Abstract
Purpose
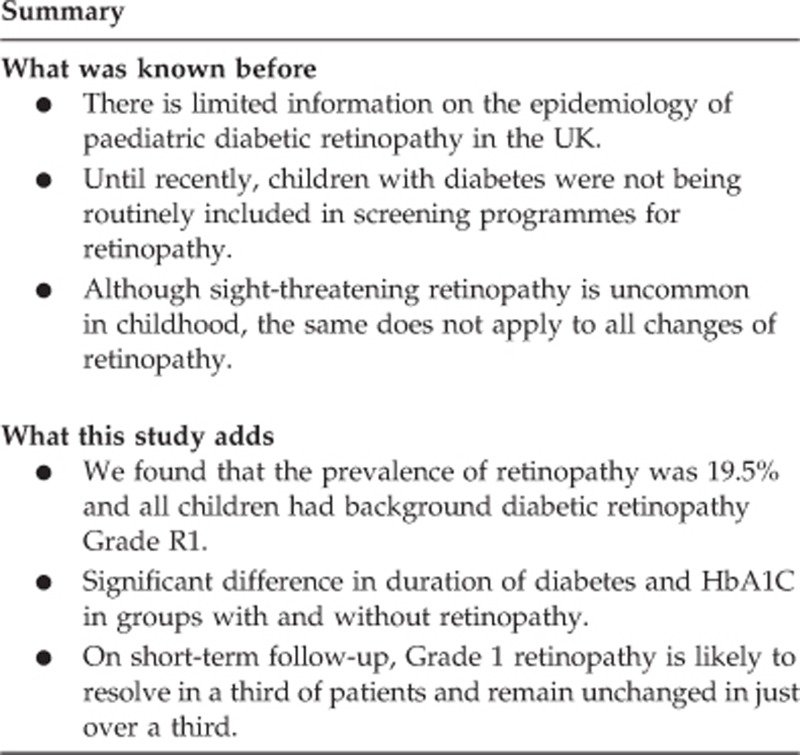
To describe the prevalence and natural history of retinopathy in a cohort of children and young people with type 1 diabetes attending a tertiary hospital diabetes clinic.
Methods
We analysed retinopathy screening data from 2008 to 2010 on all eligible children using the ‘Twinkle' diabetes database and the regional retinal screening database.
Results
A total of 88% (149/169) of eligible children were screened in 2008, median age 14 years, 52% male. The prevalence of retinopathy was 19.5% (30/149). All children had background retinopathy grade R1. There was significant difference in median (range) duration of diabetes, 7.7 years (0.6–13.7) vs 5 years (0.2–12.5) (P<0.001) and median (range) HbA1C, 9.1% (7.2–14) vs 8.6% (5.6–13.1) (P=0.02), between the groups with and without retinopathy. At 2- years follow-up, 12/30 (40%) had unchanged retinopathy grade R1, 10/30 (33.3%) showed resolution of changes (R0), 1/30 progressed to maculopathy, and 7/30 had no follow-up data. Median (range) HbA1C in 2008 and 2010 for the groups with stable vs resolved changes was similar, 9.1% (7.2–14.0) and 9.2% (7–14.0) vs 9.5% (7.8–14.0) and 9.2% (8.7–14.0). Of the 119 without retinopathy in 2008, 27 (22.5%) had developed retinopathy within 2 years, including 1 with pre-proliferative retinopathy and 1 with maculopathy. There was no significant difference in HbA1c between those who progressed to retinopathy (8.7% (7.1–13.1)) (8.7% (7.1–13.1)), and those who did not (8.6% (6.3–12.2)).
Conclusions
Prevalence of background retinopathy in our cohort was comparable to the previously published reports, with higher HbA1c and longer duration of diabetes being significant risk factors. On short-term follow-up, Grade 1 retinopathy is likely to resolve in a third of patients and remain unchanged in just over a third.
