Abstract
Introduction
MCP-1 and eotaxin-1 are encoded on chromosome 17 and have been shown to reduce hippocampal neurogenesis in mice. We investigated whether these chemokines selectively associate with memory in individuals with mild cognitive impairment (MCI) and Alzheimer's disease (AD) dementia.
Methods
MCP-1 and eotaxin-1 were assayed in controls, MCI, and AD dementia patients with varying phenotypes (n = 171). A subset of 55 individuals had magnetic resonance imaging (MRI) scans available. Composite scores for cognitive variables were created, and medial temporal lobe volumes were obtained.
Results
An interaction was noted between MCP-1 and eotaxin-1, such that deleterious associations with memory were seen when both chemokines were elevated. These associations remained significant after adding APOE genotype and comparison (non-chromosome 17) chemokines into the model. These chemokines predicted left medial temporal lobe volume and were not related to other cognitive domains.
Discussion
These results suggest a potentially selective role for MCP-1 and eotaxin-1 in memory dysfunction in the context of varied MCI and AD dementia phenotypes.
Keywords: Inflammation, Neuropsychology, Chemokines, Neuroimaging, Episodic memory
1. Introduction
The role of peripheral inflammation in the pathogenesis of neurodegenerative diseases is complex and controversial, but accumulating evidence suggests that immune-mediated processes may underlie and promote Alzheimer's disease (AD) phenotypes [1], [2]. Clarifying how and which specific inflammatory processes impact memory performance is of particular importance, as animal models have consistently linked chronic elevations in pro-inflammatory cascades with untoward cognitive outcomes and hippocampal dysfunction [3].
Within the context of pro-inflammatory process, specific chemokines have recently garnered research attention as potential modulators of memory function both in aging and AD. Monocyte chemotactic protein-1 (MCP-1; CCL2) and eotaxin-1 (CCL11) are both members of the C–C chemokine family clustered closely on the long arm of chromosome 17 and have been linked with memory impairment (eotaxin-1) [4], age at onset in familial AD (eotaxin-1) [5], senescence [6], and increased abeta pathology (MCP-1) [7] in animal models of aging and AD. In a seminal study using a parabiosis model, Villeda et al. [8] identified systemic chemokines in plasma, including MCP-1 and eotaxin-1, that correlated with reduced neurogenesis in both aged mice and heterochronic parabionts (i.e., an older mouse surgically connected to a younger mouse). Moreover, when plasma eotaxin-1 levels were increased in vivo in young mice, impaired spatial learning, and memory was observed on behavioral tasks. Although these important studies point to a possible role of MCP-1 and eotaxin-1 in aging and AD, few studies have addressed this question in humans [9], and no study has examined these markers with respect to episodic memory functions in older adults. As such, it remains unclear whether peripheral MCP-1 and eotaxin-1 levels are sensitive to verbal or visual memory difficulties in older adults, and whether this association with cognition generalizes to nonmemory domains.
The goal of this study was to assess whether MCP-1 and eotaxin-1 levels predicted memory functions in older adults with mild cognitive impairment (MCI) and Alzheimer's disease dementia. We hypothesized that higher eotaxin-1 and MCP-1 levels would be associated with poorer memory performance on both verbal and visual memory tasks. We further examined whether: (1) the association of eotaxin-1 and MCP-1 levels with cognitive functions were specific to memory and (2) chemokines outside of the chromosome 17 cluster would similarly predict memory functions. This allowed us to determine both the sensitivity and specificity of our findings. Finally, in an exploratory analysis, we further examined the association of these chemokines with medial temporal lobe structures.
