensuer R, Pap T, Raschke MJ, Ochman S. Cooperative effects in differentiation and proliferation between PDGF-BB and matrix derived synthetic peptides in human osteoblasts. BMC Musculoskelet Disord. 2011;12:263. doi: 10.1186/1471-2474-12-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Olszewska-Pazdrak B, Carney DH. Systemic administration of thrombin peptide TP508 enhances VEGF-stimulated angiogenesis and attenuates effects of chronic hypoxia. J Vasc Res. 2013;50(3):186–96. doi: 10.1159/000348250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.A Study to Evaluate the Safety and Effectiveness of Different Doses of Chrysalin in Adults Who Have a Broken Wrist. http://clinicaltrials.gov/ct2/show/results/NCT00131482. Accessed 12 Oct 2015.
- 107.Ryaby JT, Sheller MR, Levine BP, Bramlet DG, Ladd AL, Carney DH. Thrombin peptide TP508 stimulates cellular events leading to angiogenesis, revascularization, and repair of dermal and musculoskeletal tissues. J Bone Joint Surg Am. 2006;88(Suppl 3):132–9. doi: 10.2106/JBJS.F.00892. [DOI] [PubMed] [Google Scholar]
- 108.Dent-Acosta RE, Storm N, Steiner RS, San Martin J. The tactics of modern-day regulatory trials. J Bone Joint Surg Am. 2012;94(Suppl 1):39–44. doi: 10.2106/JBJS.L.00194. [DOI] [PubMed] [Google Scholar]
- 109.Yukna RA, Krauser JT, Callan DP, Evans GH, Cruz R, Martin M. Thirty-six month follow-up of 25 patients treated with combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell-binding peptide (P-15) bone replacement grafts in human infrabony defects. I. Clinical findings. J Periodontol. 2002;73(1):123–8. doi: 10.1902/jop.2002.73.1.123. [DOI] [PubMed] [Google Scholar]
- 110.Gelbart M, Friedman R, Burlui V, Rohrer M, Atkinson B. Maxillary sinus augmentation using a peptide-modified graft material in three mixtures: a prospective human case series of histologic and histomorphometric results. Implant Dent. 2005;14(2):185–93. doi: 10.1097/01.id.0000165029.86196.27. [DOI] [PubMed] [Google Scholar]
- 111.Hanks T, Atkinson BL. Comparison of cell viability on anorganic bone matrix with or without P-15 cell binding peptide. Biomaterials. 2004;25(19):4831–6. doi: 10.1016/j.biomaterials.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 112.Yang XB, Bhatnagar RS, Li S, Oreffo RO. Biomimetic collagen scaffolds for human bone cell growth and differentiation. Tissue Eng. 2004;10(7–8):1148–59. doi: 10.1089/ten.2004.10.1148. [DOI] [PubMed] [Google Scholar]
- 113.Kubler A, Neugebauer J, Oh JH, Scheer M, Zoller JE. Growth and proliferation of human osteoblasts on different bone graft substitutes: an in vitro study. Implant Dent. 2004;13(2):171–9. doi: 10.1097/01.ID.0000127522.14067.11. [DOI] [PubMed] [Google Scholar]
- 114.Yukna RA, Krauser JT, Callan DP, Evans GH, Cruz R, Martin M. Multi-center clinical comparison of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) and ABM in human periodontal osseous defects. 6-month results. J Periodontol. 2000;71(11):1671–9. doi: 10.1902/jop.2000.71.11.1671. [DOI] [PubMed] [Google Scholar]
- 115.Yukna RA, Callan DP, Krauser JT, Evans GH, Aichelmann-Reidy ME, Moore K, et al. Multi-center clinical evaluation of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) as a bone replacement graft material in human periodontal osseous defects. 6-month results. J Periodontol. 1998;69(6):655–63. doi: 10.1902/jop.1998.69.6.655. [DOI] [PubMed] [Google Scholar]
- 116.Butz F, Bachle M, Ofer M, Marquardt K, Kohal RJ. Sinus augmentation with bovine hydroxyapatite/synthetic peptide in a sodium hyaluronate carrier (PepGen P-15 Putty): a clinical investigation of different healing times. Int J Oral Maxillofac Implants. 2011;26(6):1317–23. [PubMed] [Google Scholar]
- 117.Emam H, Beheiri G, Elsalanty M, Sharawy M. Microcomputed tomographic and histologic analysis of anorganic bone matrix coupled with cell-binding peptide suspended in sodium hyaluronate carrier after sinus augmentation: a clinical study. Int J Oral Maxillofac Implants. 2011;26(3):561–70. [PubMed] [Google Scholar]
- 118.Matos S, Guerra F, Krauser JT, Figueiredo H, Marcelino JP, Sanz M. Evaluation of an anorganic bovine-derived mineral with P-15 hydrogel bone graft: preliminary study in a rabbit cranial bone model. Clin Oral Implants Res. 2012;23(6):698–705. doi: 10.1111/j.1600-0501.2011.02179.x. [DOI] [PubMed] [Google Scholar]
- 119.Sherman BP, Lindley EM, Turner AS, Seim HB, 3rd, Benedict J, Burger EL, et al. Evaluation of ABM/P-15 versus autogenous bone in an ovine lumbar interbody fusion model. Eur Spine J. 2010;19(12):2156–63. doi: 10.1007/s00586-010-1546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lindley EM, Guerra FA, Krauser JT, Matos SM, Burger EL, Patel VV. Small peptide (P-15) bone substitute efficacy in a rabbit cancellous bone model. J Biomed Mater Res B Appl Biomater. 2010;94(2):463–8. doi: 10.1002/jbm.b.31676. [DOI] [PubMed] [Google Scholar]
- 121.El-Madany I, Emam H, Sharawy M. Comparison of cellular response to anorganic bone matrix/cell binding peptide and allogenic cranial bone after sinus augmentation in rhesus monkeys. J Oral Implantol. 2011;37(2):233–45. doi: 10.1563/AAID-JOI-D-09-00081. [DOI] [PubMed] [Google Scholar]
