Abstract
Purpose
The purpose of this study was to better understand the efficacy and safety of carfilzomib, panobinostat, and elotuzumab combinations in patients with refractory/relapsed multiple myeloma(R/RMM).
Methods
We retrieved and reviewed published reports including carfilzomib, panobinostat, and elotuzumab combination regimens for patients with R/RMM.
Results
We identified 20 prospective studies that evaluated 2220 patients. Carfilzomib combination regimens produced an overall response rate (ORR ≥ PR) of 61 % in the 1211 relapsed/refractory patients. At least very good partial response (VGPR) was 29 % in patients with carfilzomib combinations. Finally, 49 % of the 597 patients achieved ORR in patients receiving panobinostat-containing combinations. At least VGPR was 16 % in patients with panobinostat combinations. Three hundred twenty-eight of these 449 patients (73 %) receiving elotuzumab-containing combinations achieved ORR. And at least VGPR was 37 %. And, the vital nonhematologic adverse events (AEs) were cardiac events and pneumonia.
Conclusion
Carfilzomib, panobinostat, and elotuzumab combination regimens produced clinical benefits in patients with R/RMM.
Keywords: Carfilzomib, Panobinostat, Elotuzumab, Multiple myeloma
To the editor
Relapsed myeloma disease is characterized by increasingly lower remission rate even following salvage therapy [1]. So, there is still an urgent need for new treatments to improve the outcomes of such patients. Carfilzomib (CFZ; a selective proteasome inhibitor), panobinostat (PAN; a pan-deacetylase inhibitor), and elotuzumab (ELO; a fully humanized monoclonal antibody against CS1 with significant anti-myeloma activity) are potent anti-myeloma agents with different mechanisms of action [2–4]. We conducted a pooled analysis to determine the efficacy and safety of carfilzomib, panobinostat, and elotuzumab combination regimens in these patients with relapsed/refractory multiple myeloma (R/RMM). The primary outcomes of the analysis were the overall response rate (ORR ≥ PR), at least very good partial response (VGPR), clinical benefit rate (CBR ≥ MR), stable disease rate (SDR), and progressive disease rate (PDR). Statistical analysis method has been shown in Appendix 1.
We identified 20 prospective studies that evaluated 2220 patients with R/RMM receiving carfilzomib-, panobinostat-, or elotuzumab-containing combinations [5–24]. Table 1 summarizes the characteristics of 20 identified clinical reports. As shown in Fig. 1a, 351 of 1211 response-evaluable R/RMM patients (29 %) who received carfilzomib combination therapy in 12 trials achieved at least a VGPR, and 739 patients (61 %) achieved OR. And 727 patients were evaluable for CBR analysis, and CBR was 74 %. And subgroup analysis indicated that the combination of carfilzomib and dexamethasone (DEX) achieved an ORR of 83 %, at least VGPR of 49 %, in those 533 response evaluable patients; in those 520 response evaluable patients, the ORR of 89 % derived from CRD (CFZ/LEN/DEX) compares favorably with that of 66.7 % from RD (LEN/DEX) [10]. Furthermore, the addition of carfilzomib to lenalidomide (LEN) and dexamethasone could improve progression free survival by 31 % [10].
Table 1.