2. Methods
2.1. Participants
A sample of 151 carefully phenotyped older adults meeting the National Institute on Aging—Alzheimer's Association (NIA-AA) criteria for either mild cognitive impairment [10] or probable dementia due to AD [11] were included in our AD group. All participants were selected from the University of California, San Francisco Memory and Aging Center database based on the availability of plasma markers of MCP-1 and eotaxin-1 as well as measures of verbal and visual recall. Both evaluations occurred within a 90-day period. MCI participants whose phenotypes suggested the presence of other primary etiologies (i.e., vascular disease; Lewy bodies) were excluded. In addition, 22 healthy controls were included as a comparison group and were selected based on availability of chemokine markers and the same memory evaluation as the MCI and AD dementia participants (see below). MCI and AD dementia participants were recruited from our Alzheimer's Disease Research Center and healthy controls were recruited from our NIH Aging and Cognition study. All patients underwent a history and physical examination, a structured caregiver interview, and neuropsychological tests (see Table 1), and diagnoses were adjudicated in a consensus conference. To confirm the robustness of our findings, we elected to include a range of validated AD phenotypes (e.g., posterior cortical atrophy, logopenic primary progressive aphasia, memory-predominant AD), severity levels (Clinical Dementia Rating Scale of 0–2), and ages (early and late onset). Amyloid imaging biomarkers, obtained via positron emission tomography imaging with Pittsburgh compound B (11C-PiB) [12] or Florbetapir F 18 (18F-AV-45) [13], were available for 57 individuals, and 55 of 57 were positive. We elected to exclude the two individuals who were amyloid negative, resulting in a final n of 149 MCI and AD dementia participants and 22 controls. Of the MCI and AD dementia phenotypes, 102 individuals had a classic memory-predominant phenotype, 28 were diagnosed with a logopenic variant of primary progressive aphasia phenotype, and 19 evidenced a posterior cortical atrophy phenotype. The study was approved by the UCSF institutional review board for human research. Informed consent for the study was provided by the participants or their assigned surrogate decision makers.
Table 1.
Participant characteristics and neuropsychological assessment
| Demographic and Cognitive Variables | Healthy controls |
MCI and AD dementia phenotypes |
|---|---|---|
| Overall group | ||
| Mean (SD) | Mean (SD) | |
| Age (y) | 75.6 (7.1) | 67.8 (10.4) |
| Gender (% female) | 50% | 55% |
| Education (y) | 17.9 | 16.5 |
| CDR (%) | 0:100% | 0.5:53.6% |
| 1.0:37.6% | ||
| 2.0:8.7% | ||
| Eotaxin levels (pg/mL) | 156.7 (48.9) | 172.3 (66.1) |
| MCP-1 levels (pg/mL) | 115.1 (19.6) | 102.3 (36.7) |
| IP-10 levels (pg/mL) | 495.4 (301.0) | 542.5 (526.9) |
| MDC levels (pg/mL) | 954.1 (319.6) | 1085.3 (502.0) |
| TARC levels (pg/mL) | 152.3 (155.0) | 190.9 (136.4) |
| MMSE | 29.2 (1.1) | 22.4 (5.4) |
| CVLT-short form 10′ Recall | 7.4 (1.9) | 2.3 (2.6) |
| CVLT-short form D-prime | 3.3 (0.4) | 1.7 (1.0) |
| Benson figure recall | 12.5 (2.1) | 3.8 (3.8) |
Abbreviations: MMSE, mini mental status examination; CVLT, California Verbal Learning Test-II.
2.2. Measures
2.2.1. Global measures
The Clinical Dementia Rating Scale (CDR) was administered to all participants to capture overall severity, and the overall score (0–2) was used as a primary covariate.
2.2.2. Verbal and visual memory
Our primary outcome variable was episodic memory and was selected due to its consistent and robust associations with early stages of Alzheimer's disease [14], [15], [16], [17]. Episodic memory was assessed with a measure of verbal and visual memory. For verbal memory, the California Verbal Learning Test-II Short Form was administered to all participants [18]; for data reduction purposes, 10-minute delayed free recall and recognition dprime were used as primary variables of interest. For visual memory, the 15-minute delayed recall of the Benson figure was used [19]. Using principal component analysis (minimum eigenvalue = 0), a single factor score for memory was created based on aforementioned verbal and visual memory measures and served as our primary outcome variable of interest.