- 122.Yukna R, Salinas TJ, Carr RF. Periodontal regeneration following use of ABM/P-1 5: a case report. Int J Periodontics Restorative Dent. 2002;22(2):146–55. [PubMed] [Google Scholar]
- 123.Suaid FA, Macedo GO, Novaes AB, Borges GJ, Souza SL, Taba M, et al. The bone formation capabilities of the anorganic bone matrix-synthetic cell-binding peptide 15 grafts in an animal periodontal model: a histologic and histomorphometric study in dogs. J Periodontol. 2010;81(4):594–603. doi: 10.1902/jop.2010.090486. [DOI] [PubMed] [Google Scholar]
- 124.Artzi Z, Kozlovsky A, Nemcovsky CE, Moses O, Tal H, Rohrer MD, et al. Histomorphometric evaluation of natural mineral combined with a synthetic cell-binding peptide (P-15) in critical-size defects in the rat calvaria. Int J Oral Maxillofac Implants. 2008;23(6):1063–70. [PubMed] [Google Scholar]
- 125.Mardas N, Stavropoulos A, Karring T. Calvarial bone regeneration by a combination of natural anorganic bovine-derived hydroxyapatite matrix coupled with a synthetic cell-binding peptide (PepGen): an experimental study in rats. Clin Oral Implants Res. 2008;19(10):1010–5. doi: 10.1111/j.1600-0501.2008.01572.x. [DOI] [PubMed] [Google Scholar]
- 126.Sarahrudi K, Mousavi M, Grossschmidt K, Sela N, Konig F, Vecsei V, et al. Combination of anorganic bovine-derived hydroxyapatite with binding peptide does not enhance bone healing in a critical-size defect in a rabbit model. J Orthop Res. 2008;26(6):759–63. doi: 10.1002/jor.20527. [DOI] [PubMed] [Google Scholar]
- 127.Kasaj A, Rohrig B, Reichert C, Willershausen B. Clinical evaluation of anorganic bovine-derived hydroxyapatite matrix/cell-binding peptide (P-15) in the treatment of human infrabony defects. Clin Oral Investig. 2008;12(3):241–7. doi: 10.1007/s00784-008-0191-y. [DOI] [PubMed] [Google Scholar]
- 128.Degidi M, Piattelli M, Scarano A, Iezzi G, Piattelli A. Maxillary sinus augmentation with a synthetic cell-binding peptide: histological and histomorphometrical results in humans. J Oral Implantol. 2004;30(6):376–83. doi: 10.1563/0720.1. [DOI] [PubMed] [Google Scholar]
- 129.Thorwarth M, Schultze-Mosgau S, Wehrhan F, Srour S, Wiltfang J, Neukam FW, et al. Enhanced bone regeneration with a synthetic cell-binding peptide--in vivo results. Biochem Biophys Res Commun. 2005;329(2):789–95. doi: 10.1016/j.bbrc.2005.01.157. [DOI] [PubMed] [Google Scholar]
- 130.Philippart P, Daubie V, Pochet R. Sinus grafting using recombinant human tissue factor, platelet-rich plasma gel, autologous bone, and anorganic bovine bone mineral xenograft: histologic analysis and case reports. Int J Oral Maxillofac Implants. 2005;20(2):274–81. [PubMed] [Google Scholar]
- 131.Thorwarth M, Schultze-Mosgau S, Wehrhan F, Kessler P, Srour S, Wiltfang J, et al. Bioactivation of an anorganic bone matrix by P-15 peptide for the promotion of early bone formation. Biomaterials. 2005;26(28):5648–57. doi: 10.1016/j.biomaterials.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 132.Vastardis S, Yukna RA, Mayer ET, Atkinson BL. Periodontal regeneration with peptide-enhanced anorganic bone matrix in particulate and putty form in dogs. J Periodontol. 2005;76(10):1690–6. doi: 10.1902/jop.2005.76.10.1690. [DOI] [PubMed] [Google Scholar]
- 133.Cakmak G, Bolukbasi S, Simsek A, Erdem O, Yilmaz G, Senkoylu A. Effect of synthetic cell-binding peptide on the healing of cortical segmental bone defects. Saudi Med J. 2006;27(6):777–80. [PubMed] [Google Scholar]
- 134.Barros RR, Novaes AB, Jr, Roriz VM, Oliveira RR, Grisi MF, Souza SL, et al. Anorganic bovine matrix/p-15 “flow” in the treatment of periodontal defects: case series with 12 months of follow-up. J Periodontol. 2006;77(7):1280–7. doi: 10.1902/jop.2006.050161. [DOI] [PubMed] [Google Scholar]
- 135.Artzi Z, Weinreb M, Tal H, Nemcovsky CE, Rohrer MD, Prasad HS, et al. Experimental intrabony and periodontal defects treated with natural mineral combined with a synthetic cell-binding Peptide in the canine: morphometric evaluations. J Periodontol. 2006;77(10):1658–64. doi: 10.1902/jop.2006.060041. [DOI] [PubMed] [Google Scholar]
- 136.Bhongade ML, Tiwari IR. A comparative evaluation of the effectiveness of an anorganic bone matrix/cell binding peptide with an open flap debridement in human infrabony defects: a clinical and radiographic study. J Contemp Dent Pract. 2007;8(6):25–34. [PubMed] [Google Scholar]
- 137.Trasatti C, Spears R, Gutmann JL, Opperman LA. Increased Tgf-beta1 production by rat osteoblasts in the presence of PepGen P-15 in vitro. J Endod. 2004;30(4):213–7. doi: 10.1097/00004770-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 138.Scarano A, Iezzi G, Petrone G, Orsini G, Degidi M, Strocchi R, et al. Cortical bone regeneration with a synthetic cell-binding peptide: a histologic and histomorphometric pilot study. Implant Dent. 2003;12(4):318–24. doi: 10.1097/01.ID.0000095467.48241.68. [DOI] [PubMed] [Google Scholar]
