Abstract
Purpose
To evaluate the neovascular age-related macular degeneration (nAMD) in patients who were morphologically poor responders to intravitreal ranibizumab (IVR) treatment using indocyanine green angiography (ICGA) for further investigation.
Methods
This was a cross-sectional, retrospective study. The patients with an initial diagnosis of nAMD who made through the clinical examination, optical coherence tomography, and fluorescein angiography imaging, and were treated with at least three monthly IVR injections that resulted with a morphological poor response, were included. ICGA was obtained from the patients and evaluated in regard to differential diagnosis of other macular diseases, which might mimic nAMD.
Results
The study included 132 eyes of 117 patients. The mean age was 67.4±9.4 years. After ICGA imaging, 13 eyes (9.8%) were diagnosed as true nAMD, 74 eyes (56.1%) as polypoidal choroidal vasculopathy (PCV), 35 eyes (26.5%) as chronic central serous chorioretinopathy (CSC), 3 eyes (2.3%) as retinal angiomatous proliferation (RAP), 3 eyes (2.3%) as choroidal neovascularization secondary to CSC, 2 eyes (1.5%) as adult-onset vitelliform macular dystrophy, and 2 eyes (1.5%) as drusenoid pigment epithelial detachment with vitelliform material, respectively. The duration between the initial diagnosis and the revised diagnosis was 15.6±10.5 months in the non-AMD group, and the mean injection number of these patients was 6.6±4.4.
Conclusions
Most of the nAMD patients who were thought to be morphologically poor responders to IVR were diagnosed as having non-AMD diseases via ICGA. A detailed differential diagnostic work-up is needed before considering these patients as poor responders.
Introduction
Neovascular age-related macular degeneration (nAMD) is a major cause of visual loss among elderly population in developed countries.1, 2 Before the era of intravitreal anti-vascular endothelial growth factor (anti-VEGF) agents, only prevention of visual loss might have been achieved in a limited number of patients despite the use of different treatment modalities.3, 4, 5, 6, 7, 8 Bevacizumab, ranibizumab, and finally aflibercept have led to the conservation of the baseline visual acuity (VA) in the vast majority of the patients and have given the chance of increasing VA significantly in approximately one third of the patients.9, 10, 11, 12 Multicenter studies have shown that ranibizumab is effective in the prevention of VA loss in up to 95% of the patients, and an improvement in VA can be achieved in up to 40% of the patients.13, 14 However, there was still a subgroup of patients who did not respond well to the IVR treatment. A new debate has begun since then, and some other treatment strategies were evaluated for this group of poor-responding patients, such as switching the drugs, shortening the injection intervals, increasing the drug dose, and using combination therapy.15 Although some of these patients did well with the alternative treatment regimens, others were still poor responders. Also, their diagnosis was questioned by several authors and various studies were designed to evaluate deeply the true diagnosis of these patients.15, 16, 17, 18, 19, 20 Enhanced depth imaging optical coherence tomography (EDI-OCT), fundus autofluorescence (FAF) imaging, and indocyanine green angiography (ICGA) were used as additional diagnostic tools in some of these studies.15, 16, 17, 18, 19, 20 Macular diseases such as polypoidal choroidal vasculopathy (PCV), central serous chorioretinopathy (CSC), and retinal angiomatous proliferation (RAP) may sometimes mimic nAMD and thus create diagnostic challenges. Polypoidal choroidal vasculopathy and RAP are usually considered as variants of nAMD; however, some authors consider them as different entities than nAMD. Likewise, although some of the PCV and RAP patients respond well to anti-VEGF monotherapy, a substantial number of these patients are indeed anti-VEGF poor responders. Only a few studies have investigated specific diseases such as PCV or chronic CSC via ICGA in anti-VEGF poor responders, and none of these studies evaluated purely the morphological poor-responding patients.16, 17, 18, 19, 20 Therefore, in this study we aimed to evaluate the patients who had a diagnosis of nAMD with a morphological poor response to IVR treatment via multimodal imaging—especially ICGA—for further differential diagnosis from all other macular diseases that mimic nAMD.
Materials and methods
In this cross-sectional, retrospective, and observational study, we reviewed the records of the nAMD patients who were treated with IVR in our clinic on an as-needed treatment regimen basis between January 2014 and December 2014. A written informed consent was obtained from all patients before the treatment and the study adhered to the tenets of the Declaration of Helsinki.