2.2.3. Other cognitive measures
To address whether the chemokines were selectively related to episodic memory, we incorporated principal component analyses of other cognitive domains using the same analytic procedure. For the visuospatial domain, our single factor score included a localization task from the visual object and space perception battery and the copy condition of the Benson figure. For the executive control domain, our single factor score included digit span backward, modified trail making test, design fluency, and phonemic fluency [16]. Finally, for the language domain, we included an abbreviated form of the Boston Naming Test (15-item) [20] and Peabody Picture Vocabulary Test (16-item).
2.2.4. APOE genotyping
APOE single-nucleotide polymorphism (SNP) genotyping was carried out by real time polymerase chain reaction (rtPCR), on an Applied Biosystems 7900HT real-time PCR machine using the Taqman SNP Genotyping Assay for rs429358 and rs7412 with identification numbers C___3084793_20 and C____904973_10, respectively (Applied Biosystems, Foster City, CA). The protocol was followed as outlined in the manufacturer's instructions, and every assay was performed in duplicate.
2.2.5. Laboratory measures of chemokines
After collection, each blood sample was centrifuged at 2000× g for 15 minutes at 4°C with the resultant plasma divided into 500 μL aliquots and stored at −80°C. All assays were conducted following the manufacturer's protocol for Human Chemokine Panel 1 V-PLEX Plus kit (Meso Scale Diagnostics, Rockville, MD). Each multiplex array was scanned using a MESO QuickPlex SQ 120. Manufacturer supplied software (Discover Workbench 4.0) was used to quantify the concentration of eotaxin-1 based on sample dilution and relative to the supplied in-assay standard curve. Nominal recovery for control levels remained between 111%–120% with a coefficient of variation (CV) <10%. Standard curve CVs for patient sample detection range remained <15% with standard sample recovery at 100% (+/− 5%) across all plates. MCP-1 and eotaxin-1 were selected as our primary chemokines of interest. We elected to focus on these two specific chemokines given the recent, striking associations with reduced hippocampal neurogenesis in parabiosis animal models [8]. We also included three comparison chemokines localized to chromosomes 4 (IP-10) and 16 (MDC; TARC) to examine sensitivity and specificity of findings. Selection of these comparison chemokines was based on non-chromosome 17 loci, availability of data (available in >80% of participants, with CVs <10%) and presence on the Meso Scale Chemokine V-PLEX kit.
2.2.6. Neuroimaging
Magnetic resonance imaging (MRI) scans were obtained on a 3.0-Tesla Siemens (Siemens, Iselin, NJ) TIM Trio scanner equipped with a 12-channel head coil located at the UCSF Neuroscience Imaging Center and were available for a subset of participants (n = 55). Whole brain images were acquired using volumetric MPRAGE sequence (repetition time, echo time, inversion time [TR/TE/TI] = 2300/2.98/900 ms, α = 9°). The field of view was 240 × 256 mm, with 1 × 1 mm in-plane resolution and 1-mm slice thickness. The T1 MPRAGE structural MR images were analyzed using the FreeSurfer 5.1 image analysis suite, which is documented and freely available for download online at: http://surfer.nmr.mgh.harvard.edu. Previous publications have provided detailed descriptions and validation of the software [21], [22], [23]. FreeSurfer is a surface-based structural MRI analysis tool that segments white matter and tessellates both gray and white matter surfaces [24]. For the purposes of this study, the T1 image for each subject was processed through FreeSurfer, version 5.1 and then individually quality checked for anatomic accuracy of white or gray matter segmentation. Common geometric inaccuracies in white matter and pial surfaces were manually corrected using the built in editing packages of FreeSurfer. Medial temporal lobe volumes served as our primary region of interest (ROI; divided into left and right hemisphere) and included the entorhinal cortex, parahippocampal gyrus, and hippocampus from the Desikan atlas [25].
2.2.7. Statistical analyses
Spearman correlations were conducted between chemokine markers and demographics. To address our primary hypothesis regarding an association between MCP-1/eotaxin-1 and memory functions, a series of general linear models were conducted with demographics (age, gender, education) and phenotype (controls, individual AD phenotypes) as nuisance covariates, MCP-1 and eotaxin-1 as covariates of interest, and the memory factor score as the target outcome variable. MCP-1 and eotaxin-1 were modeled as both individual main effects as well as an interaction. The models were repeated with (1) CDR and APOE included in the model and (2) control chemokines and ultimately applied to the three other cognitive factor scores. In exploratory analyses, we computed general linear models with medial temporal lobe volumes as the target outcome variable. SAS 9.4 was used in all analyses.