- 139.Barboza EP, de Souza RO, Caula AL, Neto LG, Caula Fde O, Duarte ME. Bone regeneration of localized chronic alveolar defects utilizing cell binding peptide associated with anorganic bovine-derived bone mineral: a clinical and histological study. J Periodontol. 2002;73(10):1153–9. doi: 10.1902/jop.2002.73.10.1153. [DOI] [PubMed] [Google Scholar]
- 140.Durrieu MC, Pallu S, Guillemot F, Bareille R, Amedee J, Baquey CH, et al. Grafting RGD containing peptides onto hydroxyapatite to promote osteoblastic cells adhesion. J Mater Sci Mater Med. 2004;15(7):779–86. doi: 10.1023/B:JMSM.0000032818.09569.d9. [DOI] [PubMed] [Google Scholar]
- 141.Huang H, Zhao Y, Liu Z, Zhang Y, Zhang H, Fu T, et al. Enhanced osteoblast functions on RGD immobilized surface. J Oral Implantol. 2003;29(2):73–9. doi: 10.1563/1548-1336(2003)029<0073:EOFORI>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 142.El-Ghannam AR, Ducheyne P, Risbud M, Adams CS, Shapiro IM, Castner D, et al. Model surfaces engineered with nanoscale roughness and RGD tripeptides promote osteoblast activity. J Biomed Mater Res A. 2004;68(4):615–27. doi: 10.1002/jbm.a.20051. [DOI] [PubMed] [Google Scholar]
- 143.Cavalcanti-Adam EA, Shapiro IM, Composto RJ, Macarak EJ, Adams CS. RGD peptides immobilized on a mechanically deformable surface promote osteoblast differentiation. J Bone Miner Res. 2002;17(12):2130–40. doi: 10.1359/jbmr.2002.17.12.2130. [DOI] [PubMed] [Google Scholar]
- 144.Dee KC, Rueger DC, Andersen TT, Bizios R. Conditions which promote mineralization at the bone-implant interface: a model in vitro study. Biomaterials. 1996;17(2):209–15. doi: 10.1016/0142-9612(96)85765-6. [DOI] [PubMed] [Google Scholar]
- 145.Hofmann S, Hilbe M, Fajardo RJ, Hagenmuller H, Nuss K, Arras M, et al. Remodeling of tissue-engineered bone structures in vivo. Eur J Pharm Biopharm. 2013;85(1):119–29. doi: 10.1016/j.ejpb.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thein-Han W, Liu J, Xu HH. Calcium phosphate cement with biofunctional agents and stem cell seeding for dental and craniofacial bone repair. Dent Mater. 2012;28(10):1059–70. doi: 10.1016/j.dental.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hayashibara T, Hiraga T, Yi B, Nomizu M, Kumagai Y, Nishimura R, et al. A synthetic peptide fragment of human MEPE stimulates new bone formation in vitro and in vivo. J Bone Miner Res. 2004;19(3):455–62. doi: 10.1359/JBMR.0301263. [DOI] [PubMed] [Google Scholar]
- 148.Rammelt S, Illert T, Bierbaum S, Scharnweber D, Zwipp H, Schneiders W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials. 2006;27(32):5561–71. doi: 10.1016/j.biomaterials.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 149.Park JW, Kurashima K, Tustusmi Y, An CH, Suh JY, Doi H, et al. Bone healing of commercial oral implants with RGD immobilization through electrodeposited poly(ethylene glycol) in rabbit cancellous bone. Acta Biomater. 2011;7(8):3222–9. doi: 10.1016/j.actbio.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 150.Priddy LB, Chaudhuri O, Stevens HY, Krishnan L, Uhrig BA, Willett NJ, et al. Oxidized alginate hydrogels for bone morphogenetic protein-2 delivery in long bone defects. Acta Biomater. 2014;10(10):4390–9. doi: 10.1016/j.actbio.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ferris DM, Moodie GD, Dimond PM, Gioranni CW, Ehrlich MG, Valentini RF. RGD-coated titanium implants stimulate increased bone formation in vivo. Biomaterials. 1999;20(23–24):2323–31. doi: 10.1016/S0142-9612(99)00161-1. [DOI] [PubMed] [Google Scholar]
- 152.Schneiders W, Reinstorf A, Pompe W, Grass R, Biewener A, Holch M, et al. Effect of modification of hydroxyapatite/collagen composites with sodium citrate, phosphoserine, phosphoserine/RGD-peptide and calcium carbonate on bone remodelling. Bone. 2007;40(4):1048–59. doi: 10.1016/j.bone.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 153.Hennessy KM, Clem WC, Phipps MC, Sawyer AA, Shaikh FM, Bellis SL. The effect of RGD peptides on osseointegration of hydroxyapatite biomaterials. Biomaterials. 2008;29(21):3075–83. doi: 10.1016/j.biomaterials.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bitschnau A, Alt V, Bohner F, Heerich KE, Margesin E, Hartmann S, et al. Comparison of new bone formation, implant integration, and biocompatibility between RGD-hydroxyapatite and pure hydroxyapatite coating for cementless joint prostheses--an experimental study in rabbits. J Biomed Mater Res B Appl Biomater. 2009;88(1):66–74. doi: 10.1002/jbm.b.31150. [DOI] [PubMed] [Google Scholar]
- 155.Elmengaard B, Bechtold JE, Soballe K. In vivo effects of RGD-coated titanium implants inserted in two bone-gap models. J Biomed Mater Res A. 2005;75(2):249–55. doi: 10.1002/jbm.a.30301. [DOI] [PubMed] [Google Scholar]
