Abstract
Recent technological innovations including bacterial artificial chromosome-based translating ribosome affinity purification (BAC-TRAP) have greatly facilitated analysis of cell type-specific gene expression in vivo, especially in the nervous system. To better study endothelial gene expression in vivo, we have generated a BAC-TRAP transgenic mouse line where the L10a ribosomal subunit is tagged with EGFP and placed under the control of the endothelium-specific Tie2 (Tek) promoter. We show that transgene expression in this line is widely, but specifically, detected in endothelial cells in several brain regions throughout pre- and postnatal development, as well as in other organs. We also show that this line results in highly significant enrichment of endothelium-specific mRNAs from brain tissues at different stages. This BAC-TRAP line therefore provides a useful genetic tool for in vivo endothelial gene profiling under various developmental, physiological, and pathological conditions.
Introduction
Blood vessels, through delivery of nutrients and oxygen and disposal of wastes, serve an essential function for organs throughout the body. It is well known that due to distinct metabolic demands by each organ, there exists close communication between the target tissue and vascular cell types throughout development and into adulthood. This is crucial during the development of an organ-specific vascular network, as well as for the function of the vasculature in adulthood in meeting local metabolic demands. This also requires that vascular endothelial cells (ECs) dynamically regulate their gene expression in response to both intrinsic and extrinsic signals.
As an energy-intense organ, supplied by a highly specialized vascular network, the brain provides one of the best examples of the close interaction between target and vascular cells. During development, brain neural progenitors (also known as radial glial cells) signal to ECs through the canonical Wnt pathway to promote central nervous system vascularization (Liebner et al. 2008; Stenman et al. 2008; Daneman et al. 2009). Subsequently, radial glial cells regulate nascent brain vessel stabilization (Ma et al. 2013; Santhosh and Huang 2015) and promote and maintain vessel maturation and integrity (McCarty et al. 2005; Proctor et al. 2005). At the postnatal stage, neural activity also regulates vessel development and patterning in the brain (Lacoste et al. 2014; Whiteus et al. 2014). In the adult brain, it is well known that blood vessels interact closely with perivascular cell types including astrocytes to form a neurovascular unit (Barres 2008; Ma et al. 2012). This includes formation of the blood brain barrier (BBB), structural and functional specializations that both protect the brain from the environment and facilitate nutrient and oxygen delivery (Abbott et al. 2006; Zlokovic 2008). Furthermore, blood vessels in the adult brain perform perfusion independent functions, such as forming a vascular niche for neural stem cells (Palmer et al. 2000; Shen et al. 2008; Tavazoie et al. 2008; Javaherian and Kriegstein 2009) as well as regulating neuronal migration (Snapyan et al. 2009; Won et al. 2013). All of these require dynamic regulation of gene expression in ECs. Finally, altered endothelial gene expression is known to play an important role in many brain diseases (Moskowitz et al. 2010; Sagare et al. 2012). Thus, determining the profiles of EC gene expression is critical to better understand target-vascular interactions, under both normal and diseased conditions.
Traditionally, several technologies have been employed to analyze cell type-specific gene expression. One approach relies on the enrichment of specific cell types through fluorescent labeling, followed by cell sorting or immune-affinity-based cell purification. Others utilize technologies, such laser capture microdissection in fixed tissues, to isolate cell types of interest. Although highly useful and informative in many studies, each of these technologies has its own limitations, especially regarding potential perturbation of endogenous gene expression during tissue/cell processing. This is especially true for the vascular system where blood flow itself plays a major role in regulating gene expression. More recently, several new technologies have been developed that allow cell type-specific tagging of ribosomes or RNAs in vivo (Heiman et al. 2008; Sanz et al. 2009; Gay et al. 2013; Zhou et al. 2013; Hupe et al. 2014). Among them, the approach of bacterial artificial chromosome-based translating ribosome affinity purification (BAC-TRAP) uses a BAC that contains an enhanced green fluorescent protein (EGFP)-tagged L10a ribosome subunit under the control of a cell type-specific promoter. This has allowed direct isolation of ribosome-associated mRNAs from specific cell types from fresh tissues, and has been used successfully to characterize gene expression of several rare cell populations in the nervous system (Doyle et al. 2008; Heiman et al. 2008; Okaty et al. 2011; Drane et al. 2014). To adopt this approach to the study of endothelial gene expression in vivo, we report here the generation and characterization of a Tie2 (Tek) gene promoter-driven endothelium-specific BAC-TRAP transgenic mouse line.