Introduction
Diabetic retinopathy is a significant cause of vision loss in developed countries and is the second most common cause of registered blindness among the adult population in the United Kingdom (UK).1 Sight-threatening diabetic retinopathy usually develops several years after diagnosis of diabetes and cumulative incidence of proliferative retinopathy, 25 years after diagnosis of type 1 diabetes in adults, has been reported to be between 10–40%.2, 3
Poor glycaemic control has been identified as a modifiable risk factor in the occurrence and progression of retinopathy in adults and adolescents,4, 5 and intensive glycaemic control has been shown to reduce the risk of progression to sight-threatening retinopathy.6 Other suggested risk factors in the development of retinopathy in adults and adolescents include modifiable factors, such as lipid abnormalities and higher body mass index,7, 8 and non-modifiable factors, such as duration of diabetes, pubertal staging, and age.9, 10 Early identification of changes of retinopathy by routine screening may allow modification of some of the risk factors, thereby preventing progression to sight-threatening retinopathy.11
Until recently, children with diabetes were not being routinely included in screening programmes for retinopathy. Although sight-threatening retinopathy is uncommon in childhood, the same does not apply to all changes of retinopathy. It is now well recognised that early changes of retinopathy can be seen in childhood and adolescence. Reported prevalence of retinopathy in this population ranges from 10–42%.12, 13, 14, 15 To the best of our knowledge there is no information on the epidemiology of paediatric diabetic retinopathy in the UK.
Current guidelines in the UK recommend that children with diabetes who are over 12 years of age or those with diabetes duration of over 5 years should be screened for diabetic retinopathy on an annual basis.16 These recommendations are in line with other international consensus guidelines.17 In the UK, national service framework for diabetes recommends that 100% of eligible patients be invited for attendance to retinopathy screening and that uptake of screening be at least 80%.18
We conducted an audit to evaluate the local performance against these standards, with an aim to describe the prevalence of retinopathy among our patients, ascertain the characteristics of patients with and without retinopathy, and describe the natural history of retinopathy in the short term in this cohort.
Materials and methods
We retrospectively collected information on all patients eligible for retinopathy screening in 2008. We used the 2004 National Institute for Clinical Excellence guidance on type 1 diabetes to define eligibility for retinopathy screening. Patients who were eligible for screening were identified from a cohort of children and young people with diabetes attending the diabetes clinic at Birmingham Children's Hospital, a tertiary paediatric centre in the West Midlands. Patients were identified using the Twinkle diabetes database, which is a national database maintained locally with a register of all paediatric diabetic patients. It contains clinical information such as date of starting insulin treatment and glycated haemoglobin (HbA1C) levels, as well as demographic information such as age and sex. All data were collected to compare the characteristics of patients with and without retinopathy.
In current practice, eligible diabetes patients are invited by letter to attend for a screening appointment. An optometrist performed the retinal screening, and obtained digital fundus photography in mydriasis, after measuring visual acuity. A trained screener then graded the photos. All positive results were subjected to a second grading and where necessary, to arbitration grading. Retinopathy was graded according to the Diabetic English Screening Programme for Retinopathy (DESP) (Appendix 1) and maculopathy was graded according to the Diabetic English Screening Programme System for Maculopathy (Appendix 2). The results of retinal screening were saved on to the Regional Retinal Screening database. Screening results for all those who were screened in 2008 and their subsequent follow-up results from 2009 and 2010 were obtained from the database.
Data were analysed using Minitab 12.0 (Statistical Software, State College, PA, USA) with two-tailed significance values P≤0.05.17 Association between retinopathy and variables was investigated using univariate analysis. For continuous variables the t-test or the Mann–Whitney U test for parametric or non-parametric data were indicated. Categorical data were analysed using the χ2 test.
Results
In 2008, there were 329 children and young people with type 1 diabetes attending the paediatric diabetes clinic at Birmingham Children's Hospital. A total of 189 were eligible for retinopathy screening, all of whom were invited to a screening appointment. Total of 149/189 (88%) attended their appointment and were screened. Median age of those screened was 14 years (range 7–18 years). This included 71 females (47.6%) and 78 males (52.4%).
The prevalence of retinopathy among those screened was 19.5% (30/149). All patients with retinopathy were graded as R1 or background retinopathy, 22 in one eye and 8 in both the eyes. Table 1 compares the characteristics of patients with and without retinopathy. There was no significant difference between the groups in age and sex distribution. Patients with retinopathy, however, had significantly higher HbA1c (P=0.02) and longer duration of diabetes (P<0.001).