3. Results
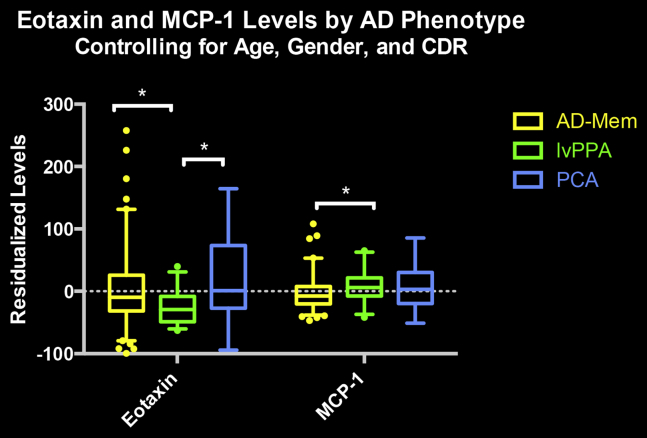
Higher levels of eotaxin-1 (rs = .198, P = .01), MCP-1 (rs = .205, P = .009), and IP-10 (rs = .189, P = .01) were associated with increasing age, with no associations found between age and MDC or TARC levels (P > .1). Controlling for age, only eotaxin-1 levels increased with higher CDR scores (rs = .272, P = .01). In terms of specific MCI and AD dementia phenotypes, on controlling for age, gender, and CDR, the language predominant phenotype (i.e., logopenic variant primary progressive aphasia) showed lower eotaxin-1 levels than memory-predominant and posterior cortical atrophy phenotypes, and marginally higher MCP-1 levels than memory-predominant phenotypes (see Fig. 1).
Fig. 1.
The figure displays eotaxin and MCP-1 levels as a function of MCI and Alzheimer's disease phenotypes. Abbreviations: AD-Mem, amnestic phenotype; lvPPA, logopenic variant of primary progressive aphasia phenotype; PCA, posterior cortical atrophy phenotype. *Significant between group difference, P <.05.
3.1. Sensitivity: Relationship between MCP-1, eotaxin-1, and memory performance
Controlling for demographics and phenotype, higher levels of MCP-1 were related to lower memory factor scores across all participants (F(1, 149) = 6.37; t = −2.52, P = .01; unstandardized beta = −0.0056; SE = 0.0022; 95% CI = −0.0100 to −0.0012). No significant effects were found for the main effect of eotaxin-1 on memory performance after controlling for aforementioned covariates (P = .69).
We next examined the interaction of both MCP-1 and eotaxin-1 levels on the memory factor score outcome (see Table 2 for full results). Controlling for demographics, phenotype, and individual MCP-1 and eotaxin-1 main effects, the interaction between MCP-1 and eotaxin-1 significantly predicted worse memory performance (F(1,143) = 4.76; t = −2.18; P = .03). This association strengthened on controlling for CDR and APOE genotype (F(1,135) = 9.12, t = −3.02, P = .003).
Table 2.
Interaction of MCP-1 and eotaxin-1 predicting episodic memory factor scores
| Analyses | General linear model results for memory factor score |
|
|---|---|---|
| t-value, unstandardized beta (standard error) |
P value | |
| 95% confidence interval | ||
| Interaction analysis (controlling for demographics and phenotype) | ||
| MCP-1 × eotaxin-1 | t = −2.18, beta = −0.00007 (SE = 0.00003) | .038 |
| 95% CI = −0.00014 to −0.000007 | ||
| Interaction analysis∗ (controlling for demographics, phenotype, CDR, and APOE) | ||
| MCP-1 × eotaxin-1 | t = −3.02, beta = −0.00009 (SE = 0.00003) | .003 |
| 95% CI = −0.00014 to −0.000030 | ||
| Interaction analysis (controlling for demographics, phenotype, CDR, APOE, and control chemokines) | ||
| MCP-1 × eotaxin-1 | t = −2.80, beta = −0.00008 (SE = 0.00003) | .006 |
| 95% CI = −0.00014 to −0.000024 | ||
NOTE. Memory Factor Scores have a mean of 0 for the entire sample.
