Abstract
Purpose
To investigate early changes in choroidal thickness (CT) and the relationship with accommodation after myopic excimer laser surgery.
Methods
We enrolled the right eye of 70 patients with myopia and without other ophthalmic or systemic diseases who were suitable for myopic excimer laser surgery. The CT was measured at the fovea and at distances of 0.5 and 2.5 mm for the following: nasal; temporal; superior; and inferior to the fovea preoperatively and at 1 month postoperatively. Other data collected included demographic information (age, sex, and refractive error), the amplitude of accommodation (AA), intraocular pressure, axial length, corneal thickness, and surgical parameters. The data were analyzed with a paired Student's t-test, stepwise linear regression, and correlation analysis.
Results
The CT was significantly thicker postoperatively compared with the preoperative CT. The AA significantly decreased postoperatively. The change in the AA was the most significant factor associated with the change in the CT at the fovea. Except for 2.5 mm inferior to the fovea, the increase in the CT at other locations was positively correlated with the decrease in the AA.
Conclusions
The CT increased following myopic excimer laser surgery and the change was most obvious when accompanied by a decrease in the AA early after the surgery.
Introduction
Myopia is a significant global public health concern throughout the world. Presently, although the age of onset of myopia is decreasing, the incidence rate of the disease is increasing.1 The choroid, which located between the retina and sclera, is uniquely situated to transfer retina-derived signals to the sclera to effect changes in ocular size.2 It is also the source of many vision-threatening diseases such as macular degeneration and polypoidal choroidal vasculopathy.3, 4 In profoundly affected myopic eyes, the choroid is thinner than in normal eyes, and it undergoes further thinning with age and increasing myopia.5, 6 A thinner choroid could be instrumental in the onset and progression of severe myopia-related diseases.7 Increasing numbers of ophthalmic scholars and clinicians are focusing on choroidopathy induced by myopia. The focus of myopia-related research has gradually shifted from the dioptric media of the anterior segment to the tissues of the posterior segment such as the choroid, to investigate the etiology of myopia.
The prevention and treatment of myopia have always been a public health concern. However, the use of refractive surgery to correct myopia is controversial. Laser in situ keratomileusis (LASIK) and laser epithelial keratomileusis (LASEK) have become common treatments for myopia. In previous studies, the safety, efficacy, and predictability of these treatments have been assessed by the change of visual function8, 9 and ocular anterior segment.9, 10 However, the change of ocular poster segment, especially choroid has rarely been evaluated following refractory surgery.
As shown in studies, the choroid expanded or thinned in myopic eyes, which has a good correlation with increasing myopia.6 This finding suggests that there may be an important relationship between myopia and choroidal thickness. During the process of myopic recovery in chick, the choroid compensated by becoming thicker.11, 12 Thinning choroidal thickness was recovered due to a reduction or neutralization of the myopiogenic stimulus to eye growth in these myopic children wearing overnight orthokeratology contact lens.13 However, the change in the choroidal thickness (CT) after correcting myopic refractive error in humans has not been reported.
The aim of the current study was to investigate whether CT, an important factor in myopia, changed following myopic excimer laser surgery and to identify the factors associated with any observed changes. The overall goal of the study is to provide a comprehensive evaluation of functional recovery levels after refractive surgery.
Materials and methods
Patients
We enrolled the right eyes of 70 patients (70 eyes) with myopia who had no other ophthalmic or systemic diseases and were eligible for myopic excimer laser surgery (LASIK or LASEK). The study took place from July 2012 to October 2013 in the Department of Ophthalmology, Shanghai First People's Hospital. The CT was measured at the fovea and at distances of 0.5 and 2.5 mm nasal, temporal, superior, and inferior to the fovea preoperatively and at 1 month postoperatively. Our study was approved by the Institutional Review Board of Shanghai First People's Hospital, Shanghai Jiao Tong University, and it adhered to the provisions of the Declaration of Helsinki for research involving human subjects. We obtained informed signed consent from all the patients before participating.
