Abstract
The role of calcitonin gene related peptide (CGRP) in neuropathic pain was investigated in a mouse model of neuropathic pain, spinal nerve L5 transection (L5Tx). Intrathecal injection (i.t) of CGRP8–37, a CGRP antagonist, significantly reduced L5Tx-induced mechanical hypersensitivity and lumbar spinal cord CCL5 expression. i.t. injection of a CCL5 neutralizing antibody significantly inhibited L5Tx-induced mechanical hypersensitivity. Further, pre-treatment with a p38-inhibitor, SB203580, was able to reduce CGRP-induced mechanical hypersensitivity, but not CGRP-induced CCL5 production. Our data indicate that CGRP can play its pro-nociceptive role through both a spinal cord CCL5-dependent, p38-independent pathway, and a p38-depenented, CCL5-independent pathway.
Keywords: Calcitonin gene-related peptide (CGRP), CCL5, Neuropathic pain, Spinal nerve L5 transection, p38, Spinal cord
Graphical abstract

1. Introduction
Defined as pain caused by a lesion or disease of the somatosensory system, neuropathic pain is one of the most devastating kinds of chronic pain. Neuroimmune interaction is believed to play an important role in the development of neuropathic pain (DeLeo et al., 2004; Ji and Suter, 2007; Milligan and Watkins, 2009; Tsuda et al., 2005).
Calcitonin gene-related peptide (CGRP) is a 37-amino acid neuropeptide within the calcitonin peptide family, and is widely distributed in both the peripheral and the central nervous systems. Produced largely by medium and small diameter primary afferent neurons, CGRP is important in modulating pain perception. Despite some controversy, studies have provided evidence that CGRP plays a pro-nociceptive role under various conditions. For example, in rodents intrathecal injection of CGRP produced significant hyperalgesia in rodents to both mechanical and thermal stimuli (Cridland and Henry, 1989; Oku et al., 1987). Administration of a CGRP antagonist has been shown to reduce both thermal and mechanical hypersensitivity in non-injured rats and rats with inflammatory pain or neuropathic pain –like behaviors (Bennett et al., 2000a; Lee and Kim, 2007; Yu et al., 1996a; Yu et al., 1996b). Further, spinal nerve ligation (SNL) markedly enhanced capsaicin-evoked CGRP release in lumbar spinal cord tissue in rats, suggesting a potentially increased functional activity of CGRP+ nociceptors following-nerve injury (Gardell et al., 2003). Previously, we have observed a significant increase of CGRP expression in the lumbar spinal cord in mice following spinal nerve L5 transection (L5Tx), a murine model of neuropathic pain. In the current study we set out to assess the role of CGRP in neuropathic pain-like behaviors following L5Tx.
It has been established that numerous chemokines are involved in the development of neuropathic pain (Gao and Ji, 2010b; White et al., 2007). For example, CCR2 knockout mice (receptor for CCL2) displayed impaired neuropathic pain responses (Abbadie et al., 2003). Blocking CCL2 (monocyte chemoattractant protein-1 (MCP-1) ) signaling via a neutralizing antibody prevented the development of SNL-induced sensory hypersensitivities (Gao et al., 2009). Further, mice either lacking CCL5 (regulated on activation, normal T cell expressed and secreted (RANTES)) or peritoneally administered with a selective CCL5 receptor antagonist, Met-RANTES, showed reduced hypersensitivity following partial sciatic nerve ligation (Liou et al., 2013; Liou et al., 2012). Injections of CCL5 either peripherally (Oh et al., 2001) or centrally (Benamar et al., 2008) induced hypersensitivities in rat. In addition, CX3CL1/CX3CR1 signaling and other chemokines, such as, CXCL1, CCL21 have also been implicated in the development of neuropathic pain (Clark and Malcangio, 2014; Zhang et al., 2013). In this study, the involvement of several chemokines in CGRP-mediated pain-like behaviors was further investigated using a chemokine multiplex assay. Result indicated a CCL5 mediated CGRP-induced mechanical hypersensitivity.
Mitogen activated protein Kinase (MAPK) pathways (including Jun N-terminal kinase (JNK), extracellular signal-regulated kinases (ERKs), and p38 MAPK) have been linked to chemokine signaling during the development of pain-like behaviors (Gao and Ji, 2010b). Associations between the p38 pathway and microglial activation, the JNK pathway and astrocyte activation, and the ERK pathway and neuronal responses, have been suggested during the development of pain state (Gao and Ji, 2010a; Gao and Ji, 2009; Ji and Suter, 2007; Jin et al., 2003; Li et al., 2010). Therefore, the involvement of MAPK pathways was also examined, which led to the detection of both CCL5-dependent/p38-independent and CCL5-independent/p38-dependent pathways mediated CGRP’s pro-nociceptive effects.
2. Material and Methods
2.1. Animals
Male and female adult BALB/c mice (7–8 weeks old) were purchased from National Cancer Institute (NCI, Frederick, MD) and were allowed to habituate to the institutional animal facility for at least one week before experimental use. All mice were group-housed with food and water ad libitum and maintained on a 12-h light/dark cycle. The Institutional Animal Care and Use Committee (IACUC) at the University of New England (UNE) approved all experimental procedures used in this study.
2.2. L5Tx and drug treatment
Spinal nerve L5 transection (L5Tx) and sham surgeries were performed on 8-week-old BALB/c mice following our previously published procedures (Cao and DeLeo, 2008). Approximately the same numbers of male and female mice were used in each group. CGRP is 37 amino acids in length. The N-terminal amino acids 1–7 are required for receptor activation and signal transduction, whereas the remainder (amino acids 8–37) of CGRP is necessary for receptor binding; therefore a polypeptide that contains only amino acids 8–37 of CGRP (CGRP8–37) acts as a CGRP receptor antagonist (Arulmani et al., 2004). To test the effects of CGRP and CCL5 in the development of L5Tx-induced behavioral hypersensitivities, we utilized CGRP8–37 (Sigma Aldrich, St. Louis, MI; 1μg/mouse) and a monoclonal neutralizing antibody against CCL5 (R&D Systems, Minneapolis, MN; 0.5μg or 2.5μg/mouse), respectively. Mice were randomly assigned to different treatment groups, and then received intrathecal injections (5 μl/mouse) (see Table 1) daily from days 6 to 13 post-surgery. This time period was chosen based on the spinal cord level of CGRP expression (peaked at day 7 and day 14 post-L5Tx) (Malon et al., 2011) and previous report (Yu et al., 1996a; Yu et al., 1996b). Intrathecal injections were performed as described previously (Malon et al., 2011). Besides vehicle-injected mice, animals that underwent L5Tx but received no i.t. injections were also used as controls. An isotype control antibody was not used as a control due to its potential “specific antibody-like” effects as shown in a previous study (Arruda et al., 2000). If behavioral tests were conducted on the same day of injection, injection was given after the behavior tests on that day. Following conclusion of the study, spinal cord tissues from treated animals were collected and sent out for chemokine analysis (Quansys Biosciences, Logan, UT) (see below section 2.5).
