Abstract
Background/Objectives:
To investigate the effect of folate status on cervical intraepithelial neoplasia (CIN) progression and its relationship with high-risk human papillomavirus (hrHPV).
Subjects/Methods:
We evaluated 20 000 sexually active women aged <65 years in Yangqu County by using a questionnaire; the subjects were also screened using the ThinPrep cytologic test (TCT). Patients with abnormal TCT results (other than glandular cell abnormalities) who were willing to provide informed consent were further diagnosed using colposcopy and histopathological examination. We investigated 247 cases of low-grade cervical squamous intraepithelial lesions (LSIL), 125 cases of high-grade cervical squamous intraepithelial lesions (HSIL) and 877 controls. A 24-item food frequency questionnaire was filled out by the investigator to estimate the consumption of dietary folate. Positivity for hrHPV from residual exfoliated cervical cells was tested; serum folate was also measured.
Results:
The hrHPV infection rate in HSIL patients (77.6%) was higher than that in LSIL (33.2%) and control (32.0%) patients. Dietary folate intakes in controls, LSIL and HSIL were 306.9±176.6, 321.8±168.0 and 314.7±193.8 μg/kcal, respectively. The levels of serum folate in controls, LSIL and HSIL were 18.2±7.9, 15.9±7.1 and 14.3±7.5 nmol/l, respectively. Increased CIN correlated with higher rates of hrHPV infection and lower levels of serum folate.
Conclusions:
Low levels of serum folate may increase the risk of CIN progression. Furthermore, potential synergy may exist between low serum folate levels and hrHPV infection to promote CIN development.
Introduction
In China, cervical cancer is the second most common cancer and cause of cancer-related death and accounts for 25% of annual global incidences(135 000 new cases) and 12% of the annual global mortality (34 000 deaths).1 Shanxi Province is a high-risk rural area; the incidence (78.23/100 000) and mortality (25.07/100 000) due to cervical cancer in Yangcheng County, within Shanxi Province, are much higher than the average incidence (9.62/100 000) and mortality (2.54/100 000) rates in China.2 Therefore, preventing the occurrence of cervical cancer in China, especially in Shanxi, is an urgent need. Cervical cancer develops from pre-existing non-invasive squamous precursor lesions, referred to as cervical intraepithelial neoplasias (CINs) or squamous intraepithelial lesions (SIL). CIN1 is referred to as low-grade SIL (LSIL), whereas CIN2 and CIN3 correspond to high-grade SIL (HSIL). CIN develops gradually, providing ample time to intervene and block the occurrence of cervical cancer. As CIN is a dynamic process, the approximate regression rates for CIN1, CIN2 and CIN3 are 60, 40 and 33%, respectively, and their corresponding rates of progression to invasive cancer are 1, 5 and 12%, respectively.3 Currently, the outcome of CIN is difficult to predict, and unnecessary excision procedures for CINs that may have otherwise naturally regressed do occur. In recent years, the excessive surgical treatment of CIN has caused a series of adverse effects, including infertility, miscarriage, premature delivery, cervical insufficiency and cesarean section in subsequent pregnancies. This has caused great physical and mental stress, especially in young patients with childbearing desires, and has thus become a focus of investigation,4, 5 as understanding the factors that predict progression of CIN is of critical value.
High-risk human papillomavirus (hrHPV) infection is by far the most important risk factor for CIN and is a critical component of its progression to cervical cancer.6 However, the lifetime risk of contracting HPV is estimated to be 80%7 at least 80% of the hrHPV infections are likely to be transient,8 whereas 5–10% of infected women will develop SIL and less than 1% will develop invasive cervical cancer.9, 10, 11 This suggests that hrHPV infection is not the only factor in CIN progression.12 Thus, identifying possible hrHPV cofactors is important for blocking the progression of CIN.
Recent research on the relationship between micronutrients and tumor progression revealed that low levels of folate, vitamin A, vitamin E and other dietary nutrients were related to the risk of cervical cancer.13, 14, 15 Folate is a water-soluble B vitamin and an important cofactor in one-carbon metabolism, where it participates in nucleotide synthesis and methylation reactions that are also responsible for tumorigenic and mutagenic effects.16 Therefore, the relationship between folate and malignant tumors has attracted greater attention.17 It is well known that neural tube defects are the second most common birth defect caused by inadequate dietary folate intake.18 China has one of the highest rates of neural tube defects in the world, with Shanxi province having the most occurrences.19 The high-risk areas of neural tube defects lie along the Taihang Mountains, as do the high-risk areas of cervical cancer.20 Our previous findings indicated that low levels of folate might increase the risk of cervical cancer,21 and further experiments in vitro have shown that increasing folate levels correlated with decreased cell proliferation and increased apoptosis in human cervical cancer cell lines.22 However, it is not clear whether folate is involved in the development of CIN.