To be included in the study, each patient was required to have all of the following criteria, age ≥50 years, to be initially diagnosed as nAMD, to have received at least three IVR injections, and an incomplete morphological response as defined below. Patients were not included in the study if they had a known retinal disease other than nAMD. All patients received three initiating doses of monthly IVR injections (0.5 mg/0.05 ml) initially. Then the patients were followed monthly. A single injection of IVR was repeated when the VA had decreased by one or more Early Treatment Diabetic Retinopathy Study (ETDRS) lines from the last visit, or when the patients had developed new onset macular hemorrhage, or evidence of subretinal fluid on OCT. The morphologically poor responder status was defined if there was persistent subretinal and/or intraretinal fluid. If the subretinal or intraretinal fluid had not disappeared after the initial three loading doses of IVR or if the subretinal fluid had persisted after three consecutive injections in any time during the follow-up period, then the patient was then categorized as a morphologically poor responder and ICGA was obtained at the same visit.
Data collected from the patients' records included age, gender, and the revised diagnosis after ICGA imaging, the time interval between the initial and revised diagnosis via ICGA, and the number of anti-VEGF injections within this time period.
All patients underwent a standardized examination including measurement of best-corrected VA (BCVA) via the ETDRS chart at 4 m, slit-lamp biomicroscopy, measurement of intraocular pressure (IOP) via applanation tonometry, and biomicroscopic fundus examination. Fundus photography, fluorescein angiography (FA) and indocyanine green angiography (HRA-2; Heidelberg Engineering, Heidelberg, Germany), and OCT imaging (Spectralis; Heidelberg Engineering, Heidelberg, Germany) were also performed. Two independent retina specialists (AO, CA) who were blinded to the clinical data simultaneously evaluated the images.
The initial diagnosis of neovascular AMD was made by eight different retina specialists who worked in our clinic using a combination of clinical examination, OCT, and FA findings of the disease. The diagnosis of neovascular AMD was made according to the following criteria, well-known clinical findings,1, 2, 3, 4 a stippled hyperfluorescence in FA representing like an occult choroidal neovascularization (CNV), subretinal fluid associated with an adjacent hyper-reflective area representing like a fibrovascular pigment epithelial detachment (PED).1, 7, 13 The final differential diagnosis was made according to known specific FA, OCT, FAF, and especially ICGA findings of the diseases. The initial diagnosis of the eyes was usually occult CNV secondary to nAMD. Only a few patients had classical CNVs, which were later diagnosed as classic CNV secondary to chronic CSC. The differential diagnosis was described in detail in the following paragraph.
Neovascular AMD with occult CNV was diagnosed when a significant hot plaque was detected in the late frames of ICGA, or when dilated choroidal vessels were not seen as a sign of CSC, or when any hot spot that may be a polyp was not seen (Figure 1).21 In fact neovascular AMD was like an exclusion diagnosis in this study. Polypoidal choroidal vasculopathy was diagnosed when polyps and/or branching vascular network were detected in ICGA (Figure 2).16 Choroidal thickness measurements via EDI-OCT was not routinely obtained by a quantitative method; however, OCT images were usually evaluated in regard of choroidal thickness with eye examination as a routine practice. Chronic CSC was diagnosed when enlarged choroidal vessels and leakage from these vessels and hyperfluorescence were detected in the early frames of ICGA, and when wash-out pattern of the leakage and hypofluorescence was detected in the late frames (Figure 2).22 Also in a detailed manner, we evaluated all of the ICGA, FA, and OCT images of the patients for the other signs of CSC, such as diffuse hyperfluorescence in FA, gravitational tracts, and pigmentary changes.22 RAP was diagnosed when a large and high retinal pigment epithelial detachment with a notch on the surface was detected and/or when angioma per se was detected in ICGA as a hot spot (Figure 3).16 CNV associated with chronic CSC was diagnosed when a classic CNV was detected with the previously described features of chronic CSC in ICGA. The patients were diagnosed as adult-onset vitelliform macular dystrophy when the specific features of pattern dystrophy were detected in FAF and OCT (Figure 4) imaging, and when a CNV was not detected in ICGA.16 The FAF patterns of adult-onset vitelliform macular dystrophy were, a diffuse round increased autofluorescence, or partial increased autofluorescence, or decrease in autofluorescence if there was no prominent yellow accumulation on examination.23 Drusenoid retinal PED associated with vitelliform-like material diagnosis was made according to the typical OCT findings, which were seen as a focal elevation of the retinal pigment epithelium contour associated with fluid but without showing a CNV on ICGA.24 Of course, there were some confusing cases that we were not able to make a definite diagnosis. In such cases, two experienced retinal specialists (AO and CA) jointly adjudicated and arrived at the most probable diagnosis.
