Abstract
Exosomes are extracellular nanovesicles. They innately possess ideal structural and biocompatible nanocarrier properties. Exosome components can be engineered at the cellular level. Alternatively, when exosome source cells are unavailable for customized exosome production, exosomes derived from a variety of biological origins can be modified post isolation which is the focus of this article. Modification of exosome surface structures allows for exosome imaging and tracking in vivo. Exosome membranes can be loaded with hydrophobic therapeutics to increase drug stability and efficacy. Hydrophilic therapeutics such as RNA can be encapsulated in exosomes to improve cellular delivery. Despite advances in post isolation exosome modification strategies, many challenges to effectively harnessing their therapeutic potential remain. Future topics of exploration include: matching exosome subtypes with nanomedicine applications, optimizing exosomal nanocarrier formulation and investigating how modified exosomes interface with the immune system. Research into these areas will greatly facilitate personalized exosome-based nanomedicine endeavors.
Keywords: : biocompatible, exosomes, fluorescent, imaging, immunotolerant, MRI, nanocarrier, nanomedicine, nanovesicles, therapeutics
Exosomes are cell-derived nanovesicles. They relay information between tissue microenvironments [1] and can influence target cell function and differentiation [2]. They contain and protect valuable mRNA and miRNA information which corresponds to source cell normal or pathogenic processes.
The use of exosomes as nanocarriers provides a number of advantages over many artificial nanovesicles. For one, exosomes naturally carry RNA, lipid, protein and metabolite cargo [1,3–5] which enables their use as drug delivery vehicles. Additionally, similar to cells, exosomes contain a deformable cytoskeleton and ‘gel-like’ cytoplasm-derived core [1]. Conceivably, these biophysical properties enhance exosome structural integrity and resistance to rupture during trafficking in vivo. This may explain in part the resistance of exosomes to osmotic lysis during hypotonic dialysis [6,7].
Exosome deformability should further facilitate exosome uptake into tissue microenvironments such as tumors via the enhanced permeability and retention effect. The average gap distance between endothelial cells in fenestrated tumor vasculature is ˜400 nm [8]. In general, nanocarriers with diameters less than 400 nm can be passively taken up by tumors. Exosomes can function essentially as nanoscale erythrocytes engaged in nanoscale diapedesis. Exosomes being <400 nm in diameter can easily traverse fenestrated capillaries to access tissue sites [1].
The near neutral, slightly negative zeta potential exhibited by exosomes [9–11] is ideal for longer circulation in vivo as demonstrated in liposomal studies [12]. Liposomes with positive zeta potentials can aggregate with negatively charged circulatory proteins reducing their circulation time [12] and access to target sites.
Additional privileged access to tunneling nanotube conduits may further allow exosomes to deliver cargo deep within tissue microenvironments [1,13]. Tunneling nanotubes can transport vesicles between cells and could provide a means for exosomal nanocarriers to uniquely move cargo up concentration gradients which is a difficult feat for synthetic nanocarriers.
Exosomes also exhibit biocompatibility properties such as immune tolerance [1]. This allows exosomes to be essentially ignored by the immune system. They can avoid adaptive immunity-mediated clearance obstacles associated with the use of synthetic nanoparticles in vivo [1]. They also express a multitude of complex targeting ligands including integrins, tetraspanins and receptors in native conformations. This includes coreceptors required for appropriate signaling in vivo [1]. Attempting to duplicate the breadth, complexity, organization and natural conformation of functional surface molecules expressed on exosomes is not currently feasible for synthetic nanovesicles from a technical or economic standpoint. Collectively, the natural properties of exosomes make them ideally suited to serve as biologic nanocarriers.
Developing means to modify exosomes to serve as nanocarriers facilitates exosome-based nanomedicine. Exosomes might be modified to serve as nanocarriers either endogenously at the cellular level or exogenously following cell culture production [1], collection from body fluids or even plant-based materials [14,15]. Endogenous exosome modification typically relies on molecular biology approaches to manipulate exosome components such as proteins at the source cell production level. Such technologies are useful for understanding exosome cell biology, function and biomarker packaging. They may also one day provide for personalized cellular exosomal nanofactories to treat disease [1]. However, exogenous exosome post isolation strategies are also required to provide knowledge as to how to interact with exosomes on the nanoscale. This is important for determining the extent to which the content and function of exosomes obtained from various biological origins can be manipulated when exosome source cells are unavailable for customized exosome production. This includes understanding how the structure and content of exosomes might be modified with artificial components to enable novel exosome-based diagnostic and therapeutic applications. For example, testing exogenous modifications to exosomes can provide information on how exosomes traffic, target and interface with specific tissue microenvironments in vivo. Such experimentation also enables the implementation of future therapeutics that have been designed with consideration to the existence of exosome trafficking and function in vivo. It further contributes to new biology inspired design strategies for synthetic nanovehicles.
Structurally, an exosome is composed of a lipid membrane bilayer expressing surface ligands and receptors [1]. The exosome lipid membrane surrounds and contains a hydrophilic core. Within the core, RNA, proteins and other source cell-derived components are found. In general, the exosomal structure provides for three exogenous modification strategies. Exosome surface molecules can be adapted to enable exosome imaging or specific targeting. Hydrophobic therapeutics can be loaded into exosome membranes. Hydrophilic drugs or therapeutic cargo can be loaded into the exosome core. Exogenous modification of one or more exosome structural components facilitates the use of exosomes for nanomedicine purposes. In the following sections, these exogenous modification strategies will be discussed. The intent is not to provide an extensive review of the literature. The purpose is to highlight examples of different post isolation modification strategies (Table 1). This includes some examples of post isolation modifications to pre-existing engineered exosome components. Future considerations for enhancing their utility will also be explored.