- 156.Elmengaard B, Bechtold JE, Soballe K. In vivo study of the effect of RGD treatment on bone ongrowth on press-fit titanium alloy implants. Biomaterials. 2005;26(17):3521–6. doi: 10.1016/j.biomaterials.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 157.Rezania A, Thomas CH, Branger AB, Waters CM, Healy KE. The detachment strength and morphology of bone cells contacting materials modified with a peptide sequence found within bone sialoprotein. J Biomed Mater Res. 1997;37(1):9–19. doi: 10.1002/(SICI)1097-4636(199710)37:1<9::AID-JBM2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 158.Shin H, Temenoff JS, Bowden GC, Zygourakis K, Farach-Carson MC, Yaszemski MJ, et al. Osteogenic differentiation of rat bone marrow stromal cells cultured on Arg-Gly-Asp modified hydrogels without dexamethasone and beta-glycerol phosphate. Biomaterials. 2005;26(17):3645–54. doi: 10.1016/j.biomaterials.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 159.Gilbert M, Shaw WJ, Long JR, Nelson K, Drobny GP, Giachelli CM, et al. Chimeric peptides of statherin and osteopontin that bind hydroxyapatite and mediate cell adhesion. J Biol Chem. 2000;275(21):16213–8. doi: 10.1074/jbc.M001773200. [DOI] [PubMed] [Google Scholar]
- 160.Wojtowicz AM, Shekaran A, Oest ME, Dupont KM, Templeman KL, Hutmacher DW, et al. Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials. 2010;31(9):2574–82. doi: 10.1016/j.biomaterials.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shekaran A, Garcia JR, Clark AY, Kavanaugh TE, Lin AS, Guldberg RE, et al. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials. 2014;35(21):5453–61. doi: 10.1016/j.biomaterials.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Reyes CD, Petrie TA, Burns KL, Schwartz Z, Garcia AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007;28(21):3228–35. doi: 10.1016/j.biomaterials.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hennessy KM, Pollot BE, Clem WC, Phipps MC, Sawyer AA, Culpepper BK, et al. The effect of collagen I mimetic peptides on mesenchymal stem cell adhesion and differentiation, and on bone formation at hydroxyapatite surfaces. Biomaterials. 2009;30(10):1898–909. doi: 10.1016/j.biomaterials.2008.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reyes CD, Garcia AJ. Alpha2beta1 integrin-specific collagen-mimetic surfaces supporting osteoblastic differentiation. J Biomed Mater Res A. 2004;69(4):591–600. doi: 10.1002/jbm.a.30034. [DOI] [PubMed] [Google Scholar]
- 165.Ceylan H, Kocabey S, Unal Gulsuner H, Balcik OS, Guler MO, Tekinay AB. Bone-like mineral nucleating peptide nanofibers induce differentiation of human mesenchymal stem cells into mature osteoblasts. Biomacromolecules. 2014;15(7):2407–18. doi: 10.1021/bm500248r. [DOI] [PubMed] [Google Scholar]
- 166.Yoo SY, Kobayashi M, Lee PP, Lee SW. Early osteogenic differentiation of mouse preosteoblasts induced by collagen-derived DGEA-peptide on nanofibrous phage tissue matrices. Biomacromolecules. 2011;12(4):987–96. doi: 10.1021/bm1013475. [DOI] [PubMed] [Google Scholar]
- 167.Culpepper BK, Phipps MC, Bonvallet PP, Bellis SL. Enhancement of peptide coupling to hydroxyapatite and implant osseointegration through collagen mimetic peptide modified with a polyglutamate domain. Biomaterials. 2010;31(36):9586–94. doi: 10.1016/j.biomaterials.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Culpepper BK, Bonvallet PP, Reddy MS, Ponnazhagan S, Bellis SL. Polyglutamate directed coupling of bioactive peptides for the delivery of osteoinductive signals on allograft bone. Biomaterials. 2013;34(5):1506–13. doi: 10.1016/j.biomaterials.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Egusa H, Kaneda Y, Akashi Y, Hamada Y, Matsumoto T, Saeki M, et al. Enhanced bone regeneration via multimodal actions of synthetic peptide SVVYGLR on osteoprogenitors and osteoclasts. Biomaterials. 2009;30(27):4676–86. doi: 10.1016/j.biomaterials.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 170.Hamada Y, Egusa H, Kaneda Y, Hirata I, Kawaguchi N, Hirao T, et al. Synthetic osteopontin-derived peptide SVVYGLR can induce neovascularization in artificial bone marrow scaffold biomaterials. Dent Mater J. 2007;26(4):487–92. doi: 10.4012/dmj.26.487. [DOI] [PubMed] [Google Scholar]
- 171.Palchesko RN, Romeo JD, McGowan KA, Gawalt ES. Increased osteoblast adhesion on physically optimized KRSR modified calcium aluminate. J Biomed Mater Res A. 2012;100(5):1229–38. doi: 10.1002/jbm.a.33303. [DOI] [PubMed] [Google Scholar]
- 172.Sun S, Yu W, Zhang Y, Zhang F. Increased preosteoblast adhesion and osteogenic gene expression on TiO2 nanotubes modified with KRSR. J Mater Sci Mater Med. 2013;24(4):1079–91. doi: 10.1007/s10856-013-4869-6. [DOI] [PubMed] [Google Scholar]
- 173.Dee KC, Andersen TT, Bizios R. Design and function of novel osteoblast-adhesive peptides for chemical modification of biomaterials. J Biomed Mater Res. 1998;40(3):371–7. doi: 10.1002/(SICI)1097-4636(19980605)40:3<371::AID-JBM5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 174.Balasundaram G, Shimpi TM, Sanow WR, Storey DM, Kitchell BS, Webster TJ. Molecular plasma deposited peptides on anodized nanotubular titanium: an osteoblast density study. J Biomed Mater Res A. 2011;98(2):192–200. doi: 10.1002/jbm.a.33105. [DOI] [PubMed] [Google Scholar]
