Abstract
Objective
There is growing evidence that fracture risk is increased in individuals with HIV and/or hepatitis C virus (HCV) infection. We systematically reviewed the literature to determine whether prevalence of osteoporosis and incidence of fracture is increased in HIV/HCV-coinfected individuals.
Design
A systematic review and meta-analysis.
Methods
A search was performed of Medline, Scopus and the Cochrane Library databases, as well as of abstracts from annual retroviral, liver and bone meetings (up to 2013) for studies with bone mineral density (BMD) or bone fracture data for HIV/HCV-coinfected individuals. Osteoporosis odds ratios (ORs) and fracture incidence rate ratios (IRRs) were estimated from studies with data on HIV-monoinfected or HIV/HCV-uninfected comparison groups.
Results
Of 15 included studies, nine reported BMD data and six reported fracture data. For HIV/HCV-coinfected, the estimated osteoporosis prevalence was 22% [95% confidence interval (95% CI) 12–31] and the crude OR for osteoporosis compared with HIV-monoinfected was 1.63 (95% CI 1.27–2.11). The pooled IRR of overall fracture risk for HIV/HCV-coinfected individuals was 1.77 (95% CI 1.44–2.18) compared with HIV-monoinfected and 2.95 (95% CI 2.17–4.01) compared with uninfected individuals. In addition to HIV/HCV-coinfection, older age, lower BMI, smoking, alcohol and substance use were significant predictors of osteoporosis and fractures across studies.
Conclusion
HIV/HCV coinfection is associated with a greater risk of osteoporosis and fracture than HIV monoinfection; fracture risk is even greater than uninfected controls. These data suggest that HIV/HCV-coinfected individuals should be targeted for fracture prevention through risk factor modification at all ages and DXA screening at age 50.
Keywords: bone, coinfection, fracture, hepatitis C virus, HIV, osteoporosis
Introduction
Hepatitis C virus (HCV) infection affects approximately 184 million individuals worldwide; in some countries, up to 75% are unaware of their status due to the latent nature of disease progression [1,2]. Complications of chronic HCV infection include osteoporosis, with prevalence ranging from 14 to 28% [3–5], and increased fracture rates [6–10]. HCV infection mediates systemic immune activation and can lead to chronic liver dysfunction, both of which have notable effects on bone health [10–13].
HIV infection and antiretroviral therapy (ART) are also associated with increased prevalence of low BMD [14,15] and increased incidence of fracture [16]. Coinfection with HCV, which occurs in approximately 20–30% of HIV-infected individuals in the United States and over 50% in certain parts of Europe [17–19], appears to have at least an additive negative effect on bone health and fracture risk, more so than monoinfection of either virus [20,21]. Fracture events dramatically affect quality of life, contributing to morbidity and mortality [22–24].
This systematic review summarizes available literature regarding the impact of HIV/HCV coinfection on osteoporosis and fracture risk. Our meta-analysis estimates the prevalence of osteoporosis and fracture incidence in HIV/HCV-coinfected individuals compared with those with HIV monoinfection and those uninfected by either virus. We also assess factors associated with osteoporosis and fracture and highlight some modifiable risk factors for clinical management of this patient population.
Materials and methods
Search strategy
Medline, Scopus and the Cochrane Library were systematically searched for studies on osteoporosis or fractures in HIV/HCV-coinfected individuals. Search terms ‘HIV’ and ‘Hepatitis C’ were used in combination with ‘osteoporosis’, ‘bone density’ and ‘fractures’. In addition, published abstracts, up to 2013, from the Conference on HIV Pathogenesis and Treatment of the International AIDS Society (IAS), the Conference on Retroviruses and Opportunistic Infections (CROI), the International AIDS Conference (AIDS), the scientific meeting of the American Society for Bone and Mineral Research (ASBMR), the scientific meeting of the American Association of Studies on Liver Disease (AASLD) and the International Conference on Viral Hepatitis (ICVH) were searched online and in printed programs when available. Search specifics are provided in Table 1. Reference lists and related citations of retrieved articles were examined further for pertinent studies.
Table 1.
Databases and search terms used for systematic review.
| Database or conference | Years | Search terms |
|---|---|---|
| Medline (OVID) | Up to August 2013 | ‘HIV’ (MeSH Terms) OR ‘HIV’ (All fields) AND ‘Hepatitis C’ (MeSH Terms) OR ‘Hepatitis C’ (All fields) AND ‘osteoporosis’ (MeSH Terms) OR ‘bone density’ (MeSH Terms) OR ‘fractures, bone’ (MeSH Terms) |
| Scopus | Up to August 2013 | (HIV OR AIDS OR human immunodef* OR acquired immun*) AND hepatitis C AND (fracture* or osteoporos* or osteopen* or osteopoen* or bone density or bone loss) |
| Cochrane Library | Up to August 2013 | ‘HIV’ OR ‘Hepatitis C’ AND ‘Bone’ |
| American Association for the Study of Liver Diseases (AASLD) | 2000–2013 | ‘HIV’ OR ‘fracture’ |
| American Society for bone and Mineral Research (ASBMR)a | 2000–2013 | ‘HIV’ OR ‘HCV’ OR ‘hepatitis’ |
| International AIDS Conference (AIDS) | 2000–2012 | ‘Bone’ OR ‘Fracture’ |
| Conference on Retroviruses and Opportunistic Infections (CROI) | 1997–2013 | ‘Bone’ OR ‘Fracture’ |
| Conference on HIV Pathogenesis and Treatment of the International AIDS Society (IAS) | 2001–2013 | ‘Bone’ OR ‘Fracture’ |
Abstracts from ASBMR 2013 were retrieved after October 2013.
Selection criteria and data extraction
Two reviewers (H.D., Y.C.) independently screened all studies by title and abstract. Studies eligible for inclusion were assessed using the full text. Inclusion criteria were English language article published through August 2013, included adult participants (aged ≥18 years) and reported data on bone mineral data (BMD) by dual-energy X-ray absorptiometry (DXA) or bone fracture for HIV/HCV-coinfected individuals. For this review, osteoporosis was defined as a BMD T-score of −2.5 or less or Z-score of −2.0 or less by DXA at either the spine or the hip [25]. Letters were eligible for inclusion, but editorials, commentaries, expert opinions, case reports and animal studies were excluded. Differences regarding a study’s eligibility were resolved by a third reviewer (M.T.Y.). The following information was extracted from included studies: author, year of publication, country, study design, sample size, sample characteristics (age, sex, race/ethnicity, BMI), ART exposure, outcome measures and findings. When available, the control group, and hepatic fibrosis or cirrhosis data were also collected. For BMD studies, DXA site was collected; for fracture studies, fracture classification method and fracture type were collected.