Table 1. . Examples of post isolation modifications to exosomes for nanomedicine purposes.
| Exosome type | Modification | Strategy | Purpose | Ref. |
|---|---|---|---|---|
| Human embryonic kidney 293T |
Engineered structure modification |
Attach fluorescent Alex Fluor® 680-Streptavidin to luciferase-biotin surface fusion protein |
Enable dual bioluminescent and fluorescent imaging |
[16] |
| Mouse 4T1 breast cancer |
Natural structure modification |
Conjugate surface protein amine groups to Azide-Fluor 545 using ‘click chemistry’ |
Enable fluorescent imaging of exosomes |
[17] |
| Mouse MSC |
Natural structure repurposing |
Transfer bioactive PD-L1 to autoreactive T cells |
Inhibit autoreactive T cells in an EAE mouse model |
[18] |
| Mouse N2a neuroblastoma |
Natural structure repurposing |
Transfer aβ aggregates on glycosaminoglycans to brain microglial cells for removal |
Evaluate a new therapeutic approach for Alzheimer's disease using a mouse model |
[19] |
| Mouse EL-4 T-cell lymphoma |
Passive loading of hydrophobic cargo |
Load anti-inflammatory curcumin into exosome membranes |
Increase curcumin stability and efficacy in a septic shock model |
[20] |
| Mouse EL-4 T-cell lymphoma |
Passive loading of hydrophobic cargo |
Load anti-inflammatory curcumin or JSI-124 into exosome membranes |
Test intranasal therapy for inflammatory brain conditions in mouse models |
[21] |
| Mouse DC |
Electroporation of hydrophilic cargo |
Load siRNA to BACE1 into exosomes expressing RVG-Lamp2b which binds to ACh receptors |
Deliver siRNA to BACE1, an Alzheimer's disease target, across the blood–brain barrier in a mouse model |
[22] |
| Mouse DC |
Electroporation of hydrophilic cargo |
Load siRNA to α-synuclein into exosomes expressing RVG-Lamp2b which binds ACh receptors |
Deliver siRNA to α-synuclein, a Parkinson's disease target, across the blood–brain barrier in a mouse model |
[23] |
| Human plasma exosomes |
Electroporation of hydrophilic cargo |
Load siRNA to MAPK-1 into exosomes |
Suppress MAPK-1 in monocytes and lymphocytes |
[24] |
| Mouse M12.4 B lymphocyte |
Electroporation of hydrophilic cargo |
Load miRNA-155 inhibitor into exosomes |
Suppress LPS-induced TNF-α production in macrophages |
[25] |
| Human MDA-MB231, HUVEC, hMSC, hESC |
Electroporation of hydrophilic cargo |
Load porphyrin model drugs into different types of exosomes |
Test encapsulation efficiency of porphyrins based on hydrophobicity and loading method |
[7] |
| Mouse immature DC |
Electroporation of hydrophilic cargo |
Load doxorubicin into Lamp2b-αV-integrin-specific iRGD peptide expressing exosomes |
Target and treat αV-integrin expressing breast tumors in mice |
[26] |
| Human MDA-MB231 breast cancer, HCT-116 colon cancer |
Electroporation of hydrophilic cargo |
Load doxorubicin into exosomes |
Improve doxorubicin efficacy and reduce cardiotoxicity in a breast cancer model |
[27] |
| Mouse aortic primary endothelial cell |
Electroporation of hydrophilic cargo |
Load siRNA to luciferase into exosomes |
Determine autocrine uptake efficiency of endothelial exosomes by endothelial cells |
[28] |
| Mouse B16-F10 melanoma |
Electroporation of hydrophilic cargo |
Load 5 nm superparamagnetic iron oxide nanoparticles (SPION5) into exosomes using trehalose pulse media |
Develop a trehalose pulse media to maximize exosome colloidal stability during cargo loading via EP |
[11] |
| Mouse B16-F10 melanoma | Electroporation of hydrophilic cargo | Load theranostic SPION5 cargo into exosomes | Track exosome homing to lymph nodes in mice using MRI | [29] |
DC: Dendritic cell; EAE: Experimental autoimmune encephalitis; LPS: Lipopolysaccharide; MSC: Mesenchymal stem cell.
Adapting exosome surface structures
Modification of exosome surface macromolecules for imaging & tracking
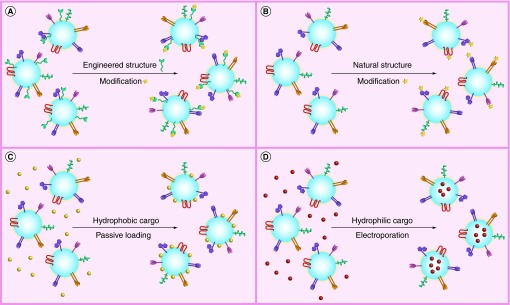
Currently, a number of reports demonstrating post isolation strategies to modify exosome surface structures have described means to more effectively track exosomes in vivo. One example of this approach, relying on modification of engineered exosomal components (Figure 1A), was recently described by Lai et al. [16]. Researchers’ engineered human embryonic kidney 293T exosomes expressing a membrane-bound Gaussian luciferase fused to a biotin receptor domain. Following isolation of the engineered exosomes, researchers complexed the biotin expressed on the exosomes with fluorescent Alex Fluor® 680-Streptavidin. This allowed the exosomes to be tracked in vivo either by bioluminescence or fluorescence. Dual labeling increased spatial and temporal imaging resolution of exosome circulatory half-life as well as enabled tracking their delivery to tumor sites in vivo.