Characteristics of included studies
| Author, year Strategy |
Age Median |
F/M (n/N) | TFD (Y) Median |
Cytogenetic F/U/M |
Drug dose mg/m2 |
Prior therapy median | Prior therapy | Regimen | ORR | PFS (m) | OS (m) | Study design | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bort | Lena | ||||||||||||
| Carfilzomib combinations for R/RMM | |||||||||||||
| Berdeja 2015 [5] | 66 | 27/17 | – | – | 20/27/36/45 | 5 (1–10) | – | – | CP | 0.67 | 7.7 | – | Phase I/II |
| Shan 2015 [6] | 64 | 12/20 | 5.9 | 10/−/− | 20/27/36/45/56 | 6 (2–12) | 31 | 32 | CPD | 0.50 | 7.2 | 20.6 | Phase I |
| Berenson 2014(1) [7] | 67 | 13/25 | 4.2 | – | 20/27/36/45 | – | – | – | ► | 0.43 | 9.9 | 15.8 | Phase I/II |
| Niesvizky 2013 [8] | 61.5 | 18/22 | 3.3 | 25/11/4 | 15/20/27 | 2 (1–3) | 30 | 28 | CRD | 0.62 | 10.2 | – | Phase Ib |
| Papadopoulos 2015 [9] | 59.5 | 5/17 | 3.6 | 14/7/1 | 20/36/45/56/70 | 4(2–9) | 21 | – | CD | 0.55 | – | – | Phase I |
| Stewart 2015 [10] | 64.0 | 181/215 | 3.0 | 48/147/201 | 20/27 | 2(1–3) | 261 | 79 | CRD | 0.87 | 26.3 | – | phase I/II |
| Wang 2013 [11] | 61.5 | 36/48 | 3.1 | 57/22/5 | 20/27 | 2 (1–5) | 65 | 59 | CRD | 0.69 | 11.8 | – | Phase II |
| Berenson 2014 (2) [12] | 63 | – | – | – | 20/45/56/70/88 | 1(1–2) | – | – | CD | 0.67 | – | – | Phase I/II |
| Dimopoulos 2015 [13] | – | – | – | – | 20/56 | – | – | – | CD | 0.77 | – | – | Phase III |
| Kaufman 2014 [14] | 64.5 | – | – | – | 20/36/45 | – | – | – | CP | 0.50 | 14.3 | – | Phase I |
| Vesole 2015 [15] | 61 | 7/10 | 4 | 3/12/2 | 15/20/27 | 4 (1–9) | 17 | 16 | QUAD | 0.53 | 12 | – | Phase I |
| Panobinostat combinations for R/RMM | |||||||||||||
| Offidani 2012 [16] | 73 | 5/7 | – | – | 15 | – | 8 | 5 | PMT | 0.41 | 14.3 | – | Phase II |
| 65 | 10/9 | – | – | 10 | – | 16 | 9 | PMT | 0.37 | 14.3 | – | Phase II | |
| Richardson 2013 [17] | 61 | 26/29 | 4.6 | 2/35/18 | 4 (2–11) | 55 | 54 | PBD | 0.34 | 5.4 | – | Phase II | |
| San-Miguel 2013 [18] | 62 | 19/43 | – | – | 10/20/25/30 | 2 (1–10) | 39 | 28 | PBD | 0.52 | – | – | Phase Ib |
| Kaufman 2014 [14] | 64.5 | – | – | – | 15-20 | – | – | – | CP | 0.50 | 14.3 | – | Phase I |
| Berenson 2014 [19] | 65 | 15/25 | – | – | 20 | 4(1–16) | – | – | PM | 0.07 | – | – | Phase I/II |
| San-Miguel 2014 [20] | 63 | 185/202 | – | – | 20 | – | 169 | 72 | PBD | 0.61 | 11 · 99 | 33 · 6 | Phase III |
| Berdeja 2015 [5] | 66 | 27/17 | – | – | 20/30 | 5 (1–10) | – | – | CP | 0.67 | 7.7 | Phase I/II | |
| Elotuzumab combinations for R/RMM | |||||||||||||
| Jakubowiak 2012 [21] | 63 | 20/18 | 3.5 | – | 2.5/4.0/10/20 | 2(1–3) | 11 | 13 | EB | 0.48 | 9.46 | – | Phase I |
| lonial 2012 [22] | 60 | – | 5.2 | 26/3/0 | 2.5/10/20 | 3(1–10) | 20 | 6 | ERD | 0.82 | – | Phase I | |
| lonial 2015 [23] | 67 | – | – | – | 10 | 2(1–4) | 219 | 16 | ERD | 0.79 | 19.4 | – | Phase III |
| Richardson 2015 [24] | 60.6 | 17/19 | 4.76 | 32/1/3 | 10 | – | 22 | – | ERD | 0.92 | 32 · 49 | – | Phase Ib-II |
| 63.3 | 13/24 | 4.96 | 27/3/7 | 20 | – | 22 | – | ERD | 0.76 | 25 · 00 | – | Phase Ib–II | |
Abbreviations: F female; M male; TFD time from diagnosis; F/U/M favor/unfavor/miss; CFZ carfilzomib; Bor bortezomib; Lena lenalidomide; CPD carfilzomib, pomalidomide, and dexamethasone; ► Replacement of bortezomib with carfilzomib from bortezomib combination therapy, CD carfilzomib, dexamethasone; CRD Carfilzomib, lenalidomide, and dexamethasone; CP carfilzomib, panobinostat; CCD carfilzomib, cyclophosphamide, and dexamethasone; QUAD carfilzomib, lenalidomide, vorinostat, and dexamethasone; PMT panobinostat melphalan prednisone; PBD panobinostat, bortezomib, and dexamethasone; EB elotuzumab bortezomib, ERD elotuzumab, lenalidomide, and dexamethasone
Fig. 1.