Outcome and data analysis
The primary outcomes for the meta-analysis were the prevalence of osteoporosis in HIV/HCV-coinfected individuals, the odds ratios (ORs) of osteoporosis in HIV/HCV-coinfection versus HIV-monoinfection and incidence rate ratios (IRRs) of all fractures in HIV/HCV-coinfected individuals compared with HIV-monoinfected or HIV/HCV-uninfected controls. Prevalence of osteoporosis was calculated by dividing the number of HIV/HCV-coinfected individuals with osteoporosis by the total HIV/HCV-coinfected study sample. If not reported, an OR was calculated by dividing the odds of osteoporosis in HIV/HCV-coinfected by the odds in HIV-monoinfected individuals. Fracture incidence rate was calculated by dividing the number of incident fractures by the period of risk and expressed as number of fractures per 1000 person-years of follow-up. Pooled IRR estimates of fractures were calculated, comparing HIV/HCV-coinfected individuals with HIV-monoinfected as well as HIV-uninfected controls. A random effects model was implemented to account for heterogeneity between studies. The I2 coefficient was used to assess statistical heterogeneity. Analyses were conducted using the Meta for package 1.9 (Wolfgang Viechtbauer) for R Software 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Selection of studies
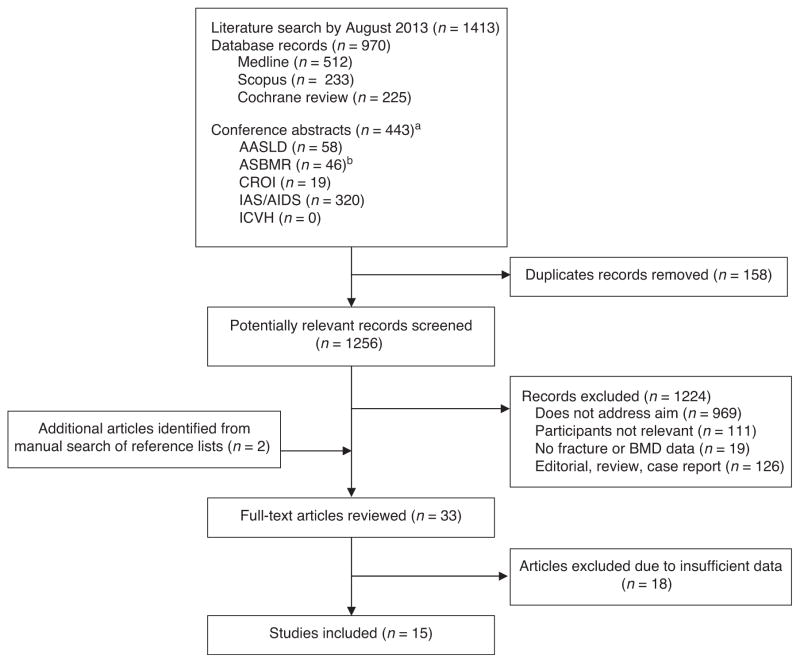
Figure 1 diagrams the literature search, in which 1256 potentially relevant records were initially identified for review. Two additional articles were identified from reference lists. After exclusion of duplicate records and studies not meeting the inclusion criteria, 33 articles remained for full-text review. Of these, 18 were excluded due to insufficient data to determine odds of osteoporosis, osteoporosis prevalence or fracture incidence among HIV/HCV-coinfected individuals. One study [26] meeting selection criteria was excluded in final analysis because a larger, more recent study [27] included much of the same population and more directly addressed our study aim. Authors of seven BMD studies [20,28–33] were contacted for additional data specific to HIV/HCV-coinfected individuals; six authors responded. Four BMD studies presented findings for viral hepatitis coinfection without differentiating between HCV and hepatitis B virus (HBV) infection; authors from three of the studies provided us with data limited to HCV coinfection [20,29,32]. One study did not provide osteoporosis data excluding HBV infection [30], but was selected for inclusion, as the majority (77%) of coinfected individuals was HIV/HCV-coinfected. Authors of two fracture studies were contacted for additional data and both responded to requests [34,35].
Fig. 1. Flow diagram of literature search and study selection.
aConferences searched included American Association for the Study of Liver Diseases (AASLD; 2000–2013), the scientific meeting of the American Society for Bone and Mineral Research (ASBMR, 2000–2013), Conference on Retroviruses and Opportunistic Infections (CROI, 1997–2013), the Conference on HIV Pathogenesis and Treatment of the International AIDS Society (IAS; 2001–2013), the International AIDS Conference (AIDS; 2002–2012) and International Conference on Viral Hepatitis (ICVH; 2011–2013).
Study characteristics
Tables 2 and 3 summarize the characteristics of the 15 studies included in this meta-analysis, nine for osteoporosis and six for fracture. Nine studies were from the United States [6,21,28,30–34,36–38], two from France [35,39] and one each from Italy [20], Denmark [40], Spain [29] and Taiwan [32]. Four studies included only women [28,31,33,34], and one included only men [38]. Of studies that included both sexes, men comprised over 60% of the sample population in most studies. The sample size of HIV/HCV-coinfected individuals varied across studies, from 22 postmenopausal women [33], to 36 950 individuals in the US Medicaid population [6]. Three studies reported data on liver fibrosis or cirrhosis [29,30,37], and one study presented data on hepatic decompensation [6].
Table 2.
Characteristics of osteoporosis studies meeting inclusion criteria (n = 9).
| Author | Country | N (Total) |
N (HIV- positive/ HCV- positive) |
Control group (N, Type) |
Sex (% men) |
Age (years) |
Race/ ethnicity (%) |
BMI (kg/m2) |
ART exposure in HIV-positive (%) |
Cirrhosis/ fibrosis (%) |
DXA site |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anastos et al. [28] | USA | 426 | 111 | 162, HIV-positive/HCV-negative | 0 | 41 (mean) | 63 B; 21 W; 16 H | 29 (mean) | 46 (current) | – | Lumbar spine and femoral neck |
| Bonjoch et al. [29]a | Spain | 671 | 208 | 373, HIV-positive/HCV-negative | 72 | 42 (mean) | – | 23 (mean) | 53 (current) | 41 (fibrosis) | Lumbar spine and femoral neck |
| El-Maouche et al. [36] | USA | 179 | 179 | None | 65 | 50 (median) | 85 B | 25 (median) | 89 (ever) | – | Total hip, lumbar spine and femoral neck |
| Lawson-Ayayi et al. [30]a | France | 626 | 208 | 357, HIV-positive/HCV-negative | 74 | 43 (median) | – | 24% (BMI ≤20 kg/m2) | – | 23 (cirrhosis) | Lumbar spine and femoral neck |
| Lo Re III et al. [20]a | Italy | 1237 | 572 | 612, HIV-positive/HCV-negative | 62 | 43 (median) | – | 6% (BMI <18.5 kg/m2); 6% (BMI ≥30 kg/m2) | 79 (current) | – | Lumbar spine and femoral neck |
| Sharma et al. [38] | USA | 389 | 151 | 79, HIV+/HCV− | 100 | 56 (mean) | 58 B; 14 W; 22 H; 6 O | 26 (mean) | 91 (ever) | – | Lumbar spine, femoral neck, and total hip |
| Sharma et al. [31] | USA | 464 | 87 | 158, HIV-positive/HCV-negative | 0 | 48 (mean) | 49 B; 10 W; 26 H; 23 O | 30 (mean) | 88 (ever) | – | Lumbar spine, femoral neck, and total hip |
| Tsai et al. [32]a | Taiwan | 320 | 80 | None | 91 | 37 (median) | – | 10% (BMI <18.5) | 94 (current) | – | Lumbar spine |
| Yin et al. [33] | USA | 187 | 22 | 70, HIV-positive/HCV-negative | 0 | 58 (mean) | 34 AA; 73 H | 29 (mean) | 79 (current) | – | Lumbar spine, femoral neck, total hip, radius |
Table 3.