Figure 1. . Post isolation modification of exosomes for nanomedicine applications.
(A) Engineered exosome membrane fusion proteins can be further modified post exosome isolation. One example is the expression of membrane-bound Gaussian luciferase fused to a biotin receptor domain [16]. This allows exosomes to be tracked in vivo either by bioluminescence or fluorescence following biotin complex formation with Alex Fluor® 680-Streptavidin. (B) Modification of natural exosome surface proteins using ‘click chemistry’ can also be used to track exosomes using fluorescent imaging [17]. (C) Exosome membranes might also be passively loaded with lipophilic fluorescent carbocyanine dyes [9] for imaging and tracking purposes and/or hydrophobic drug cargo such as curcumin [20]. (D) Electroporation can be used to load exosomes internally with hydrophilic cargo such as siRNA [22] or even theranostic superparamagnetic iron oxide cargo [29].
Alternatively, rather than using molecular cell biology approaches to enable downstream modifications of exosome surface macromolecules, ‘click chemistry’ can be used to directly attach molecules to the surface of exosomes via covalent bonds (Figure 1B). Click chemistry is defined as an azide-alkyne cycloaddition catalyzed by copper [30]. An alkyne chemical group and an azide chemical group react to form a triazole linkage [17]. The reaction is quick and efficient compared with traditional cross-linking reactions such as maleimide–thiol coupling and provides better control over the conjugation site [30]. It is also suitable for modification of biological macromolecules since it can occur in aqueous media [17].
Recently, Smyth et al. attached fluorescent molecules to the surface of mouse 4T1 breast cancer exosomes using click chemistry [17]. Exosome surface protein amine groups were functionalized with terminal alkynes followed by conjugation to Azide-Fluor 545. The resulting formulation of 1.5 alkyne groups per 150 kDa of exosome protein was found to have no significant effect on cellular uptake of exosomes or exosome size. Control exosomes labeled with fluorescent DiD lipophilic tracer and azid-fluor 545 conjugated 4T1 exosomes were 120 ± 54 and 128 ± 60 nm, respectively. These minimal alterations to exosome size with surface modification would be expected to ensure retention of naturally efficacious exosome trafficking properties.
Utilization of exosome surface molecules for therapeutic applications
Pre-existing exosome surface receptors might also be adapted for use in specific therapeutic applications. For example, Mokarizadeh et al. demonstrated that mesenchymal stem cell (MSC) exosomes could transmit membrane receptors and ligands to attenuate the function of autoreactive CD4 lymphocytes isolated from experimental autoimmune encephalitis (EAE) mice [18]. Programmed death ligand 1 (PD-L1), TFG- β and galectin-1 were transferred to T cells as demonstrated by flow cytometry. Bioactivity of the molecules inserted into T-cell membranes was confirmed by ˜50% decrease in IL-17 and IFN- γ secretion by the lymphocytes post-MSC exosome treatment.
Another direct use of pre-existing exosome surface components is to ameliorate the build-up of amyloid-β (aβ) in the brain which is known to drive the pathogenesis of Alzheimer's disease. Yuyama et al. repeatedly injected neuroblastoma (NB) exosomes into the brains of transgenic aβ expressing mice [19]. The aβ aggregates bound to glycosphingolipid glycan groups located on the surface of NB exosomes. The NB exosomes shuttled aβ aggregates to brain microglial immune cells. The microglial cells removed the NB exosomes and the exosome bound aβ aggregates. Use of exosomes to facilitate removal of aβ aggregates could become an effective approach for treating Alzheimer's disease.
Combined, these strategies for repurposing exosome surface receptors further highlight a unique advantage of exosome-based nanotherapeutics over synthetics in that exosomes innately possess natural functional receptors. Functional exosome receptors can potentially be transmitted to target cell membranes or can enable efficient exosome capture of ligands and addressing to appropriate target cells for processing. This further suggest that receptor signal transduction machinery if transmitted by the exosomes to target cells is also compatible with target cells. Such complex macromolecular machinery would be difficult to efficiently design or duplicate using existing synthetic nanomedicine approaches.
Loading exosome membranes with hydrophobic cargo
The lipid membrane bilayer of exosomes can be used for passively loading hydrophobic cargo (Figure 1C). A good example of this approach is the use of exosomes to carry the hydrophobic molecule curcumin. Curcumin is a natural polyphenol derived from Curcuma longa (Turmeric). It possesses powerful therapeutic attributes including antineoplastic, anti-inflammatory and antioxidant properties [20]. However, because it is relatively unstable and highly hydrophobic, making it poorly soluble in aqueous buffers, it has been difficult to harness its therapeutic potential for clinical purposes. To overcome curcumin stability and hydrophobic limitations, Sun et al. developed curcumin-loaded mouse lymphoma EL-4 exosomes [20]. Loading was achieved by mixing exosomes with curcumin in phosphate-buffered saline followed by isolation using sucrose density gradient centrifugation. EL-4 exosome loading increased thermal stability of curcumin and circulatory half-life in mice in vivo. Intraperitoneal administration of curcumin EL-4 exosomes in mice provided protective anti-inflammatory activity in a lipopolysaccharide (LPS)-mediated septic shock mouse model. Curcumin-loaded EL-4 exosome anti-inflammatory effects were superior to curcumin-loaded liposome controls. It follows that the presence of innate exosome macromolecules facilitated curcumin efficacy. Further, this study demonstrates the importance of selecting the appropriate exosome subtype for the nanomedicine application. Based on the previous example, some exosome subtypes may possess innate components or trafficking properties that enhance the efficacy of the therapeutic cargo.