Table 1.
List of solutions and their corresponding vehicles used in intrathecal injections.
| Solution | Drug dosage (per mouse in 5 μl) | Corresponding Vehicle |
|---|---|---|
| CGRP8–37 | 1 μg | Saline |
| α-CCL5 | 0.5μg and 2.5μg | 1xPBS |
| p38 inhibitor (SB203580) | 5 ng | 1xPBS w/0.1% DMSO |
| CGRP | 2 μg | 1xPBS |
2.3. CGRP and SB203580 treatment
To test the involvement of p38 MAPK pathway in CGRP-mediated mechanical hypersensitivity, CGRP and a p38 inhibitor, SB203580 (Sigma Aldrich) were i.t. injected to naïve animals (Table 1). SB203580 (5ng/5μl/mouse) was administered first and CGRP (2 μg/5μl/mouse) was given 30 minutes later. Animals receiving corresponding vehicles were used as controls. i.t. injections were given as described above. Behavioral test started one hour after the second injection.
2.4. Behavioral tests
As previously described (Cao et al., 2012), mechanical sensitivity was determined via von Frey test following the up-down method (Chaplan et al., 1994) and the heat sensitivity was measured via Hargreaves test using an IITC thermal plantar analgesia instrument for mice (IITC Life Science, Woodland Hills, CA). Both tests were performed with mice under non-restrained conditions. The 50% threshold force needed for paw withdrawal was calculated and used to represent mechanical sensitivity. Average of latencies (at least two tests with an interval of at least 10 min) for paw withdrawal was recorded and used to represent heat sensitivity. Mice were baseline tested twice before day 0 (surgery or CGRP injection depending on the experiment). When von Frey up-down and Hargreaves tests were performed on the same day, mice were given 1 hour to acclimate between tests to avoid potential influence between tests. The person performing the behavioral tests was blinded to the experimental groups.
2.5. Measurement of chemokine levels
Chemokine multiplex assay was performed by Quansys Biosciences. Briefly, lumbar spinal cords were collected following previously published methods (Cao et al., 2009). First, tissues were sonicated in PBS with1:100 dilution of protease inhibitor cocktail (Sigma). Then supernatants were collected following centrifugation (10000 × g at 4°C for 15 min) and stored at −80°C until assay. Prior to data collection, technicians at Quansys performed extensive pilot analyses to determine the assay buffer and sample dilutions. Total of 8 chemokines were included in the manufacturer’s pre-designed multiplex assay: CXCL1 (KC), CCL1 (TCA-3), CCL2 (MCP-1), CCL3 (MIP-1α), CCL5 (RANTES), CCL11 (Eotaxin), CCL17 (TARC), and CCL22 (MDC). In follow-up experiments, levels of CCL5 were determined via the enzyme-linked immunosorbent assay (ELISA) using a mouse DuoSet ELISA kits (R&D Systems, Minneapolis, MN, USA) following the manufacturers’ protocols. The standard series ranged from 0 – 1000 pg/ml for CCL5. Tissue levels of chemokines were normalized to the total protein levels of each sample, as determined by BCA assays (ThermoFisher Scientific, Waltham, MA).
2.6. Cytometric Bead Array (CBA) assay
Lumbar spinal cord tissue homogenates and cell lysates were collected following manufacturers recommendations (BD Biosciences, San Jose, CA). CBA assay was run following manufacturer’s guidelines with kits that contained: capture beads, detection reagent, and standards for: phospho-p38 (T180/Y182), phosphorlated-JNK1/2 (T183/Y185), and phospo-ERK1/2 (T202/Y204) flex sets. An Accuri C6 flow cytometer (BD Biosciences) was used to analyze all samples. Flow cytometry files were further analyzed using the FCAP Array™ software (version 3.0) provided by the manufacturer. Data were recorded and normalized to the total protein of each sample, which was determined through BCA assay (ThermoFisher Scientific, Rockford, IL). The CBA assay was chosen instead of Western blotting because of it being a quantitative assay and due to difficulties in interpreting Western blots of spinal cord samples (due to the overwhelming non-specific bands generated by these complex tissue homogenates). The CBA assay offers a sensitive and high-throughput approach to examine signaling pathways (Elshal and McCoy, 2006; Morgan et al., 2004) and is highly comparable to Western blotting (Schubert et al., 2009).
2.7. Primary adult spinal cord glial cultures
Primary adult mixed glia cultures were established using 8-week-old BABL/c NCI mice and maintained in complete Dulbecco’s Modified Eagle Media (cDMEM) (Lonza, Walkersville, MD) containing 50 μM 2-mercaptoethanol (2-ME, Sigma-Aldrich) in 24-well plates as we have previously described (Malon et al., 2011). Media was replaced on days 2, 4, 8, and 12 after the establishment of each culture. All cells were maintained at 37°C in a humidified atmosphere with 5% CO2. Mixed glia were treated at day 14 following the establishment of each culture with CGRP at 25, 100, 1000, and 4000 ng/ml as determined from previous experiments (Malon et al., 2011). Supernatants and cell lysate were collected 24 and 48 hours after treatment for CCL5 ELISAs and CBA respectively (see above).
2.8. Statistical analysis
All statistical analyses were performed using SigmaStat 3.5 (Systat Software, Inc.). Appropriate analysis of variance (ANOVA) with treatment and time as factors were performed followed by Student-Newman-Keuls (SNK) Post hoc analysis. All data are presented as mean ± SEM. p < 0.05 was considered as statistically significant.