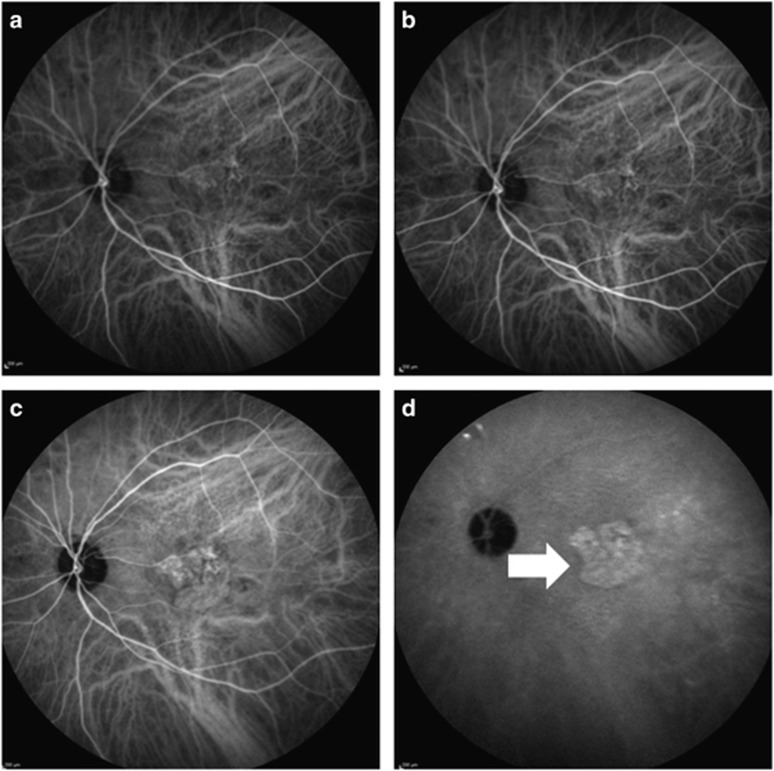
Figure 1.
The indocyanine green angiography images of a 78-year-old male neovascular age-related macular degeneration patient. (a and b). Early phases of angiography (c). The mid-phase of the angiography (d). The late phase of the angiography; white arrow shows the hot plaque formation secondary to occult choroidal neovascularization.
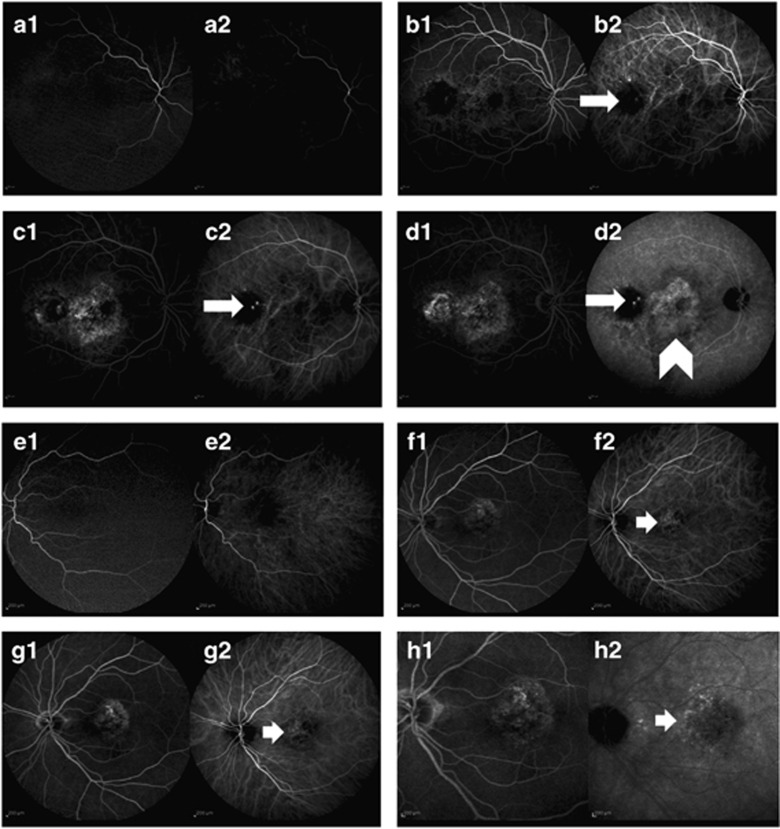
Figure 2.