We collected basic information (age, sex, and refractive error) and data on the corneal thickness, and surgical parameters (ablating depth and residual thickness of stromal bed), preoperative and postoperative intraocular pressure (IOP), amplitude of accommodation (AA). The corneal thickness was measured with a corneal pachymeter (SP3000, Tomey). The axial length was measured with intraocular len master (IOL Master; Master 500, Zeiss). The refractive error was described as equivalent power. The IOP was measured with a non-contact tonometer (Full Auto Tonometer TX-F, Canon). The IOP after surgery corrected was got by the forum (IOP corrected (mm Hg)=IOP measured−(measured corneal thickness (μm)−578 (μm)) × (5/70).14 The AA was measured by push-up test, and the CT was measured by spectral domain optical coherence tomography (SD-OCT). Two doctors measured all the ophthalmic parameters independently three times and the results were averaged.
Surgery protocol
The same doctor performed LASIK or LASEK surgery to correct the myopia. LASIK surgery was performed using a Moria 2 disposable microkeratome (head, 90 μm) to create the corneal flap (depth, 110 μm). The myopic correction was then registered on the ALLEGRTTO 2009 excimer laser and the appropriate ablation performed. LASEK surgery was performed by placing 20% ethyl alcohol within the corneal epithelial ring for 8 s. An intact epithelial flap was retracted. The ALLEGRTTO 2009 excimer laser was applied in a similar manner to the LASIK procedure.15
Spectral domain optical coherence tomography
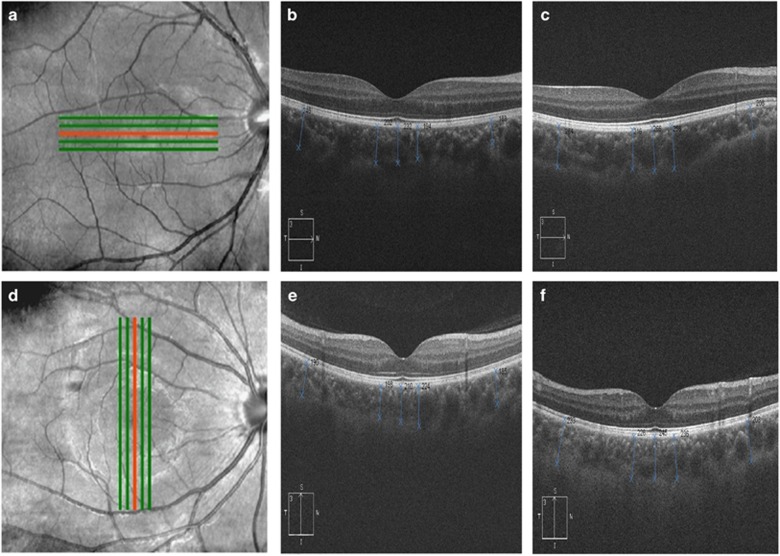
SD-OCT (Zeiss-Humphrey, Jena, Germany) with enhanced depth imaging (EDI) was used to measure the CT. Five line scanning by SD-OCT was performed at the area of fovea at the vertical and horizontal azimuth with scanning length by 6.0 mm. The OCT images for our study were selected in the middle of fovea.16 Choroidal thickness was perpendicularly measured from outermost hyper-reflective line of the retinal pigment epithelium to the choroid-scleral junction, presumed as hyper-reflective line behind large vessels of choroid at the fovea and at a distance of 0.5 and 2.5 mm nasal, temporal, superior, and inferior to the fovea (Figure 1).16, 17 All the images were taken by two doctors independently for three times to get the average, at the same time point between 0800 and 0000 hours before and after surgery in our routine clinics to avoid possible diurnal variation in the CT. When one eye was examined by OCT, preoperative patients were asked to stare at the green light (target) attached to the OCT device in order to align the test eye for the fovea and control accommodation. The distance of target was adjusted with reference to the patients' refraction. Postoperative patients were asked to look straight at another target at 5 m (infinity theoretically) to control accommodation.
Figure 1.
Choroidal measurements in myopic eyes by SD-OCT. Numbers are in μm units. (a) Five lines scanning at horizontal azimuth by SD-OCT with EDI. (b) Preoperative. (c) Postoperative. (d) Five lines scanning at vertical azimuth by SD-OCT with EDI. (e) Preoperative. (f) Postoperative. The OCT images for our study were selected in the location of the red line.