In this cross-sectional study, 20 000 women of similar backgrounds and lifestyles were investigated using a demographic characteristics-related questionnaire and screened using the ThinPrep cytologic test (TCT) and gynecologic examinations; all TCT abnormalities, except those of glandular cells, were further diagnosed by colposcopy and histopathological examination in consenting patients. All histopathologically diagnosed LSIL and HSIL patients were considered the study group, whereas those with normal cervixes were the control group. We then assessed the risk factors related to CIN grade, focusing on the relationship between folate and CIN. We aimed to determine the factors influencing CIN progression and to develop a theoretical basis for reducing the incidence of cervical cancer in Shanxi Province.
Materials and methods
Study design
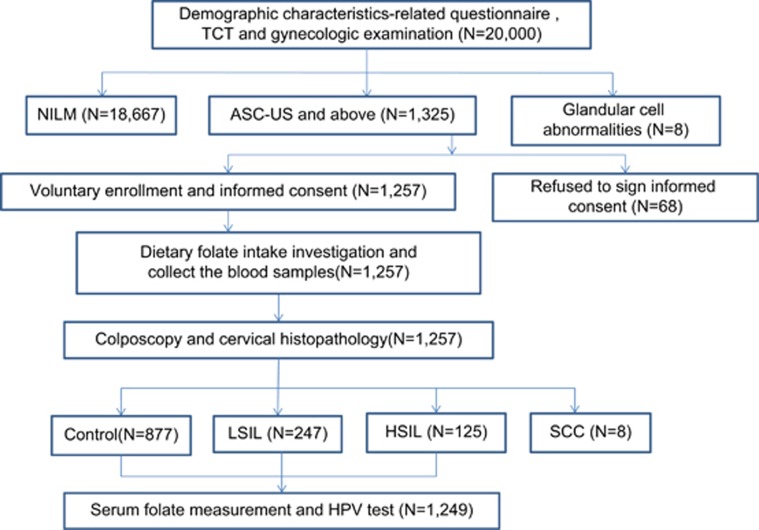
Between September and December 2014, a free cervical cancer screening program was organized for 20 000 eligible women permanently residing in Yangqu County of Taiyuan city. The inclusion criteria were as follows: sexually active woman, age <65 years, of the Han ethnic group, ⩾1 year of continuous residence in Yangqu County and willingness to participate in the screening program. The exclusion criteria included having a history of nutritional megaloblastic anemia, hemolytic disease, leukemia, liver disease, other malignant tumors and uterine surgery resection or cervical lesion treatment; moreover, those who used B vitamins within the previous 3 months were excluded. A total of 20 000 women were investigated with a demographic characteristics-related questionnaire and screened using the TCT in conjunction with gynecologic examinations. There were 1325 women diagnosed with atypical squamous cells of undetermined significance and above, among which 1257 women provided their signed, informed consent to undergo the screening program prior to enrollment. Dietary folate intake in these women was determined, and blood specimens were collected. They further underwent colposcopy and histopathological examination; all these procedures were completed under double-blind conditions. According to the final histopathological results, 247 patients were histologically diagnosed with LSIL (CIN1), 125 with HSIL (96 with CIN2 and 29 with CIN3) and 877 had normal cervixes. The final study population consisted of 1249 women, after 8 cases of invasive cervical cancer were excluded because they were beyond the scope of this study. Serum folate was also measured in all subjects, and HPV testing was performed in the residual TCT specimens (Figure 1). The study was approved by the ethics committee of the Second Hospital, Shanxi Medical University, and was registered in the Chinese Clinical Trial Register (ChiCTR), number ChiCTR-ROC-15006479.
Figure 1.
Study flow diagram. Abbreviations: TCT, ThinPrep cytologic test; NILM, negative for intraepithelial lesion or malignancy; ASC-US, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions; SCC, squamous cell carcinoma.
Data collection
Interviews
We interviewed 20 000 women face-to-face using a unified, structured demographic characteristics-related questionnaire filled out by trained and qualified investigators. Collected demographic information included age, education, occupation, reproductive patterns (including menstrual, marriage, gravidity, parity, abortion history and age at first sexual intercourse), use of tobacco, intrauterine device use, history of gynecological events and family history of cancer.
