Abstract
Psoriasis is characterized by uncontrolled proliferation and poor differentiation. Sirtuin1 (SIRT1) a class III deacetylase, crucial for differentiation in normal keratinocytes, is reduced in psoriasis. Down regulated SIRT1 levels may contribute to poor differentiation in psoriasis. In addition, the levels of early differentiation factors Keratin1 (K1) and Keratin10 (K10) are depleted in psoriasis. We attempted to study a possible effect of fructose, a SIRT1 upregulator and Propylthiouracil (PTU) to augment differentiation in psoriatic keratinocytes. Keratinocytes were cultured from lesional biopsies obtained from psoriatic patients and control cells were obtained from patients undergoing abdominoplasty. Cells were treated with fructose and PTU individually. K1 and K10 transcript levels were measured to evaluate early differentiation; SIRT1 protein expression was also studied to decipher its role in the mechanism of differentiation. The K1, K10 transcript levels, SIRT1 protein and transcript levels in fructose treated psoriatic keratinocytes were improved. This suggests keratinocyte differentiation was induced by fructose through SIRT1 upregulation. Whereas PTU induced differentiation, as confirmed by improved K1, K10 transcript levels followed a non-SIRT1 mechanism. We conclude that the use of fructose and PTU may be an adjunct to the existing therapies for psoriasis.
Keywords: Psoriasis, Fructose, Keratin1, Keratin10, SIRT1, Propylthiouracil
Highlights
-
•
Fructose induces differentiation of psoriatic keratinocytes through SIRT1 upregulation.
-
•
Propylthiouracil (PTU) improves K1 and K10 to induce differentiation in psoriasis.
-
•
Fructose and PTU can be used as adjunct to existing therapies for psoriasis.
1. Introduction
A hallmark of lesional psoriatic skin is premature keratinocyte differentiation and disturbed keratinization, altering the formation of cornified envelope in psoriasis. Human epidermal keratinocytes stratify into colonies and cells in stratum granulosum, stratum lucidum and stratum corneum gradually lose their mitotic potential to begin terminal differentiation. Differentiation is a highly organized process wherein the proteins K1, K10, profilaggrin, involucrin, loricrin, and other proteins of cornified envelope are sequentially expressed [1].
There had been a debate about the progress of differentiation pattern in psoriasis. Until 1992 it was thought that only the last step in differentiation was altered, however Bernerd et al. showed that alterations are found from the first stage itself [2]. The first step of differentiation involves heterodimerization of K1 and K10 to form cytoskeletal filaments. This keratin pair is the most abundant protein in differentiated keratinocytes [3].
Keratin K1/K10 regulates keratinocyte growth in the epidermis which is proved by in vitro experiments conducted by Paramio et al. (2001) [4]. They reported that K10 is directly involved in cell cycle control which onsets keratinocyte differentiation. Interestingly several other studies have shown that mutations either in K1/K10 or absence of K10 showed greater epidermal proliferation in the basal layer and hyperkeratosis [5], [6]. Furthermore in situ hybridization of K10 transcripts proved a delayed keratin 10 synthesis in psoriatic epidermis [2]. These studies markedly prove the essential role of K1/K10 in differentiation and controlled proliferation. Many therapies in the past targeted differentiation, in the same lines we were on the lookout for a differentiation improving factor.
A study published by Blander et al. (2009) [7] exposed the potential role of SIRT1 in inducing keratinocyte differentiation and inhibiting keratinocyte proliferation. Elevated IFN gamma levels inhibits SIRT1 expression and sensitizes psoriatic keratinocytes to IL-22 mediated inflammatory response altering epidermal differentiation [8]. Pillai and colleague (2008) found that fructose augmented SIRT1 levels in heart [9]. Since SIRT1 mediated effects are tissue specific, we attempted to increase SIRT1 levels and improve differentiation in psoriatic keratinocytes, by treating the cells with fructose. In the recent past our team has proved that PTU cleared lesions in psoriatic patients and improved differentiation as confirmed by modulated levels of involucrin [10], [11]. In this study, we have focused on the role of fructose and PTU to improve differentiation in psoriatic keratinocytes and further explored the impact of these compounds on SIRT1 levels, a promoter of keratinocyte differentiation.