Characteristics of studies reporting on bone fracture meeting inclusion criteria (n = 6).
| Author | Country | N (Total) |
N (HIV- positive/ HCV- positive) ‘coinfected’ |
N (HIV- positive /HCV- negative) ‘mono- infected’ |
N (HIV- negative /HCV- negative) ‘uninfected’ |
Sex (% men) |
Age (years) |
Race/ ethnicity (%) |
BMI (kg/m2) |
ART exposure in HIV- positive (%) |
Cirrhosis/ fibrosis (%) |
Fracture classification method |
Fracture type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Collin et al.e [35] | France | 1166 | 291 | 875 | 0 | 77 | 36 (median) | 83a | 22.0 (median) | – | – | Self-report | All grade 3 or 4 fractures |
| Hansen et al. [40] | Denmark | 31 836 | 851 | 4455 | 26 530 | 76 | 37, 31–45 (median, IQR) | 80 W | – | 78 (during study) | – | ICD Codes | All (high energy and low energy) |
| Lo Re III et al. [6] | USA | 3 520 582 | 36 950 | 95 827 | 366 829b | 71 (+/+);64 (+/−); 71 (−/−) | 42 (+/+);39 (+/−); 42 (−/−); (median) | (+/+);28 W, 40 B, 8 H, 25 O | – | – | – | ICD codes | Hip fracture |
| Maalouf et al. [27] | USA | 56 660 | 17 762 | 38 898 | 0 | 98 | 45 (+/+);44 (+/−) (median) | 35 W (+/+);49 W (+/−) | 15% (+/+);14% (+/−) (BMI<20) | 64 (current) | APRI fibrosisc; 37 (+/+);42 (+/−); APRI cirrhosisc; 52 (+/+);25 (+/−) | ICD codes | Fragility (vertebra, hip, wrist); ‘Osteoporotic’ |
| Yin et al.e [34] | USA | 2391 | 438 | 1290 | 663 | 0 | 40 (HIV-positive); 36 (HIV-negative) (mean) | – | 29 (HIV-positive); 30 (HIV-negative) (mean) | 66 (at index visit) | – | Self-report | All, fragility (fall from standing height or less) |
| Young et al.d [21] | USA | 224 490 054 | 819 | 4235 | 224 485 000 | 78 | 40, 36–46 (median, IQR) | 52 W; 33 B; 12 H; 4 O | 25 (median) | 73 (ever) | – | ICD codes (HIV−); Self/clinical report (HIV-positive) | All, fragility (wrist, vertebra, femoral neck of hip) |
A, Asian; B, black; EFV, efavirenz; H, Hispanic/Latino; IQR, interquartile range; NA, not available; O, Other; TDF, tenofovir; U, unknown; W, white. +/+, coinfected; +/−, HIV-monoinfected; −/−, uninfected. All numerical values were rounded to nearest whole number.
Unspecified; 83.1% born in France or England.
Only HIV-negative/HCV-negative control group matched to HIV-positive/HCV-positive controls.
APRI (Aspartate aminotransferase-to-platelet ratio index) score, 0.5–1.49: Fibrosis; 1.5 ≤ Cirrhosis.
Control group data from 2006, all other characteristics are from participants during 2000–2008 follow-up period.
Some unpublished data obtained from communications with author.
All studies measured BMD at a minimum of two sites by DXA and defined osteoporosis as a T-score of −2.5 or less or Z-score of −2.0 or less at the spine or hip [25,41]. A total of nine BMD studies were included for the estimate of osteoporosis prevalence of HIV/HCV-coinfected individuals, but only seven were included in the OR for osteoporosis analysis [20,28–31,33,38], as two studies did not include an HIV-monoinfected control group [32,36].
Of the six fracture studies, two did not include a HIV/HCV-uninfected control group [27,35] and two had HIV-uninfected control groups with unknown HCV status [21,40]. For fracture classification, four studies used International Classification of Diseases (ICD) coding [6,21,27,40], and three used self or clinical reports [21,34,35]; one used ICD codes for their HIV-uninfected group and patient reports for their HIV-infected group [21]. Fracture types varied: three studies included all fractures [21,34,40], one included only fragility fractures (defined as fracture of fall from standing height or less, or of the vertebra, hip or wrist) [27], one included only hip fractures [6] and one included all grade 3 or 4 fractures defined as fractures that led to severe limitation of activity or hospitalization [35].
Osteoporosis prevalence
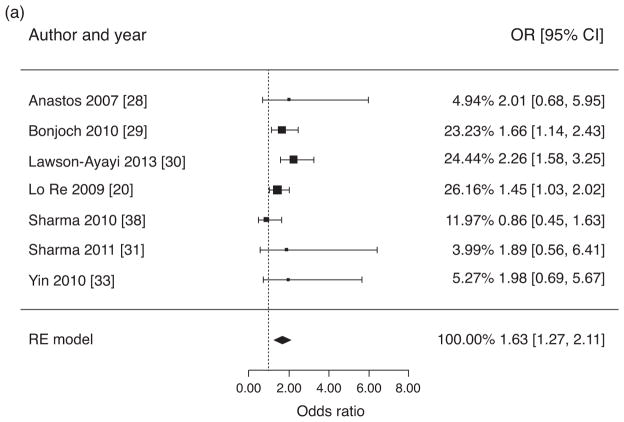
Prevalence estimates of osteoporosis in HIV/HCV-coinfected individuals ranged from 5.4 [31] to 45% [30]. The pooled prevalence estimate was 22% [95% confidence interval (95% CI) 12–31]. Figure 2a presents estimates of the OR of osteoporosis in HIV/HCV-coinfected individuals versus HIV-monoinfected controls. The pooled estimate of the crude OR for osteoporosis was 1.63 (95% CI 1.27–2.11) and the assessment for heterogeneity was not significant (Q = 7.83, P = 0.25, I2 = 32.9%). After excluding the study with postmenopausal women [33], estimated prevalence of osteoporosis in HIV/HCV-coinfected individuals remained similar at 20% (95% CI 11–30) with a crude OR for osteoporosis of 1.61 (95% CI 1.23–2.12).
