Abstract
The specialized intermediate filaments (IFs) have critical importance for the clearness and uncommon transparency of the vertebrates' lens fiber cells, although the physical mechanisms involved are poorly understood. Recently, an unusual low-scattering light transport was also described in the retinal Müller cells. Exploring the function of the IFs in the Müller cells, we have studied the morphology and distribution pattern of the IFs and other cytoskeletal filaments inside the Müller cell main processes in the foveolar part of the avian (Pied Flycatcher) retina. We found that some IFs surrounded by globular nanoparticles (that we suggest are crystallines) are present in almost every part of the Müller cells that span the retina, including the microvilli. Unlike the IFs implicated in the mechanical architecture of the cell, these IFs are not connected to any specific cellular membranes. Instead, they are organized into bundles, passing inside the cell from the endfeet to the photoreceptor, following the geometry of the processes and repeatedly circumventing numerous obstacles. We believe that all of the presently reported data effectively confirms that the model of nanooptical channels built of the intermediate filaments [Makarov et al., 2015, Khmelinskii et al., 2015] may provide a viable explanation of the Müller cell transparency.
Keywords: Intermediate filaments, avian retina, Müller cells, transparency
Introduction
The cytoskeleton and especially the intermediate filaments (IFs) are important for the clearness of some very transparent cells in the optical tract of the vertebrates. For example, any chemically or genetically provoked alterations to the specialized intermediate filaments in the eye lens fiber cells lead to the lens opacity [Tagliavini et al., 1986; Bloemendal, 1991; Marcantonio, Duncan, 1991; Rafferty et al., 1997; Matsushima et al., 1997; Clark et al., 1999; Alizadeh et al., 2002, 2003, 2004; Oka et al., 2008; Song et al., 2009]. The exact mechanisms explaining why the IFs are so important for the transparency are unknown. The classical view is that a tissue is transparent if it neither absorbs nor scatters light. The majority of organic molecules do not absorb visible light, so the primary reason for the non-transparency of organic tissues appears to be the light scattering [Tardieu, Delaye, 1988]. Light scattering is caused by discontinuities in the refractive index - the propagation direction of light is altered when it passes through the interface between the regions of different refractive indices (cytoplasm-organelles-cytoplasm, or cytoplasm-protein structure-cytoplasm). Given enough direction changes, the tissue, though non-absorbing, will be opaque [Johnsen, 2001]. Accordingly, one of the possible explanations of why the cytoskeletal elements are so important for the transparency, includes maintenance of the short-range order in the organization of the cytoplasmic proteins and organelles, thus clearing the path for the light propagation without scattering [Benedek, 1971; Delaye, Tardieu, 1983; Matsushima et al., 1997]. Another possible explanation is that some specialized intermediate filaments built of electro-conductive proteins can transmit the light energy all by themselves, working as nanoscale light guides. In this model, light enters the cells on one side, propagates through the IFs in the form of oscillations of the electron density and then is either reemitted by the IFs at the other side of the cell, or the excitation is transmitted directly to the receptor cells [Makarov et al., 2015; Khmelinskii et al., 2015]. In this model the light scattering by organelles and protein particles is of no importance, because the light energy remains contained inside the filaments during the propagation.
Recently, high transparency was also described in the retinal Müller cells (MC), with the cell processes providing cellular light guidance through the inverted retina of the vertebrates [Franze et al., 2007; Reichenbach, Bringmann, 2010; Agte et al., 2011]. It was shown previously that Müller cells have a rich ultrastructure with numerous organelles, and extensive branching of the processes [Reichenbach, 1988; Reichenbach, Bringmann, 2010].
Thus, our previously proposed model [Makarov et al., 2015; Khmelinskii et al., 2015], with nanoscale light-guides working by the quantum mechanism (10–20 µm diameter filaments, probably specialized IFs providing the light transmission), is especially relevant for the light propagation in these cells. Unfortunately, the description of the IFs in Müller cells is largely incomplete. While it is clear from the electron microscopy data that the cytoskeletal elements are abundant inside any particular compartment of the Müller cells [Wakakura, Foulds, 1988; Foulds, Reichenbach, Bringmann, 2010], immunostaining of known IFs and tubulins demonstrated that both elements are concentrated near the endfeet, becoming expressed more uniformly only after mechanical stress, retinal detachment or other retinal damage (Kivelä et al., 1986; Lewis et al., 1995; Fausett, Goldman, 2006). Here we describe the morphology of the filament bundles in the avian foveolar (fovea periphery, foveola) Müller cells of the Pied Flycatcher using the electron microscopy. We also analyze whether light might be transmitted through the complex patterns of the Müller cell processes as in a classical optical fiber, or the light guidance by cytoskeleton filaments via the quantum mechanism should be the only viable explanation.
Methods
Histological tissues were collected in the Moscow region (Russia). Four Pied flycatcher nestlings (Ficedula hypoleuca, Passerines), were taken from the nests at the second part of the nesting period and raised in artificial conditions to the age of 24, 27, 29 and 30 days. They were decapitated under dim red illumination, and incised eyeballs were put down to the fixative - 3% gluteraldehyde with 2% paraformaldehyde in 0. 15 M sodium cacodylate buffer - and stored during a month in refrigerator +4°C. The preparations were sectioned on the nasal, central and temporal parts in the permanent laboratory conditions, washed separately with 0. 15 M sodium cacodylate buffer, and post fixed in 1% osmium tetroxide OsO4 with 1. 5% K4[Fe(CN)6] for 30 min in the same buffer, washed, and incubated in 1% OsO4 for 30 min, washed in distilled water, and then incubated in a 2% aqueous solution of uranyl acetate UO2(CH3COO)2·2H2O for 1 h and washed. After dehydration through a graded series of acetone, the tissues were oriented relative to the position of the pecten and embedded in Epon-812 epoxy resin. Ultrathin sections 60 nm thick were made using the LKB Ultratome and examined with a JEM 100B and JEM 1011 electron microscopes (JEOL Ltd., Japan).
Results
The Müller cells in the Pied flycatcher retina span from the vitreous body, where the light is entering the retina, through the entire retina to the outer segment photoreceptor cone cells (Fig. 1), and thus the cell structure resembles the one previously described in other vertebrates (Reichenbach, 1988; Khokhlova et al., 2000; Reichenbach, Bringmann, 2010, Franze et al., 2007). The Müller cell bodies are located in a separate layer inside the inner nuclear layer (INL) of the retina, sending the principal processes to both inner and outer surfaces. The inner (basal) processes of the Müller cells have a special bell-shaped expanded section, a structure named “endfoot” attached to the inner limiting membrane (ILM) (Fig. 1, and Fig. 2). The number of endfeet that belong to one Müller cell may be different, depending on the zone of cell deployment. While the Müller cells usually have one endfoot only in the pit of the central fovea (Zueva et al., 2014), those away from the fovea usually have 3 – 5 endfeet, each connected to the main process. The number of the endfeet that belong to the same Müller cell grows with the distance from the central pit, reaching dozens of endfeet in one Müller cell (Cajal, 1892).
Fig. 1.