Data analysis
All the results were expressed as the mean±SD. A paired Student's t-test was used to compare differences in CT, IOP, AA preoperatively and postoperatively. We compared basic information (age, sex, refraction error), corneal thickness, axial length, and surgical parameters (ablating depth and residual thickness of stromal bed), preoperative and postoperative IOP, AA, the change of IOP (ΔIOP) (ΔIOP=IOP before surgery-IOP after surgery), the change of AA(ΔAA=AA before surgery-AA after surgery) with stepwise linear regression to determine the most significant factors associated with the change in CT (ΔCT; ΔCT=CT after surgery-CT before surgery) at the fovea.
The correlation between ΔCT and the most significant factor were assessed with correlation analysis. Statistical evaluation was performed using SPSS software 19.0 for (IBM, Armonk, NY, USA). A value of P<0.05 was considered statistically significant.
Results
Group demographics and all clinical parameters are shown in Table 1.
Table 1. Demographic and clinical information.
| Male (n) | 46 |
| Female (n) | 24 |
| Age (years) | 23.8±4 |
| Equivalent power preoperatively (D) | −4.9±0.5 |
| Equivalent power postoperatively (D) | 0.12±0.2 |
| Axial length preoperatively (mm) | 24.6±1.4 |
| Axial length postoperatively (mm) | 24.4±1.2 |
| Amplitude of accommodation preoperatively (D) | 11.2±0.9 |
| Amplitude of accommodation postoperatively (D) | 9.4±1.2 |
| IOP preoperatively (mm Hg) | 16.4± 2.6 |
| IOP postoperatively (mm Hg) | 11.3±3.0 |
| IOP preoperatively corrected (mm Hg) | 18.1±2.4 |
| IOP postoperatively corrected (mm Hg) | 19.2±4.1 |
| Ablating depth (μm) | 85.6±43.2 |
| Residual thickness of stromal bed (μm) | 428.7±83.4 |
| Corneal thickness preoperatively (μm) | 553.8±33.7 |
| Corneal thickness postoperatively (μm) | 468.1±50.5 |
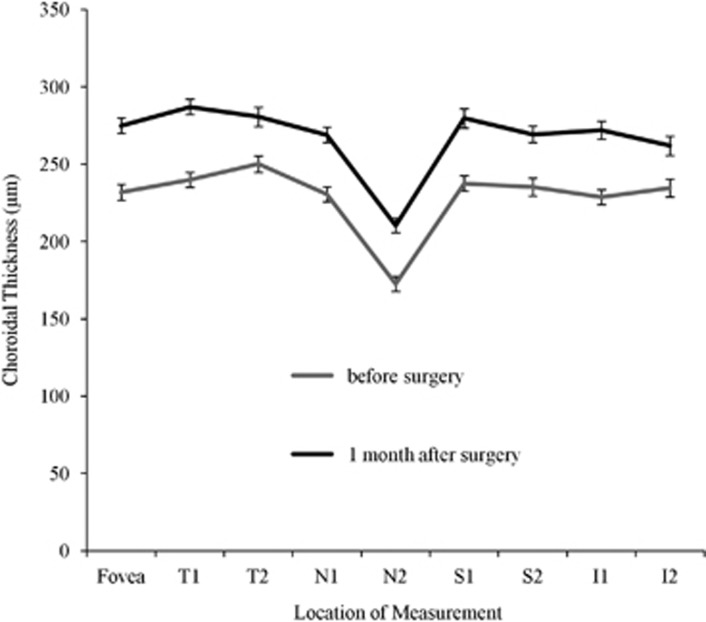
The CT at the fovea and distances of 0.5 and 2.5 mm nasal, temporal, superior, and inferior to the fovea 1 month after surgery was significantly thicker compared with the CT before surgery (P<0.05, Figure 2).
Figure 2.
Choroidal thickness measured by SD-OCT before surgery and 1 month after surgery. T1 is the location 0.5 mm temporal to the fovea. T2 is the location 2.5 mm temporal to the fovea. N1 is the location 0.5 mm nasal to the fovea. N2 is the location 2.5 mm nasal to the fovea. S1 is the location 0.5 mm superior to the fovea. S2 is the location 2.5 mm superior to the fovea. I1 is the location 0.5 mm inferior to the fovea. I2 is the location 2.5 mm inferior to the fovea.
AA significantly decreased after surgery (P<0.05). Stepwise linear regression indicated that ΔAA was the most significant factors associated with ΔCT at the fovea. The formula used was: subfoveal ΔCT (μm)=9.593+16.516 × ΔAA (D) (F=106.736, P<0.05).