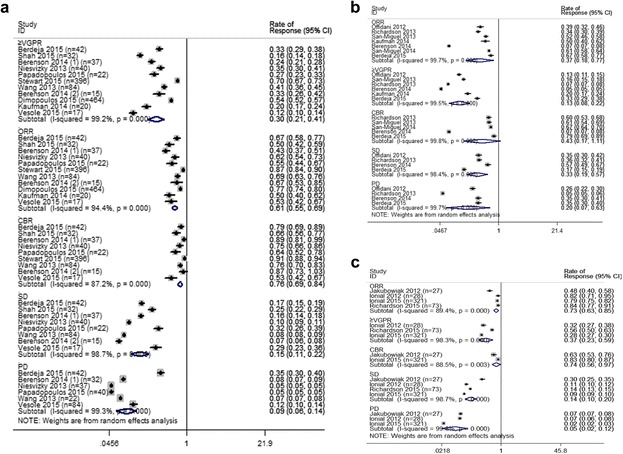
Meta-analysis of the response rate of carfilzomib (a), panobinostat (b), and elotuzumab (c) combination regimens in patients with relapsed and refractory multiple myeloma. n number of the enrolled patients, CI 95 % confidence interval, Random random effects model
Sensitivity analyses shown that the combination of panobinostat and melphalan regimen [19] differed much from the others, which contribute most to the heterogeneity. In order to strengthen the reliability of this pooled analysis, we exclude this trial. When excluding this trial, as shown in Fig. 1b, 49 % of the 597 evaluable R/RMM patients treated with panobinostat-containing combination regimens achieved an ORR, at least VGPR was achieved by 16 %, CBR by 66 %, the SDR was 28 %, and the PDR was 17 %. In those 504 response evaluable patients, the ORR of 48 % derived from PBD (PAN/BOR/DEX) regimen seems to be higher than that of bortezomib (BOR)-containing therapy in a similar population [25]. Furthermore, the addition of panobinostat to bortezomib and dexamethasone could reduce the risk of disease progression by 37 % [20].
As shown in Fig. 1c, four trials enrolling a total of 449 patients evaluated the response rate of elotuzumab-containing combination regimens for those patients with R/RMM. Three hundred twenty-eight of 449 patients (73 %) achieved ORR. And at least VGPR was 37 %, and CBR was 74 %. In the 422 response evaluable patients, the ORRs of 80 % derived from ERD (ELO/LEN/DEX) was encouraging, which compared favorably with that of 60 to 61 % reported in the two trials of RD (LEN/DEX) [26, 27].
In the pooled analysis, the most common adverse events (AEs) consisted primarily of myelosuppression (Fig. 2). And the vital nonhematologic AEs were cardiac events and pneumonia (Fig. 3). Notably, neuropathy was generally mild and infrequent in most carfilzomib trials. But 1 % of 589 patients with baseline grade 1–2 peripheral neuropathy increased to grade 3 before resolving.
Fig. 2.