Dietary folate intake investigation
On the basis of the food frequency questionnaire (FFQ) designed by Wang and Zhao,23 combined with people's eating habits in Shanxi and incorporating a list of items from the ‘China Food Composition Table'24 that were rich in folate, our team members (including epidemiologists) designed a self-administered, semiquantitative FFQ. Relying on a review of the literature, expert evaluation and a small-scale presurvey, the FFQ has been used in previous studies and is thus effectively validated.25, 26 In this study, we used the FFQ to ascertain the consumption frequency and quantity of 24 types of food in the past 5 years, including cereals, beans, fresh vegetables, fresh fruits, meats, eggs, milk and other common foods.
Biological specimens
Prior to surgery or other treatments, a 5 ml blood sample was collected in a tube that did not contain anticoagulant in the morning after overnight fasting (12 h) and centrifuged at 3000 r.p.m. for 10 min at room temperature within 10 h of collection. The serum was separated, whereupon it was drawn into a labeled cryotube and stored at −80 °C. All subjects were asked to provide two cervical tissue specimens obtained by exfoliation with a brush at gynecological examination. One sample was automatically prepared for TCT by a liquid-based device (DC-12; Dacheng Biomedical Equipment Technology Co., Ltd, Guangzhou, China). Another sample was stored at 4 °C and processed for HPV DNA testing within 2 weeks. Patients were instructed not to apply vaginal douching 3 days prior to the collection of samples or to have sexual intercourse during the preceding day. Sampling was not performed during menstrual periods. All the positive cytological samples were confirmed by biopsies.
Determination of serum folate
Serum folate levels were measured using kits (Access Folate) by Beckman Coulter (ACCESS 2 Chemiluminescence Immunoassay System; Beckman Coulter Inc., Brea, CA, USA) according to the manufacturer's instructions.
HPV genotyping by hybrimax
HPV genotyping by HybriMax was performed using an HPV GenoArray Test Kit (HybriBio Ltd, Chaozhou, China) with the residual TCT specimens. This assay can identify 21 types of HPV, including 15 high-risk types (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66 and 68) and five low-risk types (6, 11, 42, 43, 44 and CP8304) by the flow-through hybridization technique using a TC-96/G/H6 HPV DNA Amplification Analyzer and an HMM-2 fast nucleic acid molecule hybridization instrument (HybriBio Ltd).27
Statistical analysis
According to the ‘China Food Composition Table',24 24 types of food are converted to folate and contribute to folate dietary intake. Double-pass entry of all data was performed using the EpiData 3.1 software (Jens M Lauritsen, Odense, Syddan Mark, Denmark), and all statistical analyses were performed using the SPSS 19.0 statistical package for Windows (SPSS Inc., Chicago, IL, USA).
For comparison among controls, the LSIL and HSIL groups' categorical variables were compared by using the Pearson χ2 test; continuous variables were examined using the Kruskal–Wallis (K–W) test and post-hoc analysis by the Bonferroni method. Spearman's correlation coefficient was employed to assess the correlations among variables. The relative risk estimation (odds ratio (OR) with 95% confidence interval (CI)) was calculated for hrHPV infection and serum folate. The multivariate logistic regression model between serum folate and CIN was adjusted for the variables used in the study design and for the variables identified as statistically significant in the above analysis. The addition mode as well as interaction indicators (including relative excess risk of interaction, attributable proportion of interaction, the synergy index) were applied to assess qualitative and quantitative interaction. Relative excess risk of interaction and attributable proportion of interaction cannot be 0 when interactions exist, whereas synergy index cannot be 1. Attributable proportion of interaction can be used to evaluate the risk attributed to the interaction between two significant variables found on multivariate analysis; hence, their combined impact on public health is better understood. When two factors have similar prognostic trends (either both negative or positive), a stronger interaction between them correlates with a greater distance between synergy index and 1. P<0.05 was considered statistically significant.
Results
Sociodemographic factors
As shown in Table 1, there were no significant differences in marriage rates, occupation, smoking history, parity, abortion history, gynecologic disease history or family history of cancer among the control, LSIL and HSIL groups. However, there were significant differences in age, educational level, annual income, spouse smoking history, age at menarche, menopause status, age at first intercourse, gravidity and intrauterine device use. When further compared by pairs, the HSIL group subjects were younger, less likely to have received a senior education, comprised a lower number of women who are post-menopausal, had menarche at an earlier age and had higher gravidity than those in the control and LSIL groups; LSIL and HSIL group subjects were younger at first intercourse, had more smoking spouses and higher annual income than control subjects; and intrauterine device use was more common in HSIL than in control subjects but was rare in the LSIL group.