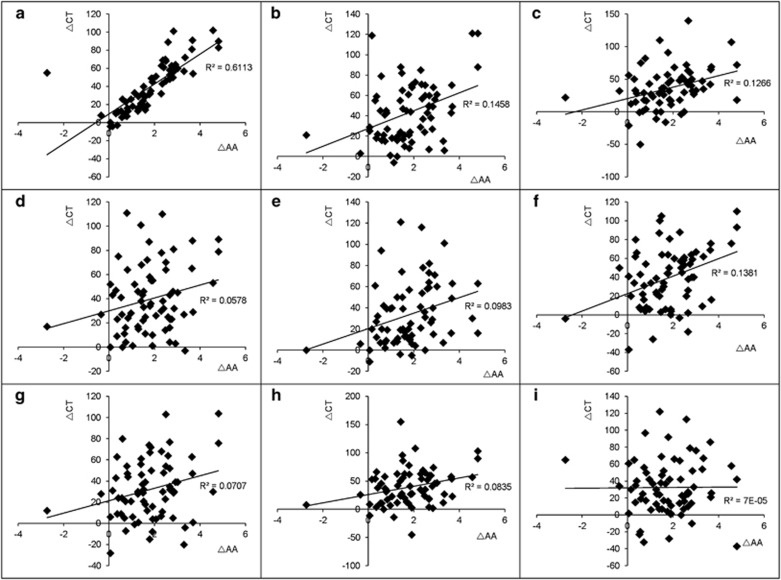
Correlation analysis showed that except 2.5 mm inferior to the fovea, the increasing in the CT at the other points examined were significantly positively associated with the decreasing of AA (Table 2, Figure 3).
Table 2. Correlation between ΔCT and ΔAA.
| Measurement of location distance to the fovea | ΔAAr (correlation coefficient) | P-value |
|---|---|---|
| 0 mm (fovea) | 0.782 | 0.000** |
| 0.5 mm temporal | 0.382 | 0.001** |
| 2.5 mm temporal | 0.356 | 0.002** |
| 0.5 mm nasal | 0.24 | 0.045* |
| 2.5 mm nasal | 0.313 | 0.008** |
| 0.5 mm superior | 0.372 | 0.002** |
| 2.5 mm superior | 0.266 | 0.026* |
| 0.5 mm inferior | 0.289 | 0.015* |
| 2.5 mm inferior | 0.061 | 0.617 |
The number of patients was 70 for all locations.
**P<0.01, *P<0.05.
Figure 3.
Correlation between ΔCT (μm) and ΔAA(D). (a) The fovea. (b) The location 0.5 mm temporal to the fovea. (c) The location 2.5 mm temporal to the fovea. (d) The location 0.5 mm nasal to the fovea. (e) The location 2.5 mm nasal to the fovea. (f) The location 0.5 mm superior to the fovea. (g) The location 2.5 mm superior to the fovea. (h) The location 0.5 mm inferior to the fovea. (i) The location 2.5 mm inferior to the fovea. ΔAA=AA before surgery−AA after surgery, ΔCT=CT after surgery−CT before surgery.
Discussion
The incidence of myopia is increasing, whereas the age of onset is gradually decreasing.1 Recently, the focus of refractive error research has shifted to the posterior segment, especially the CT, which is an important initial factor in pathologic myopia.6 A significant thinning in CT related with myopia was observed and CT in myopia had negative correlation with refractive power, axial length, and age, which was mentioned in previous studies (Table 3).5, 6, 16, 18, 19
Table 3. Choroidal thickness in myopia studies.
| Authors | Mean age (years) | Sample size | Refractive error (D) | Relationship with myopia |
|---|---|---|---|---|
| El Matri et al5 | 47.21±14.24 | 187 eyes | −13.66±5.77 | Choroid thinning in pathogenesis of choroidal neovascularization |
| Fujiwara et al6 | 59.7±17.6 | 55 eyes | −11.9±3.7 | Choroid thinning in the pathogenesis of myopic degeneration |
| Flores-Moreno et al16 | 54.4±18.2 | 120 eyes | ≥−6 | Axial length is associated with choroidal thickness in high myopia |
| Ho et al18 | 50.4±2.03 | 56 patients | −6.1 to −11 | Choroidal thickness decreases with age and severity of myopia |
| Gupta et al19 | 21.63±1.15 | 448 subjects | −8.52±1.20 | Highly myopic eyes have significantly thinner peripapillary choroid |
Excimer laser refractive surgery is a common option for correcting myopia. Our study, first reported that the choroid becomes thicker following myopic refractive surgery. Previous researches suggested that choroidal thinning is critical to the onset and progression of such choroidal diseases, such as polypoidal choroidal vasculopathy4 and central serous chorioretinopathy.20 On the basis of the findings of the present study, the CT showed a tendency to become thicker after correcting the refractive error. This may reduce the incidence of some choroidal diseases and improve patients' visual function to a certain extent.