Results remained comparable whether controlling for phenotype and CDR or simply controlling for CDR alone.
We also incorporated three control chemokines (IP-10; MDC; TARC; Only 1 additional non-chromosome 17 chemokine was available on the same multiplex; however, <80% of our participants had detectable levels of this marker, so it was not included in the analyses.) available on the same Meso Scale chemokine plex to provide a preliminary assessment of the specificity of our MCP-1/eotaxin-1 findings. After controlling for demographics, CDR, APOE, and the control chemokines, the interaction between MCP-1 and eotaxin-1 remained predictive of worse memory performance (F(1,130) = 7.83; t = −2.80; P = .006; see Table 2 for full results).
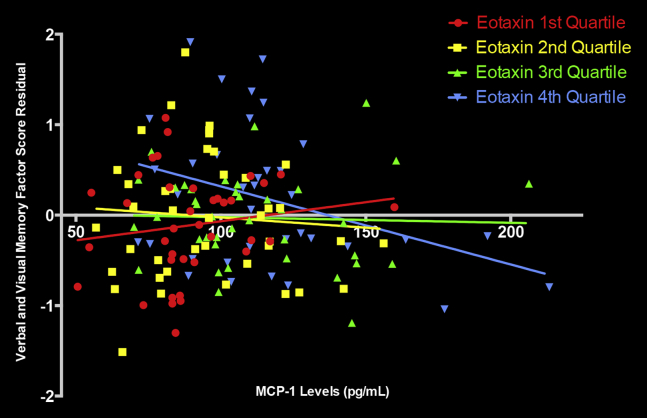
To better understand and visualize the relationship between the MCP-1 × eotaxin-1 interaction and the episodic memory factor scores, we subdivided eotaxin-1 levels into quartiles. We regressed the memory factor score over the demographic and CDR variables to create a single residual measure (i.e., the memory factor score, accounting for the aforementioned covariates). We then plotted the association between MCP-1 levels and the memory factor residual score as a function of eotaxin-1 quartiles (see Fig. 2). Results indicated that the negative association between MCP-1 and memory scores was strongest in individuals with the highest quartile of eotaxin-1 levels.
Fig. 2.
The figure demonstrates the relationship between MCP-1 and memory as a function of eotaxin-1 levels. The Verbal and Visual Memory Factory Score Residual is the memory factor score accounting for demographics and CDR.
3.2. Specificity: Relationship between MCP-1, eotaxin-1, and other cognitive domains
Controlling for demographics and phenotype, neither MCP-1 levels nor eotaxin-1 levels were significantly associated with the executive function, visuospatial, or language factors (all P's > .1). Given that the interaction between MCP-1 and eotaxin-1 resulted in stronger associations with the memory factor score, we also tested the effects of this interaction on the other cognitive domains. The interaction between MCP-1 and eotaxin-1 did not significantly predict the nonmemory factor scores (all P > .1).
3.3. Exploratory analysis: Relationship between MCP-1, eotaxin-1, and medial temporal lobe volumes
A small subset of individuals (n = 55; 8 controls, 25 MCI; 22 AD dementia) had MRI scans within 6 months of their blood draws. In an exploratory analysis, we evaluated whether MCP-1 and eotaxin-1 levels were individually associated with the medial temporal lobe volume ROI. Controlling for demographics, intracranial volume, and phenotype, both higher eotaxin-1 (F(1, 44) = 8.69; t = −2.95, P = .005; unstandardized beta = −5.43; SE = 1.84; 95% CI = −9.14 to −1.72) and MCP-1 (F(1, 46) = 4.45; t = −2.11, P = .04; unstandardized beta = −8.77; SE = 4.16; 95% CI = −17.15 to −0.40) levels were associated with smaller left medial temporal lobe volumes. Associations between the chemokines and right medial temporal lobes did not reach statistical significance but were in the expected direction (i.e., inverse associations, P's < .15). Owing to the small n of the imaging subsample, we did not explore associations with other cortical regions.