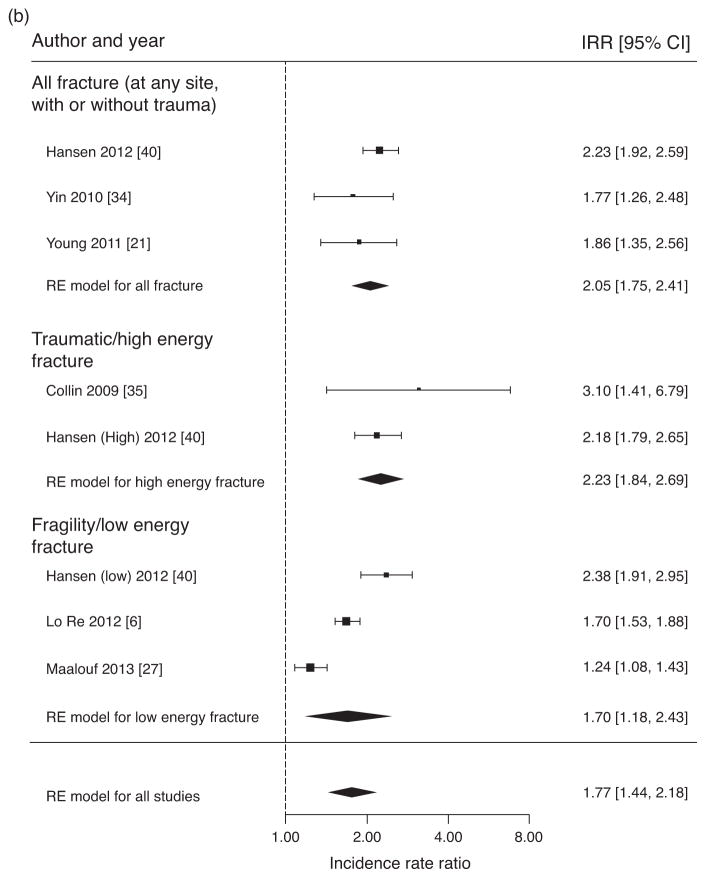
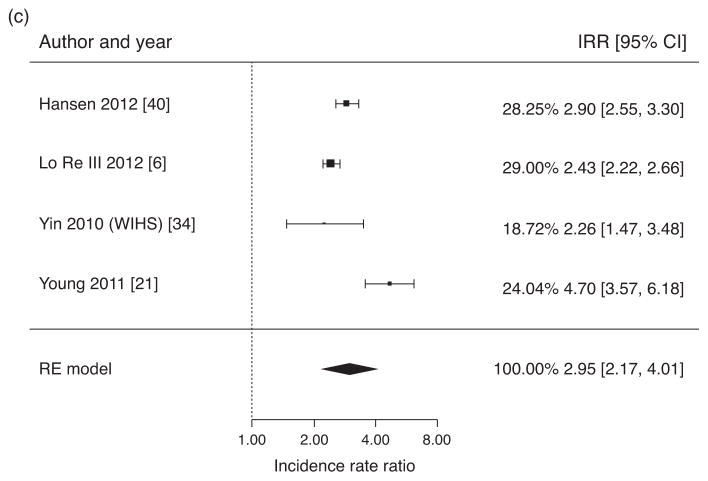
Fig. 2. Pooled ratios of osteoporosis prevalence and fracture incidence.
(a) Meta-analysis of crude odds ratios for osteoporosis (BMD T-score ≤−2.5 or Z-score ≤−2.0 at spine and/or hip) in HIV/HCV-coinfected individuals versus HIV-monoinfected individuals. (b) Meta-analysis of crude incidence rate ratios for fractures in HIV/HCV coinfected individuals versus HIV-monoinfected individuals. (c) Meta-analysis of crude incidence rate ratios for fractures in HIV/HCV-coinfected individuals versus HIV-uninfected individuals.
Fracture incidence
Figure 2b presents estimates of fracture IRR in HIV/HCV-coinfected versus HIV-monoinfected individuals, including an overall pooled estimate for all fracture studies (inclusive of all fracture definitions), and separate estimates for studies that included all fractures (at any site, with or without trauma), only traumatic/high-energy fractures and only fragility/low-energy fractures as the main outcome. The overall pooled estimate of the fracture IRR for HIV/HCV-coinfected versus HIV-monoinfected individuals was 1.77 (95% CI 1.44–2.18). The overall assessment for heterogeneity was significant (Q = 34.2, P <0.0001, I2 = 84.0%). Pooled IRRs and heterogeneity analyses by fracture categories were also assessed. The IRR for studies with all fractures as outcome was 2.05 (95% CI 1.75–2.41), and the assessment for heterogeneity was not significant (Q = 2.19, P = 0.335, I2 = 22.5%). For traumatic/high-energy fractures, the IRR was 2.23 (95% CI 1.84–2.69), and assessment for heterogeneity was not significant (Q = 0.723, P = 0.395, I2 = 0.0%). In contrast, the IRR for fragility/low-energy fractures was 1.70 (95% CI 1.18–2.43), with a significant assessment for heterogeneity (Q = 26.3, P <0.0001, I2 = 94.4%).
Figure 2c presents estimates of fracture IRR in HIV/HCV-coinfected individuals versus HIV/HCV-uninfected individuals, using eligible fracture studies and inclusive of all fracture definitions. The overall pooled estimate of fracture IRR for HIV/HCV-coinfected individuals versus HIV/HCV-uninfected individuals was 2.95 (95% CI 2.17–4.01). The assessment for heterogeneity was significant (Q = 22.6, P <0.0001, I2 = 91.8%).
Predictors of osteoporosis and fracture
Table 4 lists ORs and independent predictors of osteoporosis in multivariate models among individuals with HIV/HCV-coinfection. Several traditional risk factors were reported as significant predictors of osteoporosis in univariate analyses: older age [20,29–31,33], lower BMI [28–31,33,36], menopause [28,30,31,33], smoking [20,28,36,38] and alcohol or substance abuse [31,33,36,38]. ART exposure, durations of ART and a protease inhibitor-containing regimen were also associated with lower BMD [20,28–30,33,36,42]. In multivariate analyses of HIV/HCV-coinfected individuals, older age [20,30,31,33], lower BMI [20,28,30,31,33], postmenopausal status [28,31] and time on protease inhibitor [29,31] were associated with osteoporosis. Three studies [20,31,38] found that behavioural factors such as smoking, low physical activity and methadone use were significant predictors of osteoporosis. Results on sex differences were mixed. In two studies consisting of a sample of men and women, viral hepatitis (HBVor HCV) was an independent predictor of osteoporosis only in women in multivariate analyses [20,30]. However, in a study that included only men, HCV infection was an independent predictor of osteoporosis [38].
Table 4.
Odds of osteoporosis in HIV/hepatitis C virus coinfection versus HIV monoinfection and predictors of osteoporosis in multivariate models.
| Author | Osteoporosis OR (95% CI) | Predictors of osteoporosis in multivariate model |
|---|---|---|
| Anastos et al. [28] | 2.01 (0.68–5.95) | Lower BMI, postmenopausal status |
| Bonjoch et al. [29] | 1.66 (1.14–2.43) | NAa |
| Lawson-Ayayi et al.b [39] | 2.26 (1.58–3.24) | Viral hepatitis (HCV or HBV)c older age, MSM, low BMI <20 kg/m2 |
| Lo Re III et al.d [20] | 1.81 (1.19–2.76) | Viral hepatitis (HCV or HBV)c older age, female, lower BMI, time since HIV diagnosis, ART use, physical activity, smoking |
| Sharma et al. [38] | 0.86 (0.45–1.62) | HCV-infection, AIDS diagnosis and heroin use in past 5 years, current methadone use, baseline BMD (kg/m2) |
| Sharma et al. [31] | 1.89 (0.56–6.41) | HCV-infection, older age, lower BMI, smoking history, postmenopausal, methadone use, PI use ≥3 years |
| Yin et al. [33] | 1.98 (0.69–5.67) | Older age, lower BMI, HIV status |
ART, antiretroviral therapy; NA, not applicable; PI, protease inhibitor.