Ciliary body, ciliary muscle, and choroid are part of the accommodative apparatus of the eye. Ciliary muscle has been found to insert into regions of the anterior choroid.21 Accommodation occurs as the ciliary muscle and ciliary body move forward and inward. As reported previously, the force involved in the contraction of the ciliary muscle may be transmitted to the choroid, and this mechanical force can affect the CT.22 Previous studies confirmed that in the absence of a clear retinal image, ocular accommodation can be stimulated,22, 23 and that the force of ciliary muscle contraction applied an inward and forward pulling force, which resulted in thinning of the choroid.
Accommodation decreased early after myopic refractive surgery in our findings. We postulated that there may be two major reasons as followings: (1) poor accommodative facility and slow accommodative responsiveness were existed in myopic people with accommodative lag,24, 25 which would result in low accommodation reserve of myopia patients. (2) Accommodation demand was increased at the early postoperative period because of post apex moved backward to cornea and overcorrection early postoperatively.26 In our study, the decline in the AA had a significant positive correlation with the increase in the CT. Previous studies speculated that in the absence of a clear retinal image, ocular accommodation was stimulated. Meanwhile myopia would reduce the accommodative facility and extend the time of accommodative recovery. The accumulative effects of accommodation eventually cause the structural elongation of the eyeball in the myopic eye.22, 23, 24 Our study showed a decrease in AA postoperatively, and CT increased as AA reducing after surgery. We proposed that after correcting the refractive error, retinal images become clearer. This reduces the accommodative stimulation, which lessens axial extension, thereby relieving the load on the AA. Ultimately, the choroid becomes thicker following the decrease in the tension on the ciliary muscle and the stretching of the eyeball attached to the choroid. Besides that, we also found that there was no correlation between AA and CT at 2.5 mm inferior to the fovea. Accommodation was mainly regulated by the ciliary muscle and ligament of lens. The ciliary muscle moves forward and inward, allowing the lens equator to move away from the sclera for stretching the choroid,27 so we speculated during accommodation process, the ciliary muscle mainly stretched horizontally, which might make partition effect in the various locations at the fovea.
Previous research demonstrated that in the process of myopic optical defocus, a cascade system might be built by neurotransmitters from the retinal pigment epithelium to melanocytes in the choroid, which might induce a change in the choroid.28, 29 We speculated that structural changes in the choroid may be explained by visual information being transferred through neurotransmitters from the retina to the choroid following visual recovery after surgery. Moreover, choroid contains numerous larger blood vessels.30 Vascular permeability might have a role in change of the choroidal thickness. The protein content moved into the extracellular matrix and/or lymphatics followed by increasing vascular permeability causing a increased choroidal fluid, which will result in the choroid thicken by the restoration of vision in form deprivation myopia.31, 32 We speculate that after surgical correction of refractive errors, vascular permeability increased with the information of recovering the vision transferred from the retina, and then a large volume of liquid might move to the choroid to improve the choroidal blood flow causing reversing the increase in the CT.
As with most studies, our finding needs to be considered in light of our study limitations. First, our follow-up time was only 1 month after surgery, and further studies with a long follow-up period would be needed. Second, manual measurements were made because there is no automated software commercially available to quantify choroidal thickness. Third, there is no built-in system to correct for magnification with OCT devices in general. In the OCT system of our study, the ‘auto-all focus' feature and precise positioning for optical correction was used to minimize the magnification effects from refractory error.33 Patients in our study belonged to low and middle degree of myopia, as an alternative approach to minimize potential errors from image magnification.34 Eye magnification was corrected using Bennett's formula (axial length-corrected measurements) in previous studies.35 In our study, there was no significant difference in axial length before and after surgery and we speculated that there was no significant magnification effect in CT of the same individual before and after surgery.
