Abstract
Although Multiple Sclerosis is predominantly regarded as a disease of young adulthood, up to five percent of MS patients are diagnosed prior to age eighteen. The predominant form of MS is relapsing-remitting characterized by exacerbations of symptoms followed by periods of remission. The majority of disease modifying drugs target T cell proliferation or block migration into the central nervous system. Although these treatments reduce relapses, disease progression still occurs, warranting therapeutic strategies that protect the CNS. Biomarkers to indicate relapses would facilitate a personalized approach for add-on therapies that protect the CNS. A multiplex cytokine bead array was performed to detect T cell associated cytokines in sera from patients 6–20 years of age with pediatric onset MS clinically diagnosed in relapse or remission compared to healthy control patients. Of the 25 cytokines evaluated, 17 were increased in patients clinically diagnosed in relapse compared to sera from control patients in contrast to only 9 cytokines in the clinically diagnosed remission group. Furthermore, a linear regression analysis of cytokine levels in the remission population showed 12 cytokines to be statistically elevated as a function of disease duration, with no effect observed in the relapse population. To further explore this concept, we used a multivariable stepwise discriminate analysis and found that the following four cytokines (IL-10, IL-21, IL-23, and IL-27) are not only a significant predictor for MS, but have important predictive value in determining a relapse. Since IL-10 and IL-27 are considered anti-inflammatory and IL-21 and IL-23 are pro-inflammatory, ratios of these cytokines were evaluated using a Duncan’s multiple range test. Of the six possible combinations, increased ratios of IL-10:IL-21, IL-10:IL-23, and IL-10:IL-27 were significant suggesting levels of IL-10 to be a driving force in predicting a relapse.
Graphical abstract
1. Introduction
There are 2.5 million people diagnosed with multiple sclerosis (MS) worldwide, five percent of whom are diagnosed prior to age eighteen(1). In MS, T cells are primed against unidentified white matter or foreign antigens bound to major histocompatibility complexes (MHCs)(2). This leads to destruction of myelin, the lipid membrane of oligodendrocytes (OLs) that forms an insulating sheath around axons allowing for increased action potential speed(3). Demyelination not only impairs conduction of nerve impulses, but makes axons more vulnerable to injury contributing to motor and cognitive decline. The most common form of MS is relapsing-remitting (RRMS), whereby exacerbations of symptoms are followed by a period of remission. A relapse is defined as a clinical event involving the onset of neurological symptoms caused by CNS inflammation. The number of inflammatory events in the CNS of MS patients is far greater than the number of relapses. Current treatments in MS decrease the number of relapses, yet few eliminate relapses (4) warranting treatment strategies that protect the CNS. Predicting the time point before a relapse occurs is an important intervention interval for CNS protective strategies, thus, identifying biomarkers that predict relapses and/or disease progression highly clinically relevant.
Current studies have utilized the number (5, 6) or location of lesions observed by MRI to predict probability of a relapse(7). Assessing the number of lesions in conjunction with relapses as a function of disease duration has also been predictive of disease progression(8). However, imaging biomarkers may indicate a time point in the disease process where an intervention is no longer efficacious. Economical, easily accessible biomarkers that monitor a specific pathogenic disease process, as an early predictor of relapse, would be beneficial.
Cytokines regulate immune cell infiltration into different areas of the central nervous system resulting in different clinical manifestations in animal models of MS(9). Pro-inflammatory cytokine levels in sera from patients with MS are elevated compared to healthy controls(10–12). These data provide evidence that serum cytokine levels may represent an active disease process in patients with RRMS.
Naïve CD4+ T cells circulate between the blood and lymphatic system, and differentiate upon encountering cognate peptides presented by antigen presenting cells (APCs) in conjunction with co-stimulatory molecules and cytokine signals. Differentiated effector T cells are considered the principal orchestrators in MS pathogenesis(13, 14). CNS infiltrating effector T cells and their relevance to human disease is further confirmed by the effectiveness of disease modifying therapies that inhibit T cell proliferation/activation (e.g. IFN-β, glatiramer acetate) or bind to adhesion molecules (natalizumaub) and prevent extravasation through blood vessels into the CNS. Specific cytokines initiate the activation of discrete transcriptional regulatory programs that segregate differentiating T helper cells into one of four major subsets; Th1, Th17, Th2, and regulatory T cells (Tregs)(15).
Despite their critical function in disease initiation and resolution, to date no studies have extensively evaluated T cell associated cytokines throughout the course of disease in the pediatric RRMS population. Therefore, we evaluated a cytokine profile specifically associated with T helper signaling in sera from pediatric MS patients clinically diagnosed in remission or relapse compared to healthy controls to provide important clues about the disease process in remission distinct from relapse as well as provide biomarkers to predict relapse.
2. Methods
2.1 Clinical Samples and chart review
Serum samples were obtained with informed consent from parents or guardians of patients with pediatric onset multiple sclerosis (POMS) or healthy controls with no pathology under the approval of the Institutional Review Board No. X130307001. Patients were diagnosed with POMS based on the International Pediatric MS Study Group criteria (16, 17) and categorized clinically as being in MS relapse or remission when the sample was obtained. Patients ranged in age from 6 to 17 years of age at disease onset and from 8 to 20 years of age at time of sample collection. Control patients ranged in age from 5–19 years. Patient data are reported in Table I including corticosteroid treatment or disease modifying agents.
Table 1.