- 175.Zhang L, Hemraz UD, Fenniri H, Webster TJ. Tuning cell adhesion on titanium with osteogenic rosette nanotubes. J Biomed Mater Res A. 2010;95(2):550–63. doi: 10.1002/jbm.a.32832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schuler M, Hamilton DW, Kunzler TP, Sprecher CM, de Wild M, Brunette DM, et al. Comparison of the response of cultured osteoblasts and osteoblasts outgrown from rat calvarial bone chips to nonfouling KRSR and FHRRIKA-peptide modified rough titanium surfaces. J Biomed Mater Res B Appl Biomater. 2009;91(2):517–27. doi: 10.1002/jbm.b.31425. [DOI] [PubMed] [Google Scholar]
- 177.Nelson M, Balasundaram G, Webster TJ. Increased osteoblast adhesion on nanoparticulate crystalline hydroxyapatite functionalized with KRSR. Int J Nanomedicine. 2006;1(3):339–49. [PMC free article] [PubMed] [Google Scholar]
- 178.Balasundaram G, Webster TJ. Increased osteoblast adhesion on nanograined Ti modified with KRSR. J Biomed Mater Res A. 2007;80(3):602–11. doi: 10.1002/jbm.a.30954. [DOI] [PubMed] [Google Scholar]
- 179.Sawyer AA, Hennessy KM, Bellis SL. The effect of adsorbed serum proteins, RGD and proteoglycan-binding peptides on the adhesion of mesenchymal stem cells to hydroxyapatite. Biomaterials. 2007;28(3):383–92. doi: 10.1016/j.biomaterials.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 180.Lee JY, Choo JE, Park HJ, Park JB, Lee SC, Jo I, et al. Injectable gel with synthetic collagen-binding peptide for enhanced osteogenesis in vitro and in vivo. Biochem Biophys Res Commun. 2007;357(1):68–74. doi: 10.1016/j.bbrc.2007.03.106. [DOI] [PubMed] [Google Scholar]
- 181.Shin MK, Kim MK, Bae YS, Jo I, Lee SJ, Chung CP, et al. A novel collagen-binding peptide promotes osteogenic differentiation via Ca2+/calmodulin-dependent protein kinase II/ERK/AP-1 signaling pathway in human bone marrow-derived mesenchymal stem cells. Cell Signal. 2008;20(4):613–24. doi: 10.1016/j.cellsig.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 182.Au A, Boehm CA, Mayes AM, Muschler GF, Griffith LG. Formation of osteogenic colonies on well-defined adhesion peptides by freshly isolated human marrow cells. Biomaterials. 2007;28(10):1847–61. doi: 10.1016/j.biomaterials.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rezania A, Healy KE. Biomimetic peptide surfaces that regulate adhesion, spreading, cytoskeletal organization, and mineralization of the matrix deposited by osteoblast-like cells. Biotechnol Prog. 1999;15(1):19–32. doi: 10.1021/bp980083b. [DOI] [PubMed] [Google Scholar]
- 184.Stile RA, Healy KE. Thermo-responsive peptide-modified hydrogels for tissue regeneration. Biomacromolecules. 2001;2(1):185–94. doi: 10.1021/bm0000945. [DOI] [PubMed] [Google Scholar]
- 185.Kim YJ, Park YJ, Lee YM, Rhyu IC, Ku Y. The biological effects of fibrin-binding synthetic oligopeptides derived from fibronectin on osteoblast-like cells. J Periodontal Implant Sci. 2012;42(4):113–8. doi: 10.5051/jpis.2012.42.4.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Martino MM, Tortelli F, Mochizuki M, Traub S, Ben-David D, Kuhn GA, et al. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med. 2011;3(100):100ra189. [DOI] [PubMed]
- 187.Lee JA, Ku Y, Rhyu IC, Chung CP, Park YJ. Effects of fibrin-binding oligopeptide on osteopromotion in rabbit calvarial defects. J Periodontal Implant Sci. 2010;40(5):211–9. doi: 10.5051/jpis.2010.40.5.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kantlehner M, Schaffner P, Finsinger D, Meyer J, Jonczyk A, Diefenbach B, et al. Surface coating with cyclic RGD peptides stimulates osteoblast adhesion and proliferation as well as bone formation. Chembiochem. 2000;1(2):107–14. doi: 10.1002/1439-7633(20000818)1:2<107::AID-CBIC107>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 189.Liu Q, Limthongkul W, Sidhu G, Zhang J, Vaccaro A, Shenck R, et al. Covalent attachment of P15 peptide to titanium surfaces enhances cell attachment, spreading, and osteogenic gene expression. J Orthop Res. 2012;30(10):1626–33. doi: 10.1002/jor.22116. [DOI] [PubMed] [Google Scholar]
- 190.Mota A, Sahebghadam Lotfi A, Barzin J, Hatam M, Adibi B, Khalaj Z, et al. Human bone marrow mesenchymal stem cell behaviors on PCL/gelatin nanofibrous scaffolds modified with a collagen IV-derived RGD-containing peptide. Cell J. 2014;16(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- 191.Chang JC, Hsu SH, Chen DC. The promotion of chondrogenesis in adipose-derived adult stem cells by an RGD-chimeric protein in 3D alginate culture. Biomaterials. 2009;30(31):6265–75. doi: 10.1016/j.biomaterials.2009.07.064. [DOI] [PubMed] [Google Scholar]
- 192.Li W, Yu B, Li M, Sun D, Hu Y, Zhao M, et al. NEMO-binding domain peptide promotes osteoblast differentiation impaired by tumor necrosis factor alpha. Biochem Biophys Res Commun. 2010;391(2):1228–33. doi: 10.1016/j.bbrc.2009.12.048. [DOI] [PubMed] [Google Scholar]
- 193.Jimi E, Aoki K, Saito H, D’Acquisto F, May MJ, Nakamura I, et al. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med. 2004;10(6):617–24. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- 194.Strnad J, McDonnell PA, Riexinger DJ, Mapelli C, Cheng L, Gray H, et al. NEMO binding domain of IKK-2 encompasses amino acids 735-745. J Mol Recognit. 2006;19(3):227–33. doi: 10.1002/jmr.766. [DOI] [PubMed] [Google Scholar]