4. Discussion
Our study demonstrates that an interaction between higher MCP-1 (CCL2) and eotaxin-1 (CCL11) levels predicts worse verbal and visual episodic memory scores. The detrimental association between these chemokines and memory is strongest when both inflammatory markers were elevated. Although studies using mouse models of aging have linked MCP-1 and eotaxin-1 to memory functions, this is the first human study to show sensitivity of these chemokines to memory dysfunction in a wide range of MCI and Alzheimer's disease dementia phenotypes. Moreover, our study suggests that not only is the association of MCP-1 and eotaxin-1 levels with cognition specific to memory functions, but that these chemokines also appear to be related to left medial temporal lobe volumes.
An association between memory and chemokines, specifically eotaxin-1 and MCP-1, has been reported in mouse models of aging and Alzheimer's disease, and more recently, a human study identified a haplotype of SNPs on chromosome 17 within a chemokine gene cluster, including eotaxin-1, that associated with age of onset in familial AD [5]. Although very few studies have been conducted, they collectively suggest that eotaxin-1 and MCP-1 may be important markers for pathologic aging [8], [26]. In the one human study on MCP-1 and cognitive decline in AD, higher levels of this chemokine predicted a faster rate of cognitive decline in prodromal AD [9]; however, this study did not specifically examine its role in episodic memory functions, and the study's findings were circumscribed to CSF and not plasma. It is unclear why this study and our study differ in terms of plasma MCP-1 results and cognition, although the studies differ in terms of primary outcome variables (i.e., rate of cognitive decline versus cross-sectional memory performance). Our study provides initial evidence for a modulatory role of MCP-1 and eotaxin-1 on both verbal and visual memory, independent of APOE genotype and clinical severity level, in older adults at risk for or showing clear symptoms of AD.
Mechanisms for how circulating immune markers may relate to memory functions remain unclear. Accumulating evidence suggests the possibility of bidirectional links between the periphery and central nervous system (CNS) [27], [28], including vagal afferent pathways. As noted recently by Villeda et al. [8], neurogenic niches, including the subgranular zone of the hippocampus, are located around blood vessels, leaving open the possibility that systemic changes in proteins and inflammatory markers may alter neurogenesis in aging. Although the present study cannot directly address whether peripheral inflammatory markers reflect a separate, but mirrored CNS process, or play a direct role in CNS immune dysregulation, the results provide a translational link with animal studies that supports a role for circulating markers in AD clinical phenotypes.
An important aspect of this study was the possible specificity of the MCP-1 and eotaxin-1 findings on cognition. We attempted to address this concern in two ways, by (1) examining the association of these chemokines with other, nonmemory cognitive functions and (2) evaluating whether comparison chemokines on the MESO-plex localized to different chromosomes (4 and 16; IP-10, MDC, and TARC) were associated with episodic memory function. We found no associations between MCP-1 and eotaxin-1 with other cognitive measures nor did we find relationships between comparison chemokines and memory functions. Chemokine families tend to cluster on chromosomes 17, 4, and 16; thus, it may be that chromosome 17 chemokines represent important loci for memory dysfunction. Considering that our primary hypotheses focused on MCP-1 and eotaxin-1 levels and to reduce multiple comparisons, we did not explore other chromosome 17 chemokines in the proposed study; thus, it is important to highlight that although our results are suggestive of specificity, they should be considered preliminary. Future studies should examine the role of MCP-1 and eotaxin-1 in larger cohorts and also examine the possible role of other chromosome 17 chemokines in episodic memory function.
Our exploratory analysis in a subset of individuals also found an association between MCP-1 and eotaxin-1 with left medial temporal lobe volumes, a region of the brain known to be affected in early AD [29]. These results are consistent with the behavioral findings and are particularly encouraging given that this subanalysis was underpowered to detect small effects. Given the small sample size of individuals who obtained an MRI of the brain, we elected not to examine other regions in the brain. Thus, it is unclear whether these chemokines predict gray matter volumes in a more distributed or widespread manner.