Demographics of pediatric relapsing-remitting MS and healthy control subjects
| Clinical Information | MS | Controls | |
|---|---|---|---|
|
| |||
| Relapse (n=14) | Remission (n=26) | (n=11) | |
|
| |||
| Age Range* | 8.88–20.76 | 11.5–19.54 | 5.96–19.28 |
|
| |||
| Mean Age* ± SD | 15.28 ± 3.30 | 16.38 ± 1.94 | 13.76 ± 3.37 |
|
| |||
| Gender | 10 female | 21 female | 5 female |
| 4 male | 5 male | 6 male | |
|
| |||
| Race | 7 white | 18 white | 5 white |
| 7 black | 8 black | 5 black 1 not reported |
|
|
| |||
| Steroids | 3 Yes | 1 Yes | 11 No |
| 11 No | 25 No | ||
|
| |||
| Disease-Modifying Agents | |||
| Copaxone | 2 | 6 | None |
| Rebif | 3 | 6 | None |
| Avonex | 2 | 3 | None |
| Betaseron | 0 | 1 | None |
| Extavia | 0 | 1 | None |
| None | 7 | 9 | None |
|
| |||
| Age at symptom onset Range* | 6.56–17.62 | 7.76–17.35 | N/A |
|
| |||
| Mean Age at symptom Onset* ± SD | 13.54 ± 3.41 | 14.21 ± 2.38 | N/A |
|
| |||
| Disease Duration* | 1.74 ± 1.82 | 2.17 ± 2.11 | N/A |
Age is represented in years
2.2 Multiplex cytokine array
Serum was isolated from collected blood samples and stored at −80°C until use for multiplex cytokine analysis. Serum cytokine levels were measured using the MILLIPLEX MAP Human Th17 Magnetic Bead Panel (catalog # HT17MG-14K-PX25) and the Luminex Multi-Analyte Instrument (Luminex, Austin, TX) as per manufacturer’s instructions previously described (18), values were reported in pg/mL. Luminex multiplex suspension array technology for analysis of human samples was performed as previously described (19–21).
2.3 Statistical Analysis
Statistical analyses were performed using Graphpad Prism software (San Diego, CA) and SAS (Version 9.4). All samples categorized by disease states were compared to control samples using the two-tailed nonparametric Mann-Whitney test. A p-value of ≤ 0.05 was used to determine statistical significance with no adjustments for multiple comparisons. All of these values are reported in Table 2. DMTs were used in 60% of the patients: 50% (7/14) of patients who were experiencing a relapse were on a DMT compared to 65% (17/26) in the remission group. To assess the influence of disease modifying therapy (DMT) on cytokines levels, sera from patients on DMTs were compared to those not on DMTs using a two-tailed Mann Whitney test. No significant difference was observed in sera from patients on DMT compared to sera from patients not on DMTs. The results were thus combined and reported as their respective sub-group remission or relapse as indicated in Table 2 without adjustment for DMT. To evaluate changes in cytokine levels as a factor of disease duration within each disease group, a linear regression was used as shown in Table 3. To assess strong predictors of multiple sclerosis, a multivariable stepwise discriminate analysis was performed as shown in Tables 4, 5, and 6.
Table 2.
Statistical significance (p values) of T helper-associated cytokines in MS versus control as well as Remission versus Relapse (R vs R)
| Mean | Median | Std Dev | Minimum | Maximum | *p value |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||||||
| Cytoki ne |
Ctl | Remi ssion |
Rela pse |
Ctl | Remi ssion |
Rela pse |
Ctl | Remi ssion |
Rela pse |
Ctl | Remi ssion |
Rela pse |
Ctl | Remi ssion |
Rela pse |
Remi ssion |
Rela pse |
R vs R |
|
|
|
|||||||||||||||||||
| Th1-associated cytokines | IFNg | 0.899 | 16.830 | 5.539 | 0.00 | 0.00 | 0.00 | 2.052 | 62.064 | 10.428 | 0. 00 | 0.00 | 0.00 | 5.97 | 313.31 | 31.21 | 0.22 | 0.18 | 0.81 |
| IFNg IL-12p70 | 3.846 | 22.559 | 9.159 | 3.18 | 6.02 | 8.03 | 2.466 | 75.233 | 7.466 | 0.00 | 0.00 | 0.00 | 9.63 | 389.43 | 29.21 | 0.12 | 0.02 | 0.58 | |
| TNFb | 0.000 | 0.045 | 0.038 | 0.00 | 0.00 | 0.00 | 0.000 | 0.104 | 0.076 | 0.00 | 0.00 | 0.00 | 0.00 | 0.36 | 0.25 | 0.08 | 0.05 | 0.82 | |
| IL-2 | 0.000 | 13.125 | 8.692 | 0.00 | 0.00 | 0.00 | 0.000 | 35.776 | 15.907 | 0.00 | 0.00 | 0.00 | 0.00 | 165.80 | 55.45 | 0.09 | 0.1 | 0.88 | |
| Th2-associated cytokines | IL-4 | 0.002 | 0.033 | 0.056 | 0.00 | 0.00 | 0.00 | 0.006 | 0.102 | 0.096 | 0.00 | 0.00 | 0.00 | 0.02 | 0.50 | 0.29 | 0.39 | 0.12 | 0.22 |
| IL-33 | 0.000 | 35.352 | 27.701 | 0.00 | 3.78 | 9.12 | 0.000 | 88.523 | 52.371 | 0.00 | 0.00 | 0.00 | 0.00 | 388.38 | 165.60 | 0.06 | 0.01 | 0.49 | |