2. Materials and methods
2.1. Recruitment of patients and collection of samples
Patients (N = 7) with chronic plaque psoriasis, but otherwise in general good health who visited Saveetha Medical College Hospital, Chennai, India participated in this study. Psoriasis was confirmed by Psoriasis Area Severity Index (PASI) score by a dermatologist. None of the recruited patients had received any topical treatment for the last 2 weeks or any systemic treatment for the last 1 month. Skin from patients undergoing abdominoplasty served as control samples. Lesional biopsy specimens (5 mm) were obtained through punch biopsy from patients with chronic plaque psoriasis. The study was approved by Institutional Ethical Committee, Saveetha University (Chennai) and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from each participant included in the study.
2.2. Primary culture
The biopsy specimens were incubated overnight in 0.3% dispase (Sigma, USA) in Phosphate Buffered Saline (PBS) supplemented with 50 μg/ml Gentamicin (Life Technologies, USA) at 4 °C. After incubation the dermis layer was mechanically removed and discarded, the epidermal layer was treated with 0.25% trypsin (Life Technologies, USA) at 37 °C for 30 min. Cell dissociation was achieved by aspiration using Pasteur pipette followed by filtration (70 μm cell strainer). Keratinocytes Serum Free Medium (K-SFM) (Life Technologies, USA) was used to wash filtered keratinocytes. The viability of the cells was always > 95% as determined by Trypan blue exclusion test. To generate keratinocyte cultures, suspension of primary epidermal cells (4 × 104 cells/cm2) were plated in 100 mm petri dishes. The culture was supplemented with keratinocytes supplements such as Epidermal Growth Factor (EGF) 0.1 ng/ml, Bovine pituitary extract 25 μg/ml, and Gentamicin 50 μg/ml in K-SFM and the flasks were maintained in a humidified atmosphere containing 5% CO2 at 37 °C. The medium was changed every 2 days and the third passage keratinocytes with 70–80% confluence was used for further experiments.
2.3. Cell proliferation assay
For analyzing cell proliferation via MTT assay, keratinocytes (1 × 104 cells/well) were incubated with different concentrations of PTU (2–10 mM) and fructose (1–20 mM) for 24 h, 48 h and 72 h in 96 well plates and were incubated at 37 °C in a humidified atmosphere with 5% CO2. Stock solutions of compounds were initially dissolved in DMSO and further diluted with fresh complete medium. MTT reagent (50 μl) (5 mg/ml in PBS), was added to each well and incubated at 37 °C for 4 h. At the end of the incubation period, the supernatant was removed completely without disturbing the cell layer, 150 μl of DMSO was added and read on a microplate reader at 570 nm.
2.4. RNA isolation and real-time polymerase chain reaction (RT-PCR)
TRIzol reagent (Life Technologies, USA) was used to extract total RNA to be analyzed by real-time PCR. The expression of SIRT1, K1, K10 and β-Actin mRNA were evaluated using SYBR Green PCR reagents following the manufacturer's protocol on an Applied Biosystem Thermocycler. The forward and reverse primers used in real-time PCR were as follows: For SIRT1, forward: 5′-TCAGTGTCATGGTTCCTTTGC-3′; reverse: 5′-AATCTGCTCCTTTGCCACTCT-3′, K1 forward 5′-ATTTCTGAGCTGAATCGTGTGATC-3′ reverse 5′-CTGATGGACTGCTGCAAGTT-3′ K10 forward 5′-ATGAGCTGACCCTGACCAAG-3′ reverse 5′- TCACATCACCAGTGGACACA-3′ and for β-Actin forward: 5′-AGGCACCAGGGCGTGAT-3′; reverse 5′- GCCCACATAGGAATCCTTCTGAC-3′. The gene expression levels were determined by normalizing to β-Actin mRNA expression. The values obtained are presented as mean ± SD.