In summary, our study showed that a thin choroid due to myopia became thicker following surgical correction of a myopic refractive error. To some extent, choroidal thickening might reduce the incidence of some choroidal diseases. CT increased more when accompanied by a decrease in the AA early after the surgery. Further study will enlarge follow-up time to focus on changes in accommodation with subjective and objective methods and ocular structure changes by synchronized SD-OCT system, for better understanding of the mechanisms associated with this phenomenon.
Acknowledgments
The National Natural Science Foundation of China (Grant No.81200713); the Hospital Training Plan of Medical Talent in 2012 (Grant No.12RC06).
The authors declare no conflict of interest.
References
- Williams KM, Hysi PG, Nag A, Yonova-Doing E, Venturini C, Hammond CJ. Age of myopia onset in a British population-based twin cohort. Ophthalmic Physiol Opt 2013; 33(3): 339–345. [DOI] [PubMed] [Google Scholar]
- Summers JA. The choroid as a sclera growth regulator. Exp Eye Res 2013; 114: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus HE, Green WR. Choroidal neovascularization. Am J Ophthalmol 2004; 137(3): 496–503. [DOI] [PubMed] [Google Scholar]
- Gomi F, Tano Y. Polypoidal choroidal vasculopathy and treatments. Curr Opin Ophthalmol 2008; 19(3): 208–212. [DOI] [PubMed] [Google Scholar]
- El Matri L, Bouladi M, Chebil A, Kort F, Bouraoui R, Largueche L et al. Choroidal thickness measurement in highly myopic eyes using SD-OCT. Ophthalmic Surg Lasers Imaging 2012; 43(6 Suppl): S38–S43. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol 2009; 148(3): 445–450. [DOI] [PubMed] [Google Scholar]
- Ikuno Y, Tano Y. Retinal and choroidal biometry in highly myopic eyes with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 2009; 50(8): 3876–3880. [DOI] [PubMed] [Google Scholar]
- Tomita M, Watabe M, Yukawa S, Nakamura N, Nakamura T, Magnago T. Safety efficacy, and predictability of laser in situ keratomileusis to correct myopia or myopic astigmatism with a 750Hz scanning-spot laser system. J Cataract Refract Surg 2014; 40 M(2):251–258. [DOI] [PubMed] [Google Scholar]
- Gyldenkerne A, Ivarsen A, Hjortdal JO. Comparison of corneal shape changes and aberrations induced By FS-LASIK and SMILE for myopia. J Refract Surg 2015; 31(4): 223–229. [DOI] [PubMed] [Google Scholar]
- Gao S, Li S, Liu L, Wang Y, Ding H, Li L et al. Early changes in ocular surface and tear inflammatory mediators after small-incision lenticule extraction and femtosecond laser-assisted laser in situ keratomileusis. PLoS ONE 2014; 9(9): e107370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald ME, Wildsoet CF, Reiner A. Temporal relationship of choroidal blood flow and thickness changes during recovery from form deprivation myopia in chicks. Exp Eye Res 2002; 74(5): 561–570. [DOI] [PubMed] [Google Scholar]
- Rada JA, Palmer L. Choroidal regulation of scleral glycosaminoglycan synthesis during recovery from induced myopia. Invest ophthalmol vis sci 2007; 48(7): 2957–2966. [DOI] [PubMed] [Google Scholar]
- Swarbrick HA, Alharbi A, Watt K, Lum E, Kang P. Myopia control during orthokeratology lens wear in children using a novel study design. Ophthalmology 2015; 122(3): 620–630. [DOI] [PubMed] [Google Scholar]
- Stodtmeister R. Applanation tonometry and correction according to corneal thickness. Acta Ophthalmol Scand 1998; 76(3): 319–324. [DOI] [PubMed] [Google Scholar]
- Scerrati E. Laser in situ keratomileusis vs. laser epithelial keratomileusis (LASIK vs. LASEK). J Refract Surg 2001; 17(2 Suppl): S219–S221. [DOI] [PubMed] [Google Scholar]
- Flores-Moreno I, Lugo F, Duker JS, Ruiz-Moreno JM. The relationship between axial length and choroidal thickness in eyes with high myopia. Am J Ophthalmol 2013; 155(2): 314–319 e311. [DOI] [PubMed] [Google Scholar]
- Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol 2010; 150(3): 325–329 e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M, Liu DT, Chan VC, Lam DS. Choroidal thickness measurement in myopic eyes by enhanced depth optical coherence tomography. Ophthalmology 2013; 120(9): 1909–1914. [DOI] [PubMed] [Google Scholar]
- Gupta P, Cheung CY, Saw SM, Bhargava M, Tan CS, Tan M et al. Peripapillary choroidal thickness in young Asians with high myopia. Invest Ophthalmol Vis Sci 2015; 56(3): 1475–1481. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Hall L, Haas A, Campeas L, Yannuzzi LA, Fisher YL et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina 1996; 16(3): 203–213. [DOI] [PubMed] [Google Scholar]
- Jeon S, Lee WK, Lee K, Moon NJ. Diminished ciliary muscle movement on accommodation in myopia. Exp Eye Res 2012; 105: 9–14. [DOI] [PubMed] [Google Scholar]
- Woodman EC, Read SA, Collins MJ. Axial length and choroidal thickness changes accompanying prolonged accommodation in myopes and emmetropes. Vis res 2012; 72: 34–41. [DOI] [PubMed] [Google Scholar]
- Gao L, Zhuo X, Kwok AK, Yu N, Ma L, Wang J. The change in ocular refractive components after cycloplegia in children. Jpn J Ophthalmol 2002; 46(3): 293–298. [DOI] [PubMed] [Google Scholar]
- Pandian A, Sankaridurg PR, Naduvilath T, O'Leary D, Sweeney DF, Rose K et al. Accommodative facility in eyes with and without myopia. Invest Ophthalmol Vis Sci 2006; 47(11): 4725–4731. [DOI] [PubMed] [Google Scholar]
- Sreenivasan V, Aslakson E, Kornaus A, Thibos LN. Retinal image quality during accommodation in adult myopic eyes. Optom vis sci 2013; 90(11): 1292–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AG, Kohnen T, Ebner R, Bennett JL, Miller NR, Carlow TJ et al. Optic neuropathy associated with laser in situ keratomileusis. J Cataract Refract Surg 2000; 26(11): 1581–1584. [DOI] [PubMed] [Google Scholar]
- Croft MA, Glasser A, Heatley G, McDonald J, Ebbert T, Dahl DB et al. Accommodative ciliary body and lens function in rhesus monkeys, I: normal lens, zonule and ciliary process configuration in the iridectomized eye. Invest Ophthalmol Vis Sci 2006; 47(3): 1076–1086. [DOI] [PubMed] [Google Scholar]
- Seko Y, Tanaka Y, Tokoro T. Apomorphine inhibits the growth-stimulating effect of retinal pigment epithelium on scleral cells in vitro. Cell Biochem Funct 1997; 15(3): 191–196. [DOI] [PubMed] [Google Scholar]
- Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA. Local retinal regions control local eye growth and myopia. Science 1987; 237(4810): 73–77. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Wallman J. The multifunctional choroid. Progress Retin Eye Res 2010; 29(2): 144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendrak K, Papastergiou GI, Lin T, Laties AM, Stone RA. Choroidal vascular permeability in visually regulated eye growth. Exp Eye Res 2000; 70(5): 629–637. [DOI] [PubMed] [Google Scholar]
- Junghans BM, Crewther SG, Liang H, Crewther DP. A role for choroidal lymphatics during recovery from form deprivation myopia? Optom Vis Sci 1999; 76(11): 796–803. [DOI] [PubMed] [Google Scholar]
- Harb E, Hyman L, Gwiazda J, Marsh-Tootle W, Zhang Q, Hou W et al. Choroidal thickness profiles in myopic eyes of young adults in the Correction of Myopia Evaluation Trial Cohort. Am J Ophthalmol 2015; 160(1): 62–71 e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ctori I, Gruppetta S, Huntjens B. The effects of ocular magnification on Spectralis spectral domain optical coherence tomography scan length. Graefes Arch Clin Exp Ophthalmol 2015; 253(5): 733–738. [DOI] [PubMed] [Google Scholar]
- Moghimi S, Hosseini H, Riddle J, Lee GY, Bitrian E, Giaconi J et al. Measurement of optic disc size and rim area with spectral-domain OCT and scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci 2012; 53(8): 4519–4530. [DOI] [PubMed] [Google Scholar]