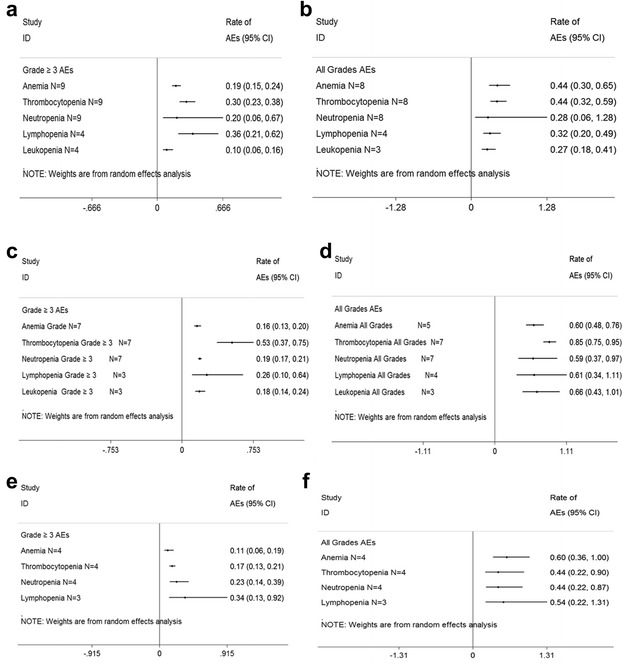
Meta-analysis of hematologic adverse events (AEs) with variable carfilzomib/panobinostat/elotuzumab-containing combination regimens in patients with multiple myeloma. a ≥Grade 3 hematologic AEs with carfilzomib combination regimens in patients with relapsed and refractory multiple myeloma. b All grades hematologic AEs with carfilzomib combination regimens in patients with relapsed and refractory multiple myeloma. c ≥Grade 3 hematologic AEs with panobinostat combination regimens in patients with relapsed and refractory multiple myeloma. d All grades hematologic AEs panobinostat combination regimens in patients with relapsed and refractory multiple myeloma. e ≥Grade 3 hematologic AEs with elotuzumab combination regimens in patients with relapsed and refractory multiple myeloma. f All grades hematologic AEs with elotuzumab combination regimens in patients with relapsed and refractory multiple myeloma. N number of the included trials, CI 95 % confidence interval, Random random effects model
Fig. 3.
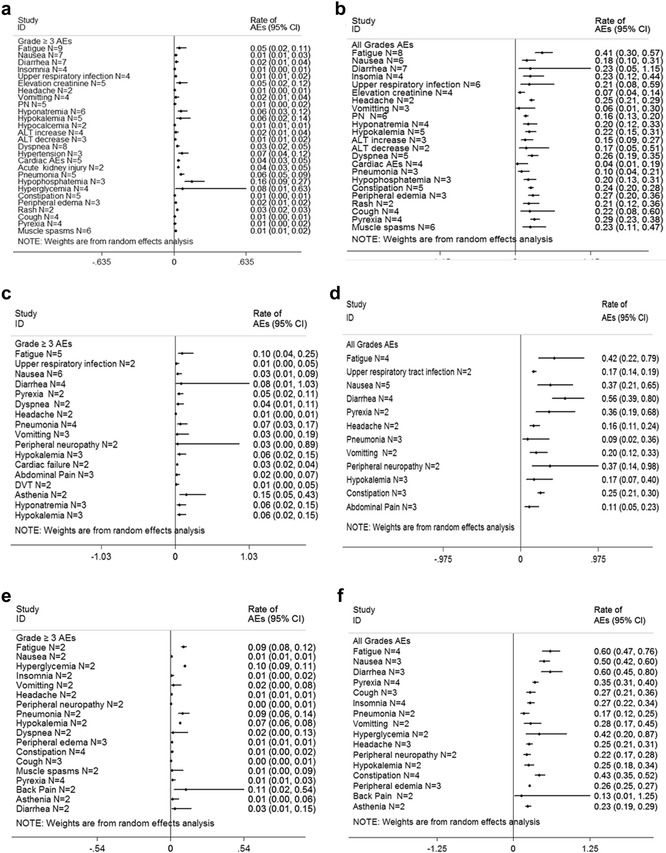
Meta-analysis of nonhematologic adverse events (AEs) with variable carfilzomib/panobinostat/elotuzumab-containing combination regimens in patients with multiple myeloma. a ≥Grade 3 nonhematologic AEs with carfilzomib combination regimens in patients with relapsed and refractory multiple myeloma. b All grades nonhematologic AEs with carfilzomib combination regimens in patients with relapsed and refractory multiple myeloma. c ≥Grade 3 nonhematologic AEs with panobinostat combination regimens in patients with relapsed and refractory multiple myeloma. d All grades nonhematologic AEs panobinostat combination regimens in patients with relapsed and refractory multiple myeloma. e ≥Grade 3 nonhematologic AEs with elotuzumab combination regimens in patients with relapsed and refractory multiple myeloma. f All grades nonhematologic AEs with elotuzumab combination regimens in patients with relapsed and refractory multiple myeloma. N number of the included trials, CI 95 % confidence interval, Random random effects model
When interpreting our results, there are some limitations that should be considered. The first and major problem is that we used abstracted data. A meta-analysis of individual patient data might more clearly define the treatment benefits of these agents and allow time-to-event analyses of progression-free and overall survival. Secondly, as is often the case with meta-analysis, the effect of heterogeneity needs to be taken into account. Finally, the quality of a meta-analysis is always subject to the quality of included studies. Eighteen of the 20 trials included in this pooled analysis were no-RCTs. And, three of them reported interim analyses, and it is unclear whether these results would change when their final analyses are conducted.