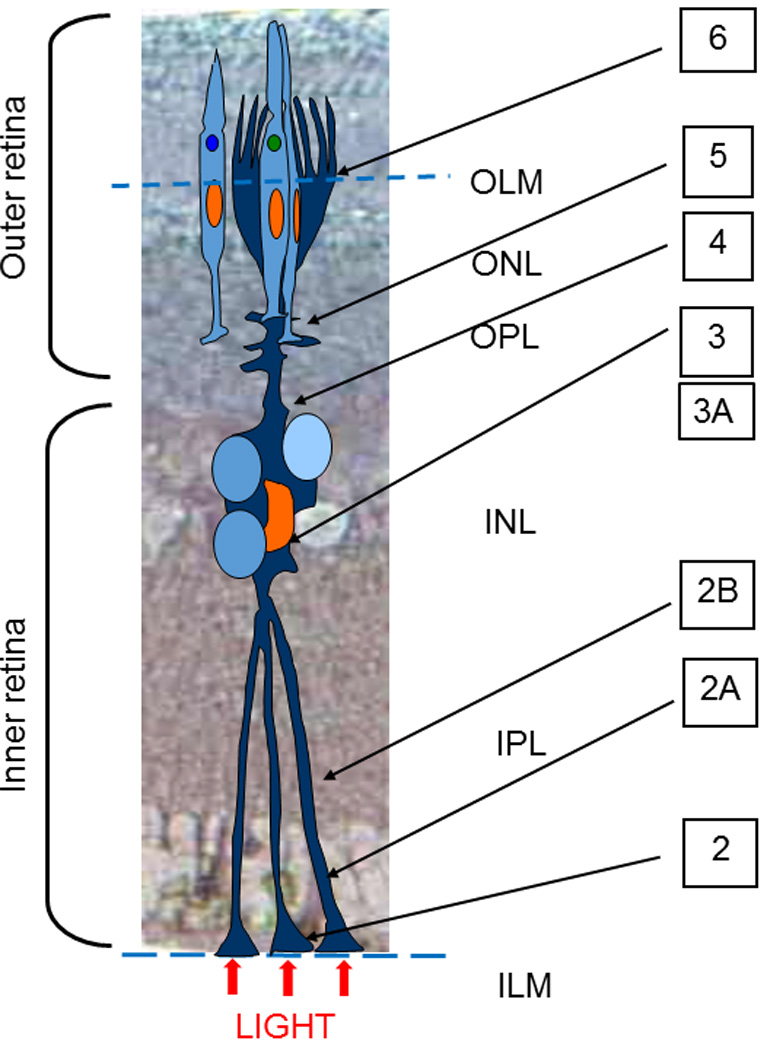
Sketch of a Müller cell alignment in the layers of Pied Flycatcher retina; OLM – Outer Limiting Membrane, ONL- outer nuclear layer, OPL- outer plexiform layer, INL- inner nuclear layer, IPL-inner plexiform layer, ILM-inner limiting membrane. Note the locations of the cross-sections of the electron microphotographs presented in the other Figures, marked by the figure numbers.
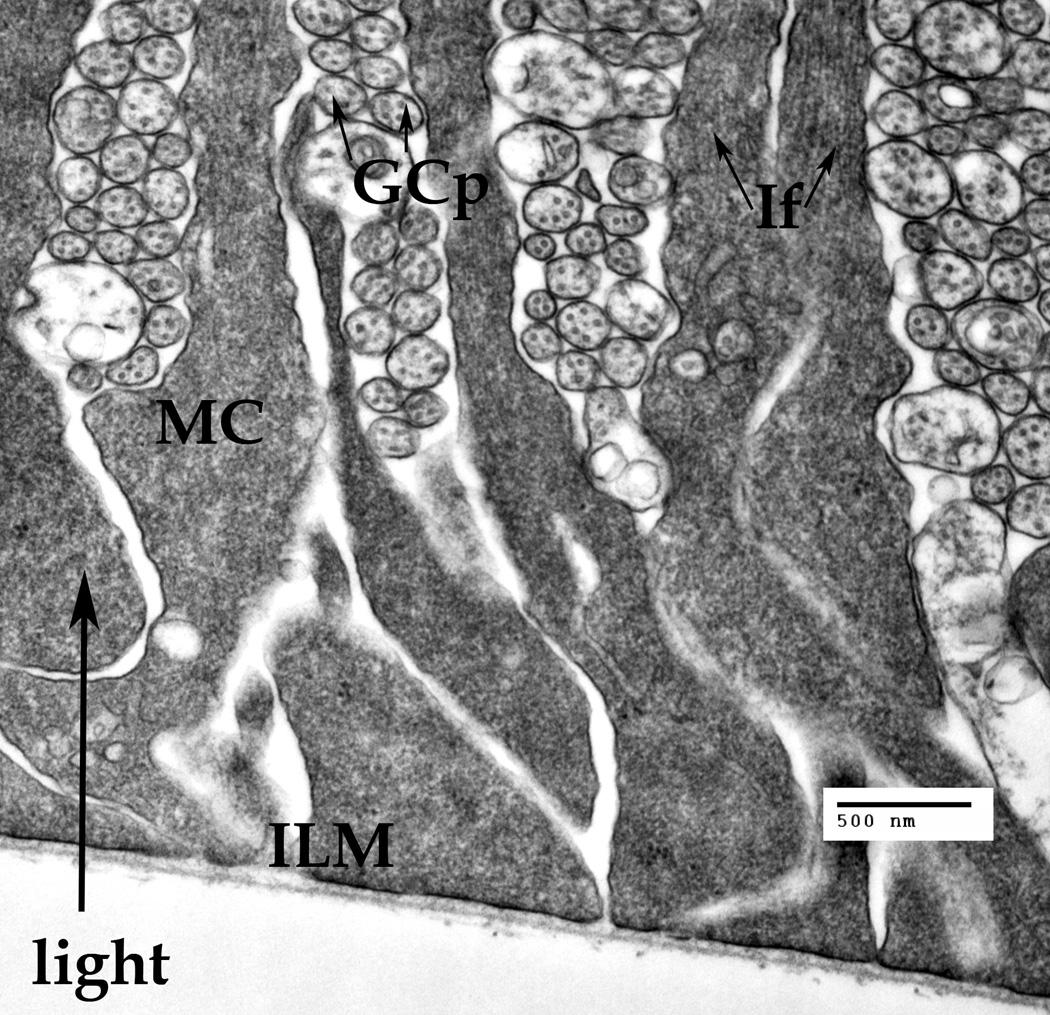
Fig. 2.
A cluster of the Müller cells' endfeet attached to the basal membrane thus forming the inner limiting membrane (ILM). The ganglion cell processes (GCp) are passing between the Müller cell endfeet. Cross-cut neuronal microtubules (arrows) are visible inside the GCps. The intermediate filaments (IF, arrows) are visible only in the narrow part of the Müller endfeet.