2.5. Western blot
Solubilized protein samples (40 μg; measured and equalized in each fraction using the Bio-Rad RC-DC protein assay; Bio-Rad) were separated by SDS-PAGE and transferred onto PVDF membrane (GE Healthcare, UK). Membranes were blocked with 3% (w/v) milk protein in Tris-buffered saline containing 0.1% Tween-20, and then incubated overnight with rabbit polyclonal anti-SIRT1 antibody (sc-15404, 1:1000 dilution, Santa Cruz Technologies, USA). Detection of bands was achieved by using the chemiluminescent substrate Super Signal West Pico (Pierce, Rockford, IL, USA). Blot is representative of three separate blots and densitometry was determined. Reference protein measurements were made with rabbit polyclonal anti-β-actin primary antibody in a 3% (w/v) Tris-buffered saline (1:1000 dilution, Santa Cruz Technologies, CA, USA).
2.6. Statistics
All values are represented as mean ± S.D. of the three measurements. A one-way analysis of variance test for post hoc multiple comparisons was used to determine significance. Probability values < 0.05 were considered significant.
3. Results
MTT assay (Fig. 1A and B) shows percentage of live cells from control, untreated and treated psoriatic keratinocytes at 24 h, 48 h and 72 h. PTU treatment (2–10 mM) and fructose treatment (1–20 mM) decreased proliferation of psoriatic keratinocytes when compared to untreated cells. A dose of 4 mM of PTU and 5 mM of fructose was used for further experiments. Control keratinocytes obtained from abdominoplasty patients were treated with fructose and PTU independently and in combination. We measured the percentage of live cells. No significant changes were seen between the treated and untreated control cells.
Fig. 1.
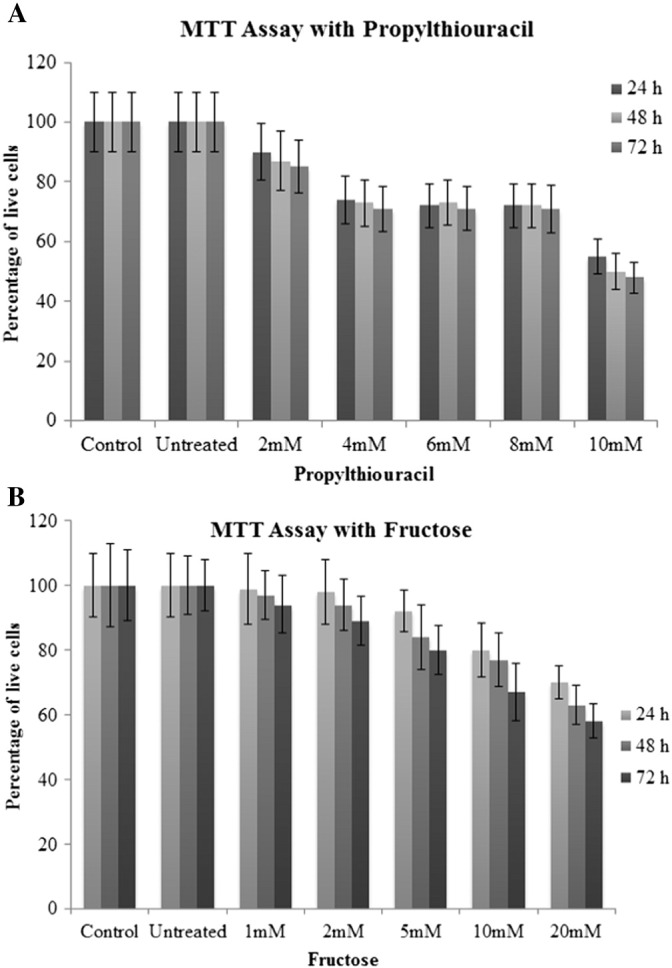
A. MTT Assay showing percentage of viable cells in control, untreated, 2–10 mM PTU treated psoriatic keratinocytes. All values are expressed as mean ± SD.
B. MTT Assay showing percentage of viable cells in control, untreated, 1–20 mM fructose treated psoriatic keratinocytes. All values are expressed as mean ± SD.