Results and Discussion
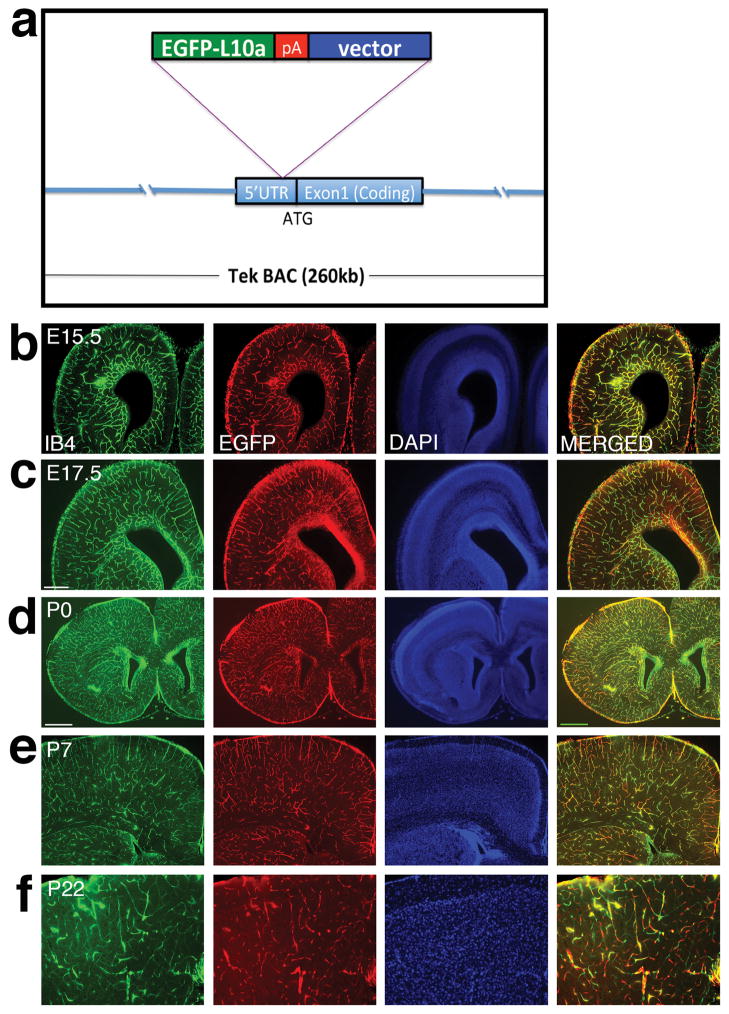
To generate an endothelium-specific BAC-TRAP mouse line, we employed a BAC containing the Tie2 gene situated in the middle of the artificial chromosome. We inserted the coding sequence of the EGFP-L10a fusion protein, followed by a polyadenylation signal, immediately before the start codon in the 5′ untranslated region (UTR) of the Tie2 gene (Fig 1a). Previous research has shown that Tie2 expression is almost exclusively restricted to endothelial cells and early hematopoietic progenitors (Maisonpierre et al. 1993; Sato et al. 1993; Schnurch and Risau 1993; Motoike et al. 2000). This regulation, provided in this construct in the large genomic environment of a BAC, should direct, with high fidelity, transgene expression. To generate transgenic lines, we employed pro-nuclear injection. Among five founders generated, three transmitted the transgene through the germ line. Of them, lines 1 and 3 showed similar patterns of EGFP expression. We report here our detailed analysis of line 1.
Figure 1. Diagram of the Tek promoter-driven BAC-TRAP construct and its overall expression throughout different stages of brain development.
(a) Schematic diagram of the BAC-TRAP construct. The EGFP tagged L10a gene is inserted immediately before the start codon of the endothelial specific Tek gene in a BAC.
(b–f) Fixed brain sections from embryonic stages to adulthood were labeled with IB4 (green), EGFP (red), and DAPI (blue). At embryonic day 15.5 (E15.5), IB4 and EGFP staining shows a similar pattern of endothelial marker and transgene expression (b). Staining was repeated at E17.5, which shows EGFP-L10a transgene expression in the same pattern as the vascular marker (c). At postnatal day 0 (P0), these staining also shows overlapping pattern of blood vessel labeling and transgene expression (d). At P7, widespread expression of EGFP-L10a is still observed along blood vessels (e). By adulthood at P22, significant transgene expression is also observed along IB4 labeled vessels, although with a greater level of variability (f).
Scale bars, 250 μm for b, c and e, 500 μm for d, and 100 μm for f.
To characterize this transgenic line, we first employed immunohistochemistry to evaluate expression of the EGFP-L10a transgene. We used an antibody against EGFP to track transgene expression and isolectin B4 (IB4) to label blood vessels, and focused our analysis on the forebrain. At E15.5, IB4 staining revealed an emerging vascular network in the forebrain, with prominent vessels labeled in the ventricular zone (Fig. 1b). We found that EGFP staining also showed a similar network, with no ectopic staining found away from IB4 positive vessels (Fig. 1b). This suggests specific expression of EGFP-L10a in ECs. To determine EGFP expression pattern at other developmental stages, we also performed analysis at E17.5, P0, P7 and P22. At 17.5, IB4 staining showed a maturing vascular network, with vessels evenly distributed throughout much of the forebrain at a higher density than E15.5 (Fig. 1c). We found that EGFP staining matched these changes in vascular pattern, though it seemed to lag behind IB4 staining in the striatum (Fig. 1c). However, we did not observe ectopic EGFP staining away from vessels, suggesting the transgene is not misexpressed in other cell types. Similar observations were also made at P0, with nearly complete overlap between IB4 and EGFP labeling (Fig. 1d). At P7 (Fig. 1e), we also observed widespread expression of EGFP along IB4 labeled vessels. By P22 (Fig. 1f), significant EGFP expression was still observed along IB4 positive vessels, although it appeared less contiguous. The overlap with EGFP expression at P22, while at much lower levels, still confirms the restricted expression of the transgene to vessels. Thus, these results indicate that the EGFP-L10a transgene is widely and specifically expressed in brain ECs throughout pre- and postnatal stages.