- 195.Dai S, Hirayama T, Abbas S, Abu-Amer Y. The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. J Biol Chem. 2004;279(36):37219–22. doi: 10.1074/jbc.C400258200. [DOI] [PubMed] [Google Scholar]
- 196.Tomomatsu N, Aoki K, Alles N, Soysa NS, Hussain A, Nakachi H, et al. LPS-induced inhibition of osteogenesis is TNF-alpha dependent in a murine tooth extraction model. J Bone Miner Res. 2009;24(10):1770–81. doi: 10.1359/jbmr.090410. [DOI] [PubMed] [Google Scholar]
- 197.Martino MM, Briquez PS, Guc E, Tortelli F, Kilarski WW, Metzger S, et al. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science. 2014;343(6173):885–8. doi: 10.1126/science.1247663. [DOI] [PubMed] [Google Scholar]
- 198.Fukushima N, Hiraoka K, Shirachi I, Kojima M, Nagata K. Isolation and characterization of a novel peptide, osteoblast activating peptide (OBAP), associated with osteoblast differentiation and bone formation. Biochem Biophys Res Commun. 2010;400(1):157–63. doi: 10.1016/j.bbrc.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 199.Jo J, Song H, Park SG, Lee SH, Ko JJ, Park JH, et al. Regulation of differentiation potential of human mesenchymal stem cells by intracytoplasmic delivery of coactivator-associated arginine methyltransferase 1 protein using cell-penetrating peptide. Stem Cells. 2012;30(8):1703–13. doi: 10.1002/stem.1146. [DOI] [PubMed] [Google Scholar]
- 200.Suh JS, Lee JY, Choi YJ, You HK, Hong SD, Chung CP, et al. Intracellular delivery of cell-penetrating peptide-transcriptional factor fusion protein and its role in selective osteogenesis. Int J Nanomedicine. 2014;9:1153–66. doi: 10.2147/IJN.S55433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Park SH, Doh J, Park SI, Lim JY, Kim SM, Youn JI, et al. Branched oligomerization of cell-permeable peptides markedly enhances the transduction efficiency of adenovirus into mesenchymal stem cells. Gene Ther. 2010;17(8):1052–61. doi: 10.1038/gt.2010.58. [DOI] [PubMed] [Google Scholar]
- 202.Lee JY, Choo JE, Choi YS, Lee KY, Min DS, Pi SH, et al. Characterization of the surface immobilized synthetic heparin binding domain derived from human fibroblast growth factor-2 and its effect on osteoblast differentiation. J Biomed Mater Res A. 2007;83(4):970–9. doi: 10.1002/jbm.a.31351. [DOI] [PubMed] [Google Scholar]
- 203.Li Z, Hou T, Luo F, Chang Z, Wu X, Xing J, et al. Bone marrow enriched graft, modified by self-assembly peptide, repairs critically-sized femur defects in goats. Int Orthop. 2014;38(11):2391–8. doi: 10.1007/s00264-014-2388-9. [DOI] [PubMed] [Google Scholar]
- 204.Hou T, Li Z, Luo F, Xie Z, Wu X, Xing J, et al. A composite demineralized bone matrix--self assembling peptide scaffold for enhancing cell and growth factor activity in bone marrow. Biomaterials. 2014;35(22):5689–99. doi: 10.1016/j.biomaterials.2014.03.079. [DOI] [PubMed] [Google Scholar]
- 205.Ikeno M, Hibi H, Kinoshita K, Hattori H, Ueda M. Effects of self-assembling peptide hydrogel scaffold on bone regeneration with recombinant human bone morphogenetic protein-2. Int J Oral Maxillofac Implants. 2013;28(5):e283–289. doi: 10.11607/jomi.te09. [DOI] [PubMed] [Google Scholar]
- 206.Nakahara H, Misawa H, Yoshida A, Hayashi T, Tanaka M, Furumatsu T, et al. Bone repair using a hybrid scaffold of self-assembling peptide PuraMatrix and polyetheretherketone cage in rats. Cell Transplant. 2010;19(6):791–7. doi: 10.3727/096368910X508906. [DOI] [PubMed] [Google Scholar]
- 207.Ozeki M, Kuroda S, Kon K, Kasugai S. Differentiation of bone marrow stromal cells into osteoblasts in a self-assembling peptide hydrogel: in vitro and in vivo studies. J Biomater Appl. 2011;25(7):663–84. doi: 10.1177/0885328209356328. [DOI] [PubMed] [Google Scholar]
- 208.Yoshimi R, Yamada Y, Ito K, Nakamura S, Abe A, Nagasaka T, et al. Self-assembling peptide nanofiber scaffolds, platelet-rich plasma, and mesenchymal stem cells for injectable bone regeneration with tissue engineering. J Craniofac Surg. 2009;20(5):1523–30. doi: 10.1097/SCS.0b013e3181b09b7e. [DOI] [PubMed] [Google Scholar]
- 209.Misawa H, Kobayashi N, Soto-Gutierrez A, Chen Y, Yoshida A, Rivas-Carrillo JD, et al. PuraMatrix facilitates bone regeneration in bone defects of calvaria in mice. Cell Transplant. 2006;15(10):903–10. doi: 10.3727/000000006783981369. [DOI] [PubMed] [Google Scholar]