3.1. Improved K1 and K10 transcript levels in cultured psoriatic keratinocytes after treatment with fructose and PTU
Poor differentiation in psoriatic keratinocytes was apparent from the decreased K1 & K10 levels compared to keratinocytes from control samples (0.56 and 0.60 fold change respectively, p < 0.05) (Fig. 2). Fructose treatment to psoriatic keratinocytes improved K1 and K10 levels by 1.66 and 2.5 fold respectively (p < 0.001). PTU treatment was also very effective in improving differentiation in psoriatic keratinocytes and the fold change for K1 and K10 were 2.04 and 2.79 respectively (p < 0.001). Control keratinocytes obtained from abdominoplasty patients were treated with fructose and PTU independently and in combination. We measured the mRNA expression of K1 and K10. No significant changes were seen between the treated and untreated control cells.
Fig. 2.
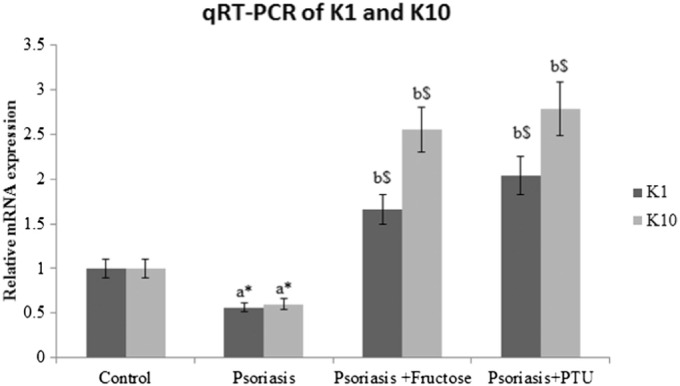
Fold change of K1 and K10 transcripts in control, psoriatic keratinocytes, fructose treated psoriatic keratinocytes and PTU treated psoriatic keratinocytes. All values are expressed as mean ± SD. ‘a’ denotes psoriasis compared to control, ‘b’ denotes psoriasis + fructose and psoriasis + PTU compared to psoriasis. ‘⁎’denotes p < 0.05, ‘$’denotes p < 0.001.
3.2. SIRT1 levels in cultured psoriatic keratinocytes was increased by fructose but not by PTU
The effect of PTU and fructose on SIRT1 mRNA transcript levels in psoriatic keratinocytes was studied by Real time PCR (Fig. 3). SIRT1 mRNA level was found to be 0.56 fold lesser in psoriatic keratinocytes than normal keratinocytes from control samples. The fold change that was brought about by fructose treatment to psoriatic keratinocytes in SIRT1 levels is 1.75 (p < 0.001); there were no significant changes in SIRT1 mRNA transcript levels in psoriatic keratinocytes upon PTU treatment.
Fig. 3.
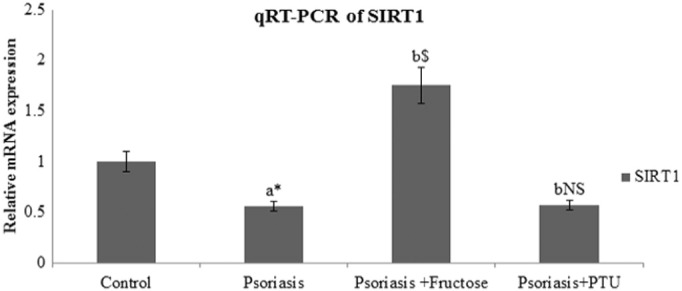
Fold change of SIRT1 transcripts in control, psoriatic keratinocytes, fructose treated psoriatic keratinocytes and PTU treated psoriatic keratinocytes. All values are expressed as mean ± SD. ‘a’ denotes psoriasis compared to control, ‘b’ denotes psoriasis + fructose and psoriasis + PTU compared to psoriasis. ‘⁎’denotes p < 0.05, ‘$’denotes p < 0.001, ‘NS’ denotes non-significant.