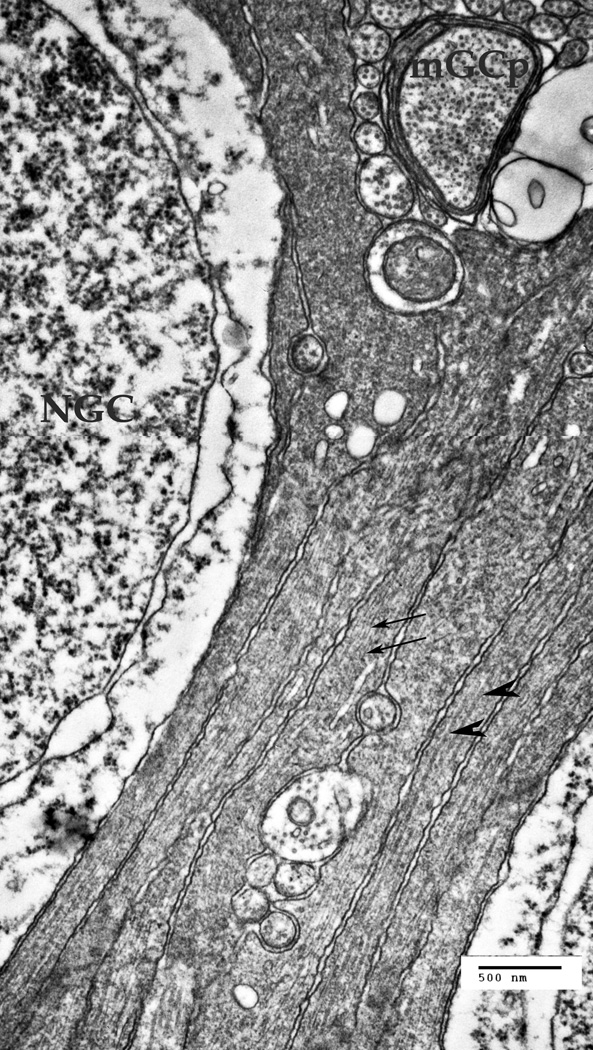
A. A cluster of the Müller cell processes at the ganglion cells' nuclear layer of the retina. NGC – the nucleus of a ganglion cell, mGCP – the myelinated process of a ganglion cell. The Müller cells processes are forced to go around other cells and their processes. Thin arrows point to the intermediate filaments in the MCs (10–18 nm), thick arrowheads point to microtubules (22–26 nm).
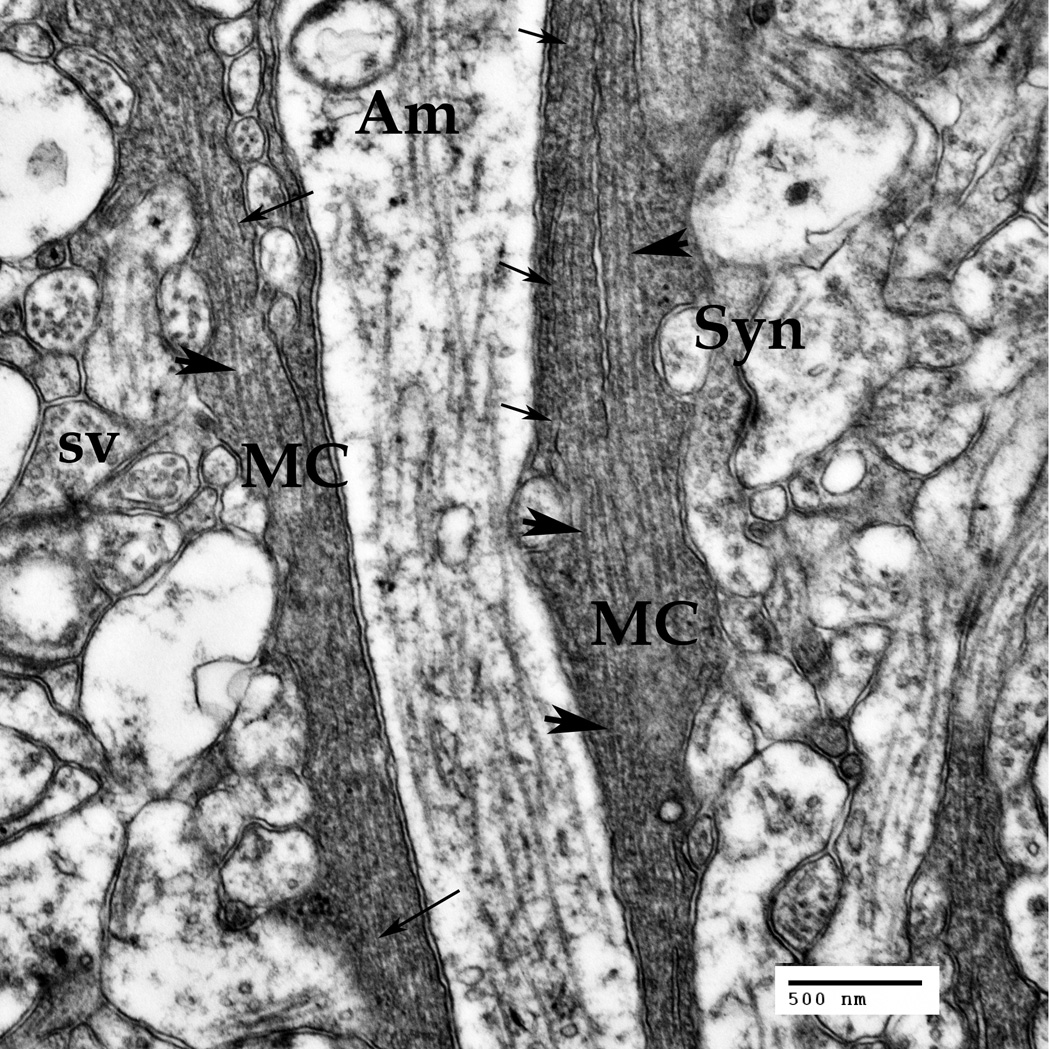
B. Müller cells' (MC) processes at the IPL level. A multitude of synapses (Syn) of ganglion, bipolar and amacrine (Am) neuronal cells are seen in this area. Synaptic vesicles (sv) are visible inside the synapses.
Thin arrows point to the intermediate filaments (10–18 nm), thick arrowheads point to microtubules (22–26 nm).
Bars: 500 nm
The endfeet are attached to the basal membrane, the endfeet bases are touching each other, covering the entire inner surface of the retina, thus forming the inner limiting membrane (ILM) (Fig. 2). The intermediate filaments (If) are visible only in the top part of the Müller endfeet (Fig. 2, arrows), about 2 microns away from the basal membrane.
The conical form of the typical endfeet allows the Müller cells to cover all of the inner surface, while allowing for the ganglion cells (GCp) processes (Fig. 2) to pass between the Müller cell processes. Additionally, the conical endfoot may work as a condenser, concentrating the photons on the IF bundle that transfers the light energy to the receptor cell.
The Müller cell processes in the ganglion nuclear layer (Fig. 2A) are passing between the ganglion cells (NGC), while the myelinated transversal processes of the ganglion cells (mGCp) and unmyelinated ganglion cell processes come from the retinal periphery to the optical disk, passing between the individual Müller cells' processes (Fig. 2A). Similarly to the previous zone, the intermediate filaments (Fig. 2A, thin arrows) and the microtubules (Fig. 2A, large arrowheads) are present inside the Müller cell processes, forming bundles of filaments that follow the form of the processes. Unlike the IFs implicated in the mechanical architecture of the cell, these IFs are seemingly disconnected from any specific cellular membranes.
Deeper in the retina, within the inner plexiform layer, the Müller cell (MC) main processes (Fig. 2B) are passing between the synaptic structures (Fig. 2B, Syn) formed by the neuronal amacrine cells, ganglion and bipolar cells in this layer of the retina. The synaptic endings may be recognized by the synaptic vesicles present inside the presynaptic structures (Fig. 2B, sv). The microtubules are sparsely distributed inside the processes of the amacrine neuronal cells (Fig. 2B, Am), being much denser inside the Müller cell processes (Fig. 2B, big arrowheads). The intermediate filaments (Fig. 2B, fine arrows) inside the Müller cell processes are going in the same direction as the microtubules, forming dense cytoskeletal bundles. These cytoskeletal bundles are reproducing all the curves of the process and evading all of the inner and outer obstacles, including the transversal neuronal processes and the synaptic sheaths (velate sheaths, truncheons).