- 210.Horii A, Wang X, Gelain F, Zhang S. Biological designer self-assembling peptide nanofiber scaffolds significantly enhance osteoblast proliferation, differentiation and 3-D migration. PLoS One. 2007;2(2):e190. doi: 10.1371/journal.pone.0000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gelain F, Bottai D, Vescovi A, Zhang S. Designer self-assembling peptide nanofiber scaffolds for adult mouse neural stem cell 3-dimensional cultures. PLoS One. 2006;1:e119. doi: 10.1371/journal.pone.0000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Garreta E, Gasset D, Semino C, Borros S. Fabrication of a three-dimensional nanostructured biomaterial for tissue engineering of bone. Biomol Eng. 2007;24(1):75–80. doi: 10.1016/j.bioeng.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 213.Sargeant TD, Aparicio C, Goldberger JE, Cui H, Stupp SI. Mineralization of peptide amphiphile nanofibers and its effect on the differentiation of human mesenchymal stem cells. Acta Biomater. 2012;8(7):2456–65. doi: 10.1016/j.actbio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Murphy MB, Blashki D, Buchanan RM, Fan D, De Rosa E, Shah RN, et al. Multi-composite bioactive osteogenic sponges featuring mesenchymal stem cells, platelet-rich plasma, nanoporous silicon enclosures, and Peptide amphiphiles for rapid bone regeneration. J Funct Biomater. 2011;2(2):39–66. doi: 10.3390/jfb2020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Anderson JM, Vines JB, Patterson JL, Chen H, Javed A, Jun HW. Osteogenic differentiation of human mesenchymal stem cells synergistically enhanced by biomimetic peptide amphiphiles combined with conditioned medium. Acta Biomater. 2011;7(2):675–82. doi: 10.1016/j.actbio.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lee JY, Choo JE, Choi YS, Suh JS, Lee SJ, Chung CP, et al. Osteoblastic differentiation of human bone marrow stromal cells in self-assembled BMP-2 receptor-binding peptide-amphiphiles. Biomaterials. 2009;30(21):3532–41. doi: 10.1016/j.biomaterials.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 217.Verheyen A, Peeraer E, Lambrechts D, Poesen K, Carmeliet P, Shibuya M, et al. Therapeutic potential of VEGF and VEGF-derived peptide in peripheral neuropathies. Neuroscience. 2013;244:77–89. doi: 10.1016/j.neuroscience.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 218.Guan J. Insulin-like growth factor-1 and its derivatives: potential pharmaceutical application for ischemic brain injury. Recent Pat CNS Drug Discov. 2008;3(2):112–27. doi: 10.2174/157488908784534630. [DOI] [PubMed] [Google Scholar]
- 219.Saito A, Suzuki Y, Kitamura M, Ogata S, Yoshihara Y, Masuda S, et al. Repair of 20-mm long rabbit radial bone defects using BMP-derived peptide combined with an alpha-tricalcium phosphate scaffold. J Biomed Mater Res A. 2006;77(4):700–6. doi: 10.1002/jbm.a.30662. [DOI] [PubMed] [Google Scholar]
- 220.Deng M, Zhang B, Wang K, Liu F, Xiao H, Zhao J, et al. Mechano growth factor E peptide promotes osteoblasts proliferation and bone-defect healing in rabbits. Int Orthop. 2011;35(7):1099–106. doi: 10.1007/s00264-010-1141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Smucker JD, Bobst JA, Petersen EB, Nepola JV, Fredericks DC. B2A peptide on ceramic granules enhance posterolateral spinal fusion in rabbits compared with autograft. Spine (Phila Pa 1976) 2008;33(12):1324–9. doi: 10.1097/BRS.0b013e3181732a74. [DOI] [PubMed] [Google Scholar]
- 222.Ezquerro IJ, Lasarte JJ, Dotor J, Castilla-Cortazar I, Bustos M, Penuelas I, et al. A synthetic peptide from transforming growth factor beta type III receptor inhibits liver fibrogenesis in rats with carbon tetrachloride liver injury. Cytokine. 2003;22(1–2):12–20. doi: 10.1016/S1043-4666(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 223.Furuya Y, Inagaki A, Khan M, Mori K, Penninger JM, Nakamura M, et al. Stimulation of bone formation in cortical bone of mice treated with a receptor activator of nuclear factor-kappaB ligand (RANKL)-binding peptide that possesses osteoclastogenesis inhibitory activity. J Biol Chem. 2013;288(8):5562–71. doi: 10.1074/jbc.M112.426080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fujita T, Fukase M, Baba H, Yamaguchi T, Takata S, Fujimi T, et al. New actions of parathyroid hormone through its degradation. J Endocrinol Invest. 1992;15(9 Suppl 6):121–7. [PubMed] [Google Scholar]
- 225.Tamai K, Takamatsu K, Kazuki K. Successful treatment of nonunion with teriparatide after failed ankle arthrodesis for Charcot arthropathy. Osteoporos Int. 2013;24(10):2729–32. doi: 10.1007/s00198-013-2367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Martin TJ, Moseley JM, Gillespie MT. Parathyroid hormone-related protein: biochemistry and molecular biology. Crit Rev Biochem Mol Biol. 1991;26(3–4):377–95. doi: 10.3109/10409239109114073. [DOI] [PubMed] [Google Scholar]
- 227.Zhao Q, Brauer PR, Xiao L, McGuire MH, Yee JA. Expression of parathyroid hormone-related peptide (PthrP) and its receptor (PTH1R) during the histogenesis of cartilage and bone in the chicken mandibular process. J Anat. 2002;201(2):137–51. doi: 10.1046/j.1469-7580.2002.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cupp ME, Nayak SK, Adem AS, Thomsen WJ. Parathyroid hormone (PTH) and PTH-related peptide domains contributing to activation of different PTH receptor-mediated signaling pathways. J Pharmacol Exp Ther. 2013;345(3):404–18. doi: 10.1124/jpet.112.199752. [DOI] [PubMed] [Google Scholar]
- 229.Wang YH, Qiu Y, Han XD, Xiong J, Chen YX, Shi HF, et al. Haploinsufficiency of endogenous parathyroid hormone-related peptide impairs bone fracture healing. Clin Exp Pharmacol Physiol. 2013;40(11):715–23. doi: 10.1111/1440-1681.12161. [DOI] [PubMed] [Google Scholar]