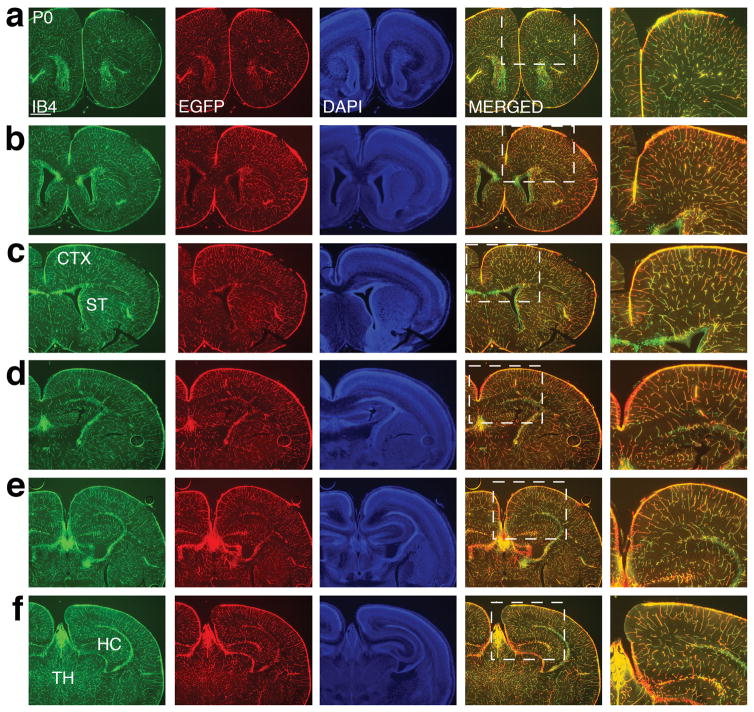
To assess potential variations in transgene expression across brain regions, we next examined the postnatal brain throughout the anterior to posterior axis (Fig. 2). In the anterior forebrain at P0 (Fig. 2a–c), IB4 staining revealed an extensive vascular network across both the cerebral cortex and the striatum, with the highest density of vessels observed in the vicinity of the lateral ventricle. We found that EGFP staining showed a similar pattern of distribution in both regions. In the posterior forebrain (Fig. 2d–f), we also observed very similar patterns of IB4 and EGFP staining in the cerebral cortex. In addition, we observed similar patterns of IB4 and EGFP staining in the hippocampus, a structure located exclusively in the posterior forebrain (Fig. 2d–f). Furthermore, in the thalamus, we found that IB4 and EGFP staining also showed a near identical pattern (Fig. 2f). In none of these brain regions did we observe any obvious ectopic EGFP expression away from IB4 positive vessels. Thus, these results indicate that throughout the anterior-posterior axis, the EGFP-L10a transgene is also widely and specifically expressed in ECs of all brain structures.
Figure 2. Consistent EGFP-L10a transgene expression in blood vessels along the anterior to posterior axis of the brain.
(a–c) Staining of P0 sections with IB4 (green), EGFP (red), and DAPI (blue) shows transgene expression in blood vessels in the anterior forebrain, including in both the cerebral cortex (CTX) and the striatum (ST).
(d–f) The same labeling in posterior sections of P0 brain also reveals a similar pattern between vessel marker and EGFP-L10a expression. TH: thalamus; HC: hippocampus.
Scale bar, 500 μm for all panels.
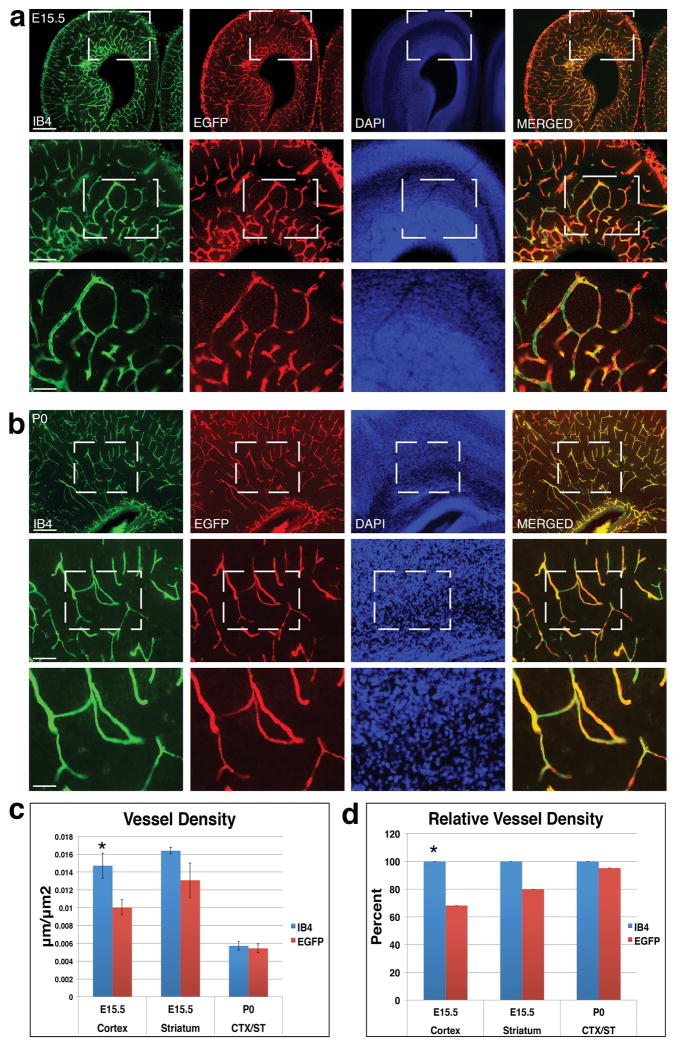
To further evaluate transgene expression, we next examined IB4 and EGFP labeling at higher magnifications (Fig. 3a–b). In the E15.5 brain (Fig. 3a), IB4 and EGFP showed a similar pattern of staining at 4x magnification. However, when examined at the higher magnifications of 10x and 20x, it became clear that some vessel segments labeled by IB4 were not positive for EGFP. While there did not appear to be any ectopic EFGP expression, not all blood vessels were labeled by EGFP along the entire length. Although it is possible that the different nature of the labeling (membrane vs. cytoplasm) may contribute to this difference, this also suggests that the EGFP-L10a transgene may be not expressed in all ECs. Similarly, in the P0 brain (Fig. 3b), at the magnifications of 10x and 20x, we observed segments of IB4 positive blood vessels that were not labeled by the EGFP antibody. However, in contrast to the embryonic brain, this difference in labeling appears to be less prominent in postnatal tissues.