Western blot analysis showed similar pattern of SIRT1 protein levels in psoriatic keratinocytes before and after treatment with PTU and fructose (Fig. 4A). Elevated SIRT1 protein level is evident in the fructose treated psoriatic keratinocytes (p < 0.001) compared to untreated cells as measured by densitometry analysis (Image J software) whereas PTU treatment did not elevate SIRT1 protein levels significantly (Fig. 4B). Control keratinocytes obtained from abdominoplasty patients were treated with fructose and PTU independently and in combination. We measured the mRNA expression and protein levels of SIRT1. No significant changes were seen between the treated and untreated control cells.
Fig. 4.
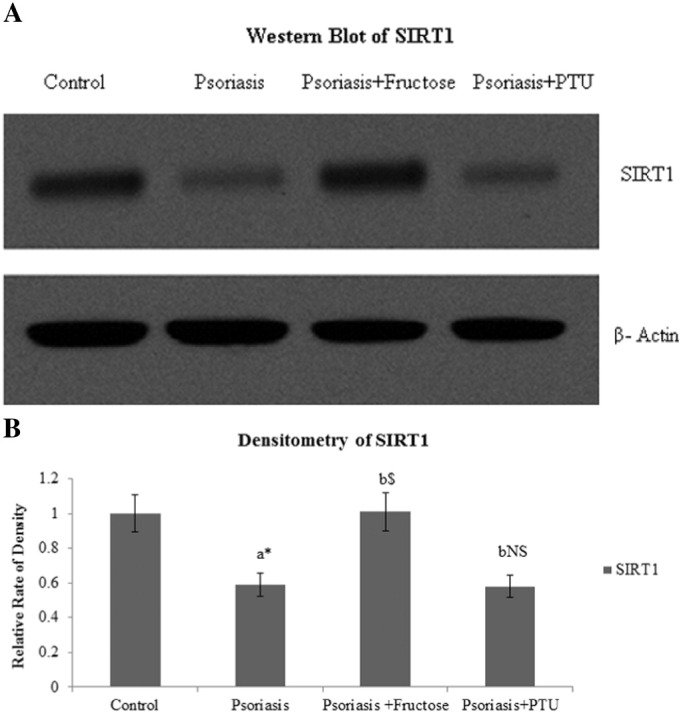
A. Western blot showing SIRT1 protein levels in control, psoriatic keratinocytes, fructose treated psoriatic keratinoctyes and PTU treated psoriatic keratinocytes.
B Relative densities of SIRT1 in control, psoriatic keratinocytes, fructose treated psoriatic keratinocytes and PTU treated psoriatic keratinocytes. All values are expressed as mean ± SD. ‘a’ denotes psoriasis compared to control, ‘b’ denotes psoriasis + fructose and psoriasis + PTU compared to psoriasis. ‘⁎’denotes p < 0.05, ‘$’denotes p < 0.001, ‘NS’ denotes non-significant.
4. Discussion
Several attempts have been made in the recent past to improve differentiation in psoriatic keratinocytes that include NO donor, monomethylfumarate, peroxisome proliferator activator receptors, cognate ligands and delphinidin [12], [13], [14], [15]. In this study, fructose and PTU were used to improve differentiation in psoriatic keratinocytes. In psoriatic keratinocytes, levels of K1 and K10 transcripts were significantly low compared to control samples. This is consistent with previous reports which describe a characterized decrease in K1, K10 in hyperproliferative epidermis [2], [16].
Decreased SIRT1 levels is the primary reason for the diminished differentiation in psoriasis. Conversely fructose treatment to psoriatic keratinocytes resulted in increased SIRT1 levels (Fig. 2) and improved K1, K10 transcript levels; PTU treatment did not produce a significant change in SIRT1 levels but managed to improve K1 and K10 levels. When Blander et al. (2009) studied the effect of SIRT1 on differentiation pattern in keratinocytes, they found that SIRT1 expression paralleled differentiation marker levels and reduced keratinocyte proliferation rates [7]. The quest for a new drug probably lead to the use of PTU in treating psoriasis. Elias et al. postulated that this drug can potentially reduce the cytokine signals that lead to keratinocyte proliferation [17]. Halting of proliferation in psoriatic keratinocytes by PTU commences differentiation. In our earlier study we reported that PTU down regulated abnormal keratinocyte differentiation protein, involucrin [11]. Presently we declare that PTU improves expression of normal keratinocyte differentiation markers (K1/K10) confirming the mechanism of PTU and its clinical efficacy in psoriasis treatment. In addition PTU is said to promote differentiation of vascular smooth muscle cells via PTEN induction [18].
