Abstract
Purpose
Acute submacular haemorrhage secondary to wet age-related macular degeneration (AMD) has a poor prognosis for which there is currently no ‘gold standard' treatment. We evaluated the efficacy of early treatment using intravitreal triple therapy of tissue plasminogen activator (tPA), expansile gas, and an anti-VEGF agent.
Methods
This retrospective case series included eight patients presenting with acute submacular haemorrhage involving the fovea. All patients received treatment with 50 μg (0.05 ml) tPA, 0.3 ml 100% perfluoropropane (C3F8), and an anti-VEGF agent (0.05 mg Ranibizumab or 1.25 mg Bevacizumab in 0.05 ml) administered via intravitreal injection. An anterior chamber paracentesis post injection or vitreous tap was performed before injection to prevent retinal vascular occlusion secondary to raised intra-ocular pressure. Outcomes assessed were visual acuity, change in macular morphology, and complications.
Results
Patients presented promptly with delay between symptom onset and clinic review being 1.9±0.6 days (mean±SD). Treatment was delivered quickly with interval from presentation to treatment being 1.1±1.2 days. Symptom onset to treatment was 3.0±1.0 days. Subfoveal haemorrhage was effectively displaced in all patients. LogMAR visual acuity improved from 1.67±0.47 at presentation to 0.63±0.33 at final follow-up (P<0.0001), a mean of 7.9±4.8 months after treatment. Central retinal thickness improved from 658.1±174.2 μm at presentation to 316.6±142.4 μm at final follow-up (P=0.0028).
Conclusions
Early treatment of submacular haemorrhage using intravitreal tPA, C3F8, and anti-VEGF was effective in significantly improving visual acuity in this series of patients who presented soon after symptom onset. Treatment was well tolerated in this group of elderly and potentially frail patients.
Introduction
Acute submacular haemorrhage is a potentially devastating complication of neovascular age-related macular degeneration (AMD). The natural history of this condition is severe, with few patients showing much improvement in visual acuity.1, 2 Accumulation of blood in the subretinal space has been shown in experimental models to cause photoreceptor damage within 24 h,3 and this may be because of shearing of photoreceptor outer segments, impaired transport of nutrients, and direct iron toxicity derived from haemoglobin.2, 3, 4 Therefore, timely intervention is required.
There is currently no ‘gold standard' treatment for acute submacular haemorrhage. Monotherapy using an anti-VEGF agent has demonstrated some success in stabilising and/or moderately improving visual acuity.5, 6 However, in many countries such as the United Kingdom where strict eligibility criteria exist regarding the use of anti-VEGF agents, such treatment may not be authorised for these patients as their presenting visual acuity is often too poor.
For nearly two decades, the use of tissue plasminogen activator (tPA) has become more widespread in the treatment of patients with acute submacular haemorrhage. This 527 amino acid polypeptide catalyses breakdown of plasminogen to plasmin, the latter being the principal enzyme involved in lysis of clots. Initial reports described its administration intravitreally in conjunction with expansile gas to lyse and displace subfoveal haemorrhage.7 Multiple subsequent reports demonstrated visual gains,8, 9, 10 although many studies were undertaken before the advent of anti-VEGF agents and therefore treatment did not address the underlying causative pathology. Alternatively, tPA may been administered via vitrectomy and subretinal injection with or without expansile gas,11, 12 with a recent review concluding that treatment of submacular haemorrhage with vitrectomy, subretinal tPA, intravitreal gas, and anti-VEGF therapy resulted in greatest visual improvement.13 However, many patients presenting with submacular haemorrhage are elderly and frail and therefore a less invasive therapeutic approach may be desirable.
There have been a few recent case series describing the use of combination therapy using intravitreal tPA, expansile gas, and an anti-VEGF agent for the treatment of acute submacular haemorrhage with good visual outcomes.14, 15, 16, 17 In these series, mean duration of symptoms ranged from 6 days17 to 11.25 days.16 Furthermore, one study only included patients with small submacular haemorrhages of one to three disc diameters,14 whereas another study only included patients with more extensive haemorrhage of between 4 and 10 disc diameters.15
In view of the short timescale within which photoreceptor damage occurs, we assessed whether prompt treatment of patients presenting with subfoveal macular haemorrhage of any presenting size was effective in improving visual outcomes. All patients were treated with intravitreal injection of tPA and C3F8 to lyse and displace haemorrhage along with an anti-VEGF agent to address underlying pathology.