Following the success of the original curcumin-loaded exosome concept the researchers proceeded to test the therapeutic approach to treat three inflammatory brain conditions including myelin oligodendrocyte glycoprotein peptide induce EAE, LPS-induced brain inflammation and GL26 brain tumors in mice [21]. The researchers tested intranasal administration of exosomes loaded with curcumin or another hydrophobic compound JSI-124 (cucurbitacin I). JSI-124 is an inhibitor of signal transducer and activator of transcription 3 (STAT3). Both curcumin and JSI-124 were loaded with mixing. Administration of both exosome variants resulted in protection from LPS-induced brain inflammation as well as reduced EAE progression and GL26 tumor growth. The results supported a mechanism of therapeutic efficacy whereby selective delivery of the exosomal cargo to brain microglial cells resulted in decreased inflammation through increased microglial immune cell apoptosis. Interestingly, the exact route of exosome trafficking to the brain was not discovered. The authors speculated based on the rapid 1 h brain delivery time that the exosomes likely traveled along the olfactory pathway. The mechanism of action may be bulk flow transport within perivascular or perineuronal channels. These studies serve to highlight the promising potential for exosomal nanocarriers to uniquely access tissue microenvironments previously inaccessible to artificial nanocarriers.
Encapsulating cargo in exosomes
RNA cargo
A large number of exogenous exosome modification strategies have focused on exosome mediated siRNA delivery. This is largely based on the natural ability of exosomes to relay mRNA and miRNA content between cells [31]. Exogenous loading of siRNA into exosomes has predominately relied on electroporation (EP) approaches to introduce transient pores into exosome membranes (Figure 1D). A first example of the EP approach was reported by Alvarez-Erviti et al. whereby dendritic cell exosomes were cleverly engineered to express the exosomal Lamp2b protein fused to the neuron-specific rabies viral glycoprotein (RVG) peptide [22]. Exosomal surface expression of RVG allows the exosomes to specifically target the acetylcholine receptor. The authors demonstrated that electroporetic loading of the exosomes with siRNA to BACE1, an Alzheimer's disease target, reduced BACE1 mRNA and protein expression in brain cortical tissues after systemic exosome administration. The study highlights the potential use of exosomal nanocarriers to naturally deliver therapeutics across the blood–brain barrier. This has not been previously achievable with the use of other systemically administered macromolecular therapeutics.
In a similar study, Cooper et al. loaded exosomes with α-synuclein siRNA [23]. Accumulation of α-synuclein aggregates in the brain is a pathological finding in Parkinson's disease. Intravenous administration of the α-synuclein siRNA-loaded exosomes expressing RVG protein on their surface resulted in brain-specific uptake. A week after injection, the therapeutic exosomes were found to decrease protein aggregates in the brain including those associated with dopaminergic neurons of the substantia nigra which participate in Parkinson's disease pathogenesis. This study further confirms the utility of exosomes as nanocarriers for moving cargo across the blood–brain barrier.
Other applications of exosome-based siRNA nanocarriers have also been described. Wahlgren et al. compared the efficiency of two methods for loading plasma-derived exosomes with siRNA to MAPK-1 [24]. The two loading methods included chemical transfection using HiPerFect commercial transfection reagent and EP. The chemical transfection approach resulted in the generation of contaminating MAPK-1 siRNA containing micelles that formed inseparable complexes with the exosomes. The chemical transfection approach was thus abandoned as a strategy for loading the exosomes with siRNA. EP was used instead. EP loading efficiency was found to be dependent on exosome concentration and applied EP voltage. The researchers discovered that plasma-derived exosomes loaded with siRNA to MAPK-1 were capable of suppressing MAPK-1 mRNA levels in monocytes and lymphocytes.
In another study, Momen-Heravi et al. loaded B-cell-derived exosomes with miRNA-155 inhibitor using EP [25]. When cells are stimulated with LPS, miRNA-155 enhances their production of TNF-α. The exosomes modified with miRNA-155 inhibitor were able to significantly reduce TNF-α production by macrophages treated with LPS. This could be a viable strategy for reducing the negative inflammatory component of a variety of disease processes. Interestingly, isolation of the modified B-cell exosomes was performed using anti-CD63 immunomagnetic beads. Immunomagnetic exosome isolation is well suited to concentrate exosome subtypes for biomarker studies. However, removal of the exosomes from the magnetic beads can require stringent chemical conditions that could potentially disrupt exosome macromolecular structures and impede downstream functional studies depending on the application. The process may also omit legitimately modified exosomes lacking or minimally expressing CD63. For some exosomes types, CD63 is not expressed on all exosomes [32] and markers presumably specific to exosomes may also be found on other types of vesicles [33].
This study further highlights the importance of choosing the correct exosome subtype for the therapeutic application of interest. Otherwise, unmodified exosomes, may innately carry conflicting RNA cargo that could confound experimental results. In this instance, B-cell exosomes loaded with miRNA-155 inhibitor did not naturally contain significant levels of miRNA-155 prior to modification.
Other cargo
Exosome encapsulation can increase cellular delivery of hydrophilic molecules besides RNA. Fuhrmann et al. tested passive and active loading processes for encapsulating porphyrin model drugs of differing water solubilities [7] within ˜150 nm exosomes. No difference in loading efficiency was observed comparing passive incubation to EP for loading exosomes with hydrophobic porphyrin. In contrast, the EP loading efficiency for intermediately hydrophobic (more hydrophilic than hydrophobic) and hydrophilic porphyrins was higher than passive loading. EP loading efficiency was also influenced by exosome zeta potential (electrokinetic mobility). Exosomes with higher negative zeta potentials were more efficiently loaded with porphyrins using EP.