The simultaneous fluorescein and indocyanine green angiography images of a 77-year-old male polypoidal choroidal vasculopathy patient. First images (1) are fluorescein angiography images, second images (2) are indocyanine green angiography images. (a1 and a2). Early phases of angiography (b1 and b2). A polyp like hot spot is prominent in the early phase of indocyanine angiography (white arrow) (c1 and c2). Two polyps are seen in the mid-phase of indocyanine green angiography (d1 and d2). White arrow shows two polyps in the late phase of indocyanine green angiography and white arrow head shows a hot plaque. The simultaneous fluorescein and indocyanine green angiography images of a 72-year-old female chronic central serous choroidopathy patient. First images (1) are fluorescein angiography images, second images (2) are indocyanine green angiography images. (e1 and e2). Early phases of angiography (f1 and f2). Dilated choroidal vessels are prominent in the early phase of indocyanine angiography (white arrow) (g1 and g2). Leakage around the dilated choroidal vessels in the mid-phase of indocyanine green angiography (h1 and h2). Wash-out pattern without a hot plaque in the late phase of indocyanine green angiography (white arrow).
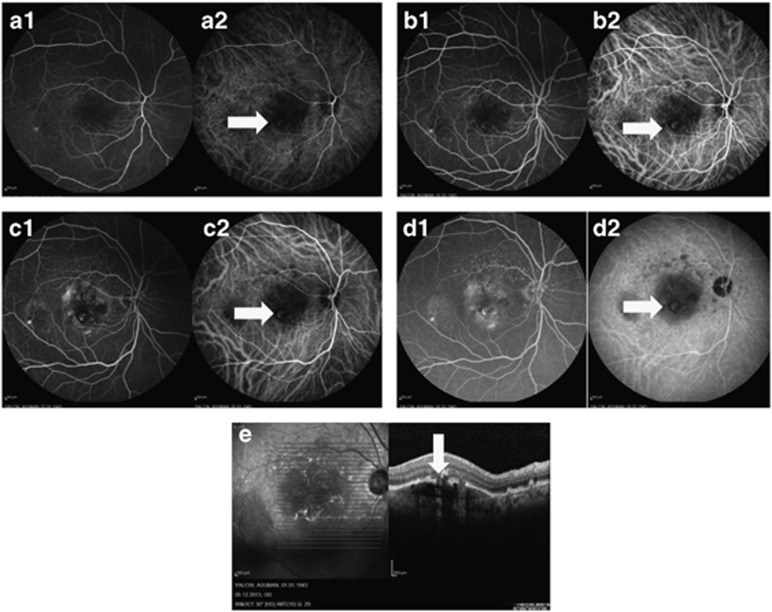
Figure 3.
The simultaneous fluorescein and indocyanine green angiography images of a 71-year-old female retinal angiomatous proliferation patient. First images (1) are fluorescein angiography images, second images (2) are indocyanine green angiography images (a1 and a2, b1 and b2, c1 and c2, and d1 and d2). A prominent angioma is seen in all phases of indocyanine green angiography (white arrows) (e). White arrows shows a notch in the retinal pigment epithelium at the lesion, which represents the angioma.
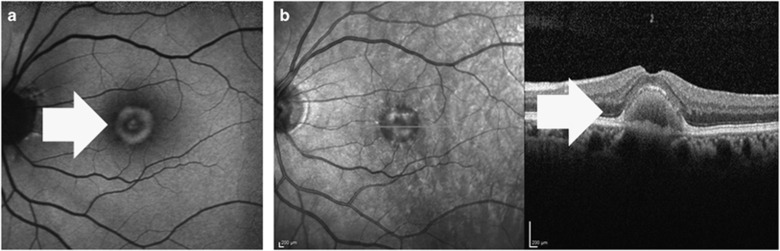
Figure 4.
(a) The autofluorosence image of a 66-year-old male adult-onset vitelliform macular dystrophy patient. White arrow shows the vitelliform lesion (b), which is seen as a hyper-reflective lesion beneath the retina pigment epithelium at the fovea (white arrow) in the optical coherence tomography image.
Primary outcome measures of this study were the initial and revised diagnosis of nAMD eyes with a morphologic poor response to IVR treatment, the relative distribution of the mimicking diseases, the time interval from the initial diagnosis to the revised diagnosis, and the number of injections received before the revised diagnosis.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software (version 16.0, SPSS Inc., Chicago, IL, USA). Data were first analyzed for normality using Kolmogorov–Smirnov test. The continuous variables were expressed as means±SD. The categorical variables were expressed as number (n) and percentages (%).