Table 1. Comparison of patient characteristics between the groups with and without retinopathy at baseline (2008).
| Variable | Retinopathy | No retinopathy |
|---|---|---|
| Age in years, median (range) | 14.5 (9–18) | 14 (7–18) |
| % Male | 46.7% | 53.8% |
| HbA1C %, median (range) | 9.1 (7.2–14) | 8.7 (7.1–13.1) |
| Duration of diabetes in years, median (range) | 7.7 (0.6–13.7) | 5.0 (0.2–12.5) |
Two-year follow-up data were available for 122/149 (81.8%). Of the 30 patients with retinopathy in 2008, retinopathy remained unchanged (R1 or background retinopathy) in 12/30 (40%) and resolved in 10/30 (33.3%). One patient had developed M1 or maculopathy in addition to background retinopathy. There were no follow-up data available for 7/30. Table 2 compares the median HbA1C in 2008 and 2010 between those with stable changes of retinopathy and those with resolution of changes. There was no significant change in median HbA1C from 2008 to 2010 in either group. The one patient who had progressed to develop maculopathy had a mean HbA1C of 9.9% in 2008 and 10.3% in 2010. Median HbA1C of those patients whose follow-up information on retinopathy is not available, was also similar and is shown in Table 2.
Table 2. Diabetic control (HbA1C) of the retinopathy group patients with stable and resolved changes on follow-up.
| Patient group | HbA1C % 2008, median (range) | HbA1C % 2010, median (range) |
|---|---|---|
| Stable | 9.1 (7.2–14) | 9.2 (7–14) |
| Resolved | 9.5 (7.8–14) | 9.2 (8.7–14) |
| No follow-up data | 8.8 (7.8–14) | 9.6 (8.3–10.3) |
Overall mean HbA1C for this cohort from January 2015 to May 2015 is now 8.1%.
Of the 119 children without retinopathy in 2008, follow-up data were available for 99 (83.2%). Of these, 27 (27.3%) had developed retinopathy at 2-year follow-up. Most of them (25/27 or 92.5%) had R1 or background retinopathy, 15 in one eye and 10 in both the eyes. One patient had R2 or pre-proliferative retinopathy in both the eyes and one had M1 or maculopathy as well as R1. Median HbA1C of this group in 2008 and 2010 was not significantly different compared with the median HbA1C of the 72 patients who did not have retinopathy in 2008 and remained free of retinopathy in 2010 (Table 3).
Table 3. Diabetic control (HbA1C) of the 2008 screening cohort without retinopathy (n=119) categorized by retinopathy on follow-up.
| Patient group | HbA1C % 2008, median (range) | HbA1C % 2010, median (range) |
|---|---|---|
| Progressed to retinopathy | 8.7 (7.2–13.1) | 9.0 (6.9–14) |
| Remained retinopathy free | 8.6 (6.3–12.2) | 9.0 (6.2–14) |
Discussion
The prevalence of retinopathy in our cohort (19.5%) is comparable to what has been previously described in literature from other parts of the world.12, 13, 14, 15 To our knowledge, this is the first report of prevalence rate of retinopathy among a cohort of children and young people with type 1 diabetes in the UK. Our retinopathy screening rates met with the national service framework standards against which we audited our performance. However, as all eligible patients had not been screened, it is possible that our reported prevalence rate is an underestimate. Also, not all children with diabetes were screened. Screening was limited to those who were considered to be at high risk, in accordance with national guidelines. It is possible that those with poor glycaemic control, but with diabetes duration of <5 years, could have early changes of diabetic retinopathy.