Did not conduct a multivariate analysis.
Study included HIV/viral hepatitis (HBV or HCV) coinfected individuals; 77% were HIV/HCV-coinfected.
Predictor only in coinfected women.
Study included HIV/viral hepatitis (HBV or HCV) coinfected individuals; additional data specific to HIV/HCV-coinfected individuals provided by authors to calculate the OR reported here.
Table 5 lists crude fracture incidence rates from the HIV/HCV-coinfected group of each study as well as predictors of fracture from studies that included HCV-infected individuals in their multivariate analysis. Across the six fracture studies, risk factors beyond HIV-infection for fracture in univariate analyses included HCV-infection [21,27,34,35], older age [6,21,27,34], smoking [21,27,34], white race [27,34], alcohol or substance abuse [21,35], diabetes [21,27] and low BMI [21,27]. Other risk factors that varied across studies included history of intravenous drug or opiate use [34], hormone replacement therapy or oral contraceptives [34], selective serotonin reuptake inhibitor use [21], elevated serum creatinine [34], estimated glomerular filtration rate (eGFR) less than 60 ml/min per 1.73 m2 [27], high DBP [34], menopause [34], history of prior fracture [34] and peripheral neuropathy [21]. Components of HIV disease predictive of fracture were baseline CD4+ T-cell count [35], baseline AIDS diagnosis [21], nadir CD4+ T cells count less than 200 cells/μl [21], overall ART use [6] and cumulative ART use [27]. Three studies found an association between fracture risk and severity of liver disease, measured by hepatic decompensation [6], cirrhosis [27] and high APRI score [27].
Table 5.
IRs of fractures in HIV/HCV coinfection and predictors of fracture.
| Author | Crude incidence rate per 1000 person-years in HIV/HCV-coinfection (95% CI) [fracture type] | Predictors of fractures from multivariate analysis [population of predictor analysis] |
|---|---|---|
| Hansen et al. [40] | 39.15 (34.54–44.39) [all fractures] | NAa |
| Yin et al. [34] | 26.78 (20.40–35.14)e [all fractures] | HCV-infection, older age, white race, high serum creatinine [HIV-infected and uninfected] |
| Young et al. [21] | 62.27 (47.33–81.94)b [all fractures] | HCV-infectionc, older agec, BMI <18.5 kg/m2c, substance abuse, nadir CD4+ cell count <200 cells/μl, diabetes [all HIV-infected] |
| Collin et al. [35] | 7.18 (4.08–12.64)e [high-grade fractures] | HCV-coinfection, excessive alcohol drinking [all HIV-infected] |
| Lo Re III et al. [6] | 3.75 (3.48–4.06) [only hip fractures] | Older age, hepatic decompensationd [all patients, HIV-infected and uninfected] |
| Maalouf et al. [27] | 2.57 (2.33–2.84) [fragility fractures] | HCV-coinfection, older age, white race, tobacco use, low BMI (<20 kg/m2), cirrhosis, APRI score [all HIV-infected] |
ADI, AIDS-defining illness; APRI, aspartate aminotransferase-to-platelet ratio index.
Predictors not reported because all HCV-infected individuals were excluded from final analysis.
Extrapolated from data on Table 4 of Young et al. [21], Risk Factors for Bone Fracture Among 5054 HOPS Patients followed during the contemporary. HAART Era, 2002–2008.
Remained significant when analysis was limited to only fragility fractures.
Hepatic decompensation was unique to HCV-monoinfected group analysis.
Calculated from unpublished data obtained from authors.
Fracture predictors that remained significant in multivariate analysis included HCV-coinfection [21,27,34,35], older age [6,21,27,34], white race [27,34], alcohol or substance abuse [21,35], low BMI [21,27], smoking [27], elevated serum creatinine [34] and diabetes [21]. Nadir CD4+ T-cell count less than 200 cells/μl remained a significant predictor of fracture after adjustment only in one study [21]. Severity of liver disease as defined by hepatic decompensation [6], cirrhosis [27] and high APRI score [27] also remained significantly associated with fracture. One study included HCV infection in two multivariate models, one with a calculated liver fibrosis score (APRI) and the other with ICD-9 cirrhosis; HCV infection remained a significant predictor of fracture after adjustments in both models [27].
Discussion
Our review found that HIV/HCV-coinfected individuals have modestly increased risk for osteoporosis and fractures compared with HIV-monoinfected controls, and substantially higher risk than uninfected controls. After reported adjustments for traditional risk factors such as older age, white race, alcohol or substance use, lower BMI, smoking history and/or severity of liver disease, viral hepatitis remained an independent predictor of osteoporosis mostly in women, and of fractures in both men and women. Although the test for heterogeneity was significant for the overall pooled IRR estimate of fractures, heterogeneity was no longer significant when comparing studies that used either all fractures or traumatic/high-energy fractures as the main outcome.
Our pooled estimate of osteoporosis prevalence of 22% in HIV/HCV-coinfected individuals is higher than those reported in studies of HIV or HCV-monoinfected individuals. Previous meta-analyses have reported an osteoporosis prevalence of 15% among HIV-monoinfected individuals [15]. Among HCV-monoinfected individuals, studies have estimated an osteoporosis prevalence of 5–13% [43–45]. Several studies in this analysis did not detect a significant OR of osteoporosis in HIV/HCV-coinfected versus HIV-monoinfected groups [28,31,33,38]. However, in multivariate analyses, HCV or viral hepatitis coinfection remained an independent predictorof osteoporosis in four studies [20,30,31,38]. One notable finding is that the association between HIV/HCV coinfection and osteoporosis may be stronger in women than in men. HCV infection was significantly associated with osteoporosis in multivariate analysis in a single-sex study including only men [38] and anther including only women [31]; however, two larger studies that included both coinfected men and women found that viral hepatitis infection was a predictor of osteoporosis only in women [20,30]. This sexual disparity is unlikely to be solely due the effect of postmenopausal bone loss, as exclusion of the postmenopausal women study [33] did not change the prevalence estimates or crude OR; other sex-specific physiological mechanisms should be examined in future studies.
HCV infection was a prominent predictor of fracture in both HIV-infected and uninfected groups in the majority of reviewed fracture studies. HCV infection remained significant after adjustments in all four studies that included it in their multivariate models [21,27,34,35]. Lo Re et al. [6] did not present a multivariate analysis, but performed analysis stratified by sex, demonstrating that in both men and women, HIV/HCV-coinfection had higher adjusted hazard ratios for fracture than HIV-monoinfected, HCV-monoinfected, and HIV/HCV-uninfected individuals of matching sex. Similarly, Hansen et al. [40] did not present a multivariate analysis, but found a higher fracture risk in HIV/HCV coinfection than in HIV monoinfection and population controls, with IRRs of 2.2 (95% CI 1.9–2.6) and 2.9 (95% CI 2.5–3.4), respectively.