Results
One hundred and thirty two out of 1024 eyes were found to be morphologically poor responders to IVR treatment and included in the study. The mean age of the patients was 67.4±9.4 years (ranging from 52 to 88). Fifty-five patients (47%) were women and 62 patients (53%) were men. Seventeen of the eyes (12.8%) were diagnosed early poor responders after the initial three injections, and 115 eyes (87.2%) were late poor responders.
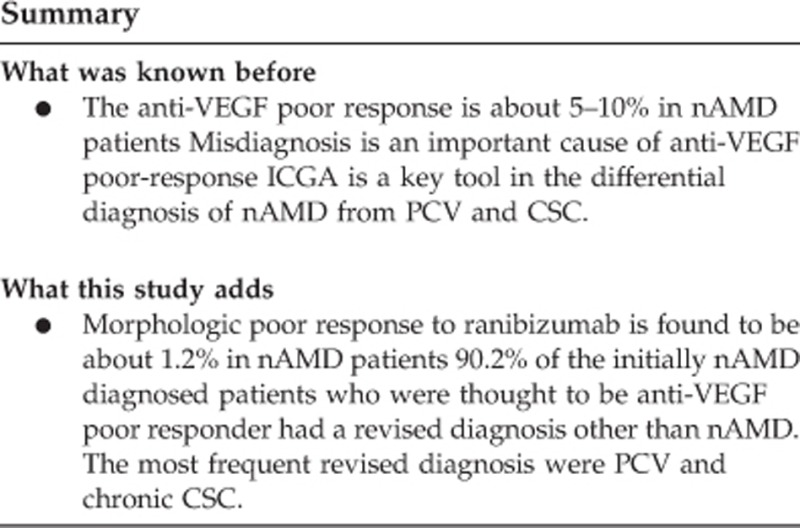
After ICGA imaging and a detailed examination done by using other multimodal imaging tools, 13 eyes (9.8%) were diagnosed as having true nAMD, and 119 eyes (90.2%) were diagnosed as having non-AMD diseases. The revised diagnosis of the non-AMD eyes were as follows, 74 eyes (56.1%) had PCV, 35 eyes (26.5%) had chronic CSC, 3 eyes (2.3%) had RAP, 3 eyes (2.3%) had CNV secondary to CSC, 2 eyes (1.5%) had adult-onset vitelliform macular dystrophy, and 2 eyes (1.5%) had drusenoid PED associated with vitelliform-like material (Table 1).
Table 1. The distrubution of the revised diagnosis after multimodal imaging.
| Total n, (%) | |
|---|---|
| nAMD (reconfirmation) | 13 (9.8) |
| PCV | 74 (56.1) |
| Chronic CSC | 35 (26.5) |
| RAP | 3 (2.3) |
| CNV secondary to CSC | 3 (2.3) |
| VD | 2 (1.5) |
| Drusenoid PED | 2 (1.5) |
| Total n, (%) | 132 (100) |
Abbreviations: CNV, choroidal neovascularization; CSC, central serous chorioretinopathy; nAMD, neovascular age-related macular degeneration; n, number; PED, pigment epithelial detachment; PCV, polypoidal choroidal vasculopathy; RAP, retinal angiomatous proliferation; VD, adult-onset vitelliform macular dystrophy.
The mean duration between the initial and the revised diagnosis was 15.6±10.5 months (ranging between 4 and 48 months) in the non-AMD eyes, and the mean injection number of these eyes was 6.6±4.4 (ranging between 3 and 24).