| IL-5 | 0.132 | 8.523 | 1.989 | 0.00 | 0.37 | 0.61 | 0.381 | 20.406 | 4.369 | 0.00 | 0.00 | 0.00 | 1.27 | 80.53 | 16.45 | 0.04 | 0.04 | 0.73 | |
| IL-10 | 0.224 | 3.846 | 9.379 | 0.00 | 1.36 | 4.07 | 0.443 | 7.900 | 10.956 | 0.00 | 0.00 | 0.00 | 1.36 | 39.35 | 31.86 | 0.01 | 0.004 | 0.06 | |
| IL-13 | 13.933 | 35.379 | 36.059 | 13.87 | 29.01 | 32.39 | 14.649 | 39.575 | 30.234 | 0.00 | 0.00 | 0.00 | 43.85 | 154.51 | 93.57 | 0.1 | 0.04 | 0.59 | |
| IL-25/IL-17E | 0.017 | 0.388 | 0.324 | 0.00 | 0.04 | 0.10 | 0.025 | 0.990 | 0.621 | 0.00 | 0.00 | 0.00 | 0.06 | 4.48 | 2.10 | 0.1 | 0.01 | 0.45 | |
| Th17- associated cytokines | IL_6 | 1.711 | 10.817 | 8.930 | 0.00 | 0.00 | 0.00 | 5.674 | 32.219 | 17.254 | 0.00 | 0.00 | 0.00 | 18.82 | 158.42 | 47.73 | 0.11 | 0.22 | 0.78 |
| IL-1β | 1.127 | 3.237 | 4.410 | 0.00 | 0.00 | 0.00 | 3.739 | 8.042 | 7.818 | 0.00 | 0.00 | 0.00 | 12.40 | 35.69 | 25.35 | 0.15 | 0.15 | 0.76 | |
| IL-23 | 0.073 | 1.600 | 0.871 | 0.00 | 0.18 | 0.24 | 0.107 | 4.309 | 1.593 | 0.00 | 0.00 | 0.00 | 0.30 | 18.31 | 4.92 | 0.05 | 0.005 | 0.44 | |
| IL-17A | 0.374 | 14.068 | 6.851 | 0.00 | 0.00 | 3.23 | 0.738 | 56.682 | 11.461 | 0.00 | 0.00 | 0.00 | 2.26 | 289.55 | 42.02 | 0.35 | 0.02 | 0.09 | |
| IL-17F | 0.012 | 0.036 | 0.034 | 0.01 | 0.02 | 0.03 | 0.006 | 0.056 | 0.028 | 0.00 | 0.00 | 0.01 | 0.02 | 0.28 | 0.10 | 0.07 | 0.006 | 0.46 | |
| IL-21 | 5.885 | 18.304 | 18.219 | 4.70 | 13.59 | 14.43 | 3.983 | 22.093 | 12.998 | 0.00 | 0.00 | 4.23 | 14.45 | 111.44 | 51.30 | 0.02 | 0.001 | 0.55 | |
| IL-22 | 0.000 | 0.090 | 0.073 | 0.00 | 0.00 | 0.00 | 0.000 | 0.237 | 0.179 | 0.00 | 0.00 | 0.00 | 0.00 | 0.89 | 0.56 | 0.27 | 0.1 | 0.58 | |
| Cytokines associated with multiple T helper cells | TNFa | 12. 145 | 19.554 | 20.434 | 10. 98 | 15.28 | 17.16 | 6.895 | 18.602 | 13.241 | 0. 00 | 0.00 | 2.32 | 23. 16 | 96.58 | 58.94 | 0.1 | 0.03 | 0.38 |
| GM- CSF | 0.000 | 0.097 | 0.000 | 0.00 | 0.00 | 0.00 | 0.000 | 0.366 | 0.000 | 0. 00 | 0.00 | 0.00 | 0.00 | 1.80 | 0.00 | 0.78 | 0.91 | 0.20 | |
| CCL20/ MIP3a | 11. 883 | 27.122 | 26.546 | 11. 19 | 20.46 | 24.86 | 6.075 | 21.577 | 9.926 | 2. 99 | 0.00 | 10.52 | 20. 85 | 104.90 | 44.78 | 0.004 | 0.0003 | 0.33 | |
| IL-9 | 0.000 | 7.895 | 6.152 | 0.00 | 0.00 | 0.00 | 0.000 | 22.648 | 9.131 | 0.00 | 0.00 | 0.00 | 0.00 | 112.26 | 22.67 | 0.04 | 0.1 | 0.92 | |
| IL-15 | 0.228 | 7.713 | 9.675 | 0.00 | 0.00 | 1.52 | 0.757 | 20.265 | 14.839 | 0.00 | 0.00 | 0.00 | 2.51 | 91.59 | 46.98 | 0.11 | 0.01 | 0.20 | |
| IL-27 | 0.189 | 1.109 | 1.066 | 0.13 | 0.59 | 0.66 | 0.185 | 1.530 | 0.948 | 0.00 | 0.21 | 0.36 | 0.52 | 6.98 | 3.46 | 0.0002 | <0.0001 | 0.32 | |
| IL-28A | 0.018 | 0.185 | 0.153 | 0.00 | 0.11 | 0.08 | 0.034 | 0.281 | 0.228 | 0.00 | 0.00 | 0.00 | 0.10 | 1.05 | 0.86 | 0.006 | 0.01 | 0.82 | |
| IL-31 | 0.005 | 0.184 | 0.126 | 0.00 | 0.02 | 0.04 | 0.008 | 0.537 | 0.240 | 0.00 | 0.00 | 0.00 | 0.02 | 2.67 | 0.75 | 0.01 | 0.002 | 0.60 | |
Table 3.
Linear regression analyses: duration of disease (years) and cytokine levels (pg/mL).
| Cytokine | MS Remission | MS Relapse | ||
|---|---|---|---|---|
| Th1-associated cytokines | ||||
| R2 | P value | R2 | P value | |
| IFNγ | 0.002 | 0.82 | 0.001 | 0.89 |
| IL-12p70 | 0.000 | 0.98 | 0.082 | 0.32 |
| TNFβ | 0.140 | 0.05 | 0.000 | 0.95 |
| IL-2 | 0.057 | 0.24 | 0.015 | 0.67 |
| Th2-associated cytokines | ||||
| IL-4 | 0.274 | 0.006 | 0.007 | 0.76 |
| IL-33 | 0.211 | 0.02 | 0.002 | 0.87 |
| IL-5 | 0.015 | 0.55 | 0.007 | 0.78 |
| IL-10 | 0.239 | 0.01 | 0.044 | 0.47 |
| IL-13 | 0.102 | 0.11 | 0.014 | 0.69 |
| IL-25/IL-17E | 0.238 | 0.01 | 0.003 | 0.84 |
| Th17-associated cytokines | ||||
| IL-6 | 0.034 | 0.37 | 0.001 | 0.90 |
| IL-1β | 0.244 | 0.01 | 0.013 | 0.70 |
| IL-23 | 0.202 | 0.02 | 0.006 | 0.79 |
| IL-17A | 0.000 | 0.94 | 0.009 | 0.74 |
| IL-17F | 0.302 | 0.003 | 0.012 | 0.70 |
| IL-21 | 0.003 | 0.79 | 0.078 | 0.33 |
| IL-22 | 0.177 | 0.03 | 0.000 | 0.99 |
| Cytokines associated with multiple T helper cells | ||||
| TNFα | 0.025 | 0.44 | 0.012 | 0.71 |
| GM-CSF | 0.000 | 0.90 | 0.002 | 0.43 |
| CCL20/MIP3α | 0.018 | 0.50 | 0.003 | 0.84 |
| IL-9 | 0.015 | 0.55 | 0.023 | 0.60 |
| IL-15 | 0.287 | 0.004 | 0.004 | 0.82 |
| IL-27 | 0.188 | 0.03 | 0.001 | 0.91 |
| IL-28A | 0.083 | 0.15 | 0.000 | 0.99 |
| IL-31 | 0.275 | 0.006 | 0.005 | 0.81 |
Table 4.