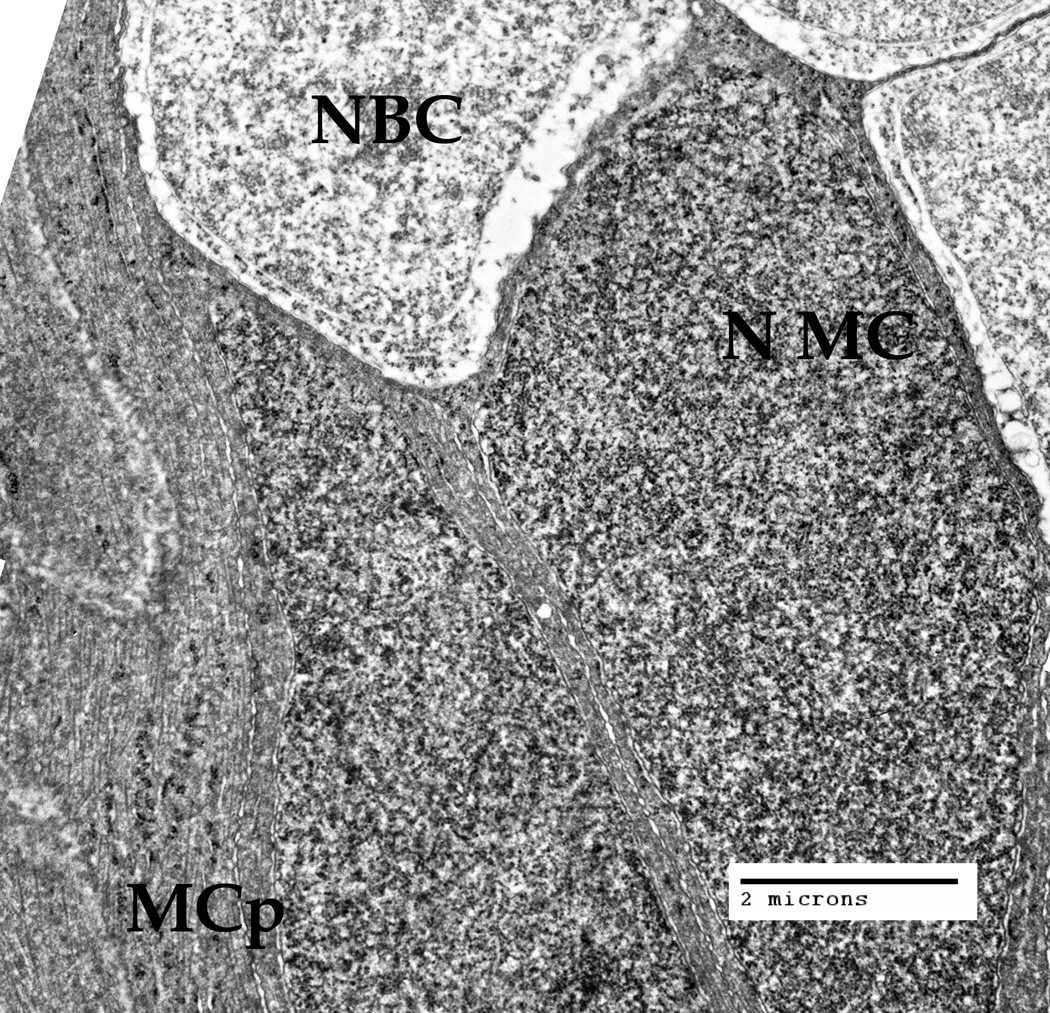
The nuclei of the Müller cells are located quite close to each other and very densely in the inner nuclear layer (Fig. 1, INL), where the nuclei of the different retinal cells are concentrated [Reichenbach, Bringmann, 2010]. We were interested to see how the cytoskeletal bundles of the intermediate filaments (Fig. 3A, thin arrows) and microtubules (Fig. 3A, large arrowheads) are passing between these obstacles (Fig. 3, Fig. 3A).
Fig. 3.
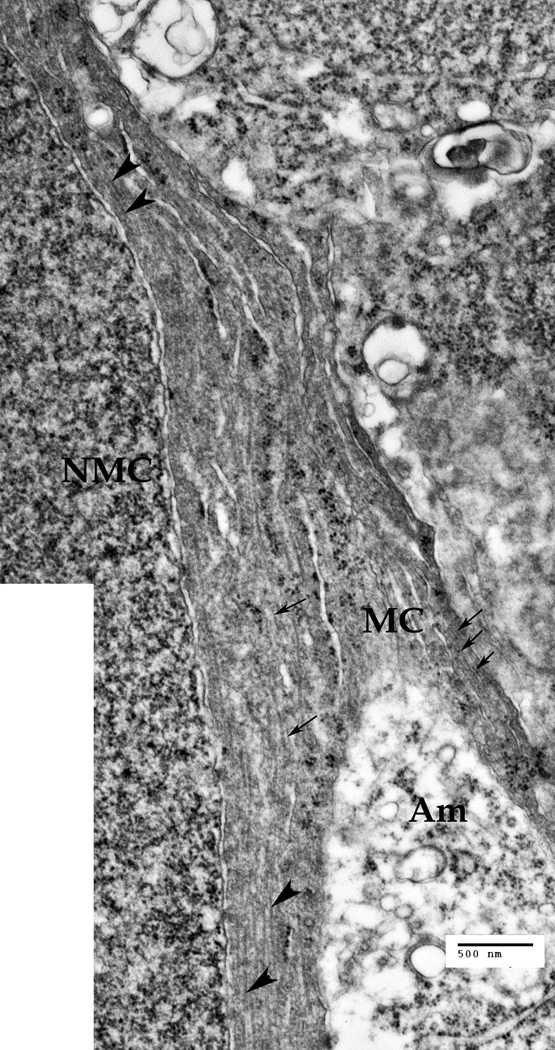
The inner nuclear layer (INL), where the nuclei of the Müller cells (NMC) and also the nuclei of the bipolar cells (NBC) are located, at low magnification (Bar: 2 µm). The cytoplasm nearby the MC nucleus, and the processes of other Müller cells, contain cytoskeletal bundles (intermediate filaments and microtubules) that go around the nuclei of both cell types.
A. The INL zone at a larger magnification. Microtrubules (large arrowheads) and intermediate filaments (thin arrows) are present inside the Müller cell (MC) processes and in the vicinity of the Müller cell nucleus (NMC). Cytoskeletal bundles are going around the NMC and Amacrine cell (Am) process, as well as around the nuclei of the other cells present. Bar: 500 nm
The Müller cells processes (Fig. 4, MC) in the outer plexiform leyer (OPL) are first passing between the horizontal and the bipolar cells. The bipolar cell processes (Fig. 4, BCp) in the foveola region of the avian retina are usually perpendicular to the Müller cell processes (Fig. 4). This allows us to identify the cell types and clearly see the difference between the cytoskeletal bundles in the neurons (BCp) and the Müller cells. Also, there are some smaller nanoparticles around both the microtubules and the IFs (Fig. 4). The intermediate filaments are usually surrounded by small particles, especially well visible in Fig. 4, and we suggest these may be the chaperone globular proteins like crystallines (see the Discussion).