Differences in the hydrophobicity of porphyrins also influenced exosomal EP loading efficiency. The EP loading efficiency for the intermediately hydrophobic porphyrin was higher than the more hydrophilic porphyrin. Hypothetically, the intermediately hydrophobic porphyrin may more effectively juxtaposition itself nearer to the exosome membrane than the hydrophilic porphyrin before and after the EP process resulting in more efficient loading and/or more efficient post-EP retention. Future experiments comparing a wider hydrophobicity range of intermediately hydrophobic porphyrins could provide further insight into the findings.
Comparing passive to active processes for loading exosomes with porphyrins of intermediate hydrophobicity (more hydrophilic than hydrophobic) revealed additional findings. Encapsulation via hypotonic dialysis or treatment with the surfactant saponin (0.01%) significantly increased the loading efficiency of MDA exosomes over 11-fold. Loading through size extrusion or EP did not significantly influence loading efficiency compared with passive loading. Further, EP or saponin treatment did not significantly influence exosome size distribution or zeta potential. Extrusion and dialysis influenced size distribution indicated by peak broadening. This suggest that these methods may have influenced the structural integrity of the exosomes conceivably resulting in fragmentation and/or aggregation effects. The extrusion method also resulted in an increase in exosome negative zeta potential compared with passive, EP, saponin and dialysis loading methods. Both EP and saponin methods resulted in a four-fold increase in cellular drug uptake versus free drug. Overall, EP or saponin might be useful methods for encapsulating hydrophilic cargo in exosomes and the method of choice may depend on the exosome-based nanomedicine application. Comparing the exosome sizing curves post saponin versus EP treatment more closely revealed that the EP sizing curve best overlapped the sizing curve for passive treatment. This could have implications for exosome modifications where size-dependent trafficking in vivo is an issue. Further, EP might be preferred for applications where saponin biocompatibility is a concern.
Exosomes can also be used as immunotolerant nanocarriers for water-soluble chemotherapeutic cargo such as doxorubicin [26]. Tian et al. engineered immune tolerant immature dendritic cell (iDC) exosomes to express a chimeric fusion protein of exosomal Lamp2b and αV-integrin-specific iRGD peptide. Exosomes were then loaded with doxorubicin using EP. The encapsulation efficiency was 20%. The iDC exosomes were able to target and accumulate in αV-integrin expressing breast tumors in mice and inhibit their growth. In contrast, no effect on tumor growth was observed for equivalent dosing of free doxorubicin or nontargeted doxorubicin containing exosomes. Tumor growth inhibition resulted in no observed toxicity which validated the use of iDC exosomes as biocompatible nanocarriers.
More recently, Toffoli et al. demonstrated in a mouse breast cancer model that loading doxorubicin into exosomes maintained doxorubin chemotherapeutic efficacy while reducing doxorubicin cardiotoxicity [27]. Exosome-mediated delivery of doxorubin reduced accumulation of doxorubicin in the heart by approximately 40%.
Future perspective
Inherent structural and biocompatible properties of exosomes make them ideally suited as therapeutic nanocarriers with great potential to serve a multitude of nanomedicine applications. Nevertheless, many challenges to effectively harnessing their therapeutic potential remain. In general, three topics of exploration to be pursued in the coming years include: selecting the appropriate exosome subtype for the nanomedicine application, optimizing formulation of exosomal nanocarriers and investigating how modified exosomes interface with the immune system.
Selecting the appropriate exosome subtype for the nanomedicine application
Natural exosome function remains mysterious given the breadth of natural cargo permissible in exosomes including mRNA, miRNA, proteins, receptors, ligands, lipid microdomains and others [1]. Continued efforts toward elucidating the endogenous function of exosome subtypes is crucial and must be performed in parallel with modification strategies to ensure therapeutic efficacy. This issue is of particular importance when designing exosomal nanocarriers from potentially pathogenic tumor exosome subtypes given that pathogenic cargo (miRNA etc.), if not identified and neutralized, may persist beyond nanocarrier modifications [1] and impede therapeutic efficacy in certain applications. For example, exosomes have been demonstrated to carry pro-angiogenic factors [34]. Therefore, using tumor exosomes to carry angiogenesis inhibitors to tumor neovasculature may be impeded if the modified exosomes still retain proangiogenic cargo. In contrast, retention of tumor antigens might be beneficial for the development of tumor exosome-based immunotherapies [35]. Certain exosome subtypes might also possess natural properties beneficial to a number of cell types. MSC exosomes in particular appear to play a role in restoring tissue homeostasis in general by facilitating tissue repair and regeneration [36].
Specific therapeutic applications will require selection of the appropriate exosome subtype for nanocarrier conversion. DC exosomes for instance express the tetraspanin CD9 on their surface [37]. This allows DC exosomes to avoid the endocytic pathway and undergo membrane fusion with cellular membranes. Utilization of DC exosomes to deliver cargo to target cells might be useful for delivering pH-sensitive therapeutics that would otherwise be rapidly inactivated within lysosomes. Additionally, differential expression of antigen presentation and costimulatory molecules on mature and immature DC exosome subtypes can dictate whether baseline DC function is immunostimulatory or immunosuppressive [38]. Baseline DC exosome subtype function becomes particularly relevant if one is developing a new tumor vaccine versus an innovative treatment for autoimmune disease.