Discussion
The treatment response to anti-VEGF agents in nAMD patients has been an evolving subject in the medical retina era. Several studies have been conducted in this important topic and the terminology for anti-VEGF treatment response has been defined in detail.15, 16, 25 The response to anti-VEGF agents has been categorized either as functional or morphological response.15 Amoaku et al15 have recently proposed to classify patients with nAMD into four categories with regard to their functional response based on the change in VA, which were as follows, good responders (>5 ETDRS letter gain from the baseline), suboptimal responders (gain of 0–5 letters from the baseline), poor responders (stable or <4 letters loss from the baseline), and nonresponders (>5 letters loss from the baseline). Morphological responses of the patients were defined according to their disease activity and divided into two categories, good responders in whom no disease activity was detected, and suboptimal or poor responders in whom only a partial regression of disease activity was evident. The authors have also evaluated the treatment response according to treatment phases, which were addressed as primary and secondary responses.15 Primary response was usually determined after the three initial loading doses at month 4, and secondary response was determined at any time during the follow-up period after the first 4 months.15
In the pivotal multicenter studies, ~5% of the patients were found to be unresponsive to anti-VEGF treatment, and showed sustained VA loss despite treatment; these patients have been called as nonresponders.11, 13, 14, 26 The sustained VA loss has been attributed to several factors, such as fibrous scar formation, pigmentary anomalies, geographic atrophy, RPE tear, retinal thinning, and thickening.26 Also some of the baseline characteristics were found to be associated with sustained VA loss, which were presence of non-foveal geographic atrophy, larger CNV area, poor baseline VA, <20/50 baseline VA in the fellow eye, and intraretinal fluid not at the foveal center.26
The patients' clinical characteristics, lesion type, and misdiagnosis have been evaluated thoroughly and constitute now the main discussed topics in anti-VEGF nonresponse literature.15, 16, 26 Factors affecting drug pharmacodynamics and pharmacokinetics such as receptor downregulation, autoantibody formation, change in drug distribution, and absorption have also been shown to be related to nonresponse.15, 25, 26
Suboptimal treatment of the patients with less frequent treatment regimens is another important clinical factor which can affect the treatment success and underlie nonresponse. Real-life studies showed that many of the patients were undertreated especially in the PRN treatment regimens.25 Also disease chronicity, change of cytokine profile during the treatment, and chronic inflammation were shown to be important clinical factors.15, 26
It is worth to recall here that some lesion types of nAMD are less responsive to anti-VEGF treatment. Polypoidal choroidal vasculopathy and RAP are usually considered as subtypes of nAMD; however, nowadays they are being considered by some as different clinical entities. Some of the PCV and RAP lesions respond well to anti-VEGF monotherapy, but in considerable number of the patients different treatment modalities such as photodynamic therapy and combination therapy are required. Taken together, all these data refer to the important role of detailed differential diagnostic work-up in the event of nonresponse. It is important to exclude all these factors by using available imaging tools in an individual patient before considering nonresponse.15, 16, 27, 28, 29
In a study by Manoj, poor-responding patients' clinical diagnosis was reported to be revised in 46.2%. And 79.2% of these patients were diagnosed as having PCV. The remaining patients were diagnosed as having RAP and adult-onset vitelliform macular dystrophy.16 However, ICGA was not used in all of the patients in the study. The authors reported that ICGA and autofluorescence imaging were done according to the clinicians' discretion. In our study, we obtained ICGA from all of our anti-VEGF poor-responding patients. As ICGA has been shown to be a valuable imaging modality in this group of patients;15, 18, 19, 20 autofluorescence and EDI-OCT were obtained only when needed. ICGA is particularly useful in the differential diagnosis of PCV, chronic CSC, and RAP, which are usually misdiagnosed as nAMD.15 Our study demonstrate that with the use of ICGA imaging, only 9.8% of our morphologically poor-responding patients were found to exhibit nAMD, whereas 90.2% of them were misdiagnosed. The revised diagnosis were PCV in 56.1% of the eyes, chronic CSC in 26.5 of the eyes, RAP in 2.3% of the eyes, and CNV secondary to CSC in 2.3% of the eyes. Interestingly, the patients which required additional photodynamic therapy represented 97% of the misdiagnosed patients. The true number of morphologically poor-responding eyes was 13, which represented only 1.2% of the 1024 ranibizumab-treated eyes during the study period. Indeed, the morphological poor response that was 132/1024 (12.8%) was found to be 13/1024 (1.2%) after the use of ICGA, which led to a tenfolds decrease in the misdiagnosis of these non-AMD patients. In another study by Hatz and Prünte the ranibizumab poor-responding patients who required at least eight injections were evaluated via ICGA. In that study the prevalence of PCV and RAP was found to be 16.8% and 11.4%, respectively. In the Hatz and Prunte study, the prevalence of PCV was lower than our study, because of the difference the poor-response criterion. The authors described the poor-response status as ‘need for frequent intravitreal injections during OCT-guided dosing'. However our criterion was persistent intraretinal and/or subretinal fluid 1 month after the last intravitreal injection.