Materials and methods
This retrospective case series included eight consecutive patients presenting with acute submacular haemorrhage involving the fovea. In all cases haemorrhage was secondary to neovascular AMD. At presentation, best corrected visual acuity (BCVA) of all patients was measured using a Snellen chart and comprehensive ophthalmic assessment performed including slit-lamp examination, applanation tonometry, and indirect fundus examination. Colour fundus photographs and spectral domain ocular coherence tomography (OCT) scans (Nidek, Co. Ltd, Aichi, Japan) were also taken.
Treatment was administered under topical anaesthesia in the operating theatre. Eyelids, eyelashes, and the periocular region were cleaned with povidone–iodine, a surgical drape applied, and an eyelid speculum positioned. In four cases, a vitreous tap was done before treatment and in four cases one or more anterior chamber paracenteses were performed after treatment. All patients received three separate but consecutive intravitreal injections of 50 μg tPA (Alteplase, in 0.05 ml volume), an anti-VEGF agent (0.05 mg Ranibizumab or 1.25 mg Bevacizumab in 0.05 ml), and 0.3 ml 100% perfluoropropane (C3F8). Perfusion of the central retinal artery was checked via indirect ophthalmoscopy in the operating theatre following administration of injections. Patients were advised to posture face down for 5 days.
In the United Kingdom, strict guidelines exist as to whether patients are eligible for Ranibizumab therapy based on their presenting visual acuity. Therefore, patients presenting with acute submacular haemorrhage with a Snellen visual acuity of 6/96 or better were given Ranibizumab, whereas patients presenting with poorer visual acuity received Bevacizumab, with the understanding that this is an off-label treatment in this setting.
Patients were reviewed on postoperative day 1, then at approximately 1 month and subsequently every 1–2 months. Continuation of anti-VEGF injections was advised according to clinical need. Colour fundal photographs were taken at each visit and OCT scans performed to monitor macular morphology. Outcomes assessed were BCVA, change in central retinal thickness, and complications. Snellen visual acuity was converted to logarithm of the minimal angle of resolution (LogMAR) visual acuity for analysis and comparison with previous studies. Count fingers visual acuity was allocated a LogMAR value of 1.98 and hand movement visual acuity a value of 2.28.18 Size of macular haemorrhage was measured from colour fundal photographs using ImageJ software (http://imagej.nih.gov). In some cases, haemorrhage extended beyond the area captured in photographs and hence the value stated may underestimate the true size. Statistical analysis was performed using Graphpad Prism software (La Jolla, CA, USA). The level of statistical significance was set at P<0.05.
Results
Regarding patient demographics and background, four were male and four were female with mean (±SD) age of 81.0±4.3 years. Five patients took antiplatelet agents, one patient was on warfarin (with international normalised ratio (INR) within the therapeutic range), and one patient took the activated factor X inhibitor rivaroxaban (Table 1). All patients were on systemic treatment for hypertension.
Table 1. Patient demographics, visual acuity, central retinal thickness and complications.
| Age | Sex | Antiplatelet/ anticoagulant | Treatment for systemic hypertension | Symptom onset to treatment (days) | Baseline VA LogMAR (Snellen) | Baseline CRT (μm) | Displacement of subfoveal haemorrhage | Final VA LogMAR (Snellen) | Final CRT (μm) | Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| 80 | F | Aspirin, clopidogrel | Yes | 2 | 1.78 (1/60) | 819 | Yes | 0.18 (6/9) | 222 | None |
| 85 | F | None | Yes | 5 | 2.28 (HM) | Yes | 0.78 (6/36) | 234 | Raised IOP, vitreous haemorrhage | |
| 84 | M | Warfarin | Yes | 3 | 1.78 (1/60) | 588 | Yes | 0.6 (6/24) | 284 | None |
| 73 | M | Aspirin | Yes | 2 | 0.78 (6/36) | 704 | Yes | 0.32 (6/12) | 209 | Vitreous haemorrhage |
| 86 | M | Aspirin | Yes | 4 | 1.3 (3/60) | 366 | Yes | 0.5 (6/18) | 316 | None |
| 78 | F | Rivaroxaban | Yes | 3 | 1.98 (CF) | 695 | Yes | 1.26 (3/60) | 613 | PED Rip |
| 79 | M | Aspirin | Yes | 3 | 1.46 (2/60) | 551 | Yes | 0.68 (6/30) | 214 | None |
| 83 | F | Aspirin | Yes | 2 | 1.98 (CF) | 884 | Yes | 0.68 (6/30) | 358 | None |
| Mean | 3 | 1.67 | 658.1 | 0.63 | 316.6 |
Patients presented to the eye clinic promptly with mean delay between symptom onset and presentation being 1.9±0.6 days. In all cases haemorrhage was secondary to wet AMD. One patient had previously treated neovascular AMD in the affected eye, one patient was known to have dry AMD, and the other patients were not known to have AMD before presentation. Submacular haemorrhage occurred in the better-seeing eye of two patients. The average size of macular haemorrhage at presentation was 6.0±5.2 disk areas (n=6 patients, range 1.7–14.3). Mean LogMAR visual acuity at presentation was 1.67±0.47 (range 0.78–2.28).