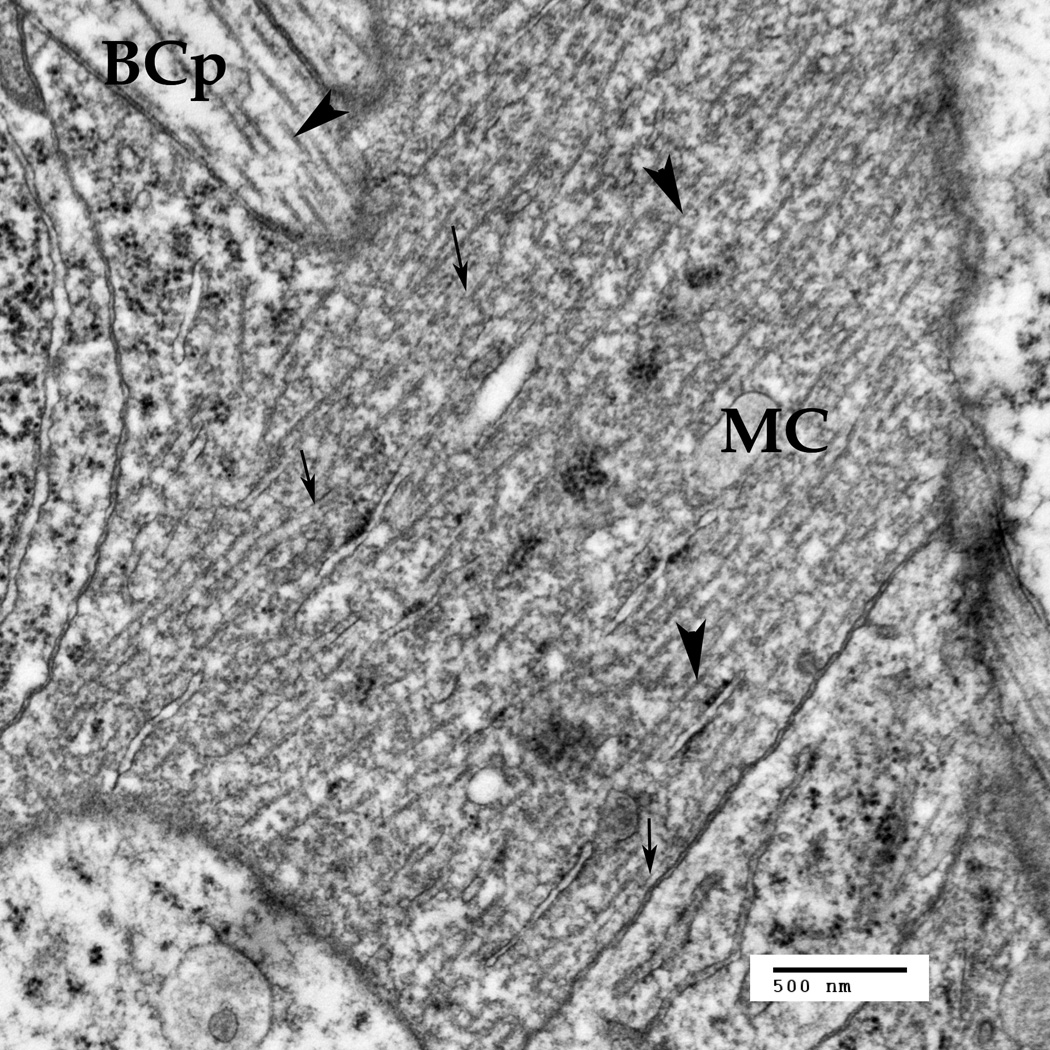
Fig. 4.
The Müller cell processes (MC) under the outer plexiform layer are crossing with the bipolar cell processes (BCp). Thin arrows point to the intermediate filaments in the MC (10–18 nm), thick arrowheads point to the microtubules (22–26 nm). Note that the microtubules and the intermediate filaments are much denser in the Müller cells, as compared to the neuron. Also, there are some smaller nanoparticles around both the microtubules and the IFs.
Still in the OPL, the Müller cells processes (Fig. 5, MCp) are next entering the zone full of synapses that mainly belong to the cone peduncles (Fig. 5, Syn). The cone peduncles may be easily recognized, as they are tightly packed with the synaptic vesicles (Fig. 5, sv). The high synaptic densities in the presynaptic zone of the photoreceptor synapses, termed the synaptic bands (sb) (Dowling, 1987), may be considered the hallmark of the cone-to-bipolar cell and cone-to-horizontal cell synapses. The cytoskeletal elements, including the intermediate filaments and the microtubules, are present in this zone as well (Fig. 5, arrows). Thin arrows indicate the intermediate filaments in the MCs (10–18 µm), while the large arrowheads indicate the microtubules (22–26 µm).
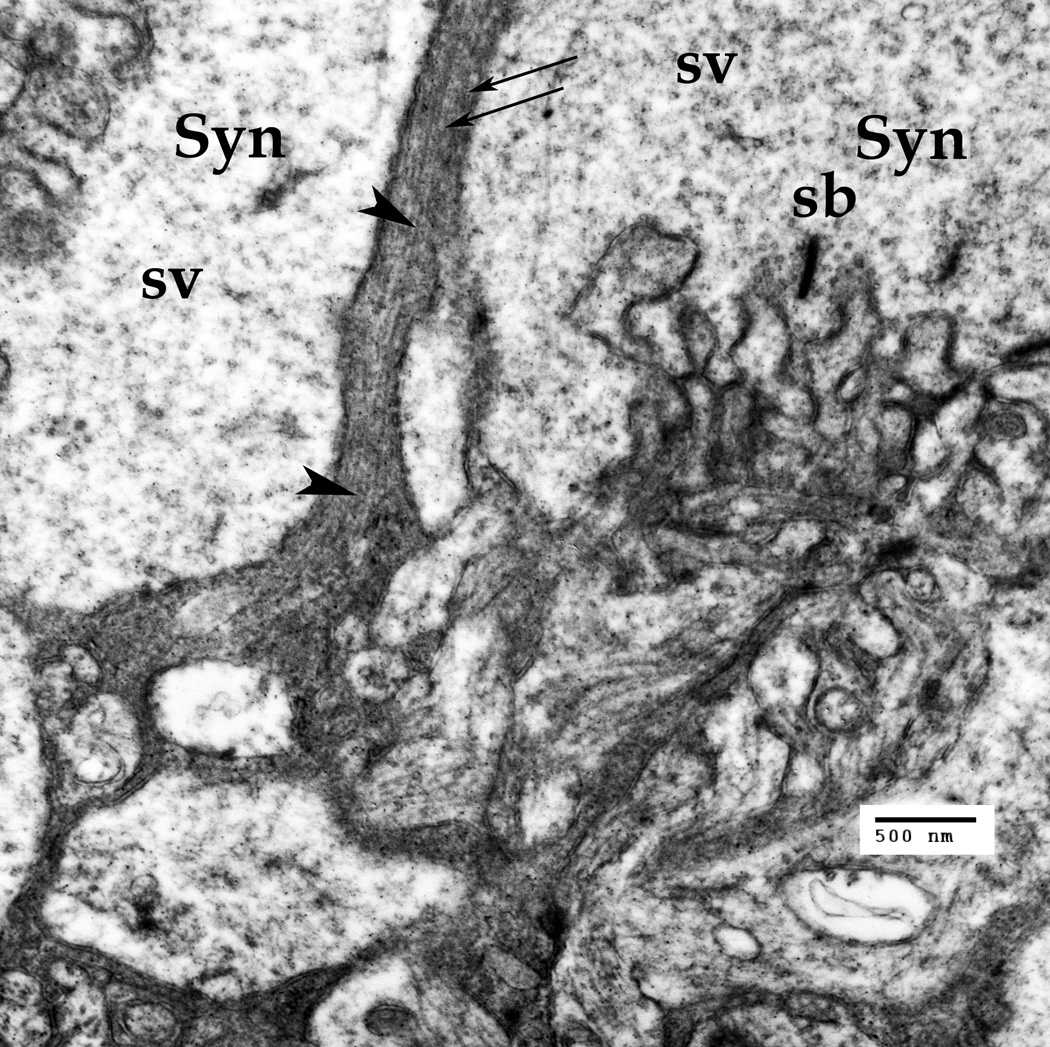
Fig. 5.