The differences in fracture rates between HIV/HCV coinfection and HIV monoinfection may be due to behaviours resulting in an increased likelihood of traumatic events leading to fracture. Our findings are supportive of this hypothesis, as we observed a higher IRR for high-energy or ‘traumatic’ fractures among HIV/HCV-coinfected individuals than low-energy or ‘fragility’ fractures. Several of our reviewed studies also found that excessive alcohol consumption or alcohol-related diagnoses were associated with a 1.6 to 2.9-fold increase in incidence fractures in HIV-infected individuals [26,35]. Two other reviewed studies reported significant associations between HCV infection and alcohol or substance use, leading the former to exclude HCV-coinfection in final analysis of predictors [6,40]. Furthermore, Hansen et al. [40] observed that HIV/HCV-coinfected, but not HIV-monoinfected, individuals had an increased risk of high-energy fracture. In a recently published study of HCV-monoinfected individuals, fracture risk decreased after adjustments for alcohol and drug use [46], yet among our reviewed studies, HCV still remained a significant predictor of fracture even after adjustments for alcohol and drug use in HIV/HCV-coinfected individuals [21,35]. Lifestyle-related factors appear to have a substantial impact on the risk of fractures in HIV/HCV-coinfected individuals, but based upon available studies, it cannot be completely attributed to alcohol and substance use.
Chronic liver disease and hepatic synthetic dysfunction are also known to have negative effects on bone remodelling [10,47,48]. HIV/HCV-coinfected patients are three times as likely to progress to cirrhosis as their HIV-monoinfected counterparts [9], despite ART [49]. For those coinfected with HIV and HCV or HBV, osteoporosis was more frequently detected in individuals with cirrhosis than those without [39]. Hepatic decompensation also appears to be a major risk factor for fracture in HIV/HCV-coinfected individuals [6,37]. However, the impact of HCV infection on osteoporosis and fractures may not be entirely attributable to hepatic decompensation. Pelazas-Gonzalez et al. [50] found no BMD difference in a small group of well nourished, noncirrhotic HIV/HCV-coinfected patients compared with HIV-negative patients. Although Womack et al. [26] found that the inclusion of FIB-4 in multivariate analysis attenuated the association between HCV-coinfection and fracture, a much larger study found that HCV coinfection remained a significant predictor of fracture after adjustment for APRI score or ICD categorized cirrhosis [27].
Some HIV management guidelines have recommended DXA screening for all HIV-infected individuals over age 50 years [14]. Although guidelines for the general population from the National Osteoporosis Foundation (NOF) recognize the risks of HIV-infection on osteoporosis and fracture, they do not yet recognize HCV infection as a risk factor for osteoporosis [25]. Our data confirm that HIV/HCV-coinfected individuals are at a significantly higher risk of fracture than HIV-monoinfected and uninfected individuals, confirming that BMD screening at age 50 is warranted. As the overall OR of osteoporosis is greater among HIV/HCV-coinfected individuals, BMD by DXA screening is an appropriate screening modality for risk of fracture in this population. Furthermore, as both large studies of Lo Re et al. [6] and Lawson-Ayayi et al. [39] found greater relative risk of fracture and lower BMD among individuals of younger age, universal recommendations for fracture prevention should be provided at all ages. On the basis of the risk factors elicited from reviewed studies, cessation of smoking and substance use, avoidance of excessive alcohol and maintaining a healthy body weight should be recommended. Counselling on adequate calcium and vitamin D intake, exercise and fall prevention should also be performed as recommended by the NOF [25].
Our review highlights important areas for future research. Successful treatment of HCV not only prevents hepatic decompensation and cirrhosis, but may also benefit bone health. In, HCV-monoinfected individuals, peginterferon and ribavirin therapy has been shown to increase BMD in patients with sustained virologic response and decrease fracture incidence after successful treatment [44,51]. As interferon therapy itself has been associated with reduced BMD [15,52], newer interferon-free directly active agents may have a better effect on bone metabolism. Although fracture risk is clearly increased in HIV/HCV-coinfected individuals, it is not clear whether DXA screening before age 50 in coinfected individuals is a cost-effective prevention method and requires further study. Lastly, although HCV and HIV infections are both associated with increased levels of pro-inflammatory cytokines that potentially promote osteoclastogenesis [8,53] or inhibit osteoblast differentiation and collagen synthesis [53], few studies have examined the role of immune activation on bone remodelling with HIV/HCV coinfection.
Our analysis has several limitations. We were only able to include studies that reported osteoporosis data for HIV/HCV infection in our analysis. We performed a thorough search of databases and conferences related to HIV, viral infections, hepatology and bone health, but the potential for publication bias cannot be ruled out. Most importantly, we observed heterogeneity in our fracture estimates, which is predominantly due to differences in fracture definitions across studies. Although most studies utilized ICD9 coding, the definition of fragility, osteoporotic and low energy fractures categorizations differed between studies. Therefore, we presented pooled estimates for studies using similar categorizations; the pooled estimates for studies including all fractures or traumatic/high-energy fractures were nonsignificant. Finally, due to limited data across studies, we were unable to adjust for important factors such as age, race, sex, weight, antiretroviral exposure, CD4+ T-cell count medications, tobacco/substance use and various comorbidities in our meta-analysis.
Conclusion
This systematic review and meta-analysis suggests an increase in osteoporosis in HIV/HCV-coinfected individuals, with pooled osteoporosis OR of 1.63 compared to HIV-monoinfected individuals. HIV/HCV-coinfection is also associated with a pooled fracture IRR of 1.77 when compared with HIV monoinfection, with higher IRR values in traumatic fractures than in fragility fractures. The overall pooled IRR estimate of HIV/HCV coinfection was 2.95 when compared with uninfected control groups. Our results confirm the importance of risk modification and DXA screening at age 50 for prevention of osteoporosis and fractures in HIV/HCV-coinfected individuals.
Acknowledgments
This work was supported in part by National Institute of Health Grant R01 AI095089 (M.T.Y.) and Training in Interdisciplinary Research to Prevent Infections Grant T32 NR013454 (Y.I.C.).
H.D. and Y.C. conducted searches, organized data, and along with M.T.Y., contacted authors for additional data. S.S. performed statistical analysis. All authors contributed to data interpretation, writing, editing and approving manuscript.
We would like to thank the following individuals and study groups for responding to our requests for unpublished data: Kathryn Anastos, MD, and the Women’s Interagency HIV Study; Anna Bonjoch, MD, Unitat VIH, Fundació Lluita contra la SIDA; Vincent Lo Re, MD, MSCE; Tiffany Ting-Fang Shih, MD, and Mao-Song Tsai, MD; Anjali Sharma, MD; Professors Catherine Leport and François Raffi of the Aproco-Copilote – ANRSCO8 Study Group.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflicts of interest
M.T.Y. has served as a consultant for Gilead and Abbvie.
The other authors have no conflicts of interest.