Number of patients with one or more of the four cytokine cluster (IL-10, IL-21, IL-23, IL-27) elevated five times above the mean of control levels
| # of four cytokines | Control | Relapse | Remission |
|---|---|---|---|
| 0 | 10 | 3 | 12 |
|
|
|||
| ≥1 | 1 | 11 | 14 |
| Percent ≥ 1 | 9% | 79% | 54% |
Table 5.
Predictive value of having one or more cytokines from the cluster elevated five times above the normative baseline as a positive predicator (PPV) of a relapse compared to none as a negative predictor of a relapse
| sensitivity | 11/14 | 79% |
| specificity | 12/26 | 46% |
| PPV | 11/25 | 44% |
| NPV | 12/15 | 80% |
Table 6.
Ratios derived from the four cytokine cluster, brackets demonstrate significance of p < 0.05 using Duncan’s multiple range test
Bracketed values indicate statistical significance
3. Results
A major underlying pathogenesis in MS is T cell infiltration into the CNS initiating an inflammatory cascade resulting in axonal demyelination and eventual neurodegeneration. In an effort to elucidate potential biomarkers to predict when relapses occur, a microarray panel of 25 cytokines associated with T cell signaling was analyzed in sera from MS patients clinically diagnosed in relapse or remission and compared to levels in healthy control sera. Demographics for all patients enrolled in the study are included in Table 1. Statistical significance for cytokine levels in serum samples from relapse compared to control as well as remission compared to control are summarized in Table 2. These results are described in the context of cytokines that regulate T cell differentiation from CD4+ naïve T cells as well as cytokines released by these effector sub-populations.
Chronic reactivity of CD4+ T cells to autoantigens play a central role in autoimmunity mainly by segregating into three major phenotypes, Th1, Th2, and Th17. Th1 cells differentiate from naïve CD4+ T cells in the presence of IL-12p70 and IFNγ to produce IL-2, IFNγ, TNFβ, and GMCSF. Patients clinically diagnosed in MS relapse had significantly increased levels of IL-12p70 and TNFβ compared to controls whereas neither of these cytokines were increased in patients diagnosed in remission (Table 2). Th2 cells differentiate from naïve CD4+ T cells in the presence of IL-4 and IL-33 to produce GMCSF, IL-4, IL-5, IL-10, IL-13, and IL-25/IL-17E. All of these cytokines with the exception of IL-4 were significantly elevated in MS relapse patients compared to control. In contrast, only IL-5 and IL-10 were statistically elevated in serum from patients in MS remission compared to controls. Th17 cells differentiate from naïve CD4+ T cells in the presence of IL-6, TGFβ (not in panel), IL-1β, and IL-23 to produce IL-17A, IL-17F, IL-21, IL-22, and GM-CSF. IL-23, IL-17A, IL-17F, and IL-21 were significantly elevated in sera from patients in MS relapse compared to control, whereas only IL-23 and IL-21 were elevated in sera from patients clinically diagnosed in remission.
Other cytokines associated with multiple T cell subsets were also assessed. TNF-α is associated with both Th1 and Th17 signaling and is elevated during MS relapse, but not MS remission compared to control sera. CCL20/MIP3a, which can recruit both Th17 cells and Tregs via the chemokine receptor CCR6 (22–25), was increased during relapse and remission. IL-9, which is released by a relatively unexplored subset of T cells, Th9, is significantly upregulated in MS remission serum samples, but not in the MS relapse subset compared to control. Both IL-15 and IL-27 are associated with a negative regulatory role on Th17 responses (26, 27) and were significantly increased in MS relapse, but only IL-27 was elevated in MS remission compared to control samples. IL-28A and IL-31 are key mediators in T-cell mediated inflammatory diseases (28, 29) and were significantly elevated in sera from both relapse and remission compared to sera from control samples.
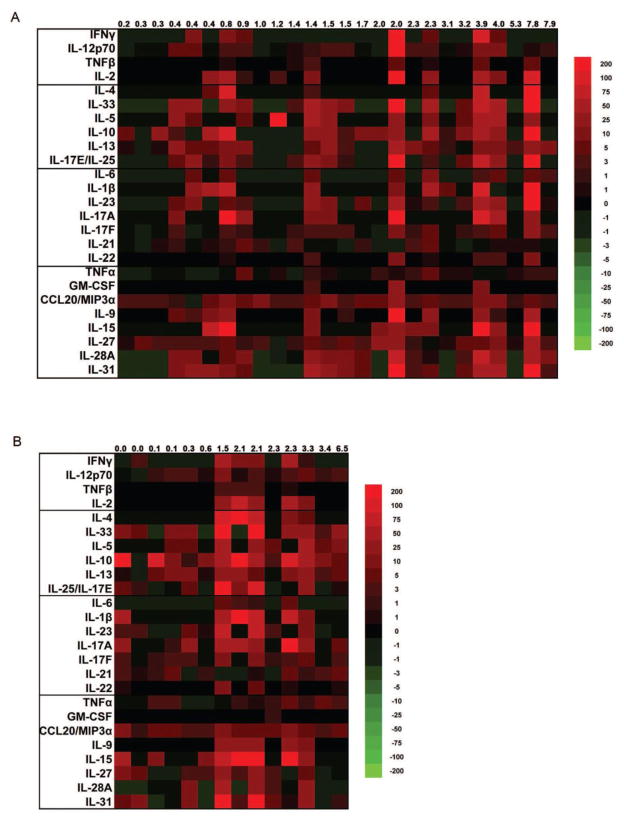
While finding predictors of relapse may have important clinical ramifications for add-on therapies, another approach in this regard is monitoring changes in T cell associated cytokines during remission as a function of disease duration. In RRMS, patients have exacerbations of symptoms followed by a remission phase where symptoms subside and clinical outcomes return to baseline. However, as disease duration increases, the remission phase of the disease may not return to baseline. Patients then advance into a secondary progressive phase precipitating a downward decline in function. To assess changes in cytokine levels during remission in relationship to disease duration, a heat map was generated by plotting cytokine levels with increasing disease duration (Fig. 1A). A linear regression analysis of cytokine levels in remission sera as a function of disease duration was performed, and coefficients of determination (R2) and p-values are reported in Table 3. Of the 25 cytokines assessed, 12 were significantly positively associated with disease duration in the remission sera. These 12 cytokines were then compared to their outcomes from Table 2. Five of the cytokines (TNF-β, IL-33, IL-25, IL-17F, and IL-15) found significant in the linear regression analysis from remission sera (Table 3) were not elevated in the remission compared to control sera, but were elevated in the relapse compared to control sera (Table 2). Another five of the cytokines (IL-10, IL-23, IL-27, IL-28A, and IL-31) found significant in the linear regression analysis from remission sera (Table 3) were elevated in the both the remission and relapse sera compared to control (Table 2). The last two cytokines (IL-4 and Il-22) were significant in the linear regression analysis from the remission sera (Table 3), but were not elevated in the remission or relapse sera compared to control. A linear regression analysis of cytokine levels in relapse sera as a function of disease duration (Table 3) demonstrated no statistically significant differences, heat map included in Figure 1B. Collectively, these data suggest that monitoring an individual’s cytokines levels during subsequent remission phases may be an important predictor of disease progression, and may potentially provide a strong rationale for a more aggressive treatment strategy.