Further, endothelial cells comprising extensive vascular networks throughout the body are ideally juxtapositioned to and participate in the pathogenicity of a number of disease microenvironments. Cancer is one such disease benefiting from pro-tumor angiogenic processes. Moreover, endothelial exosomes being readily accessible via patient plasma provide for a potentially scalable exosome-based delivery system. In a recent study, Banizs et al. reasoned that endothelial exosomes would be most appropriate for delivering foreign nucleic acid cargo to endothelial cells [28]. Endothelial CD9+CD63+ exosomes (92 ± 38 nm) were loaded with luciferase siRNA using EP. The modified exosomes reduced luciferase expression in target endothelial cells by 40%.
Without understanding the normal function of exosomes, it will be difficult to predict the function of modified exosomes. The type of exosome is important as it may effect generalizations with regards to exosome circulatory half-life studies. Liver or kidney exosome clearance for example may differ from melanoma or breast cancer exosome clearance. Liver and kidney exosomes may naturally interact in an autocrine fashion more readily with liver or kidney cells. This in turn may influence their rate of uptake and clearance compared with other exosome types.
To scale up exosome production for nanocarrier purposes, exosome rich body fluids such as plasma might be processed to produce large amounts of material such as endothelial exosomes as described previously. Alternatively, cellular culture conditions might be optimized. For example, Momen-Heravi et al. reported that culturing B lymphocytes in the presence of IL-4 and CD40 increased exosome production by over 200-fold [25]. Large numbers of exosomes might then be isolated from culture or biological fluids using advanced microfluidic isolation strategies [39]. Other potential options to scale up exosomal nanocarrier production include the use of bovine milk-derived [40] or plant-derived [14,15] exosomes following biocompatibility testing.
Nontoxic, nonhuman sources of exosomes might be used to simultaneously improve scalability and therapeutic efficacy. This could be particularly relevant in the case of developing exosome-based therapies for cancer. A recent study by Raimondo et al. demonstrated that lemon juice-derived exosome-like nanovesicles naturally induce TRAIL-mediated apoptosis in different cancer cell lines in vitro and inhibit human CML tumor growth in NOD/SCID mice in vivo [15]. Therefore, lemon juice derived or similar exosome-like nanovesicles may be converted into suitable nanocarriers to deliver cancer chemotherapeutics. The inherent anticancer properties of the lemon exosome-like nanovesicles could be additive or synergize with that of anti-cancer drug cargo. Additionally, the possibility of scalable production of lemon juice derived exosome-like nanovesicles exist.
Optimizing formulation of exosomal nanocarriers
Developing means to program exosome surface molecules will facilitate efficacy of exosomes as therapeutic nanocarriers. Earlier exosome display technology work by Delcayre et al. demonstrated that the C1C2 domains of lactadherin preferentially localize to exosome membranes [41]. Exosomes expressing lactadherin were more effective at immunizing mice against lactadherin than free lactadherin. Use of the C1C2 domain to localize other chimeric protein cargo including somatostatin receptor 2 [42] to exosome surfaces was promising as well. Additionally, other researchers have shown that proteins can be targeted to exosome surfaces via plasma membrane anchors [43]. Such strategies highlight the extent to which exosome surfaces might be specifically engineered for various applications. Undoubtedly, additional modification options will become readily available as our understanding of how exosomes are constructed at the cellular level and can be modified post isolation advances.
Investigating new strategies to remove inherent internal pathogenic exosome cargo will be advantageous as well. This might ultimately be performed using EP approaches. Beyond loading RNA cargo into exosomes, EP can also be used to electroextract innate RNA cargo [11]. Endogenous pathogenic cargo might therefore be extracted prior to loading with therapeutic cargo. Analysis of residual exosome RNA content post electroextraction can be used to monitor the successful removal of endogenous pathogenic RNA.
Cataloguing exosome cargo loading parameters will be of great benefit for formulating effective exosomal nanocarriers. For example, the EP loading efficiency of B-cell exosomes with miRNA-155 mimic was found to be dependent on voltage but not capacitance [25]. However, under certain conditions, EP can induce aggregation of siRNA cargo [44]. This occurs via complex formation between electrode-derived metal ions and hydroxide ions found in EP buffers such as OptiPrepTM. The result can be an overestimation of RNA loading efficiency given that insoluble siRNA aggregates precipitate out of solution and co-isolate with exosomes [44]. This finding is supported by another EP study demonstrating a low 15–25% loading efficiency for endothelial exosomes [28]. To reduce EP induced RNA aggregation, metal chelators such as ethylenediaminetetraacetate (EDTA) or citrate or EP cuvettes containing nonmetallic polymer electrodes can be used [44]. However, the choice of metal chelator should be made with consideration to downstream nanocarrier applications. EDTA carry over, for example, has the potential to be toxic in vivo. Alternatively, removal of hydroxide ions in the EP buffer might work as well.
When using EP to load exosomes with RNA, it is also important to consider that EP pores created in exosome membranes allow for bidirectional movement of RNA [11]. Internal exosomal RNA can move out of the exosomes while external RNA moves in. The presence of endogenous internal exosomal shuttle RNA could inhibit external RNA loading efficiency. This could occur if the pre-existing internal RNA resist vacating the exosome core or complexes with external RNA upon release. In either scenario, the movement of external RNA into the exosome core is impeded. Resistance to EP-mediated exogenous RNA loading might be further exacerbated if the exosome core is densely packed with internal RNA resulting in a more gel-like than liquid consistency [1] that resist flow.