References
- 1.Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51:729–733. doi: 10.1002/hep.23561. [DOI] [PubMed] [Google Scholar]
- 2.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 3.Loria I, Albanese C, Giusto M, Galtieri PA, Giannelli V, Lucidi C, et al. Bone disorders in patients with chronic liver disease awaiting liver transplantation. Transplant Proc. 2010;42:1191–1193. doi: 10.1016/j.transproceed.2010.03.096. [DOI] [PubMed] [Google Scholar]
- 4.Carey EJ, Balan V, Kremers WK, Hay JE. Osteopenia and osteoporosis in patients with end-stage liver disease caused by hepatitis C and alcoholic liver disease: not just a cholestatic problem. Liver Transplant. 2003;9:1166–1173. doi: 10.1053/jlts.2003.50242. [DOI] [PubMed] [Google Scholar]
- 5.Lin JC, Hsieh TY, Wu CC, Chen PJ, Chueh TH, Chang WK, et al. Association between chronic hepatitis C virus infection and bone mineral density. Calcif Tissue Int. 2012;91:423–429. doi: 10.1007/s00223-012-9653-y. [DOI] [PubMed] [Google Scholar]
- 6.Lo Re V, 3rd, Volk J, Newcomb CW, Yang YX, Freeman CP, Hennessy S, et al. Risk of hip fracture associated with hepatitis C virus infection and hepatitis C/human immunodeficiency virus coinfection. Hepatology. 2012;56:1688–1698. doi: 10.1002/hep.25866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallego-Rojo FJ, Gonzalez-Calvin JL, Muñoz-Torres M, Mundi JL, Fernandez-Perez R, Rodrigo-Moreno D. Bone mineral density, serum insulin-like growth factor I, and bone turnover markers in viral cirrhosis. Hepatology. 1998;28:695–699. doi: 10.1002/hep.510280315. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Calvin JL, Gallego-Rojo F, Fernandez-Perez R, Casado-Caballero F, Ruiz-Escolano E, Olivares EG. Osteoporosis, mineral metabolism, and serum soluble tumor necrosis factor receptor p55 in viral cirrhosis. J Clin Endocrinol Metab. 2004;89:4325–4330. doi: 10.1210/jc.2004-0077. [DOI] [PubMed] [Google Scholar]
- 9.Leslie WD, Bernstein CN, Leboff MS American Gastroenterological Association Clinical Practice. AGA technical review on osteoporosis in hepatic disorders. Gastroenterology. 2003;125:941–966. doi: 10.1016/s0016-5085(03)01062-x. [DOI] [PubMed] [Google Scholar]
- 10.Rouillard S, Lane NE. Hepatic osteodystrophy. Hepatology. 2001;33:301–307. doi: 10.1053/jhep.2001.20533. [DOI] [PubMed] [Google Scholar]
- 11.Carey EJ, Balan V, Kremers WK, Hay JE. Osteopenia and osteoporosis in patients with end-stage liver disease caused by hepatitis C and alcoholic liver disease: not just a cholestatic problem. Liver Transplant. 2003;9:1166–1173. doi: 10.1053/jlts.2003.50242. [DOI] [PubMed] [Google Scholar]
- 12.Sandler NG, et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141:1220–1230. 1230 e1–3. doi: 10.1053/j.gastro.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pacifici R. T cells: critical bone regulators in health and disease. Bone. 2010;47:461–471. doi: 10.1016/j.bone.2010.04.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McComsey GA, Tebas P, Shane E, Yin MT, Overton ET, Huang JS, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51:937–946. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 16.Shiau S, Broun EC, Arpadi SM, Yin MT. Incident fractures in HIV-infected individuals: a systematic review and meta-analysis. AIDS. 2013;27:1949–1957. doi: 10.1097/QAD.0b013e328361d241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rockstroh JK, Mocroft A, Soriano V, Tural C, Losso MH, Horban A, et al. Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiretroviral therapy. J Infect Dis. 2005;192:992–1002. doi: 10.1086/432762. [DOI] [PubMed] [Google Scholar]
- 18.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 19.Staples CT, Jr, Rimland D, Dudas D. Hepatitis C in the HIV (human immunodeficiency virus) Atlanta V.A. (Veterans Affairs Medical Center) Cohort Study (HAVACS): the effect of coinfection on survival. Clin Infect Dis. 1999;29:150–154. doi: 10.1086/520144. [DOI] [PubMed] [Google Scholar]
- 20.Lo Re V, 3rd, Guaraldi G, Leonard MB, Localio AR, Lin J, Orlando G, et al. Viral hepatitis is associated with reduced bone mineral density in HIV-infected women but not men. AIDS. 2009;23:2191–2198. doi: 10.1097/QAD.0b013e32832ec258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young B, Dao CN, Buchacz K, Baker R, Brooks JT Investigators HIVOS. Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000–2006. Clin Infect Dis. 2011;52:1061–1068. doi: 10.1093/cid/ciq242. [DOI] [PubMed] [Google Scholar]
- 22.Diamantopoulos AP, et al. Short- and long-term mortality in males and females with fragility hip fracture in Norway. A population-based study. Clin Interv Aging. 2013;8:817–823. doi: 10.2147/CIA.S45468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haentjens P, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152:380–390. doi: 10.1059/0003-4819-152-6-201003160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abrahamsen B, et al. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int. 2009;20:1633–1650. doi: 10.1007/s00198-009-0920-3. [DOI] [PubMed] [Google Scholar]
- 25.National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 2013. [Google Scholar]
- 26.Womack JA, Goulet JL, Gibert C, Brandt CA, Skanderson M, Gulanski B, et al. Physiologic frailty and fragility fracture in HIV-infected male veterans. Clin Infect Dis. 2013;56:1498–1504. doi: 10.1093/cid/cit056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maalouf N, Zhang S, Drechsler H, Brown G, Tebas P, Bedimo R. Hepatitis C co-infection and severity of liver disease as risk factors for osteoporotic fractures among HIV-infected patients. J Bone Miner Res. 2013;28:2577–2583. doi: 10.1002/jbmr.1988. [DOI] [PubMed] [Google Scholar]
- 28.Anastos K, Lu D, Shi O, Mulligan K, Tien PC, Freeman R, et al. The association of bone mineral density with HIV infection and antiretroviral treatment in women. Antiviral Ther. 2007;12:1049–1058. doi: 10.1177/135965350701200701. [DOI] [PubMed] [Google Scholar]
- 29.Bonjoch A, Figueras M, Estany C, Perez-Alvarez N, Rosales J, del Rio L, et al. High prevalence of and progression to low bone mineral density in HIV-infected patients: a longitudinal cohort study. AIDS. 2010;24:2827–2833. doi: 10.1097/QAD.0b013e328340a28d. [DOI] [PubMed] [Google Scholar]
- 30.Lawson-Ayayi S, Cazanave C, Kpozehouen A, Barthe N, Mehsen N, Hessamfar M, et al. Chronic viral hepatitis is associated with low bone mineral density in HIV-infected patients, ANRS CO 3 Aquitaine Cohort. J Acquir Immune Defic Syndr. 2013;62:430–435. doi: 10.1097/QAI.0b013e3182845d88. [DOI] [PubMed] [Google Scholar]