Figure 3. Quantitative analysis of EGFP-L10a transgene expression along vessels during development.
(a) E15.5 sections were labeled with IB4 (green), EGFP (red), and DAPI (blue), and examined at 4x, 10x and 20x magnifications. Results show that while EGFP labeling is restricted to blood vessels, there exist a small number of vessel segments that show no EGFP expression.
(b) P0 sections were stained with IB4 (green), EGFP (red), DAPI (blue), and examined at 4x, 10x and 20x magnifications. Results also show that the EFGP transgene is restricted to IB4 positive cells, while the overlap between EGFP and IB4 labeling appears higher than that at E15.5.
(c) Quantification reveals an IB4 positive vessel density (blue columns) of about 0.014, 0.016 and 0.006 μm/μm2 in the E15.5 cortex, striatum, and P0 forebrain, respectively. In comparison, the EGFP positive vessel density (red columns) is about 0.013, 0.01 and 0.006 μm/μm2, respectively. *, p < 0.05 (n = 5 for E15.5 cortex, 3 for E15.5 striatum, and 4 for P0).
(d) Relative vessel density. Normalization shows EGFP expression in about 68% of IB4 positive vessels in the E15.5 cortex, in about 79% of IB4 positive vessels in the E15.5 striatum, and in about 95% of IB4 positive vessels in the P0 brain.
Scale bars, 250 μm for 4x images (first rows in a & b), 100 μm for 10x images (second rows in a & b), and 50 μm for 20x images (third rows in a & b).
To quantitatively analyze the degree of transgene expression along vessels, we next determined vessel density separately according to IB4 or EGFP staining (Fig. 3c). At E15.5, we found that, while IB4 labeling yielded a vessel density of about 0.014 and 0.016 μm/μm2 for the cerebral cortex and striatum, respectively, EGFP staining yielded only a density of about 0.013 and 0.01 μm/μm2, respectively. In contrast, at P0, these markers yielded much closer values (both at about 0.006 μm/μm2). This is consistent with our observation of fewer IB4 positive EGFP negative vessel segments at P0. To further assess the degree of EGFP expression, we normalized the density of EGFP positive vessels against that of IB4 positive vessels (Fig. 3d). We found that at E15.5, EGFP stained about 68% of IB4 positive vessels in the cerebral cortex, and about 79% of IB4 positive vessels in the striatum. In contrast, at P0, EGFP labeled about 95% of IB4 positive vessels. While there was no significant difference in IB4 and EGFP labeling in the striatum at E15.5 and at P0, in the cortex at E15.5, EGFP staining yielded a significantly lower vessel density than IB4 labeling. This may reflect a difference in Tie2 promoter regulation in different regions of the brain. Taken together, these results indicate that the EGFP-L10a transgene is expressed in the vast majority of ECs during embryogenesis and in nearly all ECs in the early postnatal brain.
Since we have so far performed our analysis using IB4 as an endothelial marker, to determine whether the conclusion holds true when analyzed with different markers, we employed several other antibodies (Fig. 4). Using another endothelial marker in the E15.5 forebrain, we found as expected, endomucin labeling revealed a vascular network similar to that of IB4 staining (compare Fig. 4a to Fig. 1b). Importantly, the pattern of endomucin staining at this stage is also highly similar to that of EGFP (Fig. 4a). This therefore corroborates our conclusion that EGFP-L10a is widely and specifically expressed in brain ECs. Furthermore, at higher magnifications (Fig. 4b–c), similar to our observations with IB4 staining, we also observed occasional endomucin positive vessels segments that were not labeled by EGFP. Indeed, quantitative analysis showed an endomucin positive vessel density in the E15.5 forebrain of about 0.015 μm/μm2, in contrast to that of EGFP positive vessels of about 0.012 μm/μm2 (Fig. 4b). This trend (~80% of endomucin positive vessels stained positive for EGFP) is similar to that observed with IB4 staining at the same stage (Fig. 3c).