3. Results
3.1. Changes of L5Tx-induced sensory hypersensitivity following the treatment of CGRP8–37
Previously, we have shown that L5Tx induced a significant increase in CGRP expression in the lumbar spinal cord (Malon et al., 2011). To further determine the role of CGRP in the development of L5Tx-induced hypersensitivity, a CGRP antagonist, CGRP8–37 was i.t. injected (at 1 μg/5 μl) into WT BALB/c mice daily from days 6 to 13 post-L5Tx. Both mechanical and heat sensitivities were measured via the up-down method and Hargreaves test respectively. Both male and female mice were used and gender differences were also determined during data analysis. For the mechanical sensitivity, no significant gender effect was detected (Figure 1A, three-way analysis of variance (ANOVA) with time, treatment, and sex as factors, psex = 0.656), thus all data were pooled together for further analysis. Consistent with our previous report (Malon et al., 2011), compared to both non-injected and vehicle injected controls, CGRP8–37 significantly reduced L5Tx-induced mechanical hypersensitivity beginning at day 7 and continued until the end of the experiment (day 20) (Figure 1A, Two-way ANOVA, ptreatment < 0.001, ptime < 0.001 and pinteraction < 0.001; lumbar spinal cords were collected from selected mice at various time points for chemokine analysis below, thus here n = 9–45). For the Hargreaves test, initial analysis suggested a gender difference (Figure 1B, three-way ANOVA with time, treatment and sex as factors, psex = 0.023), therefore, data from male and female mice were analyzed separately. In both male and female mice CGRP8–37 did not significantly affect L5Tx-induced heat hypersensitivity (male Two-way RM ANOVA, ptreatment = 0.937, ptime < 0.001 and pinteraction = 0.068; and female Two-way RM ANOVA, ptreatment = 0.222, ptime < 0.001 and pinteraction = 0.168). Since no differences were detected in these separate analyses for males vs. for females, all data were pooled together to be shown in Figure 1B. Together, this set of data combined with our previous report, points to CGRP’s involvement in the maintenance of mechanical, but not heat, hypersensitivities following L5Tx.
Figure 1. Effects of CGRP8–37 on L5Tx-induced behavioral hypersensitivities in mice.
Wild type BALB/c mice were i.t. injected with either a CGRP antagonist (CGRP8–37, 1 μg/5 μl/mouse) or vehicle control daily from day 6 to 13 post-L5Tx. Mice were tested for mechanical (A) and heat (B) sensitivities via von Frey filaments using the up-down method and the Hargreaves test respectively. Data are presented as mean ± SEM (in A: n = 9–45 per group; in B: n = 8–12 per group). Note that in both A and B, L5Tx induced statistically significant behavioral hypersensitivities in all groups (not marked in the graphs). In addition, * indicates p < 0.05, compared between the indicated group and all other treatment groups at the same time point.
3.2. Reduction of L5Tx-induced lumbar spinal cord CCL5 following the treatment of CGRP8–37
To determine whether chemokines are involved in CGRP-mediated mechanical hypersensitivity, lumbar spinal cords were collected from individual mice in the above three groups: i.t. with CGRP8–37, i.t. with vehicle, and non-treated group, and were then analyzed for selected chemokine expression via a multiplex assay. Of the eight chemokines tested, CGRP8–37 only significantly reduced L5Tx-induced increase of spinal cord CCL5 (Figure 2A, two-way ANOVA comparing data from day 7, 14 and 20, pgroup = 0.041, ptime = 0.054, and pinteraction = 0.427). Although levels of spinal cord CCL2 and CCL11 were also significantly increased by L5Tx, they were not affected by CGRP8–37 treatment (Figure 2B, one-way ANOVA for “no i.t.” groups p < 0.001; Figure 2C, one-way ANOVA for “no i.t.” groups p = 0.049). Therefore, our data indicate that CGRP can play its pro-nociceptive role through the induction of CCL5 in lumbar spinal cord following L5Tx.
Figure 2. Effects of CGRP8–37 on lumbar spinal cord chemokine production in mice post-L5Tx.
Lumbar spinal cords were collected from mice that received no i.t. injection, CGRP8–37 (1 μg/5 μl/mouse) i.t. injection or saline i.t. injection. Chemokines, CXCL1 (KC), CCL1 (TCA-3), CCL2 (MCP-1), CCL3 (MIP-1α), CCL5 (RANTES), CCL11 (Eotaxin), CCL17 (TARC), and CCL22 (MDC) within these spinal cord samples were determined via a multiplex assay. The three chemokines, CCL5 (A), CCL2 (B) and CXCL11 (C) that were significantly induced by L5Tx are shown here. Data are presented as mean ± SEM (n = 9–45 per group). * indicates p < 0.05 compared between the indicated group and all other treatment groups at the same time point. # indicates p < 0.05 compared between the indicated group and the “Day 0” (naïve) group.
3.3. Reduction of L5Tx-induced mechanical hypersensitivity following the treatment of CCL5 neutralizing antibody
To directly test the effects of CCL5 in L5Tx-induced mechanical hypersensitivity, WT mice were i.t. treated with a CCL5 neutralizing Ab (0.5or 2.5 μg per 5 μl per mouse) daily from days 6 to 13 post-L5Tx. For all treatment groups, L5Tx induced significant mechanical hypersensitivity. Both doses of anti-CCL5 significantly reduced L5Tx-induced mechanical hypersensitivity at day 7, 10, 14, and 20 post-surgery when compared to either PBS injected or non-injected groups (Figure 3, two-way RM ANOVA, pgroup < 0.001, ptime < 0.001, and pinteraction < 0.001). Together with the data presented in Figure 2, these data further support the involvement of spinal cord CCL5 in CGRP-mediated pro-nociceptive role.
Figure 3. Effects of anti-CCL5 neutralizing antibody on L5Tx-induced mechanical hypersensitivity in mice.
Wild type BALB/c mice were i.t. injected with either one of the two doses of anti-CCL5 neutralizing antibody (αCCL5 at 0.5 and 2.5 μg/5 μl/mouse) or a vehicle control (PBS) daily from day 6 to 13 post-L5Tx. Mice were tested for mechanical sensitivities via von Frey filaments using the up-down method. Data are presented as mean ± SEM (n = 8 per group). Note that L5Tx induced statistically significant behavioral hypersensitivities in all groups at all selected times post-L5Tx (not marked in the graphs). In addition, * indicates p < 0.05, compared between the indicated group and both non-injected and PBS injected groups at the same time point.