Although not a primary goal of the study, it is worth noting that absolute levels of MCP-1 were not significantly higher in MCI and AD dementia participants nor did they correlate with clinical severity level. The literature on inflammatory markers at different stages of pathogenesis has been mixed [30], with no studies to date reporting on inflammatory levels as a function of clinical AD phenotype. To evaluate the robustness of the relationship and to increase the range in memory scores, we included a wide range of severity levels and both memory and nonmemory predominant MCI and AD dementia phenotypes. This limited the likelihood that strictly memory predominant phenotypes drove the association between chemokines and memory functions. Interestingly, differences in both eotaxin-1 and MCP-1 levels were observed between phenotypes, particularly in relation to language predominant (i.e., logopenic variant primary progressive aphasia) MCI and AD dementia phenotypes, which should be explored further in future studies.
Although our study has numerous strengths, including the use of a large MCI and AD dementia cohort and incorporation of multiple memory indices, there are limitations as well. Amyloid imaging was only available for a subset of individuals; thus, while we attempted to enrich for AD pathology by solely focusing on established phenotypes (e.g., typical memory-predominant, posterior cortical atrophy, and logopenic aphasia), it remains possible that some MCI individuals did not have underlying AD pathology. In addition, although the associations between MCP-1 and eotaxin-1 and memory functions were robust, effect sizes for the interaction remain relatively small. Whether these chemokines selectively influence hippocampal function and volume independent of AD also needs further study.
In conclusion, there is a potentially selective role for MCP-1 and eotaxin-1 in episodic memory dysfunction in the context of a wide array of MCI and Alzheimer's disease dementia phenotypes. Moreover, our study suggests that not only is the association of MCP-1 and eotaxin-1 levels with cognition specific to memory functions, but these chemokines also appear to be sensitive to left medial temporal lobe volumes. Future studies should explore possible mechanisms of action and elucidate whether chromosome 17 chemokines more broadly play an integral role in memory functions and pathological aging.
Research in context.
-
1.
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources. Although the underlying mechanisms of how MCP-1 and eotaxin relate to hippocampal function and episodic memory are in preliminary stages of evaluation, recent mouse studies suggest a potentially direct relationship between these markers and hippocampal neurogenesis. These articles have been cited.
-
2.
Interpretation: Our findings suggest a potentially sensitive and selective role for MCP-1 and eotaxin in memory consolidation across a wide range of Alzheimer's disease phenotypes, which corroborates recent animal literature.
-
3.
Future directions: The article outlines several lines of inquiry that should be addressed, including (1) the relationship between peripheral and CNS inflammation in Alzheimer's disease; (2) the role of MCP-1 and eotaxin in hippocampal function in humans, including individuals without Alzheimer's disease; (3) the role of chromosome 17 chemokines in cognitive functioning; (4) whether different AD phenotypes show different immune and inflammatory profiles.
Acknowledgments
The study described was supported by grant numbers K23 AG042492-01, P50 AG023501, and R01 AG032289 from the NIH-NIA. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Neurological Disorders and Strokes, National Institute on Aging, or NIH. This work was also supported by an Alzheimer's Association New Investigator Grant (NIRP-12-259223) and the Larry L. Hillblom Foundation.