Figure 4. Analysis of EGFP-L10a expression along endomucin labeled vessels and lack of expression in radial glia, neurons, and pericytes.
(a–c) Staining of E15.5 brain sections with endomucin (red), EGFP (green), and DAPI (blue), and examination at 4x (a), 10x (b) and 20x (c) magnifications show overlapping labeling as seen in IB4/EGFP staining.
(d) Quantification shows an endomucin positive vessel density (blue column) of about 0.015 μm/μm2 and an EGFP positive density (red column) of about 0.012 μm/μm2 in the E15.5 brain (not significant, p = 0.129, n = 5)
(e) Staining of radial glia with BLBP and transgene with EGFP antibodies at E16.5 shows that EGFP_L10a transgene is not expressed in neural progenitors.
(f) Staining of neurons with NeuN and transgene with EGFP antibodies at P0 show that EGFP_L10a transgene is not expressed in differentiated neurons.
(g) Staining of pericytes with Desmin and transgene with EGFP antibodies at E16.5 shows that pericytes do not express the EGFP-L10a transgene.
Scale bars, 250 μm for a, 100 μm for b, 50 μm for c, 15 μm for e and f, and 3 μm for g.
To further confirm specific EGFP expression in ECs, we used an antibody against brain lipid binding protein to label radial glia in the embryonic brain (Fig. 4e). This staining revealed a radial organization of neural progenitor cells, a pattern clearly distinct from that of EGFP staining. It demonstrates that the transgene is not expressed in neural progenitors. We also confirmed the lack of EGFP expression in neurons using an antibody against NeuN (Fig. 4f) and in pericytes using an antibody against Desmin (Fig. 4g). In each case, EGFP labeling reveals a pattern clearly distinct from that of the cell type marker. For example, in the case of pericyte staining, it is clear that Desmin positive cells, though adjacent to EGFP positive cells, do not express EGFP. Thus, these results indicate that the EGFP-L10a transgene is specifically expressed in brain ECs and not in radial glia, neurons or pericytes.
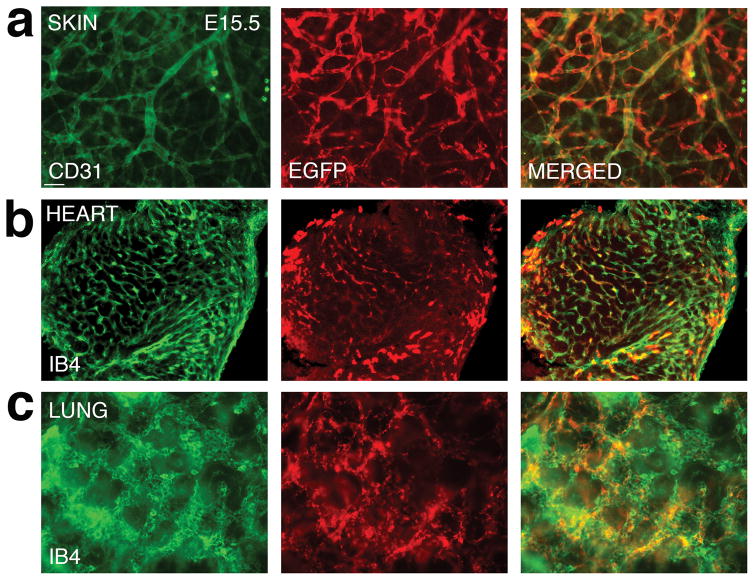
In addition to the brain, we also examined the expression of EGFP-L10a transgene in other organs, including the skin, the heart, and the lung (Fig. 5a–c). As in the brain, in the skin, we observed widespread EGFP expression along CD31 labeled blood vessels, despite a small fraction of vessel segments that appeared EGFP negative (Fig. 5a). In the heart, EGFP expression appears to be not as even, with high levels of expression only in some cells (Fig. 5b). Nonetheless, the expression remains restricted to IB4 positive cells. In the lung, on the other hand, we again observe an extensive overlap between EGFP expression and IB4 labeling (Fig. 5c), similar to that observed in the brain. Thus, these results indicate that the EGFP-L10a transgene is extensively expressed in ECs outside the nervous system.
Figure 5. Analysis of EGFP-L10a expression in the embryonic skin, heart, and lung.
(a) Staining of E15.5 skin with CD31 (green) and EGFP (red) antibodies shows that the transgene is expressed extensively along blood vessels in the skin.
(b) Staining of E15.5 heart sections with IB4 (green) and EGFP (red) antibodies shows that EGFP-L10a expression is present in the heart, and limited to IB4 positive cells.
(c) Staining of E15.5 lung sections with IB4 (green) and EGFP (red) antibodies also shows widespread expression of EGFP-L10 transgene along vessels.
Scale bars, 50 μm for a and c, and 100 μm for b.