The high-prevalence rate of retinopathy found in our cohort supports the need for screening children and young people with type 1 diabetes, in agreement with International Clinical Practice Consensus Guidelines17 and National Institute for Clinical Excellence guidelines.16 Most of our patients with retinopathy had background retinopathy, which is in keeping with what has been reported in a similar population by others.13 In the DESP criteria utilised in our study, one microaneurysm was graded as R1, which may differ from other screening programmes where multiple microaneurysms may be required for Grade R1 and this may account for the high prevalence of diabetic retinopathy in our cohort and the resolution of retinopathy. Higher HbA1C and longer duration of diabetes were identified in our cohort as significant risk factors for onset of retinopathy. This is also consistent with what has been previously reported in literature.4, 5, 8, 9, 10, 11, 12, 13 However, the latest data from RCPCH/DUK 2013/2014 showed that 14.1% of children with diabetes in England and Wales aged 12 years and older had abnormal findings on retinal screening, and their prevalence increased with age, perhaps reflecting the duration of diabetes.19
On short-term follow-up, grade 1 retinopathy remained unchanged in a third of patients and resolved in nearly a third. The only study we found, that has looked at short-term follow-up of changes of retinopathy in children and adolescents, showed a much lower resolution rate.12 They did, however, have a much higher prevalence rate of retinopathy at baseline compared to our cohort. Nearly a quarter of our patients with retinopathy had no follow-up data available. Even working on the assumption that none of them showed resolution of changes, the resolution rate in our cohort remains higher than what has been previously reported.
It has been suggested that progression of retinopathy does not change much from year to year, and that in some instances biennial screening is even warranted.20 A third of our patients showed either progression or resolution of changes on 2-year follow-up. Our data would, therefore, support screening frequency of at least two yearly. The lower prevalence rate of retinopathy in our cohort and the numbers without available follow-up data, limited our ability to identify any significant risk factors in progression or resolution of retinopathy. We did not look at our patients' HbA1C in the years preceding the audit period, and therefore, cannot comment on the influence of ‘metabolic memory' on progression of retinopathy.21 The results of our study are based on the DESP criteria, which vary slightly from other screening programmes and therefore, while relevant to other populations, is more applicable to the UK population.
In summary, our data show prevalence rate of retinopathy among a cohort of children and young people with type 1 diabetes in the UK, to be similar to what has been reported from other parts of the world. Higher HbA1C and longer duration of diabetes were significant risk factors in the development of retinopathy. On short-term follow-up, retinopathy resolved in nearly a third of the patients and remained unchanged in just over a third.
Acknowledgments
We thank the diabetes team at Birmingham Childrens' Hospital.
Appendix 1
NHS Diabetic Eye Screening Programme in England for retinopathy.
| Level | Description | Action |
|---|---|---|
| R0—No retinopathy | No retinopathy | Annual rescreen |
| R1—Background | Haemorrhages and/or microaneurysms Venous loop Any exudate in prescence of other non referable DR features Any CWS in prescence of other non referable DR features | Annual rescreen |
| R2—Pre-proliferative | Venous beading or reduplication IRMA Multiple deep, round or blot haemmorhages | Refer |
| R3—Proliferative | - New vessels (disc or elsewhere) - Preretinal or vitreous haemorrhages Preretinal fibrosis±tractional retinal detachment - Advanced retinopathy | Refer urgently |
Appendix 2
NHS Diabetic Eye Screening Programme in England for maculopathy.
| Level | Description | Action |
|---|---|---|
| M0—No maculopathy | No maculopathy | Annual rescreen |
| M1—Maculopathy | Exudate within 1DD of fovea Circinate or group of exudates within the macula Haemorraghe or microaneurysm in macula 1DD of fovea and VA 6/12 or worse Retinal thickening 1DD of centre of fovea if stereo available | Refer |
| MNR—Maculopathy non referrable | Haemmorhage or microaneurysm in macula and VA 6/9 or better | Annual rescreen |
The authors declare no conflict of interest.