In this study, we evaluated specifically the morphologically poor-responding patients. The functional poor responders were not included in the study, which was the most important limitation. Also, in fact using a three-injection threshold for defining poor response may not be suitable because some of the patients may show late responses to anti-VEGF treatment.15 Indeed, ICGA is not used as a standard imaging technique in our country. The dye is off-label and the cost of dye is not covered by social insurance. Therefore, ICGA imaging cannot be performed in all of the nAMD patients and is preserved for confusing cases such as the ones that were included in this study. Our policy is to start anti-VEGF treatment for all of nAMD confirmed with FFA and OCT first, obtain ICGA in the event of poor or nonresponse, and in patients with a possible diagnosis of PCV, chronic CSC, or RAP. In this vein, we rather prefer being more cautious about the cases who do not have a good response to three injections of anti-VEGFs and use a lower threshold for poor response in order not to delay the diagnosis of an underlying mimicking disease.
The retrospective study design was also a limitation and the treatment outcomes of the patients after the revised diagnosis were not reported. However, we are reviewing the records of these patients regarding the follow-up period after the ICGA imaging and we have planned to conduct two separate studies in which we will evaluate the treatment outcomes of misdiagnosed PCV and chronic CSC patients. On a positive side, this study was conducted in a single center and included a considerable number of nAMD patients. The imaging evaluation was done by two experienced retina specialists and ICGA was obtained from all of the patients.
In conclusion, we shown that ~90% of the morphologically ranibizumab poor-responding patients would have been classified inappropriately without using ICGA. More importantly, 97% of these patients was diagnosed as having PCV, chronic CSC, and RAP and required a different treatment modality such as photodynamic therapy. The real morphological poor-response rate was indeed around 1%. In the light of these findings we propose that all ranibizumab poor-responding patients should undergo a detailed differential diagnostic work-up including ICGA before being considered as a ‘true poor responder' and ICGA imaging might be one of the important key diagnostic tools in the evaluation of anti-VEGF nonesponse.
The authors declare no conflict of interest.
Footnotes
Author contributions
Involved in design and conduct of the study: AO, CA, RG, ZA, IP, ATY, MT; preparation and review of the study: AO, IP, ATY, MT; data collection: AO, CA, RG, ATY; and statistical analysis: AO, ZA.
References
- Soubrane G, Coscas G, Français C, Koenig F. Occult subretinal new vessels in age-related macular degeneration. Natural history and early laser treatment. Ophthalmology 1990; 97: 649–657. [DOI] [PubMed] [Google Scholar]
- Roy M, Kaiser-Kupfer M. Second eye involvement in age-related macular degeneration: a four-year prospective study. Eye (Lond) 1990; 4: 813–818. [DOI] [PubMed] [Google Scholar]
- Singerman LJ, Kalski RS. Tunable dye laser photocoagulation for choroidal neovascularization complicating age-related macular degeneration. Retina 1989; 9: 247–257. [DOI] [PubMed] [Google Scholar]
- Tornambe PE, Poliner LS, Hovey LJ, Taren D. Scatter macular photocoagulation for subfoveal neovascular membranes in age-related macular degeneration. A pilot study. Retina 1992; 12: 305–314. [DOI] [PubMed] [Google Scholar]
- Kapran Z, Ozkaya A, Uyar OM. Hemorrhagic age-related macular degeneration managed with vitrectomy, subretinal injection of tissue plasminogen activator, gas tamponade, and upright positioning. Ophthalmic Surg Lasers Imaging Retina 2013; 44: 471–476. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Kachi S, Iwata E, Ishikawa K, Terasaki H. Visual function 5 years or more after macular translocation surgery for myopic choroidal neovascularisation and age-related macular degeneration. Eye (Lond) 2012; 26: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Finana M, Murjaneh S, Mahmood S, Harding SP. Baseline clinical measures and early response predict success in verteporfin photodynamic therapy for neovascular age-related macular degeneration. Eye (Lond) 2010; 24: 1213–1219. [DOI] [PubMed] [Google Scholar]
- Hogan AC, Kilmartin DJ. Low power vs standard power transpupillary thermotherapy in patients with age-related macular degeneration and subfoveal choroidal neovascularization ineligible for photodynamic therapy. Eye (Lond) 2006; 20: 649–654. [DOI] [PubMed] [Google Scholar]