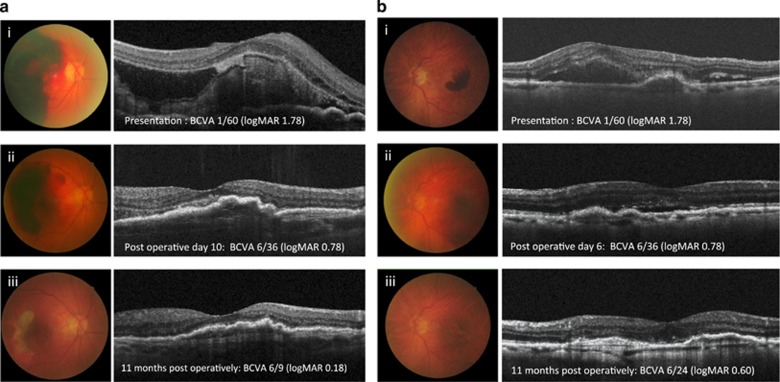
Treatment was delivered quickly with interval from presentation to treatment being 1.1±1.2 days. Therefore, the total duration of symptom onset to treatment was 3.0±1.0 days (Table 1). Anti-platelet or anticoagulant therapy was not discontinued or changed in any patient. Subfoveal haemorrhage was effectively displaced in all patients (Figure 1).
Figure 1.
Representative images of two eyes treated with intravitreal triple therapy. Fundal and OCT images from patient 1 (ai–iii) with extensive submacular haemorrhage who was treated 2 days after onset of symptoms in her right, better-seeing eye, and patient 2 (bi–iii) with more localised haemorrhage who was treated 3 days after symptom onset. Images at presentation (i), 6 days (bii) or 10 days (aii) postoperatively, and (iii) 11 months postoperatively illustrate sustained improvement with ongoing anti-VEGF treatment in both cases.
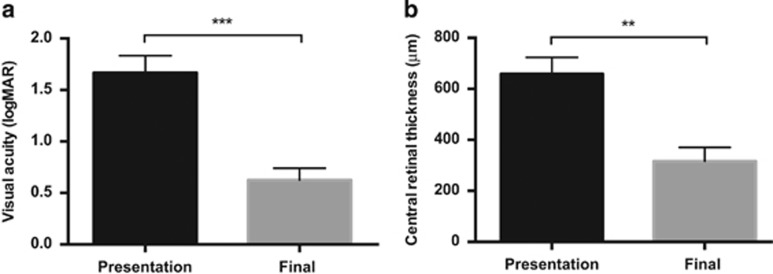
Mean LogMAR visual acuity improved from 1.67±0.47 at presentation to 0.63±0.33 at final follow-up (P<0.0001, paired t-test), a mean of 7.9±4.8 months after treatment (Figure 2a). Central retinal thickness improved from 658.1±174.2 μm at presentation to 316.6±142.4 μm at final follow-up (n=7 patients, P=0028, paired t-test; Figure 2b).
Figure 2.
Improvement in visual acuity and central retinal thickness following prompt treatment of submacular haemorrhage. Mean logMAR best corrected visual acuity (a) and central retinal thickness (b) at presentation and final follow-up are shown. **P<0.01, ***P<0.001, two-tailed, paired t-test.
Postoperatively, one patient developed vitreous haemorrhage and elevated intra-ocular pressure of 35 mm Hg, the latter settled with topical treatment using topical latanoprost and brinzolamide therapy. The patient declined further intervention for the vitreous haemorrhage that gradually improved over the 11-month follow-up period. Another patient developed postoperative vitreous haemorrhage that fully cleared within 6 weeks. A further patient with a large pigment epithelial detachment had successful displacement of submacular haemorrhage with significant improvement in visual acuity from 1.98 LogMAR to 0.60 LogMAR. A course of Ranibizumab treatment was advised; however, after the third injection the patient noted a sudden drop in acuity and was found to have a retinal pigment epithelial (RPE) rip, resulting in a final visual acuity of 1.26 LogMAR. No other adverse events such as endophthalmitis or retinal detachment occurred.