- 230.Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298(5871):240–4. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- 231.Tian G, Zhang G, Tan YH. Calcitonin gene-related peptide stimulates BMP-2 expression and the differentiation of human osteoblast-like cells in vitro. Acta Pharmacol Sin. 2013;34(11):1467–74. doi: 10.1038/aps.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bab I, Gavish H, Namdar-Attar M, Muhlrad A, Greenberg Z, Chen Y, et al. Isolation of mitogenically active C-terminal truncated pentapeptide of osteogenic growth peptide from human plasma and culture medium of murine osteoblastic cells. J Pept Res. 1999;54(5):408–14. doi: 10.1034/j.1399-3011.1999.00135.x. [DOI] [PubMed] [Google Scholar]
- 233.Gabarin N, Gavish H, Muhlrad A, Chen YC, Namdar-Attar M, Nissenson RA, et al. Mitogenic G(i) protein-MAP kinase signaling cascade in MC3T3-E1 osteogenic cells: activation by C-terminal pentapeptide of osteogenic growth peptide [OGP(10-14)] and attenuation of activation by cAMP. J Cell Biochem. 2001;81(4):594–603. doi: 10.1002/jcb.1083. [DOI] [PubMed] [Google Scholar]
- 234.Bhatnagar RS, Qian JJ, Wedrychowska A, Sadeghi M, Wu YM, Smith N. Design of biomimetic habitats for tissue engineering with P-15, a synthetic peptide analogue of collagen. Tissue Eng. 1999;5(1):53–65. doi: 10.1089/ten.1999.5.53. [DOI] [PubMed] [Google Scholar]
- 235.Nguyen H, Qian JJ, Bhatnagar RS, Li S. Enhanced cell attachment and osteoblastic activity by P-15 peptide-coated matrix in hydrogels. Biochem Biophys Res Commun. 2003;311(1):179–86. doi: 10.1016/j.bbrc.2003.09.192. [DOI] [PubMed] [Google Scholar]
- 236.Gomar F, Orozco R, Villar JL, Arrizabalaga F. P-15 small peptide bone graft substitute in the treatment of non-unions and delayed union. A pilot clinical trial. Int Orthop. 2007;31(1):93–9. doi: 10.1007/s00264-006-0087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ruoslahti E, Pierschbacher MD. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986;44(4):517–8. doi: 10.1016/0092-8674(86)90259-X. [DOI] [PubMed] [Google Scholar]
- 238.Lee JY, Choo JE, Choi YS, Park JB, Min DS, Lee SJ, et al. Assembly of collagen-binding peptide with collagen as a bioactive scaffold for osteogenesis in vitro and in vivo. Biomaterials. 2007;28(29):4257–67. doi: 10.1016/j.biomaterials.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 239.Hamada Y, Yuki K, Okazaki M, Fujitani W, Matsumoto T, Hashida MK, et al. Osteopontin-derived peptide SVVYGLR induces angiogenesis in vivo. Dent Mater J. 2004;23(4):650–5. doi: 10.4012/dmj.23.650. [DOI] [PubMed] [Google Scholar]
- 240.Park KM, Lee Y, Son JY, Bae JW, Park KD. In situ SVVYGLR peptide conjugation into injectable gelatin-poly(ethylene glycol)-tyramine hydrogel via enzyme-mediated reaction for enhancement of endothelial cell activity and neo-vascularization. Bioconjug Chem. 2012;23(10):2042–50. doi: 10.1021/bc300110b. [DOI] [PubMed] [Google Scholar]
- 241.Karagiannis ED, Urbanska AM, Sahay G, Pelet JM, Jhunjhunwala S, Langer R, et al. Rational design of a biomimetic cell penetrating peptide library. ACS Nano. 2013;7(10):8616–26. doi: 10.1021/nn4027382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jo J, Hong S, Choi WY, Lee DR. Cell-penetrating peptide (CPP)-conjugated proteins is an efficient tool for manipulation of human mesenchymal stromal cells. Sci Rep. 2014;4:4378. doi: 10.1038/srep04378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhang S. Emerging biological materials through molecular self-assembly. Biotechnol Adv. 2002;20(5–6):321–39. doi: 10.1016/S0734-9750(02)00026-5. [DOI] [PubMed] [Google Scholar]
- 244.Lee JY, Choo JE, Choi YS, Shim IK, Lee SJ, Seol YJ, et al. Effect of immobilized cell-binding peptides on chitosan membranes for osteoblastic differentiation of mesenchymal stem cells. Biotechnol Appl Biochem. 2009;52(Pt 1):69–77. doi: 10.1042/BA20070169. [DOI] [PubMed] [Google Scholar]
- 245.Mesfin A, Buchowski JM, Zebala LP, Bakhsh WR, Aronson AB, Fogelson JL, et al. High-dose rhBMP-2 for adults: major and minor complications: a study of 502 spine cases. J Bone Joint Surg Am. 2013;95(17):1546–53. doi: 10.2106/JBJS.L.01730. [DOI] [PubMed] [Google Scholar]
- 246.Latzman JM, Kong L, Liu C, Samadani U. Administration of human recombinant bone morphogenetic protein-2 for spine fusion may be associated with transient postoperative renal insufficiency. Spine (Phila Pa 1976) 2010;35(7):E231–237. doi: 10.1097/BRS.0b013e3181c71447. [DOI] [PubMed] [Google Scholar]
- 247.Simmonds MC, Brown JV, Heirs MK, Higgins JP, Mannion RJ, Rodgers MA, et al. Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann Intern Med. 2013;158(12):877–89. doi: 10.7326/0003-4819-158-12-201306180-00005. [DOI] [PubMed] [Google Scholar]
- 248.Visser R, Arrabal PM, Santos-Ruiz L, Fernandez-Barranco R, Becerra J, Cifuentes M. A collagen-targeted biomimetic RGD peptide to promote osteogenesis. Tissue Eng Part A. 2014;20(1–2):34–44. doi: 10.1089/ten.tea.2012.0610. [DOI] [PubMed] [Google Scholar]