The Müller cell processes in the outer plexiform layer (OPL) in the synaptic zone. The cone peduncles in this zone form synapses (Syn) to both horizontal and bipolar cells. The presynaptic region has specialized synaptic bands (SB), surrounded by abundant synaptic vesicles (sv). The Müller cell processes passing through this zone carry both the intermediate filaments (fine arrows) and the microtubules. Scale bar: 500 nm
The outer limiting membrane (OLM), well visible in the light-micrscope preparations of the retina, is formed by the adherent junctions between the Müller cells as well as some photoreceptor cell inner segments, forming a barrier [Reichenbach, Bringmann, 2010]. The OLM may be recognized in the electron microphotography of the retina as a dark tight junction structure (Fig. 6, OLM).
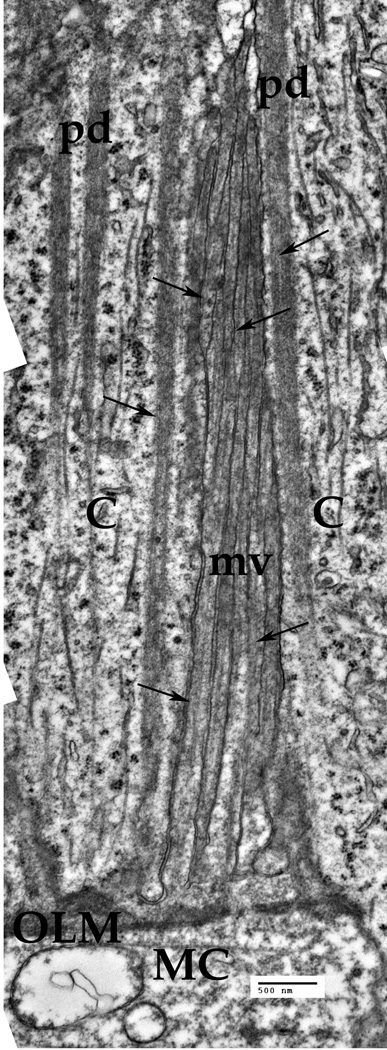
Fig. 6.
The Müller cell (MC) microvillus above the outer limiting membrane (OLM). The cylindrical microvillus (mv) stretches along the inner segments of the cones (C). The Cytoskeletal elements, mainly the IFs (arrows) are present inside each microvillus. Bar: 500 nm
Crossing the outer limiting membrane (Fig. 1, and Fig. 6), the Müller cell outer process (Fig. 6, MC) get separated into multiple specialized fine processes, the so-called microvilli, that extend into the space between the photoreceptors outer processes, frequently termed the subretinal space [Reichenbach, Bringmann, 2010]. Some 200–300 individual microvilli (Fig. 6, mv) surround the cone photoreceptor process (Fig. 6, C). Thus, the photoreceptor cells are embedded in this tassel-like structure within the subretinal space. Each microvillus is about 150 nm in diameter and 10–15 µm long (Fig. 6, mv). Some intermediate filaments are visible inside each of the individual microvilli (fine arrows), but the microtubules are rare. Interestingly, there are specialized structures inside the cones, named peridendrites [Reichenbach, Bringmann, 2010], that look like a dark-grey band, each peridendrite complimentary to a Müller cell microvillus (Fig. 6, pd). These peridendrites contain some specialized filaments as well, although these look much thinner (5–8 nm). The microtubules inside the photoreceptor cells are clearly visible in these preparations (Fig. 6).
Discussion
We used the electron microscopy to show that like in other species [as reviewed by Reichenbach, Bringmann, 2010], the Müller cells in a Pied flycatcher eye foveal zone span the retina from the vitreous body, where the light is entering the retina, to the photoreceptor cone cells. The Müller cell bodies are located in a specific inner nuclear layer (INL) inside the retina, sending principal processes (inner and outer processes) to both of its surfaces. Indeed, the electronic micrograms show that the MC endfeet (inverted cone-shaped zone of the MC adjusted to the basal membrane and the vitreous body, see the scheme on Fig. 1, and also Fig. 2) basal parts form the inner surface lining, termed the inner limiting membrane (ILM), covering the entire inner surface of the retina.
The inner processes of the MCs originating from the endfeet apical part (Fig. 2) protract away from the inner surface, usually perpendicularly to it and essentially parallel to each other (Fig. 1 and Fig. 2), on their way they change their direction several times, following around the branches of other cells and further obstacles (Fig. 2, 2A, 2B) and finally converging. Both inner and outer main processes of the Müller cells change their direction repeatedly (Fig. 3, 4 and 5), ether passing around the branches of other cells or running around the cellular nuclei. Provided the light propagation through the Müller cell processes may be explained by the laws of classical physics, a lot of it should scatter due to repeated direction changes and process branching. Even so, the high level of light transmittance was recently measured in the guinea pig retinal Müller cells, with the cell processes providing the cellular light guidance through the inverted retina, and it was proposed that other vertebrate retina share the same phenomenon [Franze et al., 2007; Reichenbach, Bringmann, 2010; Agte et al., 2011]. The inverted structure of the retina in all vertebrates, and the similarity of other retinal characteristics lead researchers to the conclusion that the Müller cell functions in both avian and mammal retinas are similar (Reichenbach, Bringmann, 2010; Fischer et al, 2010). While presently we were interested in the cytoskeletal structure in the Müller cells present in the out-of-center foveolar zone, where the Müller cells main processes are parallel to the propagation direction of the light arriving from the eye (Fig. 1), previously we have also described the detailed structure of the convexiclivate fovea in avian retinas that have a central foveal pit. The Müller cell endfeet in that specific zone are not parallel to the light propagation direction and experience a sharp bend (Zueva et al., 2014). The bends of the Müller cells processes in the pit may be very sharp, sometimes at right angles to the light propagation direction, in order to meet the photoreceptors (Zueva et al., 2014). The mechanism that explains the light transmission by the Müller cells in such extreme cases should involve the waveguide properties of these cells.