In conclusion, the results presented here show that carfilzomib, panobinostat, and elotuzumab combination regimens produced clinical benefits in patients with R/RMM and had acceptable safety profile.
Acknowledgements
We are indebted to Yanhua Sun for assistance with data analysis and critiquing the manuscript.
Funding
The authors did not receive any financial support.
Availability of data and materials
This analysis is a meta-analysis which overview and extracted data from previous published papers. These enrolled trials were shown in Table 1. All these papers can be found on-line.
Authors’ contributions
LW participated in the design of the study and performed the statistical analysis. NZ performed the statistical analysis. WX collected the data. ZS helped to draft the manuscript. LL drafted the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This pooled analysis was approved by the institutional review boards of Weifang People’s Hospital, in accordance with the Helsinki Declaration.
Abbreviations
- R/RMM
Refractory/relapsed multiple myeloma
- ORR
Overall response rate
- VGPR
Very good partial response rate
- CBR
Clinical benefit rate
- SDR
Stable disease rate
- PDR
Progressive disease rate
- CFZ
Carfilzomib
- PAN
Panobinostat
- ELO
Elotuzumab
- DEX
Dexamethasone
- BOR
Bortezomib
- LEN
Lenalidomide
Appendix 1
Methods
Literature search strategy
Medline, Embase, the Cochrane controlled trials register, the Science Citation Index, Conference proceedings from the American Society of Hematology(ASH), the European Hematology association (EHA) and the American Society of Clinical Oncology were searched for prospective trials using the medical subject headings “myeloma,” “carfilzomib,” “panobinostat,” and “elotuzumab.” Reference lists from studies selected for this review and from other published systematic reviews and practice guidelines were also hand-searched.
Selection of studies
Studies were eligible for inclusion in the meta-analysis if they met all the following criteria: (1) they were published up to February, 2016, and written in English, (2) they dealt only with patients with refractory or relapsed multiple myeloma, (3) study selection included the setting of these trials: carfilzomib, panobinostat, and elotuzumab combinations, and (4) we included studies that provided sufficient information to allow the calculation of response rate. Multiple reports of a single study were considered as one publication, and only the most recent or complete article was examined. All potentially relevant articles were reviewed by two independent investigators (L.D.W and L.P.L).
Statistical analysis
All analyses were conducted using a random effects model, which could give a more conservative evaluation of treatment effect. The heterogeneity of between-study and between-subgroup were tested using the Cochrane χ2 test. We also undertook subgroup analyses to seek the source of heterogeneity. We used a visual inspection of the funnel plot and trim and fill analyses to evaluate the influence of publication bias on the pooled RR. All meta-analyses were conducted with Stata ver.12.0 software and Review Manager version 5.1.
Contributor Information
Liping Liu, Email: liuliping200200@163.com.
Ningning Zhao, Email: 30520293@qq.com.
Wenjun Xu, Email: hantingqu@163.com.
Zhixin Sheng, Email: shengzhixin5569@126.com.
Lida Wang, Phone: +86 159 6618 2172, Email: 452697850@qq.com.