The expression levels of keratin genes are fine-tuned and appear to be regulated by large number of transcription factors [19]. Important regulatory sites of these differentiation markers are found in the promoter regions. Many keratin gene expression are regulated by transcription factors AP1, AP2 and Sp1. AP-1 is an assembly of heterodimeric protein complexes, including Jun family members namely c-Jun, JunB, JunD, and fos family of transcription factors such as c-Fos, FosB, Fra-1, and Fra-2 [20]. The most common dimer in AP-1 signalling pathway is c-Jun/c-Jun homodimers or c-Jun/c-Fos heterodimers whose transcriptional activity is regulated by post translational modification like acetylation [21]. The acetylation of transcriptional factors alters the protein-DNA and protein-protein interactions which are important regulatory mechanisms of transcription [22].
Members of the AP-1 family were found to exhibit antagonistic effects. While c-Jun is a promoter of proliferation, JunB is a suppressor of proliferation and promoter of differentiation [23]. Down regulation of JunB could be the reason for the uncontrolled proliferation and poor differentiation of keratinocytes in psoriasis [24]. It has been suggested that SIRT1 directly inhibits the transcriptional activity of AP-1 by interacting with the basic leucine zipper domains of c-Fos and c-Jun, the major components of AP-1 [25]. The improved differentiation in psoriatic keratinocytes in our results by fructose can be attributed to the improved SIRT1 levels which might have suppressed c-Fos and c-Jun, arresting proliferation but initiating differentiation. It has been reported that SIRT1 over expression does not activate JunB in skeletal muscles. Since, SIRT1 mediated effects are tissue specific, whether SIRT1 improves JunB levels in keratinocytes is yet to be identified. Of equal importance, PTU induced differentiation as confirmed by improved K1 and K10 levels, followed a non-SIRT1 mechanism due to less or no change in expression of SIRT1 in vitro. AP-2, a family of large DNA binding transcription factors also controls the balance between growth and differentiation in the epidermis [26]. AP-2 controls the expression of K10 via activation or repression of the c/ebpα, c/ebpβ, and ap-2α gene promoters [27]. Further studies are warranted with larger sample size on the role of SIRT1 in AP-2 regulated genes in differentiation and proliferation factors in keratinocytes. In conclusion, both fructose and PTU are able to induce differentiation in psoriatic keratinocytes through SIRT-1 mediated and non-SIRT-1 mechanisms respectively. Thus fructose and PTU can be used as adjunct in existing therapies for psoriasis.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Compliance with ethics guidelines
This study has been performed in accordance with the ethical standards as laid down in the 1975 Declaration of Helsinki and its later amendments or comparable ethical standards.
Transparency document
Transparency document
Acknowledgement
This work was supported by Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India. Grant No: [SR/FT/LS-125/2012].
Footnotes
Key message: A novel attempt to induce differentiation in poorly differentiated psoriatic keratinocytes.
The Transparency document associated with this article can be found, in online version.