Table 1. Characteristics among the study population (N=1249).
|
Controls (n=877) |
LSIL (n=247) |
HSIL (n=125) |
P-valuea | ||||
|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | ||
| Age (year) | 50.4 | 0.3 | 49.3 | 0.6 | 45.4 | 0.7 | 0.00 |
| Marriage (marriedb/othersc) % | 94.2/5.8 | 92.7/7.3 | 92.8/7.2 | 0.63 | |||
| Occupation (farmer/others) % | 37.1/62.9 | 36.8/63.2 | 33.6/66.4 | 0.75 | |||
| Education level (primary/junior/senior) % | 24.3/4.6/34.1 | 28.3/38.1/33.6 | 13.6/59.2/27.2 | 0.00 | |||
| Annual incomed(1/2/3)% | 42.0/46.6/11.4 | 49.0/36.8/14.2 | 42.4/40.8/16.8 | 0.04 | |||
| Smoking history (% yes) | 2.2 | 2.8 | 2.4 | 0.83 | |||
| Spouse smoking history (% yes) | 56.0 | 65.6 | 60.0 | 0.02 | |||
| Abortion history (% yes) | 55.3 | 55.5 | 53.6 | 0.93 | |||
| Gynecologic disease history (% yes) | 23.1 | 22.7 | 14.4 | 0.09 | |||
| Family history (% yes) | 11.9 | 10.9 | 8.0 | 0.44 | |||
| Age at menarche (% less than 16 years old) | 47.0 | 47.8 | 67.2 | 0.00 | |||
| Age at first intercourse (% less than 23 years old) | 43.2 | 52.6 | 48.8 | 0.02 | |||
| Gravidity (% less than 3 times) | 37.6 | 34.0 | 47.2 | 0.04 | |||
| Parity (% less than 3 times) | 72.5 | 71.7 | 82.4 | 0.05 | |||
| Menopausal status (% post) | 58.8 | 53.4 | 22.4 | 0.00 | |||
| IUD use ever (% yes) | 53.7 | 39.7 | 59.2 | 0.00 | |||
Abbreviations: LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions; IUD, intrauterine device.
P-value based on analysis of variance for continuous variables and χ2 for categorical variables.
Referred as currently married.
Including for single but having sexual activity, remarried, divorced, widowed and separated women.
Annual income can be divided into three levels: 1=less than 10 000 CNY, 2=10 000–30 000 CNY and 3=more than 30 000 CNY.
Detection of hrHPV
The rates of hrHPV infection in control, LSIL and HSIL patients were 32.0, 33.2 and 77.6%, respectively. Increased hrHPV infection rates correlated with the degree of aggravation of cervical lesions (trend χ2=67.57, P<0.001). Post-hoc analysis showed that the rate of hrHPV infection in the HSIL group was significantly higher than that in the control group (P<0.001), but a significant difference was not observed between the LSIL and control groups (P>0.05). When adjusted for age, education level, annual income, smoking spouse, age at menarche, menopause status, age at first intercourse, gravidity and intrauterine device use, hrHPV infection still produced a 5.2-fold risk of HSIL than non-infection (95% CI: 3.24–8.22) (Table 2).
Table 2. Odds ratios (95% CI) for LSIL and HSIL by hrHPV infection.
| Group | N | hrHPV infection (%) | Crude OR (95% CI) | Adjusted OR (95% CI)a |
|---|---|---|---|---|
| Control | 877 | 32.0 | Reference | Reference |
| LSIL | 247 | 33.2 | 1.05 (0.78–1.42) | 1.01 (0.73–1.39) |
| HSIL | 125 | 77.6 | 7.35 (4.72–11.45) | 5.16 (3.24–8.22) |
Abbreviations: LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions; OR, odds ratio; CI, confidence interval.
Adjusted for age, education level, annual income, spouse smoking, age at menarche, menopause, age at first intercourse, gravidity and intrauterine device.
Dietary folate intake
In the control, LSIL and HSIL groups, the daily dietary intakes of folate were 306.9±176.6, 321.8±168.0 and 314.7±193.8 μg/kcal (median±interquartile number). This did not meet normal distribution and variance homogeneity. According to the K–W test, the difference among the three groups was not statistically significant (H=0.97, P=0.62). Furthermore, the consistency test between the daily dietary intake of folate and serum folate showed that the correlation between the two was not statistically significant (r=0.05, P=0.09).