- 31.Sharma A, Cohen HW, Freeman R, Santoro N, Schoenbaum EE. Prospective evaluation of bone mineral density among middle-aged HIV-infected and uninfected women: association between methadone use and bone loss. Maturitas. 2011;70:295–301. doi: 10.1016/j.maturitas.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai MS, Hung CC, Liu WC, Chen KL, Chen MY, Hsieh SM, et al. Reduced bone mineral density among HIV-infected patients in Taiwan: prevalence and associated factors. J Microbiol Immunol Infect. 2014;47:109–115. doi: 10.1016/j.jmii.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 33.Yin M, McMahon DJ, Ferris DC, Zhang CA, Shu A, Staron R, et al. Low bone mass and high bone turnover in postmenopausal human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 2010;95:620–629. doi: 10.1210/jc.2009-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin MT, Shi Q, Hoover DR, Anastos K, Sharma A, Young M, et al. Fracture incidence in HIV-infected women: results from the Women’s Interagency HIV Study. AIDS. 2010;24:2679–2686. doi: 10.1097/QAD.0b013e32833f6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collin F, Duval X, Le Moing V, Piroth L, Al Kaied F, Massip P, et al. Ten-year incidence and risk factors of bone fractures in a cohort of treated HIV1-infected adults. AIDS. 2009;23:1021–1024. doi: 10.1097/QAD.0b013e3283292195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Maouche D, Mehta SH, Sutcliffe C, Higgins Y, Torbenson MS, Moore RD, et al. Controlled HIV viral replication, not liver disease severity associated with low bone mineral density in HIV/HCV co-infection. J Hepatol. 2011;55:770–776. doi: 10.1016/j.jhep.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maalouf NM, Zhang S, Drechsler H, Brown GR, Tebas P, Bedimo R. Hepatitis C co-infection and severity of liver disease as risk factors for osteoporotic fractures among HIV-infected patients. J Bone Miner Res. 2013;28:2577–2583. doi: 10.1002/jbmr.1988. [DOI] [PubMed] [Google Scholar]
- 38.Sharma A, Flom PL, Weedon J, Klein RS. Prospective study of bone mineral density changes in aging men with or at risk for HIV infection. AIDS. 2010;24:2337–2345. doi: 10.1097/QAD.0b013e32833d7da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawson-Ayayi S, Cazanave C, Kpozehouen A, Barthe N, Mehsen N, Hessamfar M, et al. Chronic viral hepatitis is associated with low bone mineral density in HIV-infected patients, ANRS CO 3 Aquitaine Cohort. J Acquir Immune Defic Syndr. 2013;62:430–435. doi: 10.1097/QAI.0b013e3182845d88. [DOI] [PubMed] [Google Scholar]
- 40.Hansen AB, Gerstoft J, Kronborg G, Larsen CS, Pedersen C, Pedersen G, et al. Incidence of low and high-energy fractures in persons with and without HIV infection: a Danish population-based cohort study. AIDS. 2012;26:285–293. doi: 10.1097/QAD.0b013e32834ed8a7. [DOI] [PubMed] [Google Scholar]
- 41.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 42.Fausto A, Bongiovanni M, Cicconi P, Menicagli L, Ligabo EV, Melzi S, et al. Potential predictive factors of osteoporosis in HIV-positive subjects. Bone. 2006;38:893–897. doi: 10.1016/j.bone.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Orsini LG, Pinheiro MM, Castro CH, Silva AE, Szejnfeld VL. Bone mineral density measurements, bone markers and serum vitamin D concentrations in men with chronic noncirrhotic untreated hepatitis C. PLoS One. 2013;8:e81652. doi: 10.1371/journal.pone.0081652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hofmann WP, Kronenberger B, Bojunga J, Stamm B, Herrmann E, Bucker A, et al. Prospective study of bone mineral density and metabolism in patients with chronic hepatitis C during pegylated interferon alpha and ribavirin therapy. J Viral Hepat. 2008;15:790–796. doi: 10.1111/j.1365-2893.2008.01038.x. [DOI] [PubMed] [Google Scholar]
- 45.Redondo-Cerezo E, Casado-Caballero F, Martin-Rodriguez JL, Hernandez-Quero J, Escobar-Jimenez F, Gonzalez-Calvin JL. Bone mineral density and bone turnover in noncirrhotic patients with chronic hepatitis C and sustained virological response to antiviral therapy with peginterferon-alfa and ribavirin. Osteoporos Int. 2014 doi: 10.1016/j.jhep.2014.03.007. pii: S0168-8278(14)00151-2. [Epub ahead of print] http://www.ncbi.nlm.nih.gov/pubmed/24650694. [DOI] [PubMed]
- 46.Eg Hansen AB, Haukali Omland L, Krarup H, Obel N. Fracture risk in hepatitis C virus infected persons: results from the DAN-VIR cohort study. J Hepatol. 2014 doi: 10.1016/j.jhep.2014.03.007. pii: S0168-8278(14)00151-2. [Epub ahead of print] http://www.ncbi.nlm.nih.gov/pubmed/24650694. [DOI] [PubMed]
- 47.Pignata S, Daniele B, Galati MG, Esposito G, Vallone P, Fiore F, et al. Oestradiol and testosterone blood levels in patients with viral cirrhosis and hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 1997;9:283–286. doi: 10.1097/00042737-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Hay JE. Bone disease in cholestatic liver disease. Gastroenterology. 1995;108:276–283. doi: 10.1016/0016-5085(95)90033-0. [DOI] [PubMed] [Google Scholar]
- 49.Schiefke I, Fach A, Wiedmann M, Aretin AV, Schenker E, Borte G, et al. Reduced bone mineral density and altered bone turnover markers in patients with noncirrhotic chronic hepatitis B or C infection. World J Gastroenterol. 2005;11:1843–1847. doi: 10.3748/wjg.v11.i12.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pelazas-Gonzalez R, Gonzalez-Reimers E, Aleman-Valls MR, Santolaria-Fernandez F, Lopez-Prieto J, Gonzalez-Diaz A, et al. Bone alterations in hepatitis C virus infected patients. Eur J Intern Med. 2013;24:92–96. doi: 10.1016/j.ejim.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 51.Arase Y, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Sezaki H, et al. Virus clearance reduces bone fracture in postmenopausal women with osteoporosis and chronic liver disease caused by hepatitis C virus. J Med Virol. 2010;82:390–395. doi: 10.1002/jmv.21691. [DOI] [PubMed] [Google Scholar]
- 52.Solis-Herruzo JA, Castellano G, Fernandez I, Munoz R, Hawkins F. Decreased bone mineral density after therapy with alpha interferon in combination with ribavirin for chronic hepatitis C. J Hepatol. 2000;33:812–817. doi: 10.1016/s0168-8278(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 53.Gilbert L, He X, Farmer P, Rubin J, Drissi H, van Wijnen AJ, et al. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol Chem. 2002;277:2695–2701. doi: 10.1074/jbc.M106339200. [DOI] [PubMed] [Google Scholar]