Figure 1.
Heat map depicting levels of T helper cell associated cytokines in patients with multiple sclerosis during remission (A) and relapse (B). Columns represent individual patients and are organized by increasing disease duration in years. Each box represents the percent difference between the patient and the average of healthy controls for the cytokines indicated to the left of each row, both measured in pg/mL. Red, black, and green colors represent values above, equal to, or below the average of healthy controls, respectively. Values depicted at the top of the figure represent the disease duration of each patient in years. A linear regression analysis was performed to determine which cytokines significantly increased with disease duration, and results are included in Table 3.
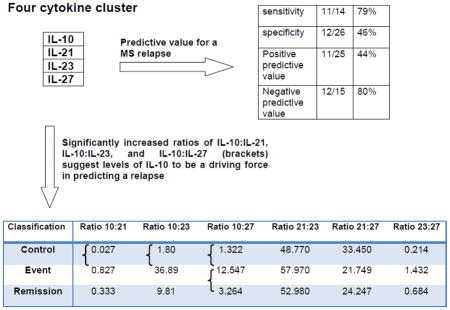
These data were further explored to assess how well cytokine data could be used to predict if a patient has MS by comparing all three subsets ie. control, relapse, and remission. Using a multivariable stepwise discriminate analysis, four cytokines (IL-23, IL-21, IL-10, and IL-27) were shown to have a predictive value for multiple sclerosis. To further evaluate the relevance of this four cytokine cluster in monitoring the remission phase as a predictor of a relapse, we assessed the number of patients with cytokine levels five times higher than the mean of the control population for each cytokine (Table 4). This table shows that 9% of the control group, 79% in the relapse group, and 54% in the remission group had a least one of the four cytokines in the cluster elevated five times higher than the mean for the control population. These values show an estimated sensitivity of 79% and a specificity of 46% (Table 5). The positive predictive value (PPV) of having one or more cytokines from the cluster elevated five times above the normative baseline as a positive predicator (PPV) of a relapse is 44%. In comparison, the probability of having none of the four cytokine cluster elevated five times above the mean of the control population as a negative predictor (Negative predictive value, NPV) of a relapse is 80%. The identification of the four cytokine cluster supports the biology of the Th17 cell as an important predictor of disease since IL-21 and IL-23 are central in the pathogenesis for Th17 differentiation and both IL-10 and IL-27 are important in the resolution of this effector state. Therefore, we next assessed the ratios derived from these cytokines using a Duncan’s multiple range test to examine the consistency of the pattern of the cytokines across the three classification groups. If a zero (below the limit of detection) was measured for a cytokine for a particular individual, the ratio used was the smallest observed value for that cytokine in the group (arguing that zero was merely below the limit of detection). This avoids excluding the observation since one cannot divide by zero and should be conservative as the ratio would exceed what is used in the analysis. Of the six possible combinations, increased ratios for IL-10:IL-21, IL-10:IL-23, and IL-10:IL-27 were significant suggesting levels of IL-10 to be a driving force in predicting a relapse (Table 6). Of note, although ratios for IL-21:IL-23, IL-21:IL-27, and IL-23:IL-27 were not statistically significant, the ratios were appropriately rank ordered between the control, relapse, and remission group.
4. Discussion
Our data show that 17 of the 25 T helper cell associated cytokines are elevated during pediatric MS relapse compared to healthy controls. In contrast, only 9 cytokines from this same cytokine profile are elevated during the remission phase compared to control subjects. Not only do these data support well-known theories that T cells are involved in MS, but they also provide evidence that serum cytokine levels have the potential to confirm diagnosis of an MS relapse. A linear regression analysis of cytokine levels in sera from patients in remission as a function of disease duration demonstrated statistical significance in 12 of the 25 cytokines from the panel. A linear regression analysis of cytokine levels in sera from patients in relapse as a function of disease duration demonstrated no statistically significant difference. Furthermore, a multivariate stepwise discriminate analysis demonstrated that four cytokines (IL-10, IL-21, IL-23, and IL-27) have a predictive value for the pediatric MS population studied. This four cytokine cluster provided a more focused group of cytokines to track within individual patients as a positive predictor of a relapse making it a potential biomarker. Since IL-10 and IL-27 are considered anti-inflammatory and IL-21 and IL-23 are pro-inflammatory, the six possible combination ratios of these cytokines were evaluated using a Duncan’s multiple range test. Increased ratios of IL-10:IL-21, IL-10:IL-23, and IL-10:IL-27 were significant suggesting levels of IL-10 may be a driving force in predicting a relapse. This study, like all studies has limitations. The focus on pediatric MS by necessity limits the sample size and a matched case/control study was not performed suggesting the possibility that matching age, gender etc. may have provided a more clear comparison. This analysis also did not control for therapeutic interventions although roughly only 50% were on DMTS. Finally, these studies warrant testing the IL-10 hypothesis in larger cohorts that include MRI data and repeat samples to compare relapse and remission period within the same pediatric patient. Since the number of inflammatory events in the CNS of MS patients is far greater than the number of relapses, it will be appropriate to evaluate serum cytokine levels with disease activity in the CNS as measured by MRI as opposed to a clinical relapse. These ideas as well as the importance of each cytokine in relationship to T cell effector functions will be explored in the following discussion.