References
- 1.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bettcher B.M., Kramer J.H. Inflammation and clinical presentation in neurodegenerative disease: a volatile relationship. Neurocase. 2013;19:182–200. doi: 10.1080/13554794.2011.654227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yirmiya R., Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Baruch K., Ron-Harel N., Gal H., Deczkowska A., Shifrut E., Ndifon W. CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc Natl Acad Sci U S A. 2013;110:2264–2269. doi: 10.1073/pnas.1211270110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lalli M.A., Bettcher B.M., Arcila M.L., Garcia G., Guzman C., Madrigal L. Whole-genome sequencing suggests a chemokine gene cluster that modifies age at onset in familial Alzheimer's disease. Mol Psychiatry. 2015;20:1294–1300. doi: 10.1038/mp.2015.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fumagalli M., d'Adda di Fagagna F. SASPense and DDRama in cancer and ageing. Nat Cell Biol. 2009;11:921–923. doi: 10.1038/ncb0809-921. [DOI] [PubMed] [Google Scholar]
- 7.Kiyota T., Yamamoto M., Xiong H., Lambert M.P., Klein W.L., Gendelman H.E. CCL2 accelerates microglia-mediated Abeta oligomer formation and progression of neurocognitive dysfunction. PLoS One. 2009;4:e6197. doi: 10.1371/journal.pone.0006197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villeda S.A., Luo J., Mosher K.I., Zou B., Britschgi M., Bieri G. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westin K., Buchhave P., Nielsen H., Minthon L., Janciauskiene S., Hansson O. CCL2 is associated with a faster rate of cognitive decline during early stages of Alzheimer's disease. PLoS One. 2012;7:e30525. doi: 10.1371/journal.pone.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klunk W.E., Engler H., Nordberg A., Wang Y., Blomqvist G., Holt D.P. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 13.Wong D.F., Rosenberg P.B., Zhou Y., Kumar A., Raymont V., Ravert H.T. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F 18) J Nucl Med. 2010;51:913–920. doi: 10.2967/jnumed.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ewers M., Walsh C., Trojanowski J.Q., Shaw L.M., Petersen R.C., J C.R., Jr. Prediction of conversion from mild cognitive impairment to Alzheimer's disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol Aging. 2012;33:1203–1214. doi: 10.1016/j.neurobiolaging.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickerson B.C., Wolk D.A., Alzheimer's Disease Neuroimaging Initiative Dysexecutive versus amnesic phenotypes of very mild Alzheimer's disease are associated with distinct clinical, genetic and cortical thinning characteristics. J Neurol Neurosurg Psychiatry. 2011;82:45–51. doi: 10.1136/jnnp.2009.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer J.H., Jurik J., Sha S.J., Rankin K.P., Rosen H.J., Johnson J.K. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Landau S.M., Harvey D., Madison C.M., Reiman E.M., Foster N.L., Aisen P.S. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delis D.C., Kramer J.H., Kaplan E., Ober B.A. 2nd ed. The Psychological Corporation; San Antonio, TX: 2000. California Verbal Learning Test. [Google Scholar]
- 19.Possin K.L., Laluz V.R., Alcantar O.Z., Miller B.L., Kramer J.H. Distinct neuroanatomical substrates and cognitive mechanisms of figure copy performance in Alzheimer's disease and behavioral variant frontotemporal dementia. Neuropsychologia. 2011;49:43–48. doi: 10.1016/j.neuropsychologia.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan E., Goodglass H., Weintraub S. Lea and Febiger; Philadelphia: 1983. The Boston Naming Test. [Google Scholar]
- 21.Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 22.Fischl B., Liu A., Dale A.M. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 23.Segonne F., Dale A.M., Busa E., Glessner M., Salat D., Hahn H.K. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 24.Segonne F., Pacheco J., Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- 25.Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Galimberti D., Schoonenboom N., Scheltens P., Fenoglio C., Venturelli E., Pijnenburg Y.A. Intrathecal chemokine levels in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2006;66:146–147. doi: 10.1212/01.wnl.0000191324.08289.9d. [DOI] [PubMed] [Google Scholar]
- 27.Baruch K., Deczkowska A., David E., Castellano J.M., Miller O., Kertser A. Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science. 2014;346:89–93. doi: 10.1126/science.1252945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louveau A., Smirnov I., Keyes T.J., Eccles J.D., Rouhani S.J., Peske J.D. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frisoni G.B., Pievani M., Testa C., Sabattoli F., Bresciani L., Bonetti M. The topography of grey matter involvement in early and late onset Alzheimer's disease. Brain. 2007;130:720–730. doi: 10.1093/brain/awl377. [DOI] [PubMed] [Google Scholar]
- 30.Bettcher B.M., Kramer J.H. Longitudinal inflammation, cognitive decline, and Alzheimer's disease: a mini-review. Clin Pharmacol Ther. 2014;96:464–469. doi: 10.1038/clpt.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]