References
- 1.Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79(7):867–874. doi: 10.4065/79.7.867. [DOI] [PubMed] [Google Scholar]
- 2.Demo SD, Kirk CJ, Aujay MA, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67(13):6383–6391. doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]
- 3.Atadja P. Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett. 2009;280:233–41. doi: 10.1016/j.canlet.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Hsi ED, Steinle R, Balasa B, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res. 2008;14:2775–84. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berdeja JG, Hart LL, Mace JR, Arrowsmith ER, Essell JH, Owera RS, Hainsworth JD, Flinn IW. Phase I/II study of the combination of panobinostat and carfilzomib in patients with relapsed/refractory multiple myeloma. Haematologica. 2015;100(5):670–6. doi: 10.3324/haematol.2014.119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah JJ, Stadtmauer EA, Abonour R, Cohen AD, Bensinger WI, Gasparetto C, Kaufman JL, Lentzsch S, Vogl DT, Gomes CL, Pascucci N, Smith DD, Orlowski RZ, Durie BG. Carfilzomib, pomalidomide, and dexamethasone for relapsed or refractory myeloma. Blood. 2015;126(20):2284–90. doi: 10.1182/blood-2015-05-643320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berenson JR, Hilger JD, Yellin O, Dichmann R, Patel-Donnelly D, Boccia RV, Bessudo A, Stampleman L, Gravenor D, Eshaghian S, Nassir Y, Swift RA, Vescio RA. Replacement of bortezomib with carfilzomib for multiple myeloma patients progressing from bortezomib combination therapy. Leukemia. 2014;28(7):1529–36. doi: 10.1038/leu.2014.27. [DOI] [PubMed] [Google Scholar]
- 8.Niesvizky R, Martin TG, Bensinger WI, Alsina M, Siegel DS, Kunkel LA, Wong AF, Lee S, Orlowski RZ, Wang M. Phase Ib dose-escalation study (PX-171-006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Clin Cancer Res. 2013;19(8):248–56. doi: 10.1158/1078-0432.CCR-12-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papadopoulos KP, Siegel DS, Vesole DH, Lee P, Rosen ST, Zojwalla N, Holahan JR, Lee S, Wang Z, Badros A. Phase I study of 30-minute infusion of carfilzomib as single agent or in combination with low-dose dexamethasone in patients with relapsed and/or refractory multiple myeloma. J Clin Oncol. 2015;33((7):732–9. doi: 10.1200/JCO.2013.52.3522. [DOI] [PubMed] [Google Scholar]
- 10.Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Oriol A, Hájek R, Rosiñol L, Siegel DS, Mihaylov GG, Goranova-Marinova V, Rajnics P, Suvorov A, Niesvizky R, Jakubowiak AJ, San-Miguel JF, Ludwig H, Wang M, Maisnar V, Minarik J, Bensinger WI, Mateos MV, Ben-Yehuda D, Kukreti V, Zojwalla N, Tonda ME, Yang X, Xing B, Moreau P, Palumbo A. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):14–52. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 11.Wang M, Martin T, Bensinger W, Alsina M, Siegel DS, Kavalerchik E, Huang M, Orlowski RZ, Niesvizky R. Phase 2 dose-expansion study (PX-171-006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Blood. 2013;122(18):3122–8. doi: 10.1182/blood-2013-07-511170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berenson JR, Klein LM, Rifkin RM, et al. Results of the dose-escalation portion of a phase 1/2 study (CHAMPION-1) investigating weekly carfilzomib in combination with dexamethasone for patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2014;32(Suppl):8594. [Google Scholar]
- 13.Dimopoulos MA, Philippe M, Antonio P, Joshua DE, Ludek P, Roman H, et al. Carfilzomib and dexamethasone (Kd) vs bortezomib and dexamethasone (Vd) in patients (pts) with relapsed multiple myeloma (RMM): results from the phase III study ENDEAVOR. J Clin Oncol. 2015;33:suppl; abstr 8509. [Google Scholar]
- 14.Kaufman J, Zimmerman T, Jakubowiak A, et al. Phase I study of the combination of carfilzomib and panobinostat for patients with relapsed and refractory myeloma: a multicenter MMRC clinical trial [abstract] Haematologica. 2013;98(suppl 1):Abstract 322. [Google Scholar]