Note that the classical theory of the light transmission by Müller cell processes has some serious difficulties, namely the diameter of the IF bundles is too low. The light incident from the vitreous body is supposedly entering the endfeet (Fig. 1, red arrows) and then the photons travel from the inner process to the cell body and then to the outer processes, where they are transferred to the cone photoreceptor outer segment, as shown on the scheme in Fig. 1. The diameter of the Müller cell main process in the Pied flycatcher almost never exceeds 300 nm (Fig. 2, Fig. 2A, Fig. 3, Fig. 3A, Fig. 5), about just enough for a classical optical waveguide that transmits visual light with the wavelengths between 700 and 400 nm. The difficulties of the classical approach become more serious noting that the secondary processes that connect the endfeet to the main process are significantly thinner, and make some sharp bends, as noted above. The same extends to the microvilli that apparently link the optical channel of the Müller cell to that of the photoreceptor, which each microvillus not exceeding 150 nm in its external diameter. The diameter values measured in other vertebrates are quite similar [Reichenbach, Bringmann, 2010]. Therefore, these cellular waveguides should operate by a different (non-classical) mechanism. This kind of waveguides with the less-than-wavelength width is known in specialized technical applications of nanooptics, and recently we had proposed the model for the nanooptical channels built of the intermediate filaments, each filament 10 to 18 nm in diameter [Makarov et al., 2015, Khmelinskii et al., 2015]. Studying the cytoplasm of the Pied flycatcher Müller cells in the outer and inner processes, we concluded that the only structures that span the cell cytoplasm all the way through from the inner membrane to the photoreceptors are the cytoskeletic bundles of intermediate filaments and microtubules. These structures in the cytoplasm reproduce the form of the cell processes, accurately following each and every of their turns, avoiding the inner obstacles, such as the endoplasmic reticulum, nucleus and mitochondria, as well as the external obstacles, such as branches of the other cells. The beaded intermediate filaments (CP49 and filensin) have the outer diameter about 10–18 nm [Quinlan et al.,1999], with some smaller nanoparticles organized around the filaments, being very important intermediate filaments in the eye lens fiber cells, determining their transparency [Tagliavini et al., 1986; Bloemendal, 1991; Marcantonio, Duncan, 1991; Rafferty et al., 1997; Matsushima et al., 1997; Clark et al., 1999; Alizadeh et al., 2002, 2003, 2004; Oka et al., 2008; Song et al., 2009]. Similarly, the transparency of the corneal cells also depends on the specialized collagen filaments [Jester, 2008; Massoudi et al., 2015].
The intermediate filaments in the glial cytoplasm (Müller cells are specialized glial cells) are most often associated with globular particles of chaperone proteins (usually termed crystallines) [Perng et al., 2008] that stabilize these long filaments. The long filaments without such chaperone proteins are unstable and may aggregate [Carver et al., 1994; Kumar, Reddy, 2009]. High concentration of crystallines were found first in the transparent (filament) cells of the eye crystal lens [Quinlan et al.,1999; Qu et al., 2012], and then in the other transparent eye cell types, like corneal cells [Jester, 2008]. High concentrations of the 25 KD and 35 KD crystallines were reported also in the Müller cells [Simirskii et al., 2003]. Thus, we suggest that the globular particles that accompany the intermediate filaments in the Müller cell processes are composed of cristallines (for example, Fig. 4, fine arrows). The presence of large amount of cristallines in the Müller cells further confirms that they belong to the class of transparent cells with specialized intermediate filaments with the function of light transmission.
Thus, all of the presently reported data effectively confirm that the model of nanooptical channels built of the intermediate filaments [Makarov et al., 2015, Khmelinskii et al., 2015] may provide a viable explanation of the Müller cell transparency.
Conclusions
The avian Müller cells of the Pyed Flycatcher in the foveola zone span the retina from the vitreous body (the basal membrane) to the outer segments of the photoreceptors, similarly to what was reported in other vertebrate species.
Same as in the other vertebrate species, the Müller cell endfeet cover all of the inner surface of the retina, making all of the light to go through the endfeet first, thus efficiently collecting the light.
The main Müller cell processes in the Pied Flycatcher, while going from the inner retina to the photoreceptor cells, are repeatedly going around numerous obstacles, thus subjecting the classical light propagation to high losses. Therefore, they should be operating as waveguides, as has been proposed earlier for the mammalian Müller cells [Franze et al., 2007; Reichenbach, Bringmann, 2010; Agte et al., 2011].
The Müller cell processes predominantly have very small diameter, branching into structures whose diameter is significantly smaller than the wavelength of the visible light. Therefore, for the Müller cells to work as waveguides, they should operate by the quantum (non-classical) mechanism.
Generally, the data on the Pied Flycatcher Müller cell organization and the cytoskeleton distribution in the retinal layers confirm our model that uses the quantum confinement to interpret the light energy transmission by the bundles of cytoskeletal filaments, each filament 10–20 nm in diameter.
Acknowledgments
Grants from the National Center for Research Resources (5 G12 RR 003035-27), from the National Institute on Minority Health and Health Disparities (8G12 MD 007583-27) to the Imaging Facilities at UCC made this publication possible. This work was in part supported by the grant from the Russian Humanitarian Scientific Foundation (#14-06-00744). The authors are also grateful to the Electron Microscopy Laboratory of the Lomonosov Moscow State University.
Literature
- 1.Agte S, Junek S, Matthias S, Ulbricht E, Erdmann I, Wurm A, Schild D, Käs JA, Reichenbach A. Müller glial cell-provided cellular light guidance through the vital guinea-pig retina. Biophys J. 2011 Dec 7;101(11):2611–2619. doi: 10.1016/j.bpj.2011.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alizadeh A, Clark JI, Seeberger T, Hess J, Blankenship T, Spicer A, FitzGerald PG. Targeted genomic deletion of the lens-specific intermediate filament protein CP49. Invest Ophthalmol Vis Sci. 2002 Dec;43(12):3722–3727. [PubMed] [Google Scholar]
- 3.Alizadeh A, Clark J, Seeberger T, Hess J, Blankenship T, FitzGerald PG. Targeted deletion of the lens fiber cell-specific intermediate filament protein filensin. Invest Ophthalmol Vis Sci. 2003;44:5252–5258. doi: 10.1167/iovs.03-0224. [DOI] [PubMed] [Google Scholar]
- 4.Alizadeh A, Clark J, Seeberger T, Hess J, Blankenship T, FitzGerald PG. Characterization of a mutation in the lens-specific CP49 in the 129 strain of mouse. Invest Ophthalmol Vis Sci. 2004 Mar;45(3):884–891. doi: 10.1167/iovs.03-0677. [DOI] [PubMed] [Google Scholar]
- 5.Benedek GB. Theory of transparency of the eye. Appl Optics. 1971;10:459–471. doi: 10.1364/AO.10.000459. [DOI] [PubMed] [Google Scholar]