- Subramanian ML, Abedi G, Ness S, Ahmed E, Fenberg M, Daly MK et al. Bevacizumab vs ranibizumab for age-related macular degeneration: 1-year outcomes of a prospective, double-masked randomised clinical trial. Eye (Lond) 2010; 24: 1708–1715. [DOI] [PubMed] [Google Scholar]
- Ozkaya A, Alkin Z, Yazici AT, Demirok A. Comparison of intravitreal ranibizumab in phakic and pseudophakic neovascular age-related macular degeneration patients with good baseline visual acuity. Retina 2014; 34: 853–859. [DOI] [PubMed] [Google Scholar]
- Brown DM, Kaiser PK, Michels M, Heier JS, Sy JP, Ianchulev T. ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1432–1444. [DOI] [PubMed] [Google Scholar]
- Grewal DS, Gill MK, Sarezky D, Lyon AT, Mirza RG. Visual and anatomical outcomes following intravitreal aflibercept in eyes with recalcitrant neovascular age-related macular degeneration: 12-month results. Eye (Lond) 2014; 28: 895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et al. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1419–1431. [DOI] [PubMed] [Google Scholar]
- CATT Research Group, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011; 364: 1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoaku WM, Chakravarthy U, Gale R, Gavin M, Ghanchi F, Gibson J et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye (Lond) 2015; 29: 721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoj S. Why does anti VEGF treatment fail in age related macular degeneration (AMD). Kerala J Ophthalmol 2011; 3: 282–286. [Google Scholar]
- Stangos AN, Gandhi JS, Nair-Sahni J, Heimann H, Pournaras CJ, Harding SP. Polypoidal choroidal vasculopathy masquerading as neovascular age-related macular degeneration refractory to ranibizumab. Am J Ophthalmol 2010; 150: 666–673. [DOI] [PubMed] [Google Scholar]
- Ilginis T, Ottosen S, Harbo Bundsgaard K, Uggerhøj Andersen C, Vorum H. Polypoidal choroidal vasculopathy in patients diagnosed with neovascular age-related macular degeneration in Denmark. Acta Ophthalmol 2012; 90: e487–e488. [DOI] [PubMed] [Google Scholar]
- Hatz K, Prünte C. Polypoidal choroidal vasculopathy in Caucasian patients with presumed neovascular age-related macular degeneration and poor ranibizumab response. Br J Ophthalmol 2014; 98: 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung AT, Yannuzzi LA, Freund KB. Type 1 (sub-retinal pigment epithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degeneration. Retina 2012; 32: 1829–1837. [DOI] [PubMed] [Google Scholar]
- Lee JE, Kim HW, Lee SJ, Lee JE. Changes of choroidal neovascularization in indocyanine green angiography after intravitreal ranibizumab injection. Retina 2015; 35: 999–1006. [DOI] [PubMed] [Google Scholar]
- Alkin Z, Ozkaya A, Agca A, Yazici AT, Demirok A. Early visual and morphologic changes after half-fluence photodynamic therapy in chronic central serous chorioretinopathy. J Ocul Pharmacol Ther 2014; 30: 359–365. [DOI] [PubMed] [Google Scholar]
- Grob S, Yonekawa Y, Eliott D. Multimodal imaging of adult-onset foveomacular vitelliform dystrophy. Saudi J Ophthalmol 2014; 28: 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre de Amorim Garcia Filho C, Yehoshua Z, Gregori G, Farah ME, Feuer W, Rosenfeld PJ. Spectral-domain optical coherence tomography imaging of drusenoid pigment epithelial detachments. Retina 2013; 33: 1558–1566. [DOI] [PubMed] [Google Scholar]
- Cazet-Supervielle A, Gozlan J, Cabasson S, Boissonnot M, Manic H, Leveziel N. Intravitreal ranibizumab in daily clinical practice for age-related macular degeneration: treatment of exudative age-related macular degeneration in real life. Ophthalmologica 2015; 234: 26–32. [DOI] [PubMed] [Google Scholar]
- Ying GS, Kim BJ, Maguire MG, Huang J, Daniel E, Jaffe GJ et al. CATT Research Group. Sustained visual acuity loss in the comparison of age-related macular degeneration treatment trials. JAMA Ophthalmol 2014; 132: 915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrejen S, Sarraf D, Mukkamala SK, Freund KB. Multimodal imaging of pigment epithelial detachment: a guide to evaluation. Retina 2013; 33: 1735–1762. [DOI] [PubMed] [Google Scholar]
- Gomi F, Oshima Y, Mori R, Kano M, Saito M, Yamashita A et al. Initial versus delayed photodynamic therapy in combination with ranibizumab for treatment of polypoidal choroidal vasculopathy: the Fujisan Study. Retina 2015; 35: 1569–1576. [DOI] [PubMed] [Google Scholar]
- Engelbert M, Zweifel SA, Freund KB. ‘‘Treat and extend'' dosing of intravitreal endothelial growth factor therapy for type 3 neovascularization/retinal angiomatous proliferation. Retina 2009; 29: 1424–1431. [DOI] [PubMed] [Google Scholar]