- 15.Vesole DH, Bilotti E, Richter JR, McNeill A, McBride L, Raucci L, Anand P, Bednarz U, Ivanovski K, Smith J, Batra V, Aleman A, Sims T, Guerrero L, Mato A, Siegel DS. Phase I study of carfilzomib, lenalidomide, vorinostat, and dexamethasone in patients with relapsed and/or refractory multiple myeloma. Br J Haematol. 2015;171(1):52–9. doi: 10.1111/bjh.13517. [DOI] [PubMed] [Google Scholar]
- 16.Offidani M, Polloni C, Cavallo F, Liberati AM, Ballanti S, Pulini S, Catarini M, Alesiani F, Corvatta L, Gentili S, Caraffa P, Boccadoro M, Leoni P, Palumbo A. Phase II study of melphalan, thalidomide and prednisone combined with oral panobinostat in patients with relapsed/refractory multiple myeloma. Leuk Lymphoma. 2012;53(9):1722–7. doi: 10.3109/10428194.2012.664844. [DOI] [PubMed] [Google Scholar]
- 17.Richardson PG, Schlossman RL, Alsina M, Weber DM, Coutre SE, Gasparetto C, Mukhopadhyay S, Ondovik MS, Khan M, Paley CS, Lonial S. PANORAMA 2: panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory myeloma. Blood. 2013;122(14):2331–7. doi: 10.1182/blood-2013-01-481325. [DOI] [PubMed] [Google Scholar]
- 18.San-Miguel JF, Richardson PG, Günther A, Sezer O, Siegel D, Bladé J, LeBlanc R, Sutherland H, Sopala M, Mishra KK, Mu S, Bourquelot PM, Victoria Mateos M, Anderson KC. Phase Ib study of panobinostat and bortezomib in relapsed or relapsed and refractory multiple myeloma. J Clin Oncol. 2013;31(29):3696–703. doi: 10.1200/JCO.2012.46.7068. [DOI] [PubMed] [Google Scholar]
- 19.Berenson JR, Hilger JD, Yellin O, Boccia RV, Matous J, Dressler K, Ghazal HH, Jamshed S, Kingsley EC, Harb WA, Noga SJ, Nassir Y, Swift RA, Vescio R. A phase 1/2 study of oral panobinostat combined with melphalan for patients with relapsed or refractory multiple myeloma. Ann Hematol. 2014;93(1):89–98. doi: 10.1007/s00277-013-1910-2. [DOI] [PubMed] [Google Scholar]
- 20.San-Miguel JF, Hungria VT, Yoon SS, Beksac M, Dimopoulos MA, Elghandour A. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15(11):1195–206. doi: 10.1016/S1470-2045(14)70440-1. [DOI] [PubMed] [Google Scholar]
- 21.Jakubowiak AJ, Benson DM, Bensinger W, Siegel DS, Zimmerman TM, Mohrbacher A, Richardson PG, Afar DE, Singhal AK, Anderson KC. Phase I trial of anti-CS1 monoclonal antibody elotuzumab in combination with bortezomib in the treatment of relapsed/refractory multiple myeloma. J Clin Oncol. 2012;30(16):1960–5. doi: 10.1200/JCO.2011.37.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lonial S, Vij R, Harousseau JL, Facon T, Moreau P, Mazumder A, Kaufman JL, Leleu X, Tsao LC, Westland C, Singhal AK, Jagannath S. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J Clin Oncol. 2012;30(16):1953–9. doi: 10.1200/JCO.2011.37.2649. [DOI] [PubMed] [Google Scholar]
- 23.Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373(7):621–31. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 24.Richardson PG, Jagannath S, Moreau P, Jakubowiak AJ, Raab MS, Facon T, et al. Elotuzumab in combination with lenalidomide and dexamethasone in patients with relapsed multiple myeloma: final phase 2 results from the randomised, open-label, phase 1b-2 dose-escalation study. Lancet Haematol. 2015;2(12):e516–27. doi: 10.1016/S2352-3026(15)00197-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar SK, Lee JH, Lahuerta JJ, International Myeloma Working Group et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26(1):149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123–32. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 27.Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357(21):2133–42. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This analysis is a meta-analysis which overview and extracted data from previous published papers. These enrolled trials were shown in Table 1. All these papers can be found on-line.