- 6.Bloemendal H. Proctor lecture. Disorganisation of membranes and abnormal intermediate filament assembly lead to cataract. Invest Ophthalmol Vis Sci. 1991;32:445–455. [PubMed] [Google Scholar]
- 7.Cajal SR. In: The structure of the retina. Thorpe SA, Glickstein M, translators. Springfield (IL): Thomas; 1892. 1972. [Google Scholar]
- 8.Carver JA, Aquilina JA, Cooper PG, Williams GA, Truscott RJ. Alpha-crystallin: molecular chaperone and protein surfactant. Biochim Biophys Acta. 1994 Feb 16;1204(2):195–206. doi: 10.1016/0167-4838(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 9.Clark JI, Matsushima H, David LL, Clark JM. Lens cytoskeleton and transparency: a model. Eye (Lond) 1999 Jun;13(Pt 3b):417–424. doi: 10.1038/eye.1999.116. [DOI] [PubMed] [Google Scholar]
- 10.Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- 11.Dowling JE. The Retina: An approachable part of the brain. Cambridge, MA: Harvard University Press; 1987. [Google Scholar]
- 12.Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Müller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006 Jun 7;26(23):6303–6313. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer AJ, Zelinka C, Scott MA. Heterogeneity of glia in the retina and optic nerve of birds and mammals. PLoS One. 2010 Jun 17;5(6):e10774. doi: 10.1371/journal.pone.0010774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franze K, Grosche J, Skatchkov SN, Schinkinger S, Foja C, Schild D, Uckermann O, Travis K, Reichenbach A, Guck J. PNAS. 2007;104:8287–8292. doi: 10.1073/pnas.0611180104. originally published online May 7, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jester JV. Corneal Crystallins and the Development of Cellular Transparency. Semin Cell Dev Biol. 2008 Apr;19(2):82–93. doi: 10.1016/j.semcdb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnsen S. Hidden in plain sight: the ecology and physiology of organismal transparency. Biol Bull. 2001 Dec;201(3):301–318. doi: 10.2307/1543609. [DOI] [PubMed] [Google Scholar]
- 17.Khokhlova TV, Zueva LV, Golubeva TB. Postnatal developmental stages in the retinal photoreceptor cells of ficedula hypoleuca. Zh Evol Biokhim Fiziol. 2000;36:354–361. [PubMed] [Google Scholar]
- 18.Kivelä T, Tarkkanen A, Virtanen I. Intermediate filaments in the human retina and retinoblastoma. An immunohistochemical study of vimentin, glial fibrillary acidic protein, and neurofilaments. Invest Ophthalmol Vis Sci. 1986 Jul;27(7):1075–1084. [PubMed] [Google Scholar]
- 19.Kondrashev SL, Gnyubkina VP, Zueva LV. Structure and spectral sensitivity of photoreceptors of two anchovy species: Engraulis japonicus and Engraulis encrasicolus. Vis. Res. 2012;68:19. doi: 10.1016/j.visres.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Kreplak L, Richter K, Aebi U, Herrmann H. Electron microscopy of intermediate filaments: teaming up with atomic force and confocal laser scanning microscopy. Methods Cell Biol. 2008;88:273–297. doi: 10.1016/S0091-679X(08)00415-9. Review. [DOI] [PubMed] [Google Scholar]
- 21.Kumar PA, Reddy GB. Modulation of alpha-crystallin chaperone activity: a target to prevent or delay cataract? IUBMB Life. 2009 May;61(5):485–495. doi: 10.1002/iub.176. [DOI] [PubMed] [Google Scholar]
- 22.Lewis GP, Matsumoto B, Fisher SK. Changes in the organization and expression of cytoskeletal proteins during retinal degeneration induced by retinal detachment. Invest Ophthalmol Vis Sci. 1995 Nov;36(12):2404–2416. [PubMed] [Google Scholar]
- 23.Marcantonio JM, Duncan G. Calcium-induced degradation of the lens cytoskeleton. BiochemSoc Trans. 1991;19:1148–1150. doi: 10.1042/bst0191148. [DOI] [PubMed] [Google Scholar]
- 24.Massoudi D, Malecaze F, Galiacy SD. Collagens and proteoglycans of the cornea: importance in transparency and visual disorders. Cell Tissue Res. 2015 Jul 24; doi: 10.1007/s00441-015-2233-5. [DOI] [PubMed] [Google Scholar]
- 25.Matsushima H, David LL, Hiraoka T, Clark JI. Loss of cytoskeletal proteins and lens cell opacification in the selenite cataract model. Exp Eye Res. 1997;64:387–395. doi: 10.1006/exer.1996.0220. [DOI] [PubMed] [Google Scholar]
- 26.Oka M, Kudo H, Sugama N, Asami Y, Takehana M. The function of filensin and phakinin in lens transparency. Mol Vis. 2008 Apr 25;14:815–822. [PMC free article] [PubMed] [Google Scholar]
- 27.Perng MD, Wen SF, Gibbon T, Middeldorp J, Sluijs J, Hol EM, Quinlan RA. Glial fibrillary acidic protein filaments can tolerate the incorporation of assembly-compromised GFAP-delta, but with consequences for filament organization and alphaB-crystallin association. Mol Biol Cell. 2008 Oct;19(10):4521–4533. doi: 10.1091/mbc.E08-03-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qu B, Landsbury A, Schönthaler HB, Dahm R, Liu Y, Clark JI, Prescott AR, Quinlan RA. Evolution of the vertebrate beaded filament protein, Bfsp2; comparing the in vitro assembly properties of a "tailed" zebrafish Bfsp2 to its "tailless" human orthologue. Exp Eye Res. 2012 Jan;94(1):192–202. doi: 10.1016/j.exer.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinlan RA, Carte JM, Sandilands A, Prescott AR. The beaded filament of the eye lens: an unexpected key to intermediate filament structure and function. Trends Cell Biol. 1996 Apr;6(4):123–126. doi: 10.1016/0962-8924(96)20001-7. [DOI] [PubMed] [Google Scholar]
- 30.Rafferty NS, Rafferty KA, Zigman S. Comparative response to UV irradiation of cytoskeletal elements in rabbit and skate lens epithelial cells. Curr Eye Res. 1997;16:310–319. doi: 10.1076/ceyr.16.4.310.10687. [DOI] [PubMed] [Google Scholar]
- 31.Reichenbach A. Bridgman, Morphology and Cellular Properties of Müller Cells as Constituents of Retinal Tissue, in the book: Müller Cells in the Healthy and Diseased Retina. Springer; 2010. [Google Scholar]
- 32.Simirskii VN, Panova IG, Sologub AA, Aleinikova KS. Localization of crystallins in Mueller cells in the grass frog retina. Ontogenez. 2003 Sep-Oct;34(5):365–370. [PubMed] [Google Scholar]
- 33.Song S, Landsbury A, Dahm R, Liu Y, Zhang Q, Quinlan RA. Functions of the intermediate filament cytoskeleton in the eye lens. J Clin Invest. 2009 Jul;119(7):1837–1848. doi: 10.1172/JCI38277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steven AC. Intermediate Filament Structure, in the book: Cellular and Molecular Biology of Intermediate Filaments. US: Springer; 1990. pp. 233–263. [Google Scholar]
- 35.Bassnett Steven, Shi Yanrong, Vrensen Gijs FJM. Biological glass: structural determinants of eye lens transparency. Phil. Trans. R. Soc. B. 2011;366:1250–1264. doi: 10.1098/rstb.2010.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tagliavini I, Gandolfi SA, Maraini G. Cytoskeleton abnormalities in human senile cataract. Curr Eye Res. 1986;5:903–910. doi: 10.3109/02713688608995170. [DOI] [PubMed] [Google Scholar]
- 37.Tardieu A, Delaye M. Eye lens proteins and transparency: from light transmission theory to solution x-ray structural analysis. Annu. Rev. Biophys. Biophys. Chem. 1988;17:47–70. doi: 10.1146/annurev.bb.17.060188.000403. [DOI] [PubMed] [Google Scholar]
- 38.Wakakura M, Foulds WS. Comparative ultrastructural study of rabbit Müller cells in vitro and in situ. Eye (Lond) 1988;2(Pt 6):664–669. doi: 10.1038/eye.1988.122. [DOI] [PubMed] [Google Scholar]
- 39.Zueva L, Makarov V, Zayas-Santiago A, Golubeva T, Korneeva E, Savvinov A, Eaton M, Skatchkov S, Inyushin M. Müller cell alignment in bird fovea: possible role in vision. J Neurosci Neuroeng. 2014 Dec 1;3(2):85–91. doi: 10.1166/jnsne.2014.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]