Serum folate levels
In the control, LSIL and HSIL groups, the serum folate levels were 18.2±7.9, 15.9±7.1 and 14.3±7.5 nmol/l (median±interquartile number), respectively. The level of serum folate among the three groups was significantly different according to the K–W test (H=59.29, P<0.001). Post-hoc analysis showed that women with HSIL had a significantly lower folate status than those with LSIL and normal cervical histology (P<0.02, adjusted test α=α/3=0.02), and women with LSIL had a significantly lower serum folate level than control (P<0.02, adjusted test α=α/3=0.02).
After serum folate levels were graded by the quartile of the control group, the data showed that LSIL and HSIL risk increased as serum folate levels decreased. In particular, serum folate correlation with risk of LSIL and HSIL continued to show a concentration–response relationship after adjustment for age, education level, annual income, smoking spouse, age at menarche, menopause status, age at first intercourse, gravidity, intrauterine device and hrHPV infection (Table 3).
Table 3. Odds ratios for LSIL and HSIL risk according to quartile of the serum folate in the control group.
| Serum folate |
LSIL |
HSIL |
||||||
|---|---|---|---|---|---|---|---|---|
| N | Crude OR (95% CI) | Adjusted OR (95% CI)a | P-trendb | N | Crude OR (95% CI) | Adjusted OR (95% CI)a | P-trendb | |
| >22.04 | 39 | Reference | Reference | 0.00 | 10 | Reference | Reference | 0.00 |
| 18.20–22.04 | 40 | 1.02 (0.63–1.64) | 1.01 (0.62–1.64) | 21 | 2.08 (0.96–4.52) | 1.82 (0.83–4.02) | ||
| 14.13–18.20 | 75 | 1.91 (1.25–2.94) | 1.85 (1.20–2.86) | 33 | 3.29 (1.58–8.83) | 3.28 (1.55–6.92) | ||
| ⩽14.13 | 93 | 2.36 (1.56–3.59) | 2.30 (1.51–3.52) | 61 | 6.05 (3.02–12.10) | 5.28 (2.60–10.73) | ||
Abbreviations: LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions; OR, odds ratio; CI, confidence interval.
Adjusted for age, education level, annual income, spouse smoking, age at menarche, menopause, age at first intercourse, gravidity, intrauterine device and hrHPV infection.
Obtained from trend χ2 test.
Interaction between serum folate and hrHPV infection in LSIL and HSIL groups
In this study, the median value (18.2 nmol/l) of serum folate in the control group was regarded as the cutoff for low serum folate. The interactions of serum folate and hrHPV infection in CIN grading by stratified analysis using the addition mode were analyzed, and results showed that the interaction indicators relative excess risk of interaction, attributable proportion of interaction and synergy index in the LSIL group were 1.15, 0.49 and 6.99, and those in the HSIL group were 11.11, 0.67 and 3.47, respectively. There was a positive net interaction between low serum folate and hrHPV infection in the LSIL and HSIL groups. The controls used for OR calculation were hrHPV-negative patients with serum folate levels higher than the median concentration. It was observed that women with hrHPV infection and serum folate lower than the median concentration were more likely to have LSIL (OR=2.34) than women with only serum folate lower than the median concentration (OR=1.60) or those with only hrHPV infection (OR=0.59); these women were also more likely to have HSIL (OR=16.61) than women with serum folate lower than the median concentration only (OR=2.40) or with hrHPV infection only (OR=4.11) (Table 4).
Table 4. Odds ratios for LSIL and HSIL risk according to 50% point value of the serum folate in the control group and hrHPV infection.
| Serum folate | hrHPV |
LSIL |
HSIL |
||||
|---|---|---|---|---|---|---|---|
| N | Crude OR (95% CI) | Adjusted OR (95% CI)a | N | Crude OR (95% CI) | Adjusted OR (95% CI)a | ||
| ⩾18.2 | − | 56 | Reference | Reference | 7 | Reference | Reference |
| ⩾18.2 | + | 21 | 0.65 (0.38–1.11) | 0.59 (0.34–1.03) | 25 | 6.15 (2.60–14.53) | 4.11 (1.71–9.88) |
| <18.2 | − | 109 | 1.69 (1.18–2.42) | 1.60 (1.11–2.31) | 21 | 2.61 (1.09–6.22) | 2.40 (1.00–5.75) |
| <18.2 | + | 61 | 2.51 (1.65–3.83) | 2.34(1.51–3.63) | 72 | 23.74 (10.62–53.11) | 16.61 (7.34–37.62) |
Abbreviations: LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions; OR, odds ratio; CI, confidence interval.