Discussion
Acute submacular haemorrhage secondary to wet AMD is known to have a poor prognosis, with few patients showing any improvement in visual acuity if untreated. Photoreceptor damage occurs rapidly, and therefore in this case series, treatment was administered promptly with mean symptom duration of 3 days, and irrespective of size of haemorrhage at presentation. We demonstrate significant gains in visual acuity following early treatment with intravitreal triple therapy of tPA, C3F8 expansile gas, and an anti-VEGF agent, with corresponding improvements in central retinal thickness on OCT scan.
There are a number of treatment options for acute submacular haemorrhage including anti-VEGF monotherapy or double therapy using intravitreal tPA and expansile gas. Our results using tPA, gas, and an anti-VEGF agent indicate higher gains in visual acuity compared with anti-VEGF therapy alone,5, 6 or tPA and gas,9, 10 consistent with previous reports.13 The improvement in visual acuity in our series was also comparable to that using vitrectomy, subretinal tPA, expansile gas, and an anti-VEGF agent, with a recent literature review concluding that the latter combination delivered the greatest visual gains.13 In view of the age and potential frailty of these patients, achieving similar outcomes using early treatment by a less invasive approach is encouraging, and a further comparative study is required to examine this.
The improvements in visual acuity that we demonstrate are greater than in other published case series using triple therapy.14, 15, 16, 17 This may be because of earlier treatment in our study as duration of symptoms in other series ranged from 6 days17 to 11.25 days.15 A further factor may be that patients had poorer baseline visual acuity in our case series, with more potential for visual gain.
In this study patients presenting with submacular haemorrhage of any size were treated, with an average of 6.0 disc areas, with effective displacement of subfoveal haemorrhage and visual improvement in all cases. One previous study only included patients with small macular haemorrhages of one to three disc diameters in size,14 and others only included large haemorrhages of greater than four disc diameters.15, 17 Therefore, as well as documenting visual gains using this approach, our study suggests that triple therapy could be used to treat all patients who present with acute submacular haemorrhage irrespective of their presenting visual acuity or size of haemorrhage. A larger study is required to establish this further.
A concern with the use of an anti-VEGF agent, tPA, and expansile gas is that of administering a high volume into the vitreous cavity that may cause complications such as retinal vascular occlusion secondary to raised intra-ocular pressure (IOP). To address this potential complication, all patients in our study had either a vitreous tap before administration of drugs or anterior chamber paracentesis following drug administration. The central retinal artery was visualised by indirect ophthalmoscopy immediately following drug delivery to ensure pulsation and good flow was seen. Furthermore, the expansile gas used in our case series was C3F8 that requires a smaller volume to be injected (0.3 ml) than the alternative SF6 (∼0.4 ml, used in the majority of previous case series using triple therapy14, 15, 16). Subsequently, none of our patients had complications of retinal vascular occlusion and only one patient had transiently elevated IOP treated with topical therapy. The use of triple therapy was safe and without significant other complications in this series.
A limitation of our study is the lack of a control group. Untreated controls were not included; however, as the natural history of this condition shows very poor outcomes, the effect of treatment is highly likely to have been beneficial. An equivalent group that was treated with longer symptom duration was also not available for comparison; however, two patients with 14-day symptom duration were also treated in our unit. One case did well with visual acuity improving from logMAR 1.98 to 0.3, whereas in the other case submacular haemorrhage was not fully displaced and there was minimal visual gain from logMAR 2.28 to 1.98. Therefore, a further study or pooled data with previous reports are required to more definitely establish the effect of early treatment.
In conclusion, in this case series patients presented soon after symptom onset, and treatment of subfoveal macular haemorrhage using intravitreal tPA, C3F8, and anti-VEGF was administered promptly, irrespective of the severity and extent of haemorrhage. Visual acuity was significantly improved following treatment with similar gains to more invasive therapeutic options. Therefore, early treatment with triple therapy could be considered as a first-line treatment for these elderly and potentially frail patients presenting with acute submacular haemorrhage.
The authors declare no conflict of interest.