3.4. L5Tx-induced activation of MAPK pathway
MAPK pathways, including p38, JNK and ERK pathways have been shown to be involved in the chronic pain state in animal models (Chen et al., 2013; Gao and Ji, 2010a; Ji and Suter, 2007; Ji et al., 2007; Li et al., 2010). We then examined the involvement of these pathways in L5Tx-induced behavioral hypersensitivity. Spinal cord tissues were collected from injured and sham-operated animals at days 0 (naïve), 1, 3, 7, 10 and 14 post-surgery and processed for the analysis of phosphorylated p38, JNK and ERK (pho-p38, pho-JNK and pho-ERK) via CBA assay. Spinal cord levels of pho-p38 gradually increased following L5Tx, which peaked at day 7 post-L5Tx, and returned to baseline by day 14 post-L5Tx (Figure 4A, two-way ANOVA, pgroup = 0.045, ptime = 0.018, and pinteraction = 0.599). L5Tx did not significantly change the levels of pho-JNK (Figure 4B, two-way ANOVA, pgroup = 0.460, ptime = 0.665, and pinteraction = 0.947). The levels of pho-ERK were too low to be detected in the majority of tissue samples.
Figure 4. Lumbar spinal cord levels of MAPK pathway proteins post-L5Tx in mice.
Lumbar spinal cords were collected from mice that underwent either L5Tx or sham surgery. MAPK pathway proteins, pho-p38 (A), pho-JNK (B), and pho-ERK (too low to be detected in most samples, data not shown) within lumbar spinal cord were determined via the CBA assay. Data are presented as mean ± SEM (n = 6–7 per group). * indicates p < 0.05 compared between the indicated group and the sham group at the same time point. # indicates p < 0.05 compared between the indicated group and the “Day 0” (naïve) group.
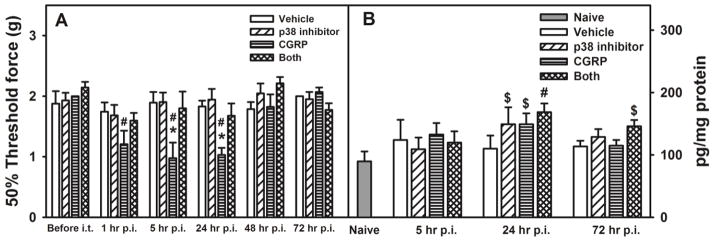
3.5. Reduction of CGRP-induced mechanical hypersensitivity by pre-treatment with a p38 inhibitor
To further test whether the p38 MAPK pathway is involved in CGRP mediated mechanical hypersensitivity, WT mice were i.t. treated with a p38 inhibitor SB203580 30 min prior to CGRP i.t. injection. Mice were randomly assigned to one of the following 4 groups: vehicle-vehicle, p38 inhibitor-vehicle, vehicle-CGRP, and p38 inhibitor-CGRP injected. We observed that one injection of CGRP significantly increased mechanical sensitivity at 1, 5, and 24 hours after CGRP injection, and injection of the p38 inhibitor before CGRP was able to reduce the CGRP-induced mechanical hypersensitivity seen at 1, 5, and 24 hours post-CGRP injection (Figure 5A, two-way RM ANOVA, pgroup = 0.009, ptime < 0.001, and pinteraction = 0.001). Furthermore, to test whether the p38 MAPK pathway is involved in CGRP-induced CCL5 production, levels of spinal cord CCL5 were quantified in mice from the above 4 groups. Single CGRP i.t. injection induced a slight increase of CCL5 in the lumbar spinal cord and this increase was not affected by SB203580. Unexpectedly, SB203580 induced a slight increase of CCL5 in the lumbar spinal cord 24 hours post-i.t. injection (Figure 5B, two-way ANOVA, pgroup = 0.404, ptime < 0.001, and pinteraction = 0.782). Thus, data suggest that CGRP/CCL5 mediated pro-nociceptive pathway is p38-independent and an additional p38-dependent/CCL5-independent pathway is possible.
Figure 5. Effects of a p38-inhibitor on CGRP-induced mechanical hypersensitivity and lumbar spinal cord levels of CCL5.
Wild type BALB/c mice were randomly assigned to the following treatment groups: vehicle/vehicle, SB 203580 (a p38-inhibitor, 5 ng/5 μl/mouse)/vehicle, vehicle/CGRP (2 μg/5 μl/mouse), and SB 203580 (5 ng/5 μl/mouse)/CGRP (2 μg/5 μl/mouse). In A, Mechanical sensitivity was tested via von Frey filaments using the up-down method. Data are shown as mean ± SEM (n = 8 per group). * indicates p < 0.05, compared between the indicated group and all other treatment groups at the same time point. # p < 0.05, compared between the indicated group and the corresponding “before i.t” group. In B, lumbar spinal cord levels of CCL5 following i.t injections were measured via ELISA. Data are shown as mean ± SEM (n = 8 per group). # indicates p < 0.05, compared between the indicated group and the “naive” group. $ indicates 0.05 < p < 0.1, compared between the indicated group and the “naive” group.
CGRP receptors have been detected on CNS glia, including both astrocytes and microglia (Moreno et al., 2002; Parsons and Seybold, 1997). To further identify the cellular source of CGRP-induced CCL5 and pho-p38, primary mixed glial cells established from adult mouse spinal cords were treated with various doses of CGRP in vitro. pho-p38, pho-JNK and pho-ERK in the cells, and CCL5 in the supernatants were measured via CBA assay and ELISA respectively, 24 and 48 hours post-treatment. Under the treatment condition we used, CGRP did not induce a significant increase in the expression of any of the MAPK pathway proteins or CCL5 release (data not shown).
4. Discussion
In this current study, we investigated the role of CGRP in peripheral nerve injury-induced neuropathic pain-like behaviors using a well-established mouse model, L5Tx. Our results indicate a novel neuroimmune pathway involved in the development of neuropathic pain behavior: peripheral nerve injury -> increased spinal cord CGRP expression (shown in our previous study (Malon et al., 2011)) -> increased spinal cord CCL5 production -> mechanical hypersensitivity (Figure 6, thick lines). It should be noted that we have performed pilot study with CGRP8–37 at both 0.5 and 1 μg/mouse and CGRP8–37 at 0.5 μg/mouse was able to reduce L5Tx-induced mechanical hypersensitivity up to day 14 post-L5Tx; however, this reduction declined at days 17 and 20 post-L5Tx. Thus, we think the effects of CGRP8–37 are dose-dependent and the effects of 1 μg of CGRP are not permanent. Interestingly, this pathway does not seem to be involved in L5Tx-induced heat hypersensitivity as measured by the Hargreaves test. Although unexpected, similar phenomena have been observed by others. For example, in a rat model of sleep disruption, mechanical and heat hypersensitivity exhibited differential sensitivity to several pharmacological agents, suggesting differing and dissociable mechanisms in mechanical and heat sensory responses (Wodarski et al., 2014). A study with mice lacking various types of prostaglandin receptors also showed disparities in the responses following mechanical vs. heat stimuli (Popp et al., 2009). Together with the current study, these studies suggest possible modality-specific sensory signal transduction pathways in nociception. Although it is well known that different types of noxious stimuli activate different transient receptor potential channels (TRP channels) (Nilius et al., 2007), studies have indicated that the interaction between different TRP channels expressed by a sensory neuron, rather than the existence of a specific TRP channel, determines the sensory neuron’s response to a certain stimulus (Belmonte and Viana, 2008). CGRP may influence the composition of TRP channels expressed by the sensory neurons and thus modify their response to mechanical stimulus specifically. CGRP may also target particular inflammatory pathways (p38- or CCL5-mediated) that control the development of mechanical hypersensitivity. One study of interest in rats found that bee venom-induced inflammation during the neonatal stage modified the mechanical, but not thermal responses, in adulthood. This modification was associated with changes in IL-1beta and cyclooxygenase 2 (COX-2) expression in the spinal cord (Li et al., 2014). Another study, which used a mouse model of second-degree burn injury, demonstrated that the voltage-gated sodium channel Na(v)1.7 was essential for lowering the threshold for the heat but not the mechanical sensitivity response after burn injury (Shields et al., 2012). It is possible that certain sodium channels are critical for mechanical sensitivity, and these channels can be modified by CGRP. Delineating the differences within these pathways may result in more specific and individualized treatment strategies for neuropathic pain patients, a patient population that exhibits a wide range of varying responses to noxious and non-noxious stimuli (Baron, 2006).
Figure 6. Summary of neuroimmune pathways that mediate L5Tx-induced mechanical hypersensitivity.
Pathways marked with solid lines are those identified in the current study. Pathways marked with dash lines are those identified in our previous studies (Malon et al., 2011).
In the current study, we also examined the involvement of various MAPK pathways in the effects mediated by CGRP. Although JNK, ERK and p38 MAPK pathways have all been associated with the development of neuropathic pain (Gao and Ji, 2010a; Gao and Ji, 2009; Gao and Ji, 2010b; Ji and Suter, 2007), we only observed a significant upregulation of spinal cord pho-p38 following L5Tx. Using a p38 pathway inhibitor, we demonstrated that CGRP’s effect on mechanical hypersensitivity can be mediated through the activation of the p38 pathway. Despite this, our data suggest that CGRP-mediated CCL5 production following L5Tx was p38-idenpendent. Thus, at least two separate pathways downstream of CGRP, a p38-dependent/CCL5-dependent pathway and a CCL5-dependent/p38 independent pathway are involved in mediating L5Tx-induced mechanical hypersensitivity (Figure 6 all solid lines). In addition, based on the previous reports on the role of CCL5 in neuropathic pain (Liou et al., 2013; Liou et al., 2012) and the fact that CCL5 was still notably elevated at day 20 post-L5Tx (Figure 2A), we expect that CCL5 is still contributing to L5Tx-induced mechanical hypersensitivity at day 20, although it may be less tightly regulated by CGRP at this time.
Previously, we have shown that microglial CD40 (a co-stimulatory molecule whose expression is increased by activated microglia) is critical in maintaining the upregulation of CGRP, i.e., CD40 signaling acts upstream of CGRP signaling (Figure 6). In that study, we also showed that CD40 supported CCL2 production in the spinal cord following L5Tx; however, CGRP and CCL2 appeared to act independently to promote L5Tx-induced mechanical hypersensitivity ((Malon et al., 2011) and current observation) (Figure 6 dash lines on the right side). In summary, we have identified several interconnected neuroimmune pathways that all contribute to the development of L5TX-induced neuropathic pain-like behaviors (Figure 6). It is very clear that a multifactorial neuroimmune network is regulating these behaviors and that further dissecting this network will lead to a better understanding of peripheral nerve injury-induced pain-like behaviors.
The involvement of CGRP in the development of neuropathic pain may depend on the peripheral nerve injury model being examined. In a previous study (Zheng et al., 2008), changes in CGRP expression were examined in three different sciatic nerve injury models: crush (SNC), ligation (SNL), and transection combined with subsequent neurorrhaphy (SNT). Both SNC and DNT induced significant increase in CGRP expression in the lumbar spinal cord, while SNL induced sustained reduction of lumbar spinal cord CGRP expression. Similar reduction was also observed with L5 spinal nerve ligation model (Kaku et al., 2007). One may predict that CGRP is more likely to contribute to the development of neuropathic pain in peripheral nerve injury models that induce significant upregulation of CGRP compared to models that result in decrease of CGRP expression.
Consistent with the above discussion regarding mechanical vs. heat sensitivity, distinct pathways may be involved in mediating different neuropathic pain-like behaviors. Many details within the neuroimmune network require further investigation. The underlying mechanisms through which CD40-expressing microglia interact with primary sensory neurons to sustain CGRP expression in the spinal cord is particular interesting, as it may provide a target for modulating neuronal response early after peripheral nerve injury, thus reducing or even preventing injury-induced central sensitization. CX3CL1/CX3CR1 signaling has been recognized as a critical link between the primary sensory neurons and spinal cord microglia during the central sensitization of neuropathic pain (Clark and Malcangio, 2014). It has been shown that peripheral nerve injury-activated microglia could further stimulate neuronal release of CX3CL1 (fractalkine) through microglial production of lysosomal protease cathepsin S (CatS) (Clark and Malcangio, 2014). Elevated CatS mRNA levels have been associated with increased CD40 mRNA levels in the plasma of patients with coronary atherosclerosis (Cerne et al., 2011). Thus, it is possible that microglia-expressed CatS serves as one of the mediators and facilitates the interaction between CD40-expressing microglia and nociceptors. Specifically, CD40 signaling may act upstream and promote microglial expression of CatS, which could further enhance CGRP release from nociceptors and the subsequent production of CCL5 in the spinal cord.
In our study, L5Tx significantly induced the production of CCL2, CCL5 and CCL11 in the lumbar spinal cord. It was somewhat unexpected that CGRP was not involved in the upregulation of CCL2 and CCL11, and its effects were specific to CCL5. Nevertheless, others have reported similar phenomena regarding CGRP’s effects. For example, CGRP was observed to specifically stimulate the production of CXCL8, but not CCL2 or CCL5 by human corneal epithelial cells (Tran et al., 2000). Conversely, CGRP was found to inhibit the production of several chemokines by human dermal microvascular endothelial cells upon lipopolysaccharide stimulation (Huang et al., 2011). Thus, CGRP’s effects on chemokines appear to be cell/tissue type-specific and these differential effects are likely to be mediated by different signaling pathways. This CGRP-specific effect on chemokine production may also explain the aforementioned sensory modality-specific response mediated by CGRP. In addition, due to the limitation of the pre-assembled multiplex assay, we only examined eight chemokines. It is possible that CGRP also regulates the production of other chemokines, such as CXCL10, CXCL12 and CXCL21, which have been implicated in the development of neuropathic pain. As such, we will investigate CGRP’s effects on other chemokines in the future.
Interestingly, CCL5 has been identified as one of the potential biomarkers for lumbar disc degeneration in humans (Grad et al., 2016). Considering that disc degeneration can often cause nerve-root damage and subsequent neuropathic pain, this recent observation may suggest that the pathophysiological changes identified in our L5Tx model could be translated to disc degeneration-associated neuropathic pain in humans. Besides targeting on CCL5, the role of CGRP in low back pain should be further explored, as appropriate blocking of CGRP responses may lead to alleviation of discogenic neuropathic pain. It is also important to note that CGRP blocking has been shown to be effective in a chronic central neuropathic pain model, spinal cord hemisection (Bennett et al., 2000b).
Glial cell activation and the subsequent inflammatory responses are well-known to be critical in the central sensitization process during the development of neuropathic pain (DeLeo et al., 2004; Ji and Suter, 2007; Milligan and Watkins, 2009; Tsuda et al., 2005). Interestingly, under our experimental conditions, we did not see a significant increase of CCL5 when CGRP was used to stimulate mixed adult glial cells in vitro. Although further experiments are needed to determine the contribution of glial cells to the CCL5 production we observe post L5Tx, other cellular sources such as neurons, should also be considered. For example, increased expression of several chemokines (CCL2, CCL5 and CXCL10) has been observed in dorsal root ganglia neurons in different rodent models of neuropathic pain (Bhangoo et al., 2007; Bhangoo et al., 2009).
Altogether, our current study demonstrated a CGRP-CCL5 mediated pathway in peripheral nerve injury-induced mechanical hypersensitivity. Together with our previous studies, we have identified a neuroimmune network that is involved in the development of peripheral nerve injury-induced mechanical hypersensitivity (summarized in Figure 6). Further delineation of the molecular/cellular mechanisms within these pathways may lead to new treatments for neuropathic pain. It should be noted that many components in this neuroimmune network are involved in maintaining normal physiological functions. For example, chemokines and CD40 are vital mediators in immune responses. Global inhibition of these factors can cause sever unwanted side effects. Based on our observations, in order to effectively inhibit the progression of neuropathic pain, multiple factors may need to be targeted simultaneously in order to completely block the advancement of pain pathways. Inhibition of CCL5 and CCL2 together, or blocking CCL2 while treating with a CGRP antagonist would be more effective in providing pain relief than inhibiting any one of these molecules alone. Further, the potential synergistic effects between individual treatments may help to reduce the side effects from each treatment alone, which has been shown when the combination treatment with clonidine (an alpha-2 adrenoceptor agonist) and p38 MAPK inhibition were used in an oxaliplatin-induced neuropathic pain model (Yeo et al., 2016). In summary, potential effective treatment for neuropathic pain should target multiple pro-nociceptive mediators and ideally, in order to reduce the side effects, should be known to specifically affect the injury or disease-induced function of these mediators.
Highlights.
CGRP contributes to spinal nerve L5 transection-induced mechanical hypersensitivity.
CGRP can play its pro-nociceptive role through both a spinal cord CCL5-dependent, p38-independent pathway, and a p38-depenented, CCL5-independent pathway.
Acknowledgments
This study was funded by a NIH/NIGMS COBRE award, P20GM103643 (PD Meng; subproject 1 PI, CAO). We appreciate the effort devoted by the staff at the Quansys Biosciences for identifying the optimal conditions to analyze our samples. We also thank Dr. Ian Meng (Biomedical Sciences Department, College of Osteopathic Medicine, University of New England) for critically reading the manuscript and providing valuable comments.
Abbreviations
- ANOVA
analysis of variance
- CatS
cathepsin S
- cDMEM
complete Dulbecco’s Modified Eagle Media
- CGRP
Calcitonin gene-related peptide
- COX-2
cyclooxygenase 2
- ELISA
enzyme-linked immunosorbent assay
- ERK
extracellular signal-regulated kinase
- IACUC
Institutional Animal Care and Use Committee
- JNK
Jun N-terminal kinase
- L5Tx
spinal nerve L5 transection
- MAPK
Mitogen activated protein Kinase
- MCP-1
monocyte chemoattractant protein-1
- NCI
National Cancer Institute
- RANTES
regulated on activation, normal T cell expressed and secreted
- SNK
Student-Newman-Keuls
- SNL
spinal nerve ligation
- TRP
transient receptor potential
- UNE
University of New England.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jennifer T. Malon, Email: jmalon@UNE.edu.
Ling Cao, Email: lcao@UNE.edu.
References
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A. 2003;100:7947– 7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda JL, Sweitzer S, Rutkowski MD, DeLeo JA. Intrathecal anti-IL-6 antibody and IgG attenuates peripheral nerve injury-induced mechanical allodynia in the rat: possible immune modulation in neuropathic pain1. Brain Research. 2000;879:216–225. doi: 10.1016/s0006-8993(00)02807-9. [DOI] [PubMed] [Google Scholar]
- Arulmani U, MaassenVanDenBrink A, Villalón CM, Saxena PR. Calcitonin gene-related peptide and its role in migraine pathophysiology. European Journal of Pharmacology. 2004;500:315–330. doi: 10.1016/j.ejphar.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Baron R. Mechanisms of disease: neuropathic pain--a clinical perspective. Nat Clin Pract Neurol. 2006;2:95–106. doi: 10.1038/ncpneuro0113. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Viana F. Molecular and cellular limits to somatosensory specificity. Mol Pain. 2008;4:14. doi: 10.1186/1744-8069-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benamar K, Geller EB, Adler MW. Elevated level of the proinflammatory chemokine, RANTES/CCL5, in the periaqueductal grey causes hyperalgesia in rats. European Journal of Pharmacology. 2008;592:93–95. doi: 10.1016/j.ejphar.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Bennett AD, Chastain KM, Hulsebosch CE. Alleviation of mechanical and thermal allodynia by CGRP8-37 in a rodent model of chronic central pain. Pain. 2000a;86:163–175. doi: 10.1016/s0304-3959(00)00242-6. [DOI] [PubMed] [Google Scholar]
- Bennett AD, Chastain KM, Hulsebosch CE. Alleviation of mechanical and thermal allodynia by CGRP8-37 in a rodent model of chronic central pain. Pain. 2000b;86:163–175. doi: 10.1016/s0304-3959(00)00242-6. [DOI] [PubMed] [Google Scholar]
- Bhangoo S, Ren D, Miller RJ, Henry KJ, Lineswala J, Hamdouchi C, Li B, Monahan PE, Chan DM, Ripsch MS, White FA. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: a mechanism for the development of chronic sensitization of peripheral nociceptors. Mol Pain. 2007;3:38. doi: 10.1186/1744-8069-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhangoo SK, Ripsch MS, Buchanan DJ, Miller RJ, White FA. Increased chemokine signaling in a model of HIV1-associated peripheral neuropathy. Mol Pain. 2009;5:48. doi: 10.1186/1744-8069-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Butler MB, Tan L, Draleau KS, Koh WY. Murine immunodeficiency virus-induced peripheral neuropathy and the associated cytokine responses. J Immunol. 2012;189:3724–3733. doi: 10.4049/jimmunol.1201313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, DeLeo JA. CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur J Immunol. 2008;38:448–458. doi: 10.1002/eji.200737485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Palmer CD, Malon JT, De Leo JA. Critical role of microglial CD40 in the maintenance of mechanical hypersensitivity in a murine model of neuropathic pain. Eur J Immunol. 2009;39:3562–3569. doi: 10.1002/eji.200939657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerne D, Stern I, Marc J, Cerne A, Zorman D, Krzisnik-Zorman S, Kranjec I. CTSS activation coexists with CD40 activation in human atheroma: evidence from plasma mRNA analysis. Clin Biochem. 2011;44:438–440. doi: 10.1016/j.clinbiochem.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen XY, Li K, Light AR, Fu KY. Simvastatin Attenuates Formalin-Induced Nociceptive Behaviors by Inhibiting Microglial RhoA and p38 MAPK Activation. The Journal of Pain. 2013;14:1310–1319. doi: 10.1016/j.jpain.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Clark AK, Malcangio M. Fractalkine/CX3CR1 signaling during neuropathic pain. Front Cell Neurosci. 2014;8:121. doi: 10.3389/fncel.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cridland RA, Henry JL. Intrathecal administration of CGRP in the rat attenuates a facilitation of the tail flick reflex induced by either substance P or noxious cutaneous stimulation. Neurosci Lett. 1989;102:241–246. doi: 10.1016/0304-3940(89)90085-2. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- Elshal MF, McCoy JP. Multiplex bead array assays: Performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–323. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics. 2010a;7:482–493. doi: 10.1016/j.nurt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009;2:11–17. doi: 10.2174/1876386300902010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010b;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardell LR, Vanderah TW, Gardell SE, Wang R, Ossipov MH, Lai J, Porreca F. Enhanced evoked excitatory transmitter release in experimental neuropathy requires descending facilitation. J Neurosci. 2003;23:8370–8379. doi: 10.1523/JNEUROSCI.23-23-08370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grad S, Bow C, Karppinen J, Luk KDK, Cheung KMC, Alini M, Samartzis D. Systemic blood plasma CCL5 and CXCL6: Potential biomarkers for human lumbar disc degeneration. Eur Cell Mater. 2016;31:1–10. doi: 10.22203/ecm.v031a01. [DOI] [PubMed] [Google Scholar]
- Huang J, Stohl LL, Zhou X, Ding W, Granstein RD. Calcitonin gene-related peptide inhibits chemokine production by human dermal microvascular endothelial cells. Brain Behav Immun. 2011;25:787–799. doi: 10.1016/j.bbi.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Suter M. p38 MAPK, microglial signaling, and neuropathic pain. Molecular Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Zhang YQ. Protein kinases as potential targets for the treatment of pathological pain. Handb Exp Pharmacol. 2007:359–389. doi: 10.1007/978-3-540-33823-9_13. [DOI] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017– 4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku R, Yokoyama M, Kobayashi H, Matsuoka Y, Sato T, Mizobuchi S, Itano Y, Morita K. Altered Response to Formalin by L5 Spinal Nerve Ligation in Rats: A Behavioral and Molecular Study. Anesthesia & Analgesia. 2007;104:936–943. doi: 10.1213/01.ane.0000258762.22607.15. [DOI] [PubMed] [Google Scholar]