Adjusted for age, education level, annual income, spouse smoking, age at menarche, menopause, age at first intercourse, gravidity and intrauterine device.
Discussion
Our previous studies have shown that low folate status was involved in the development of cervical cancer,21, 22, 28 but the role of folate in the development of CIN is still unclear. To our knowledge, this is the first large-scale population study to investigate whether folate could be clinically associated with the development of CIN. The present cross-sectional study used data derived from a large-scale cervical cancer screening program involving 20 000 people of similar living backgrounds and lifestyles in Shanxi province. We found that lower serum folate levels were significantly associated with increased LSIL and HSIL risk. Even after adjusting for hrHPV infection and other risk factors, serum folate correlation with risk of LSIL and HSIL continued to show a concentration–response relationship.
Few epidemiologic studies have investigated the association between serum folate and CIN. Most are small-scale outpatient subject cases of various living backgrounds, dietary habits and lifestyles; such results are poorly representative and inconsistent. Pathak et al.29 examined serum levels of folate in normal (n=35), SIL (n=27) and cervical cancer (n=38) patients and found a role for folate in modulating the risk of cervical cancer and HPV infection. However, another case–control study encompassing 136 control, 92 LSIL and 94 cervical cancer patients in south India indicated that serum folate levels did not predict cervical carcinogenesis but did influence B12 levels resulting in a postulated synergistic effect of promoting invasive cervical cancer (OR=7.11, 95% CI: 0.45–111.9).30 Further studies to confirm the causal relationship between folate and CIN progression will be crucial.
Decreased serum folate levels may be caused by poor intake of folate-rich foods; hence, we also investigated the self-reported FFQs to assess the relationship of dietary folate intake and CIN risk in the present study. FFQs are one of the most commonly used methods for evaluating habitual dietary intake in large-scale epidemiological studies.31, 32 In this study, daily dietary folate intake was not consistent with serum folate concentrations and was independent of CIN occurrences. This suggested that the results of the FFQ survey may be biased. On the other hand, the processes of absorption, metabolism and transport of folate after intake may be affected by various factors and should be further explored.
The mechanisms underlying the low serum folate resulting in CIN progression to cervical cancer are unclear. Epidemiological and laboratory data have established that persistent infection with hrHPV is the main risk factor for CIN progression to cervical cancer.3 In this study, HSIL patients had a significantly higher rate of hrHPV infection than did LSIL and control. After adjusting for other risk factors, hrHPV infection still produced a 5.2-fold higher risk of HSIL than non-infection. These results demonstrated the vital role of hrHPV infection in the progression of LSIL to HSIL. On the basis of this, whether folate collaborated with hrHPV in the process of CIN progression to cervical cancer is unclear. Folate deficiency may affect chromosomal stability at the FRA3B site located in the fragile histidine triad (FHIT) gene, which is prone to forming gaps or breaks on metaphase chromosomes;28 the FRA3B fragile site of FHIT is a candidate region for HPV-16 interaction.33 Despite this hypothesis, studies on the interaction of folate and hrHPV in the process of CIN progression are very few, and most are descriptive. Piyathilake et al.34 found that HPV-16-positive women with low red blood cell folate were significantly more likely to be diagnosed with CIN ⩾2 than were HPV-16-negative women with higher red blood cell folate. More recently, the same group established that a tertile increase in plasma folate resulted in a 50% (P=0.03) increase in the odds of having a higher degree of HPV-16 methylation, which provides initial evidence that folate may have an important role in maintaining a desirably high degree of methylation at specific CpG sites in the HPV E6 promoter and enhancer that are associated with CIN2+ risk.35 Using the median serum folate value among the control population as the cutoff value, simultaneous low serum folate and hrHPV infection produced a higher LSIL and HSIL risk than each alone or neither in the present study. Moreover, women with low serum folate and hrHPV infection had a greater risk of HSIL than of LSIL, implying that low serum folate had a greater role on the former than on the latter. These results suggested that low serum folate had a synergistic effect with hrHPV infection in the process of cervical cancerization.
A key strength of this study is that our subjects were from among 20 000 people with similar backgrounds, lifestyles and dietary habits who were investigated in the same area and period of time. In addition, all of the clinical examinations and questionnaires were administered under double-blind conditions. Finally, the study relied on histopathological results of multi-point biopsies under colposcopy, and all newly diagnosed CIN patients were enrolled. However, our study also has limitations. Serum folate only reflects early changes of folate levels in the body, which are affected by dietary and absorption factors; however, red blood cell folate ought to represent the storage levels of folate in the body, which are relatively stable. Therefore, it might be better to analyze the relationship between red blood cell folate and CIN simultaneously. Moreover, our data provide only the baseline information of the relationship between folate (and other relevant factors) and CIN; follow-up research is still in progress. Because of the cross-sectional nature of our study, neither causality nor the direction of association could be established. Through dynamic observation of the changes in folate levels during CIN development, however, we may derive evidence that supports or refutes a causal relationship between folate and the development of CIN.