References
- Bennett SR, Folk JC, Blodi CF, Klugman M. Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol 1990; 109(1): 33–37. [DOI] [PubMed] [Google Scholar]
- Scupola A, Coscas G, Soubrane G, Balestrazzi E. Natural history of macular subretinal hemorrhage in age-related macular degeneration. Ophthalmologica 1999; 213(2): 97–102. [DOI] [PubMed] [Google Scholar]
- Glatt H, Machemer R. Experimental subretinal hemorrhage in rabbits. Am J Ophthalmol 1982; 94(6): 762–773. [DOI] [PubMed] [Google Scholar]
- Avery RL, Fekrat S, Hawkins BS, Bressler NM. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina 1996; 16(3): 183–189. [DOI] [PubMed] [Google Scholar]
- Kim JH, Chang YS, Kim JW, Kim CG, Yoo SJ, Cho HJ. Intravitreal anti-vascular endothelial growth factor for submacular hemorrhage from choroidal neovascularization. Ophthalmology 2014; 121(4): 926–935. [DOI] [PubMed] [Google Scholar]
- Shienbaum G, Garcia Filho CA, Flynn Jr HW, Nunes RP, Smiddy WE, Rosenfeld PJ. Management of submacular hemorrhage secondary to neovascular age-related macular degeneration with anti-vascular endothelial growth factor monotherapy. Am J Ophthalmol 2013; 155(6): 1009–1013. [DOI] [PubMed] [Google Scholar]
- Heriot W. Intravitreal gas and rtPA: an outpatient procedure for subretinal hemorrhage. Vail Vitrectomy Meeting, Vail, CO, USA, 1996.
- Buhl M, Scheider A, Schonfeld CL, Kampik A. [Intra-vitreal rt-PA and gas introduction in submacular hemorrhage]. Ophthalmologe 1999; 96(12): 792–796. [DOI] [PubMed] [Google Scholar]
- Hattenbach LO, Klais C, Koch FH, Gumbel HO. Intravitreous injection of tissue plasminogen activator and gas in the treatment of submacular hemorrhage under various conditions. Ophthalmology 2001; 108(8): 1485–1492. [DOI] [PubMed] [Google Scholar]
- Chen CY, Hooper C, Chiu D, Chamberlain M, Karia N, Heriot WJ. Management of submacular hemorrhage with intravitreal injection of tissue plasminogen activator and expansile gas. Retina 2007; 27(3): 321–328. [DOI] [PubMed] [Google Scholar]
- Sandhu SS, Manvikar S, Steel DH. Displacement of submacular hemorrhage associated with age-related macular degeneration using vitrectomy and submacular tPA injection followed by intravitreal ranibizumab. Clin Ophthalmol 2010; 4: 637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillenkamp J, Surguch V, Framme C, Gabel VP, Sachs HG. Management of submacular hemorrhage with intravitreal versus subretinal injection of recombinant tissue plasminogen activator. Graefes Arch Clin Exp Ophthalmol 2010; 248(1): 5–11. [DOI] [PubMed] [Google Scholar]
- Stanescu-Segall D, Balta F, Jackson TL. Submacular hemorrhage in neovascular age-related macular degeneration: a synthesis of the literature. Surv Ophthalmol 2016; 61(1): 18–32. [DOI] [PubMed] [Google Scholar]
- Meyer CH, Scholl HP, Eter N, Helb HM, Holz FG. Combined treatment of acute subretinal haemorrhages with intravitreal recombined tissue plasminogen activator, expansile gas and bevacizumab: a retrospective pilot study. Acta Ophthalmol 2008; 86(5): 490–494. [DOI] [PubMed] [Google Scholar]
- Sacu S, Stifter E, Vecsei-Marlovits PV, Michels S, Schutze C, Prunte C et al. Management of extensive subfoveal haemorrhage secondary to neovascular age-related macular degeneration. Eye (Lond) 2009; 23(6): 1404–1410. [DOI] [PubMed] [Google Scholar]
- Guthoff R, Guthoff T, Meigen T, Goebel W. Intravitreous injection of bevacizumab, tissue plasminogen activator, and gas in the treatment of submacular hemorrhage in age-related macular degeneration. Retina 2011; 31(1): 36–40. [DOI] [PubMed] [Google Scholar]
- Papavasileiou E, Steel DH, Liazos E, McHugh D, Jackson TL. Intravitreal tissue plasminogen activator, perfluoropropane (C3F8), and ranibizumab or photodynamic therapy for submacular hemorrhage secondary to wet age-related macular degeneration. Retina 2013; 33(4): 846–853. [DOI] [PubMed] [Google Scholar]
- Lange C, Feltgen N, Junker B, Schulze-Bonsel K, Bach M. Resolving the clinical acuity categories ‘hand motion' and ‘counting fingers' using the Freiburg Visual Acuity Test (FrACT). Graefes Arch Clin Exp Ophthalmol 2009; 247(1): 137–142. [DOI] [PubMed] [Google Scholar]