The EP loading process can also result in exosome aggregation or fusion [11,28]. To overcome EP induced exosome aggregation effects, Hood et al. recently developed a biocompatible trehalose-based EP pulse media to maximize exosome colloidal stability during EP [11]. Using this unique pulse media, the researchers were able to load melanoma exosomes with 5 nm superparamagnetic iron oxide cargo (SPION5) while minimizing EP-induced aggregation effects. Subsequent investigations revealed that SPION5-loaded melanoma exosomes could be tracked to lymph nodes with MRI [29]. Because SPIONs can mediate magnetic hyperthermia, this study highlights the future potential for exosomes to be used as theranostic nanocarriers to simultaneously detect and treat tumor microenvironments using conventional clinical imaging modalities such as MRI.
Investigating how modified exosomes interface with the immune system
The immune system interfaces with all disease processes thus effecting natural and modified exosome nanocarrier utility. Ideally, the process of converting exosomes into therapeutic nanocarriers will be expected to retain naturally beneficial exosome properties such as immune tolerance while incorporating targeting selectivity for specific tissue destinations depending on the application of interest.
However the benefits of immune tolerance to exosomes must be weighed against the risk of exosome-mediated immune suppression. This is especially true for tumor exosome-based therapeutic applications. Some mechanisms of tumor exosome-mediated immune suppression include: induction of apoptosis in cytotoxic antitumor T lymphocytes, impairment of monocyte differentiation into antigen presenting dendritic cells, induction of pro-tumor myeloid-derived suppressor cells or induction of regulatory T cells [45]. In contrast, it might be preferable to use normal immunotolerant exosome subtypes. For example, the inherent macromolecules comprising the structure of normal B-cell exosomes would be expected to be better tolerated by the immune system given their role in normal B-cell functions.
The addition or modification or exosome biochemical surface structures or use of existing structures to capture other macromolecules may also influence natural exosome trafficking and function in vivo. The number of surface macromolecules modified might also influence exosomal nanocarrier immunogenicity or clearance by the liver and spleen. This is particularly true if modification results in increased aggregation of exosomes. As the average diameter of aggregates exceeds 250 nm, modified exosomes will be more readily phagocytosed by antigen presenting cells [1]. Large multi-exosome complexes might be preferentially taken up by macrophages via phagocytosis whereas individual cargo-bearing exosomes may still engage in membrane fusion resulting in differential cell signaling events. This could abrogate the intended function of engineered exosomes if their therapeutic application requires exosome-mediated cargo delivery via cell membrane fusion.
Increased phagocytosis of modified exosome aggregates would also be expected to increase the immunogenicity of modified exosome components via processing through antigen presentation pathways. Further, generalized expression of phosphatidyl serine in exosome membranes may facilitate cellular engagement with immunological lipid raft receptors and ligands found on exosomes [18] resulting in a kind of remote immune trogocytosis. Essentially, the number of surface modified macromolecules required to achieve modified exosome effectiveness in vivo must be balanced against the threshold number of modifications that will incur increased immunogenicity.
Relatedly, it will be necessary to determine the extent to which surface expressed or conjugated imaging modalities are transferred nonspecifically to antigen presenting cells via endocytosis or membrane fusion given the potential for exosomes to aggregate [11] or to carry a plethora of tetraspanins not specific to individual cells types. Exosome surface imaging structures might be recognized as foreign by monocytes, dendritic cells or other antigen presenting cells. This too could result in aberrant exosome uptake by antigen presenting cells and false trafficking trails as the APCs carry the exosome imaging materials to extramedullary antigen processing sites such as the liver, spleen or lymph nodes. Subsequent challenges with surface modified exosomes might result in memory B-cell antibody-mediated clearance of the exosomal structures before they have a chance to achieve their intended diagnostic or therapeutic purpose.
Given their inherently low peptide-dependent immunogenicity, use of nonpeptide-based fluorescent lipophilic tracers might become a good gold standard for monitoring baseline exosome interactions with immune cell subsets. Such baseline studies would provide a means to evaluate the immunogenicity of more complex peptide-based exosome surface imaging or targeting macromolecules. A related consideration is whether exosome modification diminishes exosome biocompatibility by increasing the toxicity of the modified exosomes. For example, if paraformaldehyde is used to cross-link exosome surface cargo to exosomes for in vivo use, this could result in allergic or inflammatory reactions in patients that negate the intended therapeutic efficacy of the modified exosomes.
Conclusion
Ultimately, many challenges remain with regards to exogenous formulation of effective exosomal nanocarriers. However, with continued investigations into the complex diversity of exosome structure and function, new therapeutic strategies for cancer and other diseases will be revealed. Such strategies will undoubtedly incorporate the innate biocompatible nature of exosomes and facilitate personalized exosome-based nanomedicine endeavors.
Executive summary.
Exosomes are cell-derived nanovesicles that relay complex information between cells.
Exosomes possess ideal innate structural, trafficking and biocompatible nanocarrier properties.
Developing means to modify exosomes post isolation enables exosome-based nanomedicine applications in the absence of exosome source cells.
Adapting exosome surface structures
Modifying exosome surface structures post isolation allows for exosome imaging in vivo.
Previously engineered exosome surface structures can be further modified post isolation to enable dual fluorescent and bioluminescent exosome tracking in vivo.
Pre-existing exosome surface structures can be modified using ‘click chemistry’ to enable fluorescent exosome imaging.
Exosome subtypes expressing specific surface receptors might be used to capture and redirect extracellular protein deposits for removal by the immune system.