In conclusion, low serum folate may increase the risk of CIN progression, and synergy may potentially exist between low serum folate and hrHPV infection to promote CIN development. Although children's formula is supplemented with folate nowadays, no adult food is similarly enriched in China. Therefore, in the poorer areas along the Taihang mountains in Shanxi Province, which also have high incidences of cervical cancer, it is possible that fortification of staple food items with folate may lead to the prevention of CIN progression; this remains to be further investigated in the next prospective follow-up study.
Acknowledgments
This study was supported by grants from the Special Public Welfare Industry Research of National Health and Family Planning Commission (No. 201402010) and the Youth Project of Shanxi Health and Family Planning Commission (No. 201301015).
The authors declare no conflict of interest.
References
- Shi JF, Canfell K, Lew JB, Qiao YL. The burden of cervical cancer in China: synthesis of the evidence. Int J Cancer 2012; 130: 641–652. [DOI] [PubMed] [Google Scholar]
- Zhang SK, Kang LN, Chang IJ, Zhao FH, Hu SY, Chen W et al. The natural history of cervical cancer in Chinese women: results from an 11-year follow-up study in china using a multistate model. Cancer Epidemiol Biomarkers Prev 2014; 23: 1298–1305. [DOI] [PubMed] [Google Scholar]
- Chen HC, Schiffman M, Lin CY, Pan MH, You SL, Chuang LC et al. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst 2011; 103: 1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Jiang XH, Jiang YH, Ding WC, Zhang CL, Shen H et al. Amplification and overexpression of TP63 and MYC as biomarkers for transition of cervical intraepithelial neoplasia to cervical cancer. Int J Gynecol Cancer 2014; 24: 643–648. [DOI] [PubMed] [Google Scholar]
- Koeneman MM, Kruitwagen RF, Nijman HW, Slangen BF, Van Gorp T, Kruse AJ. Natural history of high-grade cervical intraepithelial neoplasia: a review of prognostic biomarkers. Expert Rev Mol Diagn 2015; 15: 527–546. [DOI] [PubMed] [Google Scholar]
- Snijders PJ, Steenbergen RD, Heideman DA, Meijer CJ. HPV-mediated cervical carcinogenesis: concepts and clinical implications. J Pathol 2006; 208: 152–164. [DOI] [PubMed] [Google Scholar]
- Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MC et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis 2013; 40: 187–193. [DOI] [PubMed] [Google Scholar]
- Meijer CJ, Snijders PJ, van den Brule AJ. Screening for cervical cancer: should we test for infection with high-risk HPV? CMAJ 2000; 163: 535–538. [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Park JS. Clinical significance of human papillomavirus genotyping. J Gynecol Oncol 2016; 7: e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez AC, Schiffman M, Herrero R, Wacholder S, Hildesheim A, Castle PE et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst 2008; 100: 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa N, Egawa K, Griffin H, Doorbar J. Human papillomaviruses: epithelial tropisms, and the development of neoplasia. Viruses 2015; 7: 3863–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak S, Bajpai D, Banerjee A, Bhatla N, Jain SK, Jayaram HN et al. Serum one-carbon metabolites and risk of cervical cancer. Nutr Cancer 2014; 66: 818–824. [DOI] [PubMed] [Google Scholar]
- Yeo AS, Schiff MA, Montoya G, Masuk M, van Asselt-King L, Becker TM. Serum micronutrients and cervical dysplasia in Southwestern American Indian women. Nutr Cancer 2000; 38: 141–150. [DOI] [PubMed] [Google Scholar]
- Zhang X, Dai B, Zhang B, Wang Z. Vitamin A and risk of cervical cancer: a meta-analysis. Gynecol Oncol 2012; 124: 366–373. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim MK, Lee JK, Kim JH, Son SK, Song ES et al. Intakes of vitamin A, C, and E, and beta-carotene are associated with risk of cervical cancer: a case-control study in Korea. Nutr Cancer 2010; 62: 181–189. [DOI] [PubMed] [Google Scholar]