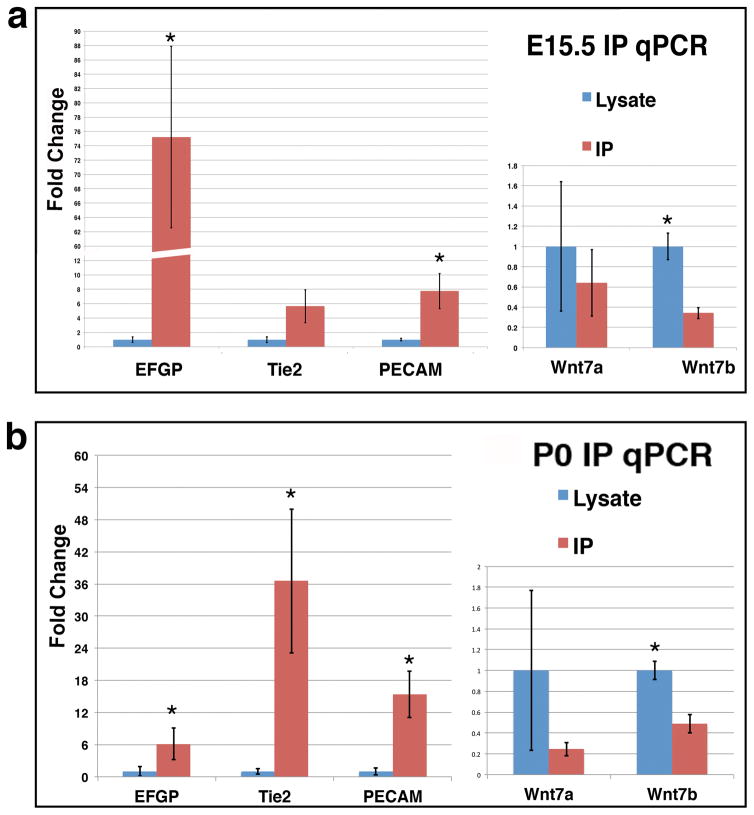
To determine whether the BAC-TRAP line is suitable for purification of endothelial specific mRNAs for in vivo gene expression analysis, we next examined enrichment of endothelium-specific transcripts following immunoprecipitation (IP) of tagged ribosomes. To this end, we employed a combination of monoclonal antibodies against EGFP for ribosome IP, following published protocols with slight modifications (Heiman et al. 2014). We performed the evaluation at two stages, E15.5 and P0. After collecting tissues from the E15.5 or P0 brains, we pulled down tagged ribosomes using the EGFP antibodies, while saving a portion of the total lysates as pre-IP controls. We then extracted RNA from both the IP samples and pre-IP lysates, and employed RT-qPCR to evaluate the amount of endothelium-specific mRNAs in each sample (Fig. 6). For endothelium-specific genes, we analyzed EGFP, Tie2, and PECAM. For neural cell genes, we analyzed Wnt7a and Wnt7b. To evaluate transcript enrichment by IP, we normalized all mRNA levels in the IP samples against those in pre-IP lysates. We found that the BAC-TRAP IP led to a significant 5–75 fold enrichment of the endothelial genes for EGFP and PECAM in the E15.5 samples, while the neural genes showed moderate to significant depletion (Fig. 6a). Similarly, in the P0 samples, we found a significant 6–36 fold enrichment for all endothelial genes and 2–5 fold depletion of neural genes (Fig. 6b). In addition, we observed minimal presence of hematopoietic genes in the IP samples, at levels 400–900 fold lower than endothelial genes (PECAM: 0.057±0.021; CD45: 0.00014±0.0008; CD48: 0.00006±0.0001; p < 0.05 both between PECAM and CD45 and between PECAM and CD48). Thus, these results indicate that this BAC-Trap line is suitable for purification and enrichment of endothelial specific mRNAs.
Figure 6. Real-time PCR confirms BAC-TRAP enrichment of endothelial specific mRNAs.
(a) RT-qPCR results of endothelial specific genes after IP (red columns) compared to those in tissue lysates (blue columns) at E15.5 show enrichment of EC genes (EGFP, Tie2, and PECAM) and depletion of neural genes (Wnt7a & b). *, p < 0.05 (n = 4 for EGFP, Tie2, and PECAM, and 3 for Wnt7a/b). (p = 0.07 for Tie2).
(b) RT-qPCR results of endothelial specific genes at P0 show a similar pattern of EC transcript enrichment and non-EC mRNA depletion. *, p < 0.05 (n = 4 for EGFP, Tie2, and PECAM, and 3 for Wnt7a/b).
Taken together, our analysis indicates that the Tie2-driven BAC-TRAP line we generated can be used to efficiently enrich endothelium-specific mRNAs and is a valuable tool for in vivo endothelial gene profiling. In comparison to other tools, this line has several advantages. First, endothelial gene expression in vivo is known to be susceptible to changes in blood flow (Chien 2007). Thus, analytical approaches that rely on EC purification will likely incur changes, while the BAC-TRAP approach allows profiling under physiological conditions of blood flow. Second, several ribosome-tagging mouse lines have been recently generated that allow gene profiling using different cre drivers (Sanz et al., 2009; Zhou et al., 2013). This approach does have the advantage of making use of already available cre lines. Our BAC-TRAP line, on the other hand, has the unique advantage of enabling analysis of endothelial genes induced by conditional mutations in non-ECs.