References
- 1.Fuchs E. Epidermal differentiation: the bare essentials. J. Cell Biol. 1990;111:2807–2814. doi: 10.1083/jcb.111.6.2807. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2116387&tool=pmcentrez&rendertype=abstract (accessed July 20, 2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernerd F., Magnaldo T., Darmon M. Delayed onset of epidermal differentiation in psoriasis. J. Investig. Dermatol. 1992;98:902–910. doi: 10.1111/1523-1747.ep12460344. ( http://www.ncbi.nlm.nih.gov/pubmed/1375620 (accessed August 19, 2015)) [DOI] [PubMed] [Google Scholar]
- 3.Cheng J., Syder A.J., Yu Q.C., Letai A., Paller A.S., Fuchs E. The genetic basis of epidermolytic hyperkeratosis: a disorder of differentiation-specific epidermal keratin genes. Cell. 1992;70:811–819. doi: 10.1016/0092-8674(92)90314-3. ( http://www.ncbi.nlm.nih.gov/pubmed/1381287 (accessed August 19, 2015)) [DOI] [PubMed] [Google Scholar]
- 4.Paramio J.M., Segrelles C., Ruiz S., Jorcano J.L. Inhibition of protein kinase B (PKB) and PKCzeta mediates keratin K10-induced cell cycle arrest. Mol. Cell. Biol. 2001;21:7449–7459. doi: 10.1128/MCB.21.21.7449-7459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frost P., Weinstein G.D., Van Scott E.J. The ichthyosiform dermatoses. II. Autoradiographic studies of epidermal proliferation. J. Investig. Dermatol. 1966;47:561–567. doi: 10.1038/jid.1966.185. ( http://www.ncbi.nlm.nih.gov/pubmed/5957926 (accessed August 19, 2015)) [DOI] [PubMed] [Google Scholar]
- 6.Reichelt J., Furstenberger G., Magin T.M. Loss of keratin 10 leads to mitogen-activated protein kinase (MAPK) activation, increased keratinocyte turnover, and decreased tumor formation in mice. J. Investig. Dermatol. 2004;123:973–981. doi: 10.1111/j.0022-202X.2004.23426.x. [DOI] [PubMed] [Google Scholar]
- 7.Blander G., Bhimavarapu A., Mammone T., Maes D., Elliston K., Reich C., Matsui M.S., Guarente L., Loureiro J.J. SIRT1 promotes differentiation of normal human keratinocytes. J. Investig. Dermatol. 2009;129:41–49. doi: 10.1038/jid.2008.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boniface K., Bernard F.-X., Garcia M., Gurney A.L., Lecron J.-C., Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J. Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. ( http://www.ncbi.nlm.nih.gov/pubmed/15749908 (accessed May 10, 2015)) [DOI] [PubMed] [Google Scholar]
- 9.Pillai J.B., Chen M., Rajamohan S.B., Samant S., Pillai V.B., Gupta M., Gupta M.P. Activation of SIRT1, a class III histone deacetylase, contributes to fructose feeding-mediated induction of the alpha-myosin heavy chain expression. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1388–H1397. doi: 10.1152/ajpheart.01339.2007. [DOI] [PubMed] [Google Scholar]
- 10.Malligarjunan H., Gnanaraj P., Subramanian S., Elango T., Dayalan H. Clinical efficacy of propylthiouracil and its influence on prolactin in psoriatic patients. Clin. Biochem. 2011;44:1209–1213. doi: 10.1016/j.clinbiochem.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Gnanaraj P., Dayalan H., Elango T., Malligarjunan H., Raghavan V., Rao R. Downregulation of involucrin in psoriatic lesions following therapy with propylthiouracil, an anti-thyroid thioureylene: immunohistochemistry and gene expression analysis. Int. J. Dermatol. 2015;54:302–306. doi: 10.1111/ijd.12565. [DOI] [PubMed] [Google Scholar]
- 12.Abeyakirthi S., Mowbray M., Bredenkamp N., van Overloop L., Declercq L., Davis P.J., Matsui M.S., Weller R.B. Arginase is overactive in psoriatic skin. Br. J. Dermatol. 2010;163:193–196. doi: 10.1111/j.1365-2133.2010.09766.x. [DOI] [PubMed] [Google Scholar]