In experimental autoimmune encephalomyelitis (EAE), an animal model of MS, Th17 cells have been implicated in playing a role in the disease (13). In patients with MS, there is evidence for IL-17-producing T cell upregulation in lesions (14, 30) and cerebrospinal fluid (CSF) (31). Here, we showed that IL-17A and IL-17F were only elevated in sera from the relapse group compared to control. Although elevated IL-17F levels have been documented in sera from adult MS patients (12), only one other adult study demonstrated an increase in IL-17A preferentially in RRMS patients during relapse as opposed to patients in remission (32). Of note, even when a single statistical outlier for IL-17A in the remission group is eliminated, it is five standard deviations above the mean which exceeds the usual statistical cutoff for outliers, IL-17A is two times higher during relapse than the mean remission value. Again, this is consistent with previous findings (32). Taken together, these data suggest the potential use of IL17A and IL-17F as biomarkers to predict relapse. The time horizon for the prediction is unknown, which is relevant for understanding the therapeutic window to intervene. This is best assessed in a longitudinal study and is not readily available from a cross-sectional survey.
Th1 cells also have a significant role in the pathogenesis of MS, which produce IFN-γ as their signature cytokine. Other serum cytokine profile studies in adult RRMS patients showed elevated levels of the Th1 signature cytokine IFN-γ (10), however our study did not observe any significant changes in either of the MS groups. Explanations for this disparity may be that the aforementioned study did not assess relapse separately from remission groups or differences between adult verses pediatric RRMS. Our study did identify, however, two cytokines involved in the development of Th1 cells IL-12p70 and TNF-β to be elevated in relapse compared to control sera and none were elevated in remission. Again, emphasizing a discerning function of Th1 cells in MS pathology with implications as a predictor of an MS relapse.
Th17 and Th1 cells are implicated in the pathogenesis of EAE, while Th2 cells have typically been shown to have anti-inflammatory functions in this setting. Interestingly, several cytokines associated with T helper type 2 development IL-33, IL-5, IL-10, IL-13, IL-25/IL-17E are elevated in the relapse group compared to control sera, whereas only IL-5 and IL-10 are elevated in the remission sera compared to control. These data provide important implications for Th2-associated cytokines in MS with their augmentation in the relapse phase of the disease, and suggest that a greater understanding of the function of these cytokines in different phases of disease may lead to new insights into the role of Th2 cells in pediatric RRMS.
IL-28A (a Type III Interferon), similar to the Type I Interferons used to treat MS, has been shown to have both potent anti-viral/pro-inflammatory properties as well immunomodulatory functions (33). In this study, it was shown here to be upregulated in both phases of the disease. The CCL20/MIP3a chemokine was found to be associated with Th17 entry specifically in the choroid plexus of mice in order to initiate experimental autoimmune encephalomyelitis (EAE), the animal model of EAE (22). Furthermore, mice deficient in CCR6, a receptor specific for the CCL20 chemokine, showed reduced T cell entry into the fifth lumbar spinal cord during the early stages of EAE induction (23). Finally, CCL20/CCR6 signaling was also shown to be required for T regulatory cell (Tregs) recruitment into the inflamed CNS during EAE (24, 25). Th1 and Th17 effector T cells have been shown to be drivers of CNS inflammation in MS, while Tregs dampen inflammation and are important for disease resolution. In our cohort, CCL20 was significantly increased both in MS relapse as well as remission. This suggests that this chemokine may have a biphasic effect during disease, in which it may enhance Th17 cell entry during relapses and promote disease resolution during remission via Treg recruitment. IL-9, which is released by a relatively unexplored subset of T cells, Th9, has been shown to promote expression of CCL20 in tumor cells as well as activate T cells in autoimmune inflammation (34–36). IL-9 is significantly upregulated in MS remission serum samples, but not in the MS relapse subset compared to control.
Although this study does not represent a complete view of the CD4+ T cell effector groups and their contribution to disease pathogenesis, collectively, the cytokine profile during relapse is distinct from the remission group. Our analysis of cytokine levels in remission sera as a function of disease duration demonstrated statistical significance in 12 of the 25 cytokines from the panel. These data suggest that monitoring cytokine concentrations in individuals during subsequent remission phases compared to baseline levels may be a useful tool in monitoring disease.
From our multivariable analyses we identified a four cytokine cluster (IL-10, IL-21, IL-23, and IL-27) as a predictor of MS and then evaluated the probability of having one or more cytokines within the cluster, elevated above baseline during remission, as an indicator of a relapse. The positive predictive value (PPV) of having one or more cytokines from the cluster elevated five times above the normative baseline during remission was 44%. While not a longitudinal assessment it is indicative of some predictive value. Moreover, having none of the cytokine cluster elevated above normative baseline during the remission phase estimated that no relapse will occur 80% of the time. This cytokine cluster should be evaluated in other pediatric RRMS populations as well as adults in a prospective manner allowing the assessment of the predictive power for elevations of these cytokines during remission and investigating more carefully the time horizon for the predictor. Since IL-10 and IL-27 are considered anti-inflammatory and IL-21 and IL-23 are pro-inflammatory, ratios of these cytokines were evaluated. Specifically, increased ratios of IL-10:IL-21, IL-10:IL-23, and IL-10:IL-27 were significant, suggesting levels of IL-10 may be a driving force in predicting a relapse. Even though it is generally assumed that effector T cells mediate immunopathology in contrast to regulatory cells which limit effector mechanisms, there are blurred boundaries in how these respective cytokines may play a role. For instance, IL-10 is secreted by the effector T cell groups Th1, Th2, and Th17 as well as by T reg cells. Therefore, increases in IL-10 during remission may be a consequence of an increased T regulatory response, whereas elevated levels of IL-10 during relapse may indicate T cell effector populations. Interestingly, experimental models and in vitro studies have shown that IL-27 upregulates IL-10 expression by T cells (37, 38), and the fact that both of these cytokines are co-upregulated with respect to disease course and duration suggest that this relationship may extend to the pediatric RRMS population. Taken together, these data support that ratios of the four cytokine cluster may be a better biomarker to define the disease state taking into account the interrelated expression of this four cytokine cluster in both disease propagation and resolution.