Materials and Methods
Generation of the Tie2 BAC-TRAP transgenic mouse
The EGFP-L10a vector (gift of Dr. N. Heintz, Rockefeller) was inserted into a Tie2-containing BAC by RecA dependent recombineering per published protocols (Gong et al. 2010). The insertion was confirmed by PCR amplification and sequencing of 5′ and 3′ flanking regions. Pro-nuclear injection of the modified BAC was performed at the transgenic facility at Roswell Park Cancer Institute (Buffalo, NY). The following primer pairs were used for genotyping: 5′ GAGCAGGAGCAGAAGATAAGCCTTGGAT 3′ (in Tie2 genomic region) and 5′ GGACTTGAAGAAGTCGTGCTGCTTCATG 3′ (in EGFP). Animal studies were performed in accordance to institutional guidelines approved at UW-Madison. The characterized line will be available to the research community upon acceptance of the manuscript.
Immunohistochemistry
Embryonic brains, skin, heart or lungs were dissected from pregnant females at E15.5 and E17.5 and postnatal brains from P0 and P7 pups. P22 mice were euthanized with isoflurane overdose and the brains were perfused with 4% paraformaldehyde (PFA). All other brains and organs were drop fixed in 4% PFA overnight. Immunohistochemistry was carried out essentially as described previously on 50 μm-thick Vibratome sections (Ma et al. 2012, 2013). Primary antibodies or reagents used include biotinylated isolectin B4 (Vector Lab), rat anti-mouse CD31 (BD Pharmingen), rabbit anti-GFP (Life Technologies), rabbit anti-Brain lipid binding protein (Millipore), rabbit anti-Desmin (Millipore), mouse anti-NeuN (Millipore) and rat anti-mouse endomucin (Affymetrix eBioscience). FITC or Cy3 conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Images were taken under a Nikon eclipse Ti microscope and processed using Adobe Photoshop CS6.
Quantification of vessel density
Blood vessels within each of the brains section, stained against IB4, EGFP or endomucin, and imaged using the Nikon microscope, were manually traced. Vessel density was calculated by dividing total vessel length by area in each section.
Ribosome immunoprecipitation (IP)
Ribosome IP was performed according to published protocols with slight modifications (Heinman et al., 2014). To prepare agarose beads, 100 μl per IP of agarose G beads (Santa Cruz Biotech) were washed 3 times with 0.15 M KCl wash buffer and resuspended in the wash buffer at a final volume of 100 μl. 25 μg each of EGFP monoclonal antibodies (19C8 & 19F7) were added to the beads and incubated with slow end-over-end mixing at room temp for 1 hr. After incubation, the antibody-bound beads were washed 3 times with 0.15 M KCl wash buffer and resuspended in a final volume of 100 μl before use. To prepare tissue lysates 1 ml of Lysis Buffer was added to each flash frozen sample. For E15.5 samples, 4 brains were pooled into one sample tube. For P0 samples, one brain was used for each. Tissues were homogenized by passage through 18 gauge needles 25 times. The homogenates were then centrifuged at 2,000 g for 10 min at 4°C, and the supernatants transferred to new tubes (S2). 1/9 volume of 10% NP-40 (for a final concentration of 1%) was then added and gently mixed. After incubation on ice for 5 min, the lysates were centrifuged at 16,000 g for 10 min at 4°C and the supernatants transferred to new tubes (S20). To pull down tagged ribosomes, the prepared antibody-bound beads were then added to each of the S20 supernatants and incubated at 4°C overnight with end-over-end mixing. On day 2, the beads were pelleted by centrifugation at 4,000g for 1 min at 4°C and washed 4 times with 1 ml of 0.35 M KCl wash buffer. Bound ribosomes were then eluted at room temperature using the RTL buffer per instructions of the Qiagen RNeasy Plus Micro kit and stored at −80°C until use.
RNA extraction
IP Samples were thawed on ice and centrifuged to remove any residual beads. Genomic DNA were removed from the supernatants using columns from the Qiagen RNeasy Plus Micro kit per manufacturer’s instructions. For final elution, 14 μl of RNase-free water were added. RNA samples were stored at −80°C before quantification and use for reverse transcription (ABI High-Capacity cDNA Reverse Transcription Kit).
Real-time PCR
Real-time PCR was performed on an ABI 7300 machine. The following real time primers were used for measuring enrichment of endothelial cell specific mRNA after IP. EGFP primers: forward, 5′ AAGCTGACCCTGAAGTTCATCTGC 3′ and reverse, 5′ CTTGTAGTTGCCGTCGTCCTTGAA 3′,
Pecam primers: forward, 5′GAGCCCAATCACGTTTCAGTTT 3′ and reverse, 5′ TCCTTCCTGCTTCTTGCTAGCT 3′, and Tie2 primers: forward, 5′ TTAGTGACATTCTCCCTCCTCA 3′ and reverse, 5′ CTTCATTTTTGCCCTGAACCTT 3′.
Wnt7a primers: forward 5′ CGACTGTGGCTGCGACAAG 3′ and reverse 5′ CTTCATGTTCTCCTCCAGGATCTTC 3′.
Acknowledgments
Supported by an NIH grant NS076729-01A1 to Z.H.