References
- Bunce C, Xing W, Wormald R. Causes of blind and partial sight certifications in England and Wales: April 2007–March 2008. Eye (Lond) 2010; 24(11): 1692–1699. [DOI] [PubMed] [Google Scholar]
- Skrivarhaug T, Fosmark DS, Stene LC, Bangstad HJ, Sandvik L, Hanssen KF et al. Low cumulative incidence of proliferative retinopathy in childhood-onset type 1 diabetes: a 24-year follow-up study. Diabetologia 2006; 49(10): 2281–2290. [DOI] [PubMed] [Google Scholar]
- Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology 2008; 115(11): 1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen BS, Sjølie A, Hougaard P, Johannesen J, Borch-Johnsen K, Marinelli K et al. A 6-year nationwide cohort study of glycaemic control in young people with type 1 diabetes. Risk markers for the development of retinopathy, nephropathy and neuropathy. Danish Study Group of Diabetes in Childhood. J Diabetes Complications 2000; 14(6): 295–300. [DOI] [PubMed] [Google Scholar]
- Danne T, Weber B, Hartmann R, Enders I, Burger W, Hovener G. Long-term glycemic control has a nonlinear association to the frequency of background retinopathy in adolescents with diabetes. Follow-up of the Berlin Retinopathy Study. Diabetes Care 1994; 17(12): 1390–1396. [DOI] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- Lyons TJ, Jenkins AJ, Zheng D, Lackland DT, McGee D, Garvey WT et al. Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest Ophthalmol Vis Sci 2004; 45: 910–918. [DOI] [PubMed] [Google Scholar]
- Dorchy H, Claes C, Vergougstraete C. Risk factors of developing proliferative retinopathy in type 1 diabetic patients: role of BMI. Diabetes Care 2002; 25: 789–799. [DOI] [PubMed] [Google Scholar]
- Donaghue KC, Fairchild JM, Chan A, Hing SJ, King J, Howard NJ et al. Diabetes microvascular complications in prepubertal children. J Pediatr Endocrinol Metab 1997; 10: 579–585. [DOI] [PubMed] [Google Scholar]
- Holl RW, Lang GE, Grabert M, Heinze E, Lang GK, Debatin KM. Diabetic retinopathy in pediatric patients with type 1 diabetes: effect of diabetes duration, prepubertal and pubertal onset of diabetes and metabolic control. J Pediatr 1998; 132: 790–794. [DOI] [PubMed] [Google Scholar]
- Jones S, Edwards RT. Diabetic retinopathy screening: a systematic review of the economic evidence. Diabet Med 2010; 27(3): 249–256. [DOI] [PubMed] [Google Scholar]
- Fairchild JM, Hing SJ, Donaghue KC, Bonney, Fung AT, Stephens MM et al. Prevalence and risk factors for retinopathy in adolescents with type 1 diabetes. Med J Aust 1994; 160: 757–762. [DOI] [PubMed] [Google Scholar]
- Falck AA, Kaar ML, Laatikainen LT. Prevalence and risk factors of retinopathy in children with diabetes: a population based study on Finnish children. Acta Ophthalmol (Copenh) 1993; 71: 801–809. [DOI] [PubMed] [Google Scholar]
- Kernell A, Dedorsson I, Johansson B, Wickström CP, Ludvigsson J, Tuvemo T et al. Prevalence of diabetic retinopathy in children and adolescents with IDDM: a population-based multicentre study. Diabetologia 1997; 40: 307–310. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. Retinopathy in young-onset diabetic patients. Diabetes Care. 1985; 8: 311–315. [DOI] [PubMed] [Google Scholar]
- NICE guidelines2004CG15 Type 1 diabetes in children and young people. [Online]. Available at http://www.nhs.uk/ipgmedia/national/nice/assets/type1diabetesinchildrenandyoungpeople.pdf.
- Kim D, Francesco C, Daniela T, Jeremy A, Knut D-J. Microvascular and macrovascular complications. Pediatr Diabetes 2009; 10(Suppl 12): 195–203. [DOI] [PubMed] [Google Scholar]
- National Service Framework for Diabetes. Department of Health, 2010. [Online]. Available at https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/198836/National_Service_Framework_for_Diabetes.pdf.
- National Paediatic Diabetes Audit Report 1: Care Processes and Outcomes. Royal College of Paediatrics and Child Health. Healthcare Quality Improvement Partnership. 2015, p 48. Available at http://www.rcpch.ac.uk/system/files/protected/page/Revised%20Sept%202014%20NPDA%20Report%201%20FINAL.pdf.
- Maguire A, Chan A, Cusumano J, Hing S, Craig M, Silink M et al. The case for biennial retinopathy screening in children and adolescents. Diabetes Care 2005; 28(3): 509–513. [DOI] [PubMed] [Google Scholar]
- Lind M, Odén A, Fahlén M, Eliasson B. The shape of the metabolic memory of HbA1c: re-analysing the DCCT with respect to time-dependent effects. Diabetologia 2010; 53(6): 1093–1098. [DOI] [PubMed] [Google Scholar]