Loading exosome membranes with hydrophobic cargo
Hydrophobic therapeutics can be passively loaded into the lipid membrane of exosomes.
Loading hydrophobic drugs into exosome membranes enhances drug stability and minimizes hydrophobicity limitations to drug efficacy.
Exosomes increase the tissue biodistribution potential of effective hydrophobic drugs.
Encapsulating cargo in exosomes
Electroporation can be used to load exosomes with RNA or other water soluble therapeutic cargo.
Exosomes can carry hydrophilic cargo across the blood–brain barrier.
Encapsulation in immunotolerant exosomes increases cellular delivery of hydrophilic cargo.
Future perspective
Many challenges to effectively harnessing the therapeutic potential of exosomal nanocarriers remain.
Matching exosome subtypes to appropriate nanomedicine applications will decrease the potential for residual endogenous exosomal cargo to influence the efficacy of exogenously loaded therapeutics.
Optimizing exosomal nanocarrier formulations will increase nanocarrier efficacy by removing undesirable innate pathogenic cargo and minimizing exosome aggregation.
Comparing how natural and modified exosomes interface with the immune system will ensure effective nanocarrier trafficking in vivo, minimize off target effects and retain desirable natural exosome immunotolerant properties.
Footnotes
Financial & competing interests disclosure
The Elsa U Pardee Foundation and NIH NIGMS grant 5R21GM107894–03 are recognized for their encouragement and financial support for this work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Hood JL, Wickline SA. A systematic approach to exosome-based translational nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012;4(4):458–467. doi: 10.1002/wnan.1174. [DOI] [PubMed] [Google Scholar]
- 2.Quesenberry PJ, Aliotta JM. Cellular phenotype switching and microvesicles. Adv. Drug Deliv. Rev. 2010;62(12):1141–1148. doi: 10.1016/j.addr.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This is an excellent review article discussing the potential for exosomes to naturally impose significant changes on target cell phenotypes and is thus highly relevant to exosome-based nanocarrier design considerations.
- 3.Viaud S, Ullrich E, Zitvogel L, Chaput N. Exosomes for the treatment of human malignancies. Horm. Metab. Res. 2008;40:82–88. doi: 10.1055/s-2007-1022548. [DOI] [PubMed] [Google Scholar]
- 4.Lakkaraju A, Rodriguez-Boulan E. Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol. 2008;18(5):199–209. doi: 10.1016/j.tcb.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience. 2015;65(8):783–797. doi: 10.1093/biosci/biv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musante L, Saraswat M, Ravida A, Byrne B, Holthofer H. Recovery of urinary nanovesicles from ultracentrifugation supernatants. Nephrol. Dial. Transplant. 2013;28(6):1425–1433. doi: 10.1093/ndt/gfs564. [DOI] [PubMed] [Google Scholar]
- 7.Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release. 2015;205:35–44. doi: 10.1016/j.jconrel.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 8.Monsky WL, Fukumura D, Gohongi T, et al. Augmentation of transvascular transport of macromolecules and nanoparticles in tumors using vascular endothelial growth factor. Cancer Res. 1999;59(16):4129–4135. [PubMed] [Google Scholar]
- 9.Hood JL, Pan H, Lanza GM, Wickline SA. Paracrine induction of endothelium by tumor exosomes. Lab. Invest. 2009;89(11):1317–1328. doi: 10.1038/labinvest.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Measuring the Size & Surface Charge of Exosomes, Microvesicles and Liposomes. www.brookhaveninstruments.com
- 11.Hood JL, Scott MJ, Wickline SA. Maximizing exosome colloidal stability following electroporation. Anal. Biochem. 2014;448:41–49. doi: 10.1016/j.ab.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao W, Zhuang S, Qi XR. Comparative study of the in vitro and in vivo characteristics of cationic and neutral liposomes. Int. J. Nanomed. 2011;6:3087–3098. doi: 10.2147/IJN.S25399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mccoy-Simandle K, Hanna SJ, Cox D. Exosomes and nanotubes: control of immune cell communication. Int. J. Biochem. Cell Biol. 2015;71:44–54. doi: 10.1016/j.biocel.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mu J, Zhuang X, Wang Q, et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014;58(7):1561–1573. doi: 10.1002/mnfr.201300729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raimondo S, Naselli F, Fontana S, et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget. 2015;6(23):19514–19527. doi: 10.18632/oncotarget.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai CP, Mardini O, Ericsson M, et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014;8(1):483–494. doi: 10.1021/nn404945r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smyth T, Petrova K, Payton NM, et al. Surface functionalization of exosomes using click chemistry. Bioconjug. Chem. 2014;25(10):1777–1784. doi: 10.1021/bc500291r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, Dalir-Naghadeh B. Phenotypic modulation of auto-reactive cells by insertion of tolerogenic molecules via MSC-derived exosomes. Vet. Res. Forum. 2012;3(4):257–261. [PMC free article] [PubMed] [Google Scholar]; •• This is a unique investigation demonstrating the potential for exosomes to transmit bioactive molecules to target cell membranes which could influence the effectiveness of strategies used to formulate exosomal nanocarriers.
- 19.Yuyama K, Sun H, Sakai S, et al. Decreased amyloid-beta pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. J. Biol. Chem. 2014;289(35):24488–24498. doi: 10.1074/jbc.M114.577213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010;18(9):1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuang X, Xiang X, Grizzle W, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011;19(10):1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 23.Cooper JM, Wiklander PB, Nordin JZ, et al. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov. Disord. 2014;29(12):1476–1485. doi: 10.1002/mds.25978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wahlgren J, De LKT, