Abstract
Background
Pelvic girdle pain (PGP) is a multifactorial condition, which can be mentally and physically compromising both during and after pregnancy. However, long-term pregnancy-related PGP has been poorly investigated. This longitudinal follow-up study uniquely aimed to describe prevalence and predictors of PGP and its consequences on women’s health and function up to 11 years after pregnancy.
Methods/Design
A postal questionnaire was sent to 530 women who participated in 1 of 3 randomized controlled studies for PGP in pregnancy. Women who reported experiencing lumbopelvic pain were offered a clinical examination. Main outcome measure was the presence of long term PGP as assessed by an independent examiner. Secondary outcomes were: working hours/week, function (the Disability Rating Index, and Oswestry Disability Index), self-efficacy (the General Self-Efficacy Scale), HRQL (Euro-Qol 5D and EQ-Visual scale), anxiety and depression, (Hospital anxiety and depression scale,) and pain-catastrophizing (Pain Catastrophizing Scale), in women with PGP compared to women with no PGP.
Results
A total of 371/530 (70 %) women responded and 37/ 371 (10 %) were classified with long-term PGP. Pregnancy-related predictors for long-term PGP were number of positive pain provocation tests (OR = 1.79), history of low back pain (LBP) (OR = 2.28), positive symphysis pressure test (OR = 2.01), positive Faber (Patrick’s) test (OR = 2.22), and positive modified Trendelenburg test (OR = 2.20). Women with PGP had significantly decreased ability to perform daily activities (p < .001), lower self-efficacy (p = 0.046), decreased HRQL (p < .001), higher levels of anxiety and depression (p < .001), were more prone to pain catastrophizing, and worked significantly fewer hours/week (p = 0.032) compared to women with no PGP.
Conclusions
This unique long-term follow up of PGP highlights the importance of assessment of pain in the lumbopelvic area early in pregnancy and postpartum in order to identify women with risk of long term pain. One of 10 women with PGP in pregnancy has severe consequences up to 11 years later. They could be identified by number of positive pain provocation tests and experience of previous LBP. Access to evidence based treatments are important for individual and socioeconomic reasons.
Keywords: Anxiety, Depression, Function, Health-related quality of life, Long-term, Pain catastrophizing, Pelvic girdle pain, Predictor, Self-efficacy
Background
Pelvic girdle pain (PGP) is a multifactorial condition with a partly unknown aetiology [1, 2] PGP can be mentally and physically compromising both during and after pregnancy [2–12]. Previous research reports a prevalence of PGP from the postpartum stage to 3 years after childbirth from 1 to 43 % [13–16], and 7 % at 6 years [17]. Disability and pain intensity in pregnancy are associated with sick leave due to pain in pregnancy and persistent pain [16, 18, 19]. Some studies have focused on prevalence, consequences, risk factors, prognostic factors [2, 9, 13, 16–18, 20–35] and protecting factors while others have evaluated treatment outcomes [36–38]. However, the prevalence predictors and consequences of long-term pregnancy-related PGP have been poorly investigated.
Guidelines for the diagnosis of PGP state that this pain is experienced between the posterior iliac crest and the gluteal fold, particularly in the vicinity of the sacroiliac joints (SIJ), separately or in conjunction with pain in the symphysis, and that pain and functional disturbances in relation to PGP must be reproducible by specific clinical tests [5]. In most studies, however, no examination during pregnancy was performed, and there is no study with more than a 3-year follow-up postpartum that has classified PGP by clinical examination [14]. Women with a pelvic girdle syndrome (PGS), i.e. an anterior and posterior pain location, have been found to have the worst prognosis, and those with isolated anterior, i.e. symphysiolysial pain, the best prognosis [13].
Suggested pregnancy-related predictors for long-term PGP include demographic variables, such as: age [2, 23, 27] and high Body Mass Index (BMI) [16], work-related variables strenuous work [28] and sick leave [29], delivery-related variables e.g. previous caesarean section [21, 26, 30], and higher fetal weight [20], PGP-related variables e.g. previous low back pain (LBP) [2, 31], hypermobility [16], severe pain [2, 16], decreased function [31], ≥8 h sleep or rest/day [20], PGS [13, 32], difficulty in performing the Active Straight Leg Raise test [33], number of positive pain provocation tests [31, 34, 39] and emotional distress [25].
Development from acute to chronic pain is complex, and more research is needed to explore why some women develop long-term PGP after delivery, which women are at greater risk, and whether prevention is possible. To our knowledge, this is the only longitudinal follow-up study describing the prevalence, predictors and consequences of PGP up to 11 years after pregnancy.
Methods
Data were obtained through one self-administered questionnaire, sent and returned by mail. Two reminders were sent. Presence of lumbopelvic pain (LPP) was assessed by one question, recommended by a modified Delphi study conducted with 28 experts in back pain research from 12 countries [40]. According to the above mentioned recommended question, women were asked whether they had experienced LPP with or without radiation into either or both legs during the past 4 weeks. The pain should have been bad enough to limit usual activities or cause changes in daily routines for more than one day. The questionnaire included information about the follow-up study and contact details for additional information.
Outcome measures
The primary outcome measure and dependent variable was the presence of long-term PGP.
Secondary outcomes were marital status, educational level, physical activity, employment, working hours/week, ability to take breaks at work, number of pregnancies, parity, caesarean section and birth weight and sex of last born baby, use of hormonal contraceptives, and menopause. Function was measured on the Disability Rating Index (DRI) [41] and the Oswestry Disability Index (ODI) [42]. The DRI [41] measures ability in 12 activities, indicated on a100 mm visual analogue scale (VAS) (where 0 = the ability to perform an activity without difficulty, and 100 = no ability whatsoever to perform the activity) [43]. An index is achieved by measuring the distance in millimeter from 0 to the women’s markings on the VAS. The mean of these measurements provides the DRI expressed in percent of the highest possible rating. Pain when ‘turning in bed’ (where 0 = no pain and 1 = pain) was also queried, since this was performed in the original RCTs [44–46]. Ten different items on perceived disability were rated on the ODI: pain intensity, personal care and lifting, walking, sitting, standing, sleeping, sexual life, social life and travelling. The items were scored from 0 to 5. The scores of all items were added, giving a possible maximum score of 50. The total score was then doubled and expressed as percentages where 0 % represents no disability, 0–20 % no or minimal disability, 20–40 % moderate disability, 40–60 % severe disability, 60–80 % crippled and 80–100 % bed bound. Health-related quality of life (HRQL) was measured with the European quality of life measure (the EuroQol- 5Dimensions (EQ-5D), and the EQ-Visual analogue Scale (EQ-VAS) [47]. The EQ- 5D [47] assesses five dimensions of HRQL: mobility, self-care, activities of daily life, pain. Levels of anxiety and depression were measured. For each dimension, the woman describes three possible levels of problems (none, mild to moderate and severe). This descriptive system contains 243 combinations or index values for state of health. The total score range is from −0.43 to 1.0, in which -0.43 is the lowest health state, and 1, the highest. For a normal population, the average value is 0.8-0.9 [48]. The EQ-5D VAS is a vertical VAS (0–100 in which 0 is the lowest conceivable health state, and 100 the optimal health state) [47]. Levels of anxiety and depression was measured on the Hospital Anxiety and Depression Scale (HADS) [49]. The HADS is a 14-item scale for detection of anxiety and depression in people with physical health problems. Seven items relate to anxiety (HADS-A) and 7 items to depression (HADS-D). Each item on the questionnaire is scored from 0-3, indicating that a person can score between a total of 0 and 21 for either anxiety or depression. A cut-off point of 8/21 for anxiety or depression has been identified [50]. For anxiety this gave a specificity of 0.78, and a sensitivity of 0.9. For depression, this gave a specificity of 0.79, and a sensitivity of 0.83 [50]. Self-efficacy was measured with the General self-efficacy Scale (GES) [51]. The GES [51] is a 9-item scale that measures self-efficacy, and the range is from 10-40 points, a sum score is usually calculated. Pain catastrophizing was measured with the Pain Catastrophizing Scale (PCS). The PCS [52] was developed as a self-report measurement tool that provided a valid index of catastrophizing in clinical and non-clinical populations [52]. The PCS is a 13-item self-report scale to measure thoughts and feelings related to pain (e.g. “when I am in pain, I worry all the time about whether the pain will end”). In the PCS, each item is rated on a 5-point scale: (in which 0 is not at all, and 4, constantly). A total score is calculated (range 0-65 points). The three subscales of magnification, rumination, and helplessness reveal different dimensions of the same underlying content. Women stating LPP were also asked questions about sleep, use of analgesia, sick-leave due to LPP, and severity of LPP in relation to work, and offered an examination.
Assessment
Women experiencing LPP in the questionnaire were contacted by telephone. During the telephone interview they could confirm the prevalence, location and severity of symptoms, and were asked about exclusion criteria i.e. on-going pregnancy and/or systemic disease. If they fulfilled the criteria and agreed on an examination, they were scheduled to one of two specially trained physiotherapists. A standardised and reliable assessment [53] was performed in order to classify the women’s LPP into the categories PGP, PGP plus LBP, or only LBP. The classification included a history of pain provocation in different positions or activities of daily living, pelvic pain provocations tests, and a standardised mechanical assessment of the lumbar spine according to Mechanical Diagnosis and Therapy [53]. This assessment also included repeated end range flexion and extension movements in standing position and/or in lying position. The pelvic pain provocation tests used were: the posterior pelvic pain provocation test (P4 test) [54], distraction test, compression test, sacral thrust [53] and the MAT-test [55]. The MAT-test: standing with one hip in abduction; perform an adduction simulating the movement to pull a mat. The MAT-test was added as a replacement for the symphysis pubis pressure test [55, 56]. The reason for the decision to use the MAT test instead of the symphysis pubic pressure test was both ethically and scientifically based. The symphysis pubic pressure test provokes severe pain that does not subside directly, and the test has been shown to be false positive in women with no PGP [56]. The MAT test has been shown to have a high percentage of agreement with palpation of the pubic symphysis. [55]. In order to use the same classification used in the original RCTs [44–46]. The Faber (Patrick's) test and modified Trendelenburg test were also performed [57]. A neurological examination was performed if the women had a history of pain radiating into the leg. Load transfer between trunk and pelvis evaluated the active straight leg test (ASLR test) [58]. A classification of PGP was defined according to European guidelines [5]. All of the following criteria had to be fulfilled:
Pain experienced between the posterior iliac crest and the gluteal fold, particularly in the vicinity of the SIJ in conjunction with/or separately in the symphysis.
Reports of duration and weight-bearing-related pain in the pelvic girdle.
Diminished endurance in standing, walking and sitting.
Positive clinical diagnostic tests, which reproduced pain in the pelvic girdle.
No nerve root syndrome
No reproducible pain and/or changed symptoms in the lumbar spine by repeated end range movement.
A classification of LBP was defined as:
Reported pain in the lumbar spine with or without radiation to the leg.
Reproducible pain and/or changed symptoms in the lumbar spine by repeated end range motion [53].
A classification of combined PGP and LBP were defined when both of the above criteria were fulfilled. Since the focus of this follow up was to study long-term PGP, women with PGP and women with PGP + LBP are hereafter presented in the same group and named ‘PGP’.
Severity of PGP
Predictors of PGP were grouped and described in 5 clusters e.g., demographic, work-related, pregnancy-related and PGP-related predictors. The demographic predictor was age at randomisation in the RCT. Work-related predictors included severity of PGP in relation to work at randomisation, ability to take breaks at work, and sick leave due to PGP at randomisation. Pregnancy-related predictors were age at menarche plus total number of pregnancies and parity at the follow-up. PGP-related predictors were: a history of LBP before randomisation, function on the DRI [41], pain when turning in bed, HRQL (EQ-5D and EQ-VAS) [47], pain intensity related to motion in the morning and evening on a 0-100 mm VAS [43], unpleasantness of PGP on a 0-100 mm VAS, the P4 test [54], Faber (Patrick's) test, the symphysis pubis pressure test, the modified Trendelenburg's test [57], and the ASLR test [59] at randomisation. However, EQ-5D, EQ-VAS and the ASLR test were not used in Elden et al. 2005 [44].
The median, CI, quartiles, means and SD were calculated when appropriate. Continuous variables are presented with mean, Standard Deviation (SD) and 95 % Confidence Interval or median, minimum and maximum, and categorical variables are presented with number and percentage. For comparison between groups Fisher´s Exact test was used for dichotomous variables, Mantel-Haenszel’s Chi Square Exact test for ordered categorical variables, Chi Square test for non-ordered categorical variables and the Mann-Whitney U-test were used for continuous variables. In order to find independent predictors to occurrence of PGP, all significant variables from the univariable step were entered into a stepwise multivariable forward logistic regression. P < 0.10 was the prerequisite entry in the stepwise model. The results of the logistic regression analysis are presented as Odds Ratio (OR) with a 95 % confidence interval for each predictor and as the area under the ROC curve (AUC) for the strongest predictor. All significance tests were two-sided and conducted at the 5 % significance level. All statistical analysis was performed with SAS System version 9, SAS Institute, Cary, NC, USA.
Results
Of 536 women in one of the three original studies, only 530 questionnaires were sent out, since six women had participated in two of the RCTs. A total of 371 (70 %) responded to the questionnaire. However 11 women were excluded due to systemic disease and 15 due to on-going pregnancy. Thirty-seven/345 (10.7 %) women were classified with PGP. The clinical classification showed that 16 women suffered from PGP, and 21 women from PGP plus LBP. Among the women with PGP; two women had only an anterior pain location i.e. symphysiolysis; nine had a posterior pain location; and five had both anterior and posterior pain compared to women “with” no PGP at follow up. Figure 1 shows the progress of participants throughout the study, and the results of the examination at follow-up.
Fig. 1.
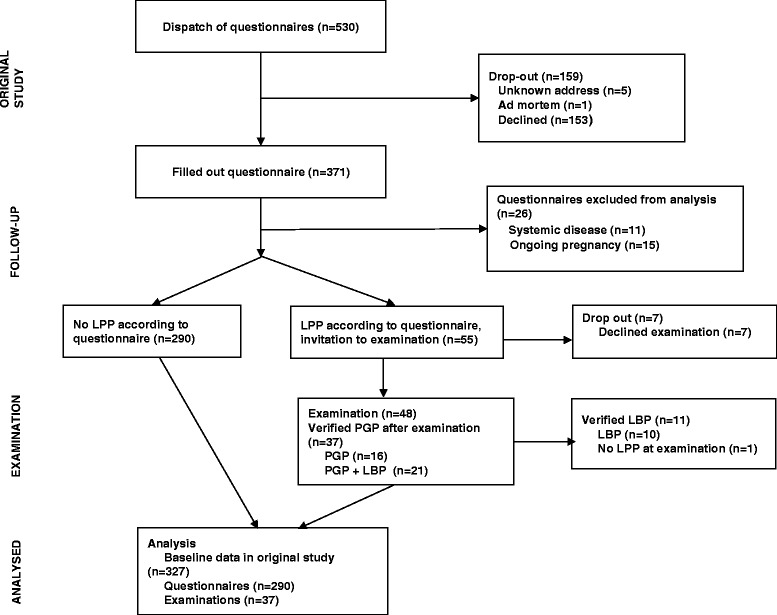
Flow-chart of the study
Table 1 shows the baseline characteristic of the study population by PGP and no PGP. More women with persistent PGP had previous LBP and the women had a higher number of positive pain provocation tests compared to women with no PGP at follow-up.
Table 1.
Characteristics of women with PGP or PGP plus LBP and women with no PGP before inclusion in RCT in pregnancy
| Variable | PGP or PGP + LBP (n = 37) | No PGP (n = 290) | p-value |
|---|---|---|---|
| Treatment in RCT | |||
| Standard treatment | 11 (29.7 %) | 77 (26.6 %) | |
| Standard treatment + Acupuncture | 14 (37.8 %) | 129 (44.5 %) | |
| Standard treatment + Specific stabilising exercises | 4 (10.8 %) | 56 (19.3 %) | |
| Standard treatment + Craniosacral therapy | 8 (21.6 %) | 28 (9.7 %) | 0.11 |
| Age, years | 30.0 (23.0; 39.0) | 31.0 (20.0; 43.0) | 0.40 |
| n = 37 | n = 289 | ||
| BMI before pregnancy | 22.6 (19.7; 34.2) | 23.2 (18.0; 38.4) | 0.99 |
| n = 23 | n = 115 | ||
| Age at menarche, years | 13.0 (10.0; 15.0) | 13.0 (9.0; 16.0) | 0.63 |
| n = 32 | n = 248 | ||
| Previous LBP | 24 (64.9 %) | 127 (44.7 %) | 0.032 |
| Women on sick-leave due to PGP | 13 (35.1 %) | 143 (50.0 %) | 0.13 |
| Severity of PGP | |||
| No complaints, PGP do not affect ability to work | 1 (2.7 %) | 5 (1.8 %) | |
| Moderate complaints, PGP only affect ability to work sporadically | 5 (13.5 %) | 67 (23.8 %) | |
| Not insignificant, cannot do some parts of my work | 15 (40.5 %) | 90 (32.0 %) | |
| Severe, can almost not work | 12 (32.4 %) | 79 (28.1 %) | |
| Severe, cannot work at all | 4 (10.8 %) | 40 (14.2 %) | 0.80 |
| Tests for assessment of PGP before inclusion in the RCT | |||
| Pain provocation tests | |||
| P4 test | 37 (100.0 %) | 283 (97.6 %) | 0.86 |
| Symphysis pressure test | 22 (59.5 %) | 121 (42.2 %) | 0.070 |
| Patrick Faber test | 27 (73.0 %) | 159 (54.8 %) | 0.051 |
| Modified Trendelenburg test | 22 (59.5 %) | 116 (40.0 %) | 0.039 |
| Number of bilateral positive pain provocation tests | |||
| 0 | 0 (0.0 %) | 3 (1.0 %) | |
| 1 | 6 (16.2 %) | 58 (20.0 %) | |
| 2 | 6 (16.2 %) | 107 (36.9 %) | |
| 3 | 10 (27.0 %) | 81 (27.9 %) | |
| 4 | 15 (40.5 %) | 41 (14.1 %) | 0.0013 |
| Functional test | |||
| ASLR test (sum of scores) | 3.00 (0.00; 8.00) | 3.00 (0.00; 10.00) | 0.35 |
| n = 23 | n = 116 | ||
| Subgroups of pelvic girdle pain | |||
| Solely symphysiolysis | 0 (0.0 %) | 5 (1.7 %) | 1.00 |
| One sided sacroiliac pain | 3 (8.1 %) | 37 (12.8 %) | 0.61 |
| One sided sacroiliac pain + symphyseal pain | 6 (16.2 %) | 39 (13.4 %) | 0.80 |
| Double sided sacroiliac pain | 12 (32.4 %) | 118 (40.7 %) | 0.43 |
| Pelvic girdle syndrome | 16 (43.2 %) | 91 (31.4 %) | 0.21 |
| Pain related to motion | |||
| In the morning, VAS | 31.0 (8.0; 92.0) | 26.5 (0.0; 96.0 | 0.089 |
| In the evening, VAS | 62.0 (5.0; 93.0) | 62.8 (6.0; 100.0) | 0.30 |
| n = 37 | n = 288 | ||
| Unpleasantness of PGP, VAS | 63.0 (20.0; 100.0) | 73.0 (0.0; 100.0) | 0.068 |
| n = 30 | n = 200 | ||
| DRI | 50.0 (23.0; 100.0) | 59.0 (11.0; 100.0) | 0.11 |
| n = 37 | n = 279 | ||
| EQ-VAS | 40.0 (25.0; 100.0) | 50.0 (20.0; 99.0) | 0.37 |
| n = 23 | n = 113 | ||
| EQ-5D score | 0.620 (-0.016; 0.760) | 0.620 (-0.074; 0.796) | 0.23 |
| n = 23 | n = 112 | ||
| Education level | |||
| Primary school | 0 (0.0 %) | 5 (2.0 %) | |
| Secondary school | 11 (33.3 %) | 64 (25.3 %) | |
| College | 5 (15.2 %) | 21 (8.3 %) | |
| University degree | 17 (51.5 %) | 163 (64.4 %) | 0.37 |
| No or rare ability to take rest breaks at work | 4 (13.8 %) | 68 (29.8 %) | 0.18 |
| Physical activity ≥30 minutes during leisure before pregnancy, days/week | |||
| 0 | 3 (9.1 %) | 8 (3.2 %) | |
| 1 | 1 (3.0 %) | 20 (7.9 %) | |
| 2 | 3 (9.1 %) | 43 (17.1 %) | |
| 3 | 7 (21.2 %) | 57 (22.6 %) | |
| 4 | 5 (15.2 %) | 31 (12.3 %) | |
| 5 | 3 (9.1 %) | 37 (14.7 %) | |
| 6 | 2 (6.1 %) | 13 (5.2 %) | |
| 7 | 9 (27.3 %) | 43 (17.1 %) | 0.35 |
For comparison between groups Fisher’s Exact test was used for ichotomous variables and the Mantel-Haenszel Chi Square Exact test was used for ordered categorical variables and the Mantel-Haenszel Chi Square test was used for ordered categorical variables and Chi Square Exact test was used for non-ordered categorical variables and Chi Square test was used for non-ordered categorical variables and the Mann-Whitney U-test was used for continuous variables
PGP pelvic girdle pain, LBP Low back pain, RCT Randomized controlled trial, BMI Body mass index, P4-test Posterior pelvic pain provocation test, ASLR-test Active straight leg test, VAS visual analoge scale. DRI Disability Rating Index; EQ-5D European Quality of Life measure – five dimensions; EQ-VAS European Quality of Life measure – visual analog scale. For categorical variables n (%) is presented. For continuous variables Mean (SD) / Median (Min; Max) / n = is presented
Table 2 shows the characteristics of the study population at follow-up. Women with PGP worked significantly fewer hours/week compared to women with no PGP at follow-up. In addition, sleep was disturbed in 26/37 (70 %) women, and 19/35 (54 %) women reported that their PGP was affected by the menstrual cycle (data not shown).
Table 2.
Characteristics of women with PGP or PGP plus LBP and women with no PGP at follow-up
| Variable | PGP or PGP + LBP (n = 37) | No PGP (n = 290) | p-value |
|---|---|---|---|
| Age, years | 37.7 (4.9) | 39.4 (5.7) | 0.059 |
| 38.0 (29.0; 49.0) | 40.0 (24.0; 54.0) | ||
| n = 37 | n = 290 | ||
| Time from randomisation to follow-up (years) | 7.35 (4.02) | 8.41 (3.80) | 0.27 |
| 5.00 (2.00; 12.00) | 11.00 (1.00; 13.00) | ||
| Working hours/week | 35.7 (7.6) | 37.1 (6.5) | 0.032 |
| 38.0 (19.0; 60.0) | 40.0 (1.0; 49.0) | ||
| n = 29 | n = 221 | ||
| Marital status | |||
| Single | 3 (8.6 %) | 24 (9.5 %) | |
| Married/cohabitating | 32 (91.4 %) | 229 (90.5 %) | 1.00 |
| Number of pregnancies | |||
| 1 | 4 (12.1 %) | 18 (7.2 %) | |
| 2 | 8 (24.2 %) | 89 (35.6 %) | |
| 3 | 15 (45.5 %) | 69 (27.6 %) | |
| 4 | 6 (18.2 %) | 73 (29.2 %) | |
| 5 | 0 (0.0 %) | 1 (0.4 %) | 0.56 |
| Parity | |||
| 1 | 5 (14.7 %) | 30 (12.0 %) | |
| 2 | 20 (58.8 %) | 139 (55.4 %) | |
| 3 | 8 (23.5 %) | 75 (29.9 %) | |
| 4 | 1 (2.9 %) | 6 (2.4 %) | |
| 5 | 0 (0.0 %) | 1 (0.4 %) | 0.52 |
| Caesarean section | 4 (14.3 %) | 48 (19.0 %) | 0.76 |
| Birthweight last born baby, grams | 3633 (492) | 3692 (490) | 0.43 |
| 3548 (2995; 4615) | 3700 (2129; 5000) | ||
| n = 30 | n = 274 | ||
| Sex of last born baby, boy | 15 (50 %) | 140 (51 %) | 0.925 |
| n = 30 | n = 275 |
For comparison between groups Fisher’s Exact test was used for dichotomous variables and the Mantel-Haenszel Chi Square Exact test was usedfor ordered categorical variables and the Mantel-Haenszel Chi Square test was used for ordered categorical variables and Chi Square Exact test was used for non-ordered categorical variables and Chi Square test was used for non-ordered categorical variables and the Mann-Whitney
U-test was used for continuous variables
PGP pelvic girdle pain, LBP Low back pain, For categorical variables n (%) is presented. For continuous variables Mean (SD) / Median (Min; Max) / n = is presented
Table 3 shows results of measurements of function (DRI, ODI), HRQL (EQ-5D, EQ-VAS, anxiety and depression (HADS), and pain catastrophizing (PCS) in women with PGP and women with no PGP at follow-up. Women classified with PGP had a significantly decreased ability to perform daily activities, lower self-efficacy, and decreased HRQL compared to women without PGP. Moreover, they had significantly higher levels of anxiety, depression and pain catastrophizing than women with no PGP. The time interval between original randomisation and follow-up did not impact the outcome measures assessed.
Table 3.
Function (ODI), health related quality of life (EQ-5D, EQ-VAS), Anxiety and depression (HADS) and pain catastrophisation (PCS) in women PGP or PGP plus LBP and women with no PGP at follow-up
| Variable | PGP or PGP + LBP (n = 37) | No pain (n = 290) | p-value |
|---|---|---|---|
| ODI | 22.0 (6; 42) | 4.00 (0; 58) | <.001 |
| n = 31 | n = 243 | ||
| EQ-5D | 0.725 (0.414; 1) | 0.848 (0.222; 1) | <.001 |
| n = 25 | n = 234 | ||
| EQ -VAS | 76.5 (50; 100) | 86.0 (0; 100) | <.001 |
| n = 30 | n = 230 | ||
| HADS-A, sum of scores | 66 (0; 14) | 3 (0; 22) | <.001 |
| n = 32 | n = 241 | ||
| HADS-A > 8 | 10 (31.3 %) | 24 (10.0 %) | 0.0044 |
| HADS-D, sum of scores | 2.50 (0; 12) | 1.00 (0; 12) | <.001 |
| n = 32 | n = 241 | ||
| HADS-D, >8 | 2 (6.3 %) | 4 (1.7 %) | 0.30 |
| PCS score | 15.5 (0; 35) | 6 (0; 52) | <.001 |
| n = 32 | n = 237 | ||
| PCS magnification | 3.50 (0; 8) | 1 (0; 12) | <.001 |
| n = 32 | n = 241 | ||
| PCS helplessness | 6 (0; 17) | 2 (0; 24) | <.001 |
| n = 32 | n = 241 | ||
| PCS rumination | 5.50 (0; 11) | 2 (0; 16) | 0.0036 |
| n = 32 | n = 241 | ||
| GSE (half scale) | 33.0 (24.0; 40.0) | 35.0 (10.0; 40.0) | 0.046 |
| n = 32 | n = 239 | ||
| DRI index | 30.8 (2.8; 78.6) | 4.62 (0; 55.85) | <.001 |
| n = 32 | n = 243 | ||
| DRI, Dressing | 4 (0; 100) | 1 (0; 26) | <.001 |
| n = 33 | n = 241 | ||
| DRI, Outdoor walks | 11 (0; 90) | 1.50 (0; 71) | <.001 |
| n = 33 | n = 242 | ||
| DRI, Climbing stairs | 12 (0; 100) | 2 (0; 77) | <.001 |
| n = 33 | n = 243 | ||
| DRI, Sitting for a longer time | 30 (0; 80) | 3 (0; 90) | <.001 |
| n = 33 | n = 243 | ||
| DRI, Get up from chair | 13 (0; 100) | 2 (0; 77) | <.001 |
| n = 33 | n = 243 | ||
| DRI, Standing bent | 39 (0; 100) | 3 (0; 94) | <.001 |
| n = 33 | n = 243 | ||
| DRI, Carrying a bag | 22 (0; 97) | 2 (0; 69) | <.001 |
| n = 33 | n = 242 | ||
| DRI, Running | 38 (1; 100) | 3 (0; 100) | <.001 |
| n = 33 | n = 243 | ||
| DRI, Light work | 17 (0; 90) | 2 (0; 79) | <.001 |
| n = 33 | n = 243 | ||
| DRI, Turn in bed | 49 (2; 100) | 3 (0; 98) | <.001 |
| n = 33 | n = 242 | ||
| DRI, Heavy work | 47 (0; 98.) | 4 (0; 86) | <.001 |
| n = 33 | n = 242 | ||
| DRI, Lifting heavy | 26 (2; 100) | 3 (0; 75) | <.001 |
| n = 33 | n = 243 | ||
| DRI, Sports | 17 (0; 100) | 2 (0; 65) | <.001 |
| n = 33 | n = 243 |
For comparison between groups Fisher’s Exact test was used for dichotomous variables and the Mann-Whitney U-test was used for continuous variables
PGP Pelvic girdle pain, LBP Low back pain, ODI Oswestry disability index, EQ-5D EuroQol-5 dimensions, EQ-VAS EuroQol-visual analogue scale, HADS-A Hospitality anxiety depression score-anxiety, HADS-D Hospitality anxiety depression score-depression, PCS Pain catastrophisation scale, GES General self-efficacy scale. For categorical variables n (%) is presented. For continuous variables Median (Min; Max) / n = is presented
Figure 2 shows the result of the univariable logistic regressions with variables measured in pregnancy from the 37 women with PGP compared to the results from the 290 women with no PGP. Earlier LBP (Odds Ratio, OR = 2.28), a positive symphysis pressure test (OR = 2.01), positive Faber (Patrick’s) test (OR = 2.22), a positive modified Trendelenburg test (OR = 2.20), and a high number of bilateral positive pain provocation tests (OR = 1.79) were predictors for long-term PGP. In the stepwise multivariable logistic regression the variable of a high number of bilateral positive pain provocation tests were entered into the model and resulted in an OR = 1.79 with a 95 % CI of 1.25-2.57 a P-value of 0.0015, and an area under the ROC-Curve of 0.65 with a 95 % CI of 0.55-0.75. After that no other variable was entered in to the model.
Fig. 2.
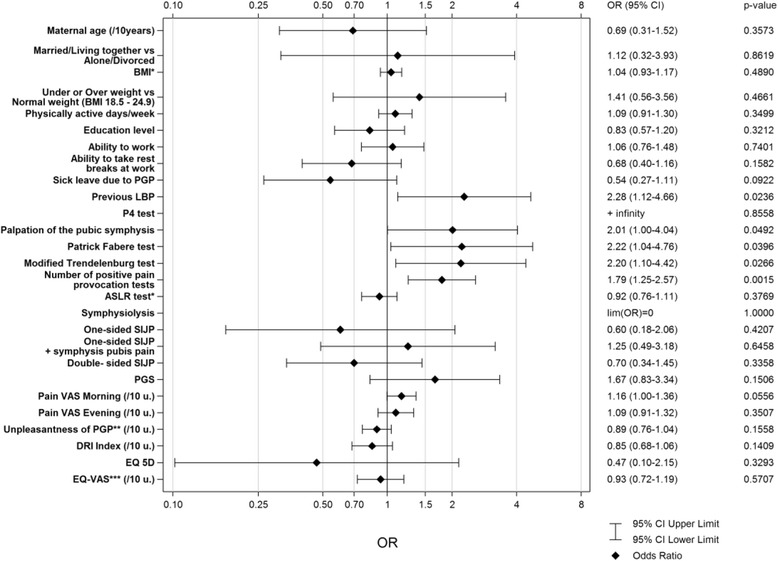
Association between baseline characteristics in pregnancy and the occurrence of PGP 2, 6 and 11 years after pregnancy. Result of the univariable logistic regressions with variables measured in pregnancy
There was no association between characteristics registered at the follow-up such as: age at menarche, total number of pregnancies, parity, caesarean section (yes/no), birth weight (grams) or sex of last born baby and the occurrence of PGP 2, 6 and 11 years after pregnancy (data not shown).
Discussion
Main findings
Ten percent of women classified with PGP in pregnancy had PGP with considerable consequences on health and function in daily life up to a decade later. Pregnancy-related predictors for long-term PGP were: a history of LBP before index pregnancy, a high number of positive pain provocation tests, a positive symphysis pressure test, and a modified Trendelenburg or Patrick’s test. Some of the predictors were already known from shorter follow-up studies such as a history of LBP before pregnancy [2, 9, 27, 31] and a high number of positive pain provocation tests in pregnancy [31, 34], but Faber (Patrick's) test, the modified Trendelenburg’s and the symphysis pubic pressure tests as predictors are new findings. However, the Faber (Patrick's) test, as a predictor must be interpreted with caution since this is not a specific test for PGP.
In contrast to other follow-up studies [9, 12, 31], HRQL, decreased function, and high pain intensity were not shown to be significant pregnancy-related predictors. This may be explained by shorter follow-up periods in those studies, and that women with long-term PGP may have adjusted and confined their lives to decrease pain exacerbation. Also, a recently published syndrome-specific instrument may have been better for identifying PGP-specific functional limitations than the DRI [60]. That work-related factors such as strenuous work [28] and sick leave [29] were not confirmed as predictors may be interpreted by changes in work-related circumstances during the long time elapsed, and that sick leave is influenced by many factors, not only the severity of PGP. [19] Moreover, our study did not confirm age as a predictor for long-term PGP [23, 27]. However, age has been suggested as a bimodal factor due to conflicting results [2]. Neither did our study confirm that higher fetal weight [20] could predict long-term PGP. Nonetheless, the mean weight of the fetuses in the aforementioned study (3531 gram) was lower than the mean weight of the fetuses in both the PGP group and the non-PGP group in our study (3633, and 3692 grams, respectively). Moreover, other studies have [21, 26, 30] described an association between elective caesarean section and LBP 3 and 6 months after delivery, respectively. However, their results must be interpreted with caution because of a relatively small study sample [21], and that the reasons for caesarean delivery or vaginal delivery were not explored. Also, none of these studies used a medical examination for classification of PGP [20, 21, 26].
It is not surprising that long-term PGP, in common with many other chronic pain conditions, was associated with decreased HRQL, function, and psychosocial factors i.e. disturbed sleep, anxiety, depression, self-efficacy and pain catastrophizing [61, 62]. Anxiety and depression may act as facilitators of pain nociception, making the pain worse, and pain, in turn, may act as facilitators of anxiety and depression [63]. Sleep disturbance is also an important factor of chronic pain to identify and address [20]. However, as these factors were not measured in pregnancy no conclusions can be drawn regarding their predictive value and role in the development from acute to chronic PGP.
The results presented are also in line with earlier publications of persistent LBP and PGP in a shorter perspective. However, the prevalence of PGP was higher (10 %) in our study than in a 6-year postpartum follow-up study (7 %) [17]. The higher rate in our study can be explained by differences in the study populations, i.e. the women in our study may have had more severe PGP in pregnancy since they sought treatment, and that women participating in RCT 2-3 [45, 46] fulfilled the inclusion criteria of evening pain of at least 50/100. Two of the women had only symphysiolysis. Although this is a small number of women, there is a need to reconsider if women with isolated symphysiolysis recover completely as previously suggested [13].
Strengths and limitations
There is a relative paucity of literature in this area. The topic is very important clinically, as pregnancy-associated musculoskeletal pain is highly prevalent and associated with significant morbidity that has a major socioeconomic impact on physical medicine as well as obstetric services.
The main strength of this work lies in that it is based on the follow-up of relatively robust randomised controlled trials, plus a relatively good response rate (71 %) from the original participants despite the time interval of up to 11 years. It is noteworthy that 26 of 37 respondents in the PGP group (70 %) and 213 of 290 respondents in the no PGP group (73 %) were in the active arms of the original trials, suggesting that fewer women randomised to the control arms (standard treatment only) completed the questionnaire. However, a majority of the women reporting LPP responded after the first questionnaire. None reporting LPP responded after reminder 2, which confirms the assumption that these women did not respond to the questionnaire because of the resolution of their LPP rather than neglect or other reasons. Prompt responses may also reflect the fact that many of these women receive insufficient care from healthcare provider’s barriers they encounter in seeking help [4, 64]. This can also be due to postpartum PGP being a neglected area of research. This is unacceptable both for the individual experiencing PGP and for the societal consequences of long-term pregnancy-related PGP. There was no evidence of bias caused by participant dropout on the prognostic factors or outcome; therefore, our findings are unlikely to be substantially influenced by loss at follow-up.
There is a potential for greater confounding in the group followed up at 11 years (the majority). These women are ultimately likely to be older and more likely to have had intercurrent pregnancies. However, there was no statistical significant difference in the time interval from randomisation to follow-up between women with PGP and women without PGP.
The reason for using both the ODI and the DRI for function was to be able to compare function measured in pregnancy with function measured at follow-up. The DRI was used in all the RCTs [44–46] but the ODI was added in Elden et al. 2008 [45] and Elden et al. 2013 [46] because some activities in the DRI e.g. running, lifting heavy objects and participating in sports was found inappropriate for women with PGP. The topics e.g. ability to care for oneself, ability to walk, ability to sit, sexual function, ability to stand, social life, sleep quality, and ability to travel in the ODI complements the DRI. The topics reflect both decreased body functions, activities in daily life and symptoms (pain), which are included in the European Guidelines of diagnosis and treatment for PGP [5] and reported in both quantitative [65] and qualitative studies describing women’s reported difficulties of life by women with PGP [8, 66–68] and their partners [69].
Other major strengths of this study are the relatively large sample of women classified with PGP in pregnancy [5] and baseline registration of pain and function before inclusion in the RCTs. In addition the examination of women with self-reported LPP was performed by skilled physiotherapists using the same classification at follow-up as in pregnancy, and the wide range of predictors measured by validated instruments. Another strength was that false positive pain provocation tests at follow-up were minimized by the identification of lumbar pain and the centralization phenomenon [70]. Furthermore, the use of pain provocation tests minimized false negative cases [7].
Other strengths are that many of the instruments used in this study have shown good internal consistency, test-retest reliability, and construct validity when used with a sample of participants with PGP postpartum [12, 17, 37, 71].
A weakness of our study was that the sample size (in the women with PGP, n = 37) is not of sufficient number to rule out a type II error for the small difference in the primary and secondary outcome variables measured. Moreover, BMI, breastfeeding and emotional distress shown to influence the prognosis of PGP, were not included [16, 24, 25, 35]. It may also be the case that predictors of PGP outcome are not fixed at specific time points (i.e. baseline registration before inclusion in an RCT) but are more fluid in nature and change, and evolve during different periods of the women’s experiences of PGP. Future studies with more frequent follow-ups will better identify such patterns. In addition, the study design could not control for other potential comorbid health conditions that may be accounting for an individual's PGP. Although care was taken to exclude any patients with current systemic disease (n = 11), the possibility of other conditions being present at some stage in the intervening years remains. Although parity did not differ significantly between participants with PGP versus those without PGP at follow-up, it remains unclear whether or not any of these intervening pregnancies were complicated by musculoskeletal pain conditions and whether there may have been a differential effect between the women with or without PGP. We have earlier shown that 99 % of women in the RCT performed 11 years ago [72] reported resolution of their pain by 12 weeks. Therefore pain reported 11 years later in these women may be new-onset (i.e. have nothing to do with the index pregnancy), or may indicate recurrence, i.e. is unlikely to reflect persistent pain. However, the low prevalence shown 12 weeks after pregnancy [72] might have reflected that the women had not yet resumed with their daily activities. To definitively associate the presence of PGP 11 years later with the index pregnancy without careful review of relevant events in between would potentially be misleading. Thus, the generalizability (external validity) of the study results may be limited to this population of women.
Interpretation
PGP is closely related to pregnancy, and most commonly debuts in pregnancy. Thus, the debut of PGP is easy to identify and screen. Based on the results of our study we suggest that the classification of pregnant women with self-reported LPP include an examination of both the lumbar spine and the pelvic girdle, and that the P4 test is used to identify women with posterior pelvic pain, and the MAT-test for anterior pelvic pain, as these tests are reliable and valid for posterior and anterior PGP [5, 7, 55]. In additon, more pelvic pain provocation tests can be used to identify women with severe PGP, which in turn will indicate that these women run a higher risk of long-term PGP. The women should thereafter be offered structured and individualised treatment depending on the classification of their condition. Promising interventions for PGP during pregnancy are acupuncture, a semi-elastic belt, and specific stabilizing exercises [1, 44, 73]. Since it has been shown that improvement from persistent PGP level off around 6 months postpartum [74], It is of great importance that these women are followed up. Women that are not symptom free by that time most probably need individualised treatment depending on the classification of their condition in order to prevent long term pain [75, 76].
Conclusions
In conclusion, this unique long-term longitudinal study highlights the importance of assessment and classification of LPP in pregnancy and at postpartum follow-up for the prediction of long-term PGP. One of ten women classified with PGP during pregnancy have PGP with severe consequences not only on women’s everyday life but also on their family life and economy up to 11 years later. Previous LBP and a high number of positive pain provocation tests are pregnancy-related predictors lasting more than a decade. The severity of PGP in pregnancy thus seems important to identify early, which is possible in most countries since these women are in frequent contact with the health care system. More attention should be paid to pregnant women with a history of LBP and many positive pain provocation tests and women with remaining PGP at follow-up postpartum. Focus should be to minimize the symptoms and consequences of PGP for the women. Further research of this major health problem is needed.
Abbreviations
ASLR, Active Straight Leg Raising; BMI, Body Mass Index; LBP, Low Back Pain; LPP, Lumbopelvic pain; OR, Odds Ratio; P4 test, The posterior pelvic pain provocation test; PGP, Pelvic Girdle Pain; PGS, Pelvic Girdle Syndrome; RCT, Randomised Controlled Trial; SIJ, Sacroiliac joints.
Acknowledgments
Funding
This study was supported by grants from the Foundation of the Health and Medical care committee of the Region of Vastra Gotaland, Sweden: VGFOUREG-294331 (H.C.O), VGFOUREG-294871 (H.C.O), and VGFOUREG-381421 (H.E).
Availability of data and materials
The data supporting the findings presented in this paper is kept in an anonymous file at the archive at the Sahlgrenska University Hospital Trust according to hospital policy and rules and regulations stated by the Regional Committee for Medical Research Ethics.
Authors’ contributions
HE, AG, GKW, MFO, and HCO All participated in the design, funding applications, interpretation of the results, and drafting of the article. HE, AG and GKW contributed to data collection. All authors read and approved the final manuscript. HE and HCO are guarantors.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All women who decided to participate in the study provided written consent to the questionnaire on the first page before completing the rest of the questionnaire. The ethics committee at University of Gothenburg, Gothenburg, Sweden approved the study in May, 2012 (Dnr 193-12).
Contributor Information
Helen Elden, Email: helen.elden@gu.se.
Annelie Gutke, Email: annelie.gutke@neuro.gu.se.
Gunilla Kjellby-Wendt, Email: gunilla.kjellby-wendt@vgregion.se.
Monika Fagevik-Olsen, Email: monika.fagevik-olsen@vgregion.se.
Hans-Christian Ostgaard, Email: hans-christian.ostgaard@vgregion.se.
References
- 1.Pennick V, Liddle SD. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database Syst Rev. 2013;8:CD001139. doi: 10.1002/14651858.CD001139.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Wu WH, Meijer O, Uegaki K, Mens J, van Dieen J, Wuisman PI, Ostgaard H. Pregnancy-related pelvic girdle pain (PPP), I: Terminology, clinical presentation, and prevalence. Eur Spine J. 2004;13:575–589. doi: 10.1007/s00586-003-0615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutke A, Ostgaard HC, Oberg B. Pelvic girdle pain and lumbar pain in pregnancy: a cohort study of the consequences in terms of health and functioning. Spine (Phila Pa 1976) 2006;31:E149–155. doi: 10.1097/01.brs.0000201259.63363.e1. [DOI] [PubMed] [Google Scholar]
- 4.Skaggs C, Prather H, Gross G, George J, Thompson P, Nelson D. Back and pelvic pain in an underserved United States pregnant population: a preliminary descriptive survey. Journal of manipulative and physiological therapeutics. 2007;30:130–134. doi: 10.1016/j.jmpt.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Vleeming A, Albert HB, Ostgaard HC, Sturesson B, Stuge B. European guidelines for the diagnosis and treatment of pelvic girdle pain. Eur Spine J. 2008;17:794–819. doi: 10.1007/s00586-008-0602-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermani E, Mittal R, Weeks A. Pelvic girdle pain and low back pain in pregnancy: a review. Pain Pract. 2010;10:60–71. doi: 10.1111/j.1533-2500.2009.00327.x. [DOI] [PubMed] [Google Scholar]
- 7.Kanakaris NK, Roberts C, Giannoudis P. Pregnancy-related pelvic girdle pain: an update. BMC Med. 2011;9:15. doi: 10.1186/1741-7015-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elden H, Lundgren I, Robertson E. Life’s pregnant pause of pain: pregnant women’s experiences of pelvic girdle pain related to daily life: a Swedish interview study. Sex Reprod Healthc. 2013;4:29–34. doi: 10.1016/j.srhc.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Bergstrom C, Persson M, Mogren I. Pregnancy-related low back pain and pelvic girdle pain approximately 14 months after pregnancy - pain status, self-rated health and family situation. BMC pregnancy and childbirth. 2014;14:48. doi: 10.1186/1471-2393-14-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engeset J, Stuge B, Fegran L. Pelvic girdle pain affects the whole life--a qualitative interview study in Norway on women’s experiences with pelvic girdle pain after delivery. BMC Res Notes. 2014;7:686. doi: 10.1186/1756-0500-7-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wuytack F, Curtis E, Begley C. Experiences of First-Time Mothers With Persistent Pelvic Girdle Pain After Childbirth: Descriptive Qualitative Study. Phys Ther. 2015;95:1354–1364. doi: 10.2522/ptj.20150088. [DOI] [PubMed] [Google Scholar]
- 12.Gutke A, Lundberg M, Ostgaard HC, Oberg B. Impact of postpartum lumbopelvic pain on disability, pain intensity, health-related quality of life, activity level, kinesiophobia, and depressive symptoms. Eur Spine J. 2011;20:440–448. doi: 10.1007/s00586-010-1487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albert H, Godskesen M, Westergaard J. Prognosis in four syndromes of pregnancy-related pelvic pain. Acta Obstet Gynecol Scand. 2001;80:505–510. doi: 10.1080/j.1600-0412.2001.080006505.x. [DOI] [PubMed] [Google Scholar]
- 14.Noren L, Ostgaard S, Johansson G, HC O: Lumbar back and posterior pelvic pain during pregnancy: a 3-year follow-up. Eur Spine J. 2002, 11:267-71. [DOI] [PMC free article] [PubMed]
- 15.Rost CC, Jacqueline J, Kaiser A, Verhagen AP, Koes BW. Prognosis of women with pelvic pain during pregnancy: a long-term follow-up study. Acta Obstet Gynecol Scand. 2006;85:771–777. doi: 10.1080/00016340600626982. [DOI] [PubMed] [Google Scholar]
- 16.Mogren IM. BMI, pain and hyper-mobility are determinants of long-term outcome for women with low back pain and pelvic pain during pregnancy. Eur Spine J. 2006;15:1093–1102. doi: 10.1007/s00586-005-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostgaard HC, Zetherstrom G, Roos-Hansson E. Back pain in relation to pregnancy: a 6-year follow-up. Spine (Phila Pa 1976) 1997;22:2945–2950. doi: 10.1097/00007632-199712150-00018. [DOI] [PubMed] [Google Scholar]
- 18.Mogren I. Perceived health, sick leave, psychosocial situation, and sexual life in women with low-back pain and pelvic pain during pregnancy. Acta Obstet Gynecol Scand. 2006;85:647–656. doi: 10.1080/00016340600607297. [DOI] [PubMed] [Google Scholar]
- 19.Gutke A, Olsson CB, Vollestad N, Oberg B, Wikmar LN, Robinson HS. Association between lumbopelvic pain, disability and sick leave during pregnancy - a comparison of three Scandinavian cohorts. J Rehabil Med. 2014;46:468–474. doi: 10.2340/16501977-1801. [DOI] [PubMed] [Google Scholar]
- 20.den Berg SG S-v, Hendriksen IJ, Bruinvels DJ, Twisk JW, van Mechelen W, van Poppel MN. Predictors for postpartum pelvic girdle pain in working women: the Mom@Work cohort study. Pain. 2012;153:2370–2379. doi: 10.1016/j.pain.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Mogren I. Does caesarean section negatively influence the post-partum prognosis of low back pain and pelvic pain during pregnancy. Eur Spine J. 2007;16:115–121. doi: 10.1007/s00586-006-0098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutke A, Ostgaard H, Oberg B. Predicting persistent pregnancy-related low back pain. Spine. 2008;33:E386–393. doi: 10.1097/BRS.0b013e31817331a4. [DOI] [PubMed] [Google Scholar]
- 23.Sjodahl J, Gutke A, Oberg B. Predictors for long-term disability in women with persistent postpartum pelvic girdle pain. Eur Spine J. 2013;22:1665–1673. doi: 10.1007/s00586-013-2716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjelland EK, Owe KM, Stuge B, Vangen S, Eberhard-Gran M. Breastfeeding and pelvic girdle pain: a follow-up study of 10 603 women 18 months after delivery. BJOG. 2014;122:1765–1771. doi: 10.1111/1471-0528.13118. [DOI] [PubMed] [Google Scholar]
- 25.Bjelland EK, Stuge B, Engdahl B, Eberhard-Gran M. The effect of emotional distress on persistent pelvic girdle pain after delivery: a longitudinal population study. BJOG. 2013;120:32–40. doi: 10.1111/1471-0528.12029. [DOI] [PubMed] [Google Scholar]
- 26.Bjelland EK, Stuge B, Vangen S, Stray-Pedersen B, Eberhard-Gran M. Mode of delivery and persistence of pelvic girdle syndrome 6 months postpartum. Am J Obstet Gynecol. 2013;208(298):e291–297. doi: 10.1016/j.ajog.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Turgut F, Turgut M, Cetinsahi M. A prospective study of persistent back pain after pregnancy. Eur J Obstet Gynecol Reprod Biol. 1998;80:45–48. doi: 10.1016/S0301-2115(98)00080-3. [DOI] [PubMed] [Google Scholar]
- 28.Ostgaard HC, Andersson GB. Postpartum low-back pain. Spine (Phila Pa 1976) 1992;17:53–55. doi: 10.1097/00007632-199201000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Ansara D, Cohen MM, Gallop R, Kung R, Schei B. Predictors of women’s physical health problems after childbirth. Journal of psychosomatic obstetrics and gynaecology. 2005;26:115–125. doi: 10.1080/01443610400023064. [DOI] [PubMed] [Google Scholar]
- 30.Mukkannavar P, Desai BR, Mohanty U, Kulkarni S, Parvatikar V, Daiwajna S. Pelvic girdle pain in Indian postpartum women: a cross-sectional study. Physiother Theory Pract. 2013;30:123–130. doi: 10.3109/09593985.2013.816399. [DOI] [PubMed] [Google Scholar]
- 31.Robinson H, Mengshoel A, Veierod M, Vollestad N. Pelvic girdle pain: potential risk factors in pregnancy in relation to disability and pain intensity three months postpartum. Man Ther. 2010;15:522–528. doi: 10.1016/j.math.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Verstraete EH, Vanderstraeten G, Parewijck W. Pelvic Girdle Pain during or after Pregnancy: a review of recent evidence and a clinical care path proposal. Facts Views Vis Obgyn. 2013;5:33–43. [PMC free article] [PubMed] [Google Scholar]
- 33.Vollestad NK, Stuge B. Prognostic factors for recovery from postpartum pelvic girdle pain. Eur Spine J. 2009;18:718–726. doi: 10.1007/s00586-009-0911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronchetti I, Vleeming A, van Wingerden JP. Physical characteristics of women with severe pelvic girdle pain after pregnancy: a descriptive cohort study. Spine (Phila Pa 1976) 2008;33:E145–151. doi: 10.1097/BRS.0b013e3181657f03. [DOI] [PubMed] [Google Scholar]
- 35.Biering K, Nohr EA, Olsen J, Andersen AM, Hjollund NH, Juhl M. Pregnancy-related pelvic pain is more frequent in women with increased body mass index. Acta Obstet Gynecol Scand. 2011;90:1132–1139. doi: 10.1111/j.1600-0412.2011.01141.x. [DOI] [PubMed] [Google Scholar]
- 36.Gutke A, Sjodahl J, Oberg B. Specific muscle stabilizing as home exercises for persistent pelvic girdle pain after pregnancy: a randomized, controlled clinical trial. J Rehabil Med. 2010;42:929–935. doi: 10.2340/16501977-0615. [DOI] [PubMed] [Google Scholar]
- 37.Stuge B, Laerum E, Kirkesola G, Vollestad N. The efficacy of a treatment program focusing on specific stabilizing exercises for pelvic girdle pain after pregnancy: a randomized controlled trial. Spine. 2004;29:351–359. doi: 10.1097/01.BRS.0000090827.16926.1D. [DOI] [PubMed] [Google Scholar]
- 38.Ferreira P. Specific stabilising exercise improves pain and function in women with pelvic girdle pain following pregnancy. Aust J Physiother. 2004;50:259. doi: 10.1016/S0004-9514(14)60120-3. [DOI] [PubMed] [Google Scholar]
- 39.Robinson H, Mengshoel A, Veierod M, Vollestad N. Pelvic girdle pain, clinical tests and disability in late pregnancy. Man Ther. 2010;15:280–285. doi: 10.1016/j.math.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Dionne CE, Dunn KM, Croft PR, Nachemson AL, Buchbinder R, Walker BF, Wyatt M, Cassidy J, Rossignol M, Leboeuf-Yde C, et al. A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine. 2008;33:95–103. doi: 10.1097/BRS.0b013e31815e7f94. [DOI] [PubMed] [Google Scholar]
- 41.Salen BA, Spangfort E, Nygren AL, Nordemar R. The Disability Rating Index: an instrument for the assessment of disability in clinical settings. J Clin Epidemiol. 1994;47:1423–1435. doi: 10.1016/0895-4356(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 42.Fairbank J, Couper J, Davies J, O’Brien J. The Oswestery low back pain disability questionnaire. Physiotherapy. 1994;66:271–273. [PubMed] [Google Scholar]
- 43.Huskinsson E. Measurement of pain. Lancet. 1974;2(7889):1127–31. [DOI] [PubMed]
- 44.Elden H, Ladfors L, Olsen MF, Ostgaard HC, Hagberg H. Effects of acupuncture and stabilising exercises as adjunct to standard treatment in pregnant women with pelvic girdle pain: randomised single blind controlled trial. BMJ. 2005;330:761–764. doi: 10.1136/bmj.38397.507014.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elden H, Fagevik-Olsen M, Ostgaard HC, Stener-Victorin E, Hagberg H. Acupuncture as an adjunct to standard treatment for pelvic girdle pain in pregnant women: randomised double-blinded controlled trial comparing acupuncture with non-penetrating sham acupuncture. BJOG. 2008;115:1655–1668. doi: 10.1111/j.1471-0528.2008.01904.x. [DOI] [PubMed] [Google Scholar]
- 46.Elden H, Ostgaard HC, Glantz A, Marciniak P, Linner AC, Olsen MF. Effects of craniosacral therapy as adjunct to standard treatment for pelvic girdle pain in pregnant women: a multicenter, single blind, randomized controlled trial. Acta Obstet Gynecol Scand. 2013;92:775–782. doi: 10.1111/aogs.12096. [DOI] [PubMed] [Google Scholar]
- 47.Rabin R, De Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 48.Burstrom K, Johannesson M, Rehnberg C. Deteriorating health status in Stockholm 1998-2002: results from repeated population surveys using the EQ-5D. Qual Life Res. 2007;16:1547–1553. doi: 10.1007/s11136-007-9243-z. [DOI] [PubMed] [Google Scholar]
- 49.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 50.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/S0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 51.Love J, Moore CD, Hensing G: S Validation of the Swedish translation of the General Self-Efficacy scale. 2012, 21:1249-53. [DOI] [PubMed]
- 52.Sullivan MJL, Bishop SR, Pivic J: The Pain Catastrophizing scale Psychol Assess. 1995, 524-532.
- 53.Gutke A, Kjellby-Wendt G, Oberg B. The inter-rater reliability of a standardised classification system for pregnancy-related lumbopelvic pain. Man Ther. 2010;15:13–18. doi: 10.1016/j.math.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Ostgaard HC, Zetherstrom G, Roos-Hansson E. The posterior pelvic pain provocation test in pregnant women. Eur Spine J. 1994;3:258–260. doi: 10.1007/BF02226575. [DOI] [PubMed] [Google Scholar]
- 55.Fagevik Olsen M, Elden H, Gutke A. Evaluation of self-administered tests for pelvic girdle pain in pregnancy. BMC Musculoskelet Disord. 2014;15:138. doi: 10.1186/1471-2474-15-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fagevik Olsen M, Gutke A, Elden H, Nordenman C, Fabricius L, Gravesen M, Lind A, Kjellby-Wendt G. Self-administered tests as a screening procedure for pregnancy-related pelvic girdle pain. Eur Spine J. 2009;18:1121–1129. doi: 10.1007/s00586-009-0948-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Albert H, Godskesen M, Westergaard J. Evaluation of clinical tests used in classification procedures in pregnancy-related pelvic joint pain. Eur Spine J. 2000;9:161–166. doi: 10.1007/s005860050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mens JM, Vleeming A, Snijders CJ, Koes BW, Stam HJ. Validity of the active straight leg raise test for measuring disease severity in patients with posterior pelvic pain after pregnancy. Spine (Phila Pa 1976) 2002;27:196–200. doi: 10.1097/00007632-200201150-00015. [DOI] [PubMed] [Google Scholar]
- 59.Mens JM, Vleeming A, Snijders C, Koes BW, Stam HJ. Reliability and validity of the active straight leg raise test in posterior pelvic pain since pregnancy. Spine. 2001;26:1167–1171. doi: 10.1097/00007632-200105150-00015. [DOI] [PubMed] [Google Scholar]
- 60.Stuge B, Garratt A, Krogstad Jenssen H, Grotle M. The pelvic girdle questionnaire: a condition-specific instrument for assessing activity limitations and symptoms in people with pelvic girdle pain. Phys Ther. 2011;91:1096–1108. doi: 10.2522/ptj.20100357. [DOI] [PubMed] [Google Scholar]
- 61.Linton SJ, Nicholas MK, MacDonald S, Boersma K, Bergbom S, Maher C, Refshauge K. The role of depression and catastrophizing in musculoskeletal pain. Eur J Pain. 2011; 15(4):416-22. doi:10.1016/j.ejpain.2010.08.009. Epub 2010 Sep 29. [DOI] [PubMed]
- 62.Linton SJ. A review of psychological risk factors in back and neck pain. Spine (Phila Pa 1976). 2000;25(9):1148-56. [DOI] [PubMed]
- 63.Baliki MN, Apkarian AV. Nociception, Pain, Negative Moods, and Behavior Selection. Neuron. 2015;87:474–491. doi: 10.1016/j.neuron.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wuytack F, Curtis E, Begley C. The health-seeking behaviours of first-time mothers with persistent pelvic girdle pain after childbirth in Ireland: A descriptive qualitative study. Midwifery. 2015;31:1104–1109. doi: 10.1016/j.midw.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 65.Mens JM, Vleeming A, Stoeckart R, Stam HJ, Snijders CJ: Understanding peripartum pelvic pain. Implications of a patient survey. Spine (Phila Pa 1976). 1996, 21:1363-1369; discussion 1369-1370. [DOI] [PubMed]
- 66.Elden H, Lundgren I, Robertson E: The pelvic ring of pain: Pregnant women’s experiences of severe pelvic girdle pain. Clinical Nursing Studies: 2014, 2 (2): ISSN 2014; 2.
- 67.Persson M, Winkvist A, Dahlgren L, Mogren I: “Struggling with daily life and enduring pain”: a qualitative study of the experiences of pregnant women living with pelvic girdle pain BMC pregnancy and childbirth. 2013 May, 13:111. [DOI] [PMC free article] [PubMed]
- 68.Engeset J, Stuge B, Fegran L. Pelvic girdle pain affects the whole life--a qualitative interview study in Norway on women's experiences with pelvic girdle pain after delivery. BMC Res Notes. 2014;7:686. doi:10.1186/1756-0500-7-686. [DOI] [PMC free article] [PubMed]
- 69.Elden H, Lundgren I, Robertson E. Demanding and challenging: Men’s experiences of living with a pregnant woman with pelvic girdle pain: An interview study. Clinical Nursing Studies. 2014;2:17–29. [Google Scholar]
- 70.Laslett M, Young SB, Aprill CN, McDonald B. Diagnosing painful sacroiliac joints: A validity study of a McKenzie evaluation and sacroiliac provocation tests. Aust J Physiother. 2003;49:89–97. doi: 10.1016/S0004-9514(14)60125-2. [DOI] [PubMed] [Google Scholar]
- 71.Grotle M, Garratt AM, Krogstad Jenssen H, Stuge B. Reliability and construct validity of self-report questionnaires for patients with pelvic girdle pain. Phys Ther. 2012;92:111–123. doi: 10.2522/ptj.20110076. [DOI] [PubMed] [Google Scholar]
- 72.Elden H, Hagberg H, Olsen MF, Ladfors L, Ostgaard HC. Regression of pelvic girdle pain after delivery: follow-up of a randomised single blind controlled trial with different treatment modalities. Acta Obstet Gynecol Scand. 2008;87:201–208. doi: 10.1080/00016340701823959. [DOI] [PubMed] [Google Scholar]
- 73.Gutke A, Betten C, Degerskar K, Pousette S, Fagevik Olsen M. Treatments for pregnancy-related lumbopelvic pain: a systematic review of physiotherapy modalities. Acta Obstet Gynecol Scand. 2015;94:1156–7. [DOI] [PubMed]
- 74.Ostgaard HC, Roos-Hansson E, Zetherstrom G: Regression of back and posterior pelvic pain after pregnancy. Spine (Phila Pa 1976). 1996, 21:2777-2780. [DOI] [PubMed]
- 75.Lund I, Lundeberg T, Lonnberg L, Svensson E: Decrease of pregnant women's pelvic pain after acupuncture: a randomized controlled single-blind study. Acta Obstet Gynecol Scand. 2006;85:12-19. [DOI] [PubMed]
- 76.Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, Fu R, Dana T, Kraegel P, Griffin J et al. In: Noninvasive Treatments for Low Back Pain. edn. Rockville (MD); 2016. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings presented in this paper is kept in an anonymous file at the archive at the Sahlgrenska University Hospital Trust according to hospital policy and rules and regulations stated by the Regional Committee for Medical Research Ethics.