- Ferrari A, de Carvalho AM, Steluti J, Teixeira J, Marchioni DM, Aguiar S Jr. Folate and nutrients involved in the 1-carbon cycle in the pretreatment of patients for colorectal cancer. Nutrients 2015; 7: 4318–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollset SE, Clarke R, Lewington S, Ebbing M, Halsey J, Lonn E et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet 2013; 381: 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbard A, Benoist JF, Blom HJ. Neural tube defects, folic acid and methylation. Int J Environ Res Public Health 2013; 10: 4352–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren AG. Prevention of neural tube defects with folic acid: the Chinese experience. World J Clin Pediatr 2015; 4: 41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Tian YH, Zhang FR, Zhong XY, Zhang BL, Tan M et al. Variation of plasma folate levels in adults between some areas and different seasons in China. Chin J Prev Med 2002; 36: 308–310. [PubMed] [Google Scholar]
- Wang JT, Ding L, Jiang SW, Hao J, Zhao WM, Zhou Q et al. Folate deficiency and aberrant expression of DNA methyltransferase 1 were associated with cervical cancerization. Curr Pharm Des 2014; 20: 1639–1646. [DOI] [PubMed] [Google Scholar]
- Ding L, Ma J, Zhou Q, Wang J. Effect of folate on the proliferation of human cervical cancer cell and relationship with HPV16. Wei Sheng Yan Jiu 2013; 42: 748–753. [PubMed] [Google Scholar]
- Wang JL, Zhao WH. Application of simplified food frequency questionnaires on dietary assessment. Clin J Prev Contr Chron Non-commun Dis 2000; 8: 29–31. [Google Scholar]
- Yang YX, Wang GY, Pan XC (eds). China Food Composition Table, 1st edn. Peking University Medical Press: Beijing, China, 2002.
- Wang JT, Ma XC, Cheng YY, Ding L, Zhou Q. A case-control study on the association between folate and cervical cancer. Zhonghua Liu Xing Bing Xue Za Zhi 2006; 27: 424–427. [PubMed] [Google Scholar]
- Ma XC, Wang JT, Cheng YY, Yan JW, Zhou Q. Case-control study on relationship between dietary factors and cervical cancer. Chin J Public Health 2005; 21: 312–314. [Google Scholar]
- Tao P, Zheng W, Wang Y, Bian M. Sensitive HPV genotyping based on the flow-through hybridization and gene chip. J Biomed Biotechnol 2012; 2012: 938780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai LX, Wang JT, Ding L, Jiang SW, Kang HJ, Gao CF et al. Folate deficiency and FHIT hypermethylation and HPV 16 infection promote cervical cancerization. Asian Pac J Cancer Prev 2014; 15: 9313–9317. [DOI] [PubMed] [Google Scholar]
- Pathak S, Bhatla N, Singh N. Cervical cancer pathogenesis is associated with one-carbon metabolism. Mol Cell Biochem 2012; 369: 1–7. [DOI] [PubMed] [Google Scholar]
- Ragasudha PN, Thulaseedharan JV, Wesley R, Jayaprakash PG, Lalitha P, Pillai MR. A case-control nutrigenomic study on the synergistic activity of folate and vitamin B12 in cervical cancer progression. Nutr Cancer 2012; 64: 550–558. [DOI] [PubMed] [Google Scholar]
- Bingham SA, Luben R, Welch A, Wareham N, Khaw KT, Day N. Are imprecise methods obscuring a relation between fat and breast cancer? Lancet 2003; 362: 212–214. [DOI] [PubMed] [Google Scholar]
- Kaaks R, Ferrari P. Dietary intake assessments in epidemiology: can we know what we are measuring? Ann Epidemiol 2006; 16: 377–380. [DOI] [PubMed] [Google Scholar]
- Thorland EC, Myers SL, Gostout BS, Smith DI. Common fragile sites are preferential targets for HPV16 integrations in cervical tumors. Oncogene 2003; 22: 1225–1237. [DOI] [PubMed] [Google Scholar]
- Piyathilake CJ, Macaluso M, Brill I, Heimburger DC, Partridge EE. Lower red blood cell folate enhances the HPV-16-associated risk of cervical intraepithelial neoplasia. Nutrition 2007; 23: 203–210. [DOI] [PubMed] [Google Scholar]
- Piyathilake CJ, Macaluso M, Chambers MM, Badiga S, Siddiqui NR, Bell WC et al. Folate and vitamin B12 may play a critical role in lowering the HPV 16 methylation-associated risk of developing higher grades of CIN. Cancer Prev Res (Phila) 2014; 7: 1128–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]