This work was supported by an NIH grant NS076729-01A1 to Z.H. We thank Dr. N. Heintz (Rockefeller) for the EGFP-L10 and RecA plasmids and Dr. E. Bresnik (UW-Madison) for access to an ABI realtime PCR machine.
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, Bupp S, Shrestha P, Shah RD, Doughty ML, et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135:749–762. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane L, Ainsley JA, Mayford MR, Reijmers LG. A transgenic mouse line for collecting ribosome-bound mRNA using the tetracycline transactivator system. Frontiers in molecular neuroscience. 2014;7:82. doi: 10.3389/fnmol.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay L, Miller MR, Ventura PB, Devasthali V, Vue Z, Thompson HL, Temple S, Zong H, Cleary MD, Stankunas K, et al. Mouse TU tagging: a chemical/genetic intersectional method for purifying cell type-specific nascent RNA. Genes Dev. 2013;27:98–115. doi: 10.1101/gad.205278.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Kus L, Heintz N. Rapid bacterial artificial chromosome modification for large-scale mouse transgenesis. Nature protocols. 2010;5:1678–1696. doi: 10.1038/nprot.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman M, Kulicke R, Fenster RJ, Greengard P, Heintz N. Cell type-specific mRNA purification by translating ribosome affinity purification (TRAP) Nature protocols. 2014;9:1282–1291. doi: 10.1038/nprot.2014.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, Suarez-Farinas M, Schwarz C, Stephan DA, Surmeier DJ, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupe M, Li MX, Gertow Gillner K, Adams RH, Stenman JM. Evaluation of TRAP-sequencing technology with a versatile conditional mouse model. Nucleic Acids Res. 2014;42:e14. doi: 10.1093/nar/gkt995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaherian A, Kriegstein A. A stem cell niche for intermediate progenitor cells of the embryonic cortex. Cereb Cortex. 2009;19(Suppl 1):i70–77. doi: 10.1093/cercor/bhp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste B, Comin CH, Ben-Zvi A, Kaeser PS, Xu X, da Costa LF, Gu C. Sensory-related neural activity regulates the structure of vascular networks in the cerebral cortex. Neuron. 2014;83:1117–1130. doi: 10.1016/j.neuron.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Kwon HJ, Huang Z. A functional requirement for astroglia in promoting blood vessel development in the early postnatal brain. PLoS One. 2012;7:e48001. doi: 10.1371/journal.pone.0048001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Kwon HJ, Johng H, Zang K, Huang Z. Radial glial neural progenitors regulate nascent brain vascular network stabilization via inhibition of Wnt signaling. PLoS Biol. 2013;11:e1001469. doi: 10.1371/journal.pbio.1001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, Goldfarb M, Yancopoulos GD, Gao G. Distinct rat genes with related profiles of expression define a TIE receptor tyrosine kinase family. Oncogene. 1993;8:1631–1637. [PubMed] [Google Scholar]
- McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J, Hynes RO. Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoike T, Loughna S, Perens E, Roman BL, Liao W, Chau TC, Richardson CD, Kawate T, Kuno J, Weinstein BM, et al. Universal GFP reporter for the study of vascular development. Genesis. 2000;28:75–81. doi: 10.1002/1526-968x(200010)28:2<75::aid-gene50>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Okaty BW, Sugino K, Nelson SB. A quantitative comparison of cell-type-specific microarray gene expression profiling methods in the mouse brain. PloS one. 2011;6:e16493. doi: 10.1371/journal.pone.0016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Proctor JM, Zang K, Wang D, Wang R, Reichardt LF. Vascular development of the brain requires beta8 integrin expression in the neuroepithelium. J Neurosci. 2005;25:9940–9948. doi: 10.1523/JNEUROSCI.3467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare AP, Bell RD, Zlokovic BV. Neurovascular dysfunction and faulty amyloid beta-peptide clearance in Alzheimer disease. Cold Spring Harbor perspectives in medicine. 2012:2. doi: 10.1101/cshperspect.a011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhosh D, Huang Z. Regulation of the nascent brain vascular network by neural progenitors. Mech Dev. 2015 doi: 10.1016/j.mod.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13939–13944. doi: 10.1073/pnas.0907143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TN, Qin Y, Kozak CA, Audus KL. Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci U S A. 1993;90:9355–9358. doi: 10.1073/pnas.90.20.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurch H, Risau W. Expression of tie-2, a member of a novel family of receptor tyrosine kinases, in the endothelial cell lineage. Development. 1993;119:957–968. doi: 10.1242/dev.119.3.957. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, Gravel C, Berthod F, Gotz M, Barker PA, et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteus C, Freitas C, Grutzendler J. Perturbed neural activity disrupts cerebral angiogenesis during a postnatal critical period. Nature. 2014;505:407–411. doi: 10.1038/nature12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won C, Lin Z, Kumar TP, Li S, Ding L, Elkhal A, Szabo G, Vasudevan A. Autonomous vascular networks synchronize GABA neuron migration in the embryonic forebrain. Nature communications. 2013;4:2149. doi: 10.1038/ncomms3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Zhang Y, Ma Q, Gu F, Day DS, He A, Zhou B, Li J, Stevens SM, Romo D, et al. Interrogating translational efficiency and lineage-specific transcriptomes using ribosome affinity purification. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15395–15400. doi: 10.1073/pnas.1304124110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]