- 13.Helwa I., Patel R., Karempelis P., Kaddour-Djebbar I., Choudhary V., Bollag W.B. The antipsoriatic agent monomethylfumarate has antiproliferative, prodifferentiative, and anti-inflammatory effects on keratinocytes. J. Pharmacol. Exp. Ther. 2015;352:90–97. doi: 10.1124/jpet.114.218818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramot Y., Mastrofrancesco A., Camera E., Desreumaux P., Paus R., Picardo M. The role of PPARγ-mediated signalling in skin biology and pathology: new targets and opportunities for clinical dermatology. Exp. Dermatol. 2015;24:245–251. doi: 10.1111/exd.12647. [DOI] [PubMed] [Google Scholar]
- 15.Chamcheu J.C., Pal H.C., Siddiqui I.A., Adhami V.M., Ayehunie S., Boylan B.T., Noubissi F.K., Khan N., Syed D.N., Elmets C.A., Wood G.S., Afaq F., Mukhtar H. Prodifferentiation, anti-inflammatory and antiproliferative effects of delphinidin, a dietary anthocyanidin, in a full-thickness three-dimensional reconstituted human skin model of psoriasis. Skin Pharmacol. Physiol. 2015;28:177–188. doi: 10.1159/000368445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishida-Yamamoto A., Senshu T., Takahashi H., Akiyama K., Nomura K., Iizuka H. Decreased deiminated keratin K1 in psoriatic hyperproliferative epidermis. J. Investig. Dermatol. 2000;114:701–705. doi: 10.1046/j.1523-1747.2000.00936.x. [DOI] [PubMed] [Google Scholar]
- 17.Elias A.N., Nanda V.S., Barr R.J. Effect of PTU on IL-12 and IL-10 in psoriasis. J. Drugs Dermatol. 2003;2:645–648. ( http://www.ncbi.nlm.nih.gov/pubmed/14711144 (accessed June 28, 2016)) [PubMed] [Google Scholar]
- 18.Chen W.-J., Pang J.-H.S., Lin K.-H., Lee D.-Y., Hsu L.-A., Kuo C.-T. Propylthiouracil, independent of its antithyroid effect, promotes vascular smooth muscle cells differentiation via PTEN induction. Basic Res. Cardiol. 2010;105:19–28. doi: 10.1007/s00395-009-0045-z. [DOI] [PubMed] [Google Scholar]
- 19.Springer US; Boston, MA: 2006. Intermediate Filaments. [Google Scholar]
- 20.Foletta V.C. Transcription factor AP-1, and the role of Fra-2. Immunol. Cell Biol. 1996;74:121–133. doi: 10.1038/icb.1996.17. [DOI] [PubMed] [Google Scholar]
- 21.Vries R.G., Prudenziati M., Zwartjes C., Verlaan M., Kalkhoven E., Zantema A. A specific lysine in c-Jun is required for transcriptional repression by E1A and is acetylated by p300. EMBO J. 2001;20:6095–6103. doi: 10.1093/emboj/20.21.6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo J., Li M., Tang Y., Laszkowska M., Roeder R.G., Gu W. Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 2004;101:2259–2264. doi: 10.1073/pnas.0308762101. ( http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=356938&tool=pmcentrez&rendertype=abstract (accessed August 19, 2015)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaulian E., Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 24.Zenz R., Wagner E.F. Jun signalling in the epidermis: from developmental defects to psoriasis and skin tumors. Int. J. Biochem. Cell Biol. 2006;38:1043–1049. doi: 10.1016/j.biocel.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Zhang R., Chen H.-Z., Liu J.-J., Jia Y.-Y., Zhang Z.-Q., Yang R.-F., Zhang Y., Xu J., Wei Y.-S., Liu D.-P., Liang C.-C. SIRT1 suppresses activator protein-1 transcriptional activity and cyclooxygenase-2 expression in macrophages. J. Biol. Chem. 2010;285:7097–7110. doi: 10.1074/jbc.M109.038604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufman C.K., Sinha S., Bolotin D., Fan J., Fuchs E. Dissection of a complex enhancer element: maintenance of keratinocyte specificity but loss of differentiation specificity. Mol. Cell. Biol. 2002;22:4293–4308. doi: 10.1128/MCB.22.12.4293-4308.2002. ( http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=133856&tool=pmcentrez&rendertype=abstract (accessed August 19, 2015)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maytin E.V., Lin J.C., Krishnamurthy R., Batchvarova N., Ron D., Mitchell P.J., Habener J.F. Keratin 10 gene expression during differentiation of mouse epidermis requires transcription factors C/EBP and AP-2. Dev. Biol. 1999;216:164–181. doi: 10.1006/dbio.1999.9460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document