- Lee SE, Kim JH. Involvement of substance P and calcitonin gene-related peptide in development and maintenance of neuropathic pain from spinal nerve injury model of rat. Neuroscience Research. 2007;58:245–249. doi: 10.1016/j.neures.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Li K, Lin T, Cao Y, Light AR, Fu KY. Peripheral Formalin Injury Induces 2 Stages of Microglial Activation in the Spinal Cord. The Journal of Pain. 2010;11:1056–1065. doi: 10.1016/j.jpain.2010.01.268. [DOI] [PubMed] [Google Scholar]
- Li M, Chen H, Tang J, Chen J. Neonatal bee venom exposure induces sensory modality-specific enhancement of nociceptive response in adult rats. Pain Med. 2014;15:986–997. doi: 10.1111/pme.12296. [DOI] [PubMed] [Google Scholar]
- Liou JT, Mao CC, Ching-Wah Sum D, Liu FC, Lai YS, Li JC, Day YJ. Peritoneal administration of Met-RANTES attenuates inflammatory and nociceptive responses in a murine neuropathic pain model. J Pain. 2013;14:24–35. doi: 10.1016/j.jpain.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Liou JT, Yuan HB, Mao CC, Lai YS, Day YJ. Absence of C-C motif chemokine ligand 5 in mice leads to decreased local macrophage recruitment and behavioral hypersensitivity in a murine neuropathic pain model. Pain. 2012;153:1283–1291. doi: 10.1016/j.pain.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Malon JT, Maddula S, Bell H, Cao L. Involvement of calcitonin gene-related peptide and CCL2 production in CD40-mediated behavioral hypersensitivity in a model of neuropathic pain. Neuron Glia Biol. 2011;7:117–128. doi: 10.1017/S1740925X12000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno MJ, Terron JA, Stanimirovic DB, Doods H, Hamel E. Characterization of calcitonin gene-related peptide (CGRP) receptors and their receptor-activity-modifying proteins (RAMPs) in human brain microvascular and astroglial cells in culture. Neuropharmacology. 2002;42:270–280. doi: 10.1016/s0028-3908(01)00176-9. [DOI] [PubMed] [Google Scholar]
- Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J, Lowe L, Chen R, Shivraj L, Agadir A, Campos R, Ernst D, Gaur A. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clinical Immunology. 2004;110:252–266. doi: 10.1016/j.clim.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and Glycoprotein120 Produce Pain Hypersensitivity by Directly Exciting Primary Nociceptive Neurons. The Journal of Neuroscience. 2001;21:5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku R, Satoh M, Fujii N, Otaka A, Yajima H, Takagi H. Calcitonin gene-related peptide promotes mechanical nociception by potentiating release of substance P from the spinal dorsal horn in rats. Brain Research. 1987;403:350–354. doi: 10.1016/0006-8993(87)90074-6. [DOI] [PubMed] [Google Scholar]
- Parsons AM, Seybold VS. Calcitonin gene-related peptide induces the formation of second messengers in primary cultures of neonatal rat spinal cord. Synapse. 1997;26:235–242. doi: 10.1002/(SICI)1098-2396(199707)26:3<235::AID-SYN5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Popp L, Haussler A, Olliges A, Nusing R, Narumiya S, Geisslinger G, Tegeder I. Comparison of nociceptive behavior in prostaglandin E, F, D, prostacyclin and thromboxane receptor knockout mice. Eur J Pain. 2009;13:691–703. doi: 10.1016/j.ejpain.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Schubert R, Geiger H, Zielen S, Baer PC. Simultaneous detection of ERK-, p38-, and JNK-MAPK phosphorylation in human adipose-derived stem cells using the Cytometric Bead Array technology. Journal of Immunological Methods. 2009;350:200–204. doi: 10.1016/j.jim.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Shields SD, Cheng X, Uceyler N, Sommer C, Dib-Hajj SD, Waxman SG. Sodium channel Na(v)1.7 is essential for lowering heat pain threshold after burn injury. J Neurosci. 2012;32:10819–10832. doi: 10.1523/JNEUROSCI.0304-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran MT, Ritchie MH, Lausch RN, Oakes JE. Calcitonin gene-related peptide induces IL-8 synthesis in human corneal epithelial cells. J Immunol. 2000;164:4307–4312. doi: 10.4049/jimmunol.164.8.4307. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci U S A. 2007;104:20151–20158. doi: 10.1073/pnas.0709250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarski R, Schuh-Hofer S, Yurek DA, Wafford KA, Gilmour G, Treede RD, Kennedy JD. Development and pharmacological characterization of a model of sleep disruption-induced hypersensitivity in the rat. Eur J Pain. 2014 doi: 10.1002/ejp.580. [DOI] [PubMed] [Google Scholar]
- Yeo JH, Yoon SY, Kim SJ, Oh SB, Lee JH, Beitz AJ, Roh DH. Clonidine, an alpha-2 adrenoceptor agonist relieves mechanical allodynia in oxaliplatin-induced neuropathic mice; potentiation by spinal p38 MAPK inhibition without motor dysfunction and hypotension. International Journal of Cancer. 2016;138:2466–2476. doi: 10.1002/ijc.29980. [DOI] [PubMed] [Google Scholar]
- Yu LC, Hansson P, Brodda-Jansen G, Theodorsson E, Lundeberg T. Intrathecal CGRP8-37-induced bilateral increase in hindpaw withdrawal latency in rats with unilateral inflammation. Br J Pharmacol. 1996a;117:43–50. doi: 10.1111/j.1476-5381.1996.tb15152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LC, Hansson P, Lundeberg T. The calcitonin gene-related peptide antagonist CGRP8-37 increases the latency to withdrawal responses bilaterally in rats with unilateral experimental mononeuropathy, an effect reversed by naloxone. Neuroscience. 1996b;71:523–531. doi: 10.1016/0306-4522(95)00428-9. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Cao DL, Zhang X, Ji RR, Gao YJ. Chemokine contribution to neuropathic pain: respective induction of CXCL1 and CXCR2 in spinal cord astrocytes and neurons. Pain. 2013;154:2185–2197. doi: 10.1016/j.pain.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng LF, Wang R, Xu YZ, Yi XN, Zhang JW, Zeng ZC. Calcitonin gene-related peptide dynamics in rat dorsal root ganglia and spinal cord following different sciatic nerve injuries. Brain Research. 2008;1187:20–32. doi: 10.1016/j.brainres.2007.10.044. [DOI] [PubMed] [Google Scholar]