The T cell is a known critical mediator in MS providing a strong rationale to evaluate T cell associated cytokine signaling as a biomarker for relapse as well as an indicator of disease progression for personalized medicine approaches to clinical care. While it is not clear if signs of inflammation in the serum can be an early predictor of an ensuing disease process in the brain providing an important therapeutic time interval, based on the critical role of T cells and the evidence provided here that their cytokine profile in remission is distinct from relapse, it seems a likely endeavor. To advance such a concept, it would be beneficial to evaluate T cell cytokine profiles described here with lesion development assessed by MRI imaging in a larger population. It should also be noted that our study investigated cytokines from whole sera and not from distinct cellular sources. Although CD4+ T cells are essential for MS pathogenesis, can express numerous cytokine receptors, and are capable of secreting copious amounts of cytokines upon stimulation, other immune cell populations play a role in dictating the cytokine milieu in MS. Future studies to ascertain the relevant cellular responders to and sources of the cytokines measured here may further enhance the prognostic value of serum cytokine analysis in MS and other neuro-inflammatory disorders such as transverse myelitis and neuromyelitis optica.
Highlights.
A linear regression analysis of serum cytokine levels in the pediatric remission population showed 12 cytokines to be statistically elevated as a function of disease duration, with no effect observed in the relapse population
A multivariable stepwise discriminate analysis revealed a four cytokine cluster (IL-10, IL-21, IL- 23, and IL-27) to have important predicative value in determining a relapse
Since IL-10 and IL-27 are considered anti-inflammatory and IL-21 and IL-23 are pro- inflammatory, ratios of these cytokines were evaluated using a Duncan’s multiple range test. Of the six possible combinations, increased ratios of IL-10:IL-21, IL-10:IL-23, and IL-10:IL-27 were significant suggesting levels of IL-10 to be a driving force in predicting a relapse
Acknowledgments
This work was funded by NINDS P30-NS069324, The National Multiple Sclerosis Society RG 4587-A-1, The Civitan International Research Foundation, The Mike L. Jezdimir Transverse Myelitis Foundation, The University of Alabama Health Services Foundation - General Endowment Fund, Child Neurology Foundation Swaiman Scholarship CY2013, The National Science Foundation 1355183, and T32 AI007051 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Renoux C, Vukusic S, Confavreux C. The natural history of multiple sclerosis with childhood onset. Clinical neurology and neurosurgery. 2008;110:897–904. doi: 10.1016/j.clineuro.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Hemmer B, Archelos JJ, Hartung HP. New concepts in the immunopathogenesis of multiple sclerosis. Nat Rev Neurosci. 2002;3:291–301. doi: 10.1038/nrn784. [DOI] [PubMed] [Google Scholar]
- 3.Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith B, Carson S, Fu R, McDonagh M, Dana T, Chan BKS, Thakurta S, Gibler A. Drug Class Review: Disease-modifying Drugs for Multiple Sclerosis: Final Update 1 Report. Portland (OR): 2010. [PubMed] [Google Scholar]
- 5.Morgan CJ, Ranjan A, Aban IB, Cutter GR. The magnetic resonance imaging 'rule of five': predicting the occurrence of relapse. Mult Scler. 2013;19:1760–1764. doi: 10.1177/1352458513485147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neuteboom RF, Ketelslegers IA, Boon M, Catsman-Berrevoets CE, Hintzen RQ S Dutch Study Group on Childhood Multiple, and E. Acute Disseminated. Barkhof magnetic resonance imaging criteria predict early relapse in pediatric multiple sclerosis. Pediatric neurology. 2010;42:53–55. doi: 10.1016/j.pediatrneurol.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Filippi M, Agosta F. Imaging biomarkers in multiple sclerosis. Journal of magnetic resonance imaging : JMRI. 2010;31:770–788. doi: 10.1002/jmri.22102. [DOI] [PubMed] [Google Scholar]
- 8.Sormani MP, Li DK, Bruzzi P, Stubinski B, Cornelisse P, Rocak S, De Stefano N. Combined MRI lesions and relapses as a surrogate for disability in multiple sclerosis. Neurology. 2011;77:1684–1690. doi: 10.1212/WNL.0b013e31823648b9. [DOI] [PubMed] [Google Scholar]
- 9.Pierson E, Simmons SB, Castelli L, Goverman JM. Mechanisms regulating regional localization of inflammation during CNS autoimmunity. Immunological reviews. 2012;248:205–215. doi: 10.1111/j.1600-065X.2012.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martins TB, Rose JW, Jaskowski TD, Wilson AR, Husebye D, Seraj HS, Hill HR. Analysis of proinflammatory and anti-inflammatory cytokine serum concentrations in patients with multiple sclerosis by using a multiplexed immunoassay. American journal of clinical pathology. 2011;136:696–704. doi: 10.1309/AJCP7UBK8IBVMVNR. [DOI] [PubMed] [Google Scholar]
- 11.Farhadi N, Oryan S, Nabiuni M. Serum levels of melatonin and cytokines in multiple sclerosis. Biomedical journal. 2014;37:90–92. doi: 10.4103/2319-4170.125885. [DOI] [PubMed] [Google Scholar]
- 12.Kallaur AP, Oliveira SR, Colado Simao AN, Delicato de Almeida ER, Kaminami Morimoto H, Lopes J, de Carvalho Jennings Pereira WL, Marques Andrade R, Muliterno Pelegrino L, Donizete Borelli S, Kaimen-Maciel DR, Reiche EM. Cytokine profile in relapsingremitting multiple sclerosis patients and the association between progression and activity of the disease. Molecular medicine reports. 2013;7:1010–1020. doi: 10.3892/mmr.2013.1256. [DOI] [PubMed] [Google Scholar]
- 13.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 14.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer MT, Weaver CT. Autoimmunity: increasing suspects in the CD4+ T cell lineup. Nature immunology. 2010;11:36–40. doi: 10.1038/ni.1802. [DOI] [PubMed] [Google Scholar]
- 16.Krupp LB, Tardieu M, Amato MP, Banwell B, Chitnis T, Dale RC, Ghezzi A, Hintzen R, Kornberg A, Pohl D, Rostasy K, Tenembaum S, Wassmer E G. International Pediatric Multiple Sclerosis Study. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19:1261–1267. doi: 10.1177/1352458513484547. [DOI] [PubMed] [Google Scholar]
- 17.Krupp LB, Banwell B, Tenembaum S MSSG. International Pediatric. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68:S7–12. doi: 10.1212/01.wnl.0000259422.44235.a8. [DOI] [PubMed] [Google Scholar]
- 18.Singh N, Husain S, Limaye AP, Pursell K, Klintmalm GB, Pruett TL, Somani J, Stosor V, del Busto R, Wagener MM, Steele C. Systemic and cerebrospinal fluid T-helper cytokine responses in organ transplant recipients with Cryptococcus neoformans infection. Transplant immunology. 2006;16:69–72. doi: 10.1016/j.trim.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Myers RC, Dunaway CW, Nelson MP, Trevor JL, Morris A, Steele C. STAT4-dependent and -independent Th2 responses correlate with protective immunity against lung infection with Pneumocystis murina. J Immunol. 2013;190:6287–6294. doi: 10.4049/jimmunol.1300431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris A, Hillenbrand M, Finkelman M, George MP, Singh V, Kessinger C, Lucht L, Busch M, McMahon D, Weinman R, Steele C, Norris KA, Gingo MR. Serum (1-->3)-beta-D-glucan levels in HIV-infected individuals are associated with immunosuppression, inflammation, and cardiopulmonary function. J Acquir Immune Defic Syndr. 2012;61:462–468. doi: 10.1097/QAI.0b013e318271799b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun HY, Singh N, Cacciarelli TV, Wannstedt C, Wagener MM, Steele C. Dysregulated expression of T-helper cell responses and susceptibility to infections in high-risk liver transplant recipients. Transplant immunology. 2008;20:68–72. doi: 10.1016/j.trim.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nature immunology. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 23.Arima Y, Harada M, Kamimura D, Park JH, Kawano F, Yull FE, Kawamoto T, Iwakura Y, Betz UA, Marquez G, Blackwell TS, Ohira Y, Hirano T, Murakami M. Regional neural activation defines a gateway for autoreactive T cells to cross the blood-brain barrier. Cell. 2012;148:447–457. doi: 10.1016/j.cell.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, Martin-Orozco N, Kang HS, Ma L, Panopoulos AD, Craig S, Watowich SS, Jetten AM, Tian Q, Dong C. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181:8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villares R, Cadenas V, Lozano M, Almonacid L, Zaballos A, Martinez AC, Varona R. CCR6 regulates EAE pathogenesis by controlling regulatory CD4+ T-cell recruitment to target tissues. Eur J Immunol. 2009;39:1671–1681. doi: 10.1002/eji.200839123. [DOI] [PubMed] [Google Scholar]
- 26.Pandiyan P, Yang XP, Saravanamuthu SS, Zheng L, Ishihara S, O'Shea JJ, Lenardo MJ. The role of IL-15 in activating STAT5 and fine-tuning IL-17A production in CD4 T lymphocytes. J Immunol. 2012;189:4237–4246. doi: 10.4049/jimmunol.1201476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nature immunology. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 28.Siebler J, Wirtz S, Weigmann B, Atreya I, Schmitt E, Kreft A, Galle PR, Neurath MF. IL-28A is a key regulator of T-cell-mediated liver injury via the T-box transcription factor T-bet. Gastroenterology. 2007;132:358–371. doi: 10.1053/j.gastro.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 29.Castellani ML, Felaco P, Galzio RJ, Tripodi D, Toniato E, De Lutiis MA, Fulcheri M, Caraffa A, Antinolfi P, Tete S, Felaco M, Conti F, Pandolfi F, Theoharides TC, Shaik-Dasthagirisaheb YB. IL-31 a Th2 cytokine involved in immunity and inflammation. International journal of immunopathology and pharmacology. 2010;23:709–713. doi: 10.1177/039463201002300304. [DOI] [PubMed] [Google Scholar]
- 30.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 31.Ishizu T, Osoegawa M, Mei FJ, Kikuchi H, Tanaka M, Takakura Y, Minohara M, Murai H, Mihara F, Taniwaki T, Kira J. Intrathecal activation of the IL-17/IL-8 axis in opticospinal multiple sclerosis. Brain. 2005;128:988–1002. doi: 10.1093/brain/awh453. [DOI] [PubMed] [Google Scholar]
- 32.Brucklacher-Waldert V, Stuerner K, Kolster M, Wolthausen J, Tolosa E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain. 2009;132:3329–3341. doi: 10.1093/brain/awp289. [DOI] [PubMed] [Google Scholar]
- 33.Li M, Liu X, Zhou Y, Su SB. Interferon-lambdas: the modulators of antivirus, antitumor, and immune responses. Journal of leukocyte biology. 2009;86:23–32. doi: 10.1189/jlb.1208761. [DOI] [PubMed] [Google Scholar]
- 34.Lu Y, Hong S, Li H, Park J, Hong B, Wang L, Zheng Y, Liu Z, Xu J, He J, Yang J, Qian J, Yi Q. Th9 cells promote antitumor immune responses in vivo. The Journal of clinical investigation. 2012;122:4160–4171. doi: 10.1172/JCI65459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Nourbakhsh B, Cullimore M, Zhang GX, Rostami A. IL-9 is important for T-cell activation and differentiation in autoimmune inflammation of the central nervous system. Eur J Immunol. 2011;41:2197–2206. doi: 10.1002/eji.201041125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nowak EC, Weaver CT, Turner H, Begum-Haque S, Becher B, Schreiner B, Coyle AJ, Kasper LH, Noelle RJ. IL-9 as a mediator of Th17-driven inflammatory disease. The Journal of experimental medicine. 2009;206:1653–1660. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinemann C, Heink S, Petermann F, Vasanthakumar A, Rothhammer V, Doorduijn E, Mitsdoerffer M, Sie C, Prazeres da Costa O, Buch T, Hemmer B, Oukka M, Kallies A, Korn T. IL-27 and IL-12 oppose pro-inflammatory IL-23 in CD4+ T cells by inducing Blimp1. Nature communications. 2014;5:3770. doi: 10.1038/ncomms4770. [DOI] [PubMed] [Google Scholar]
- 38.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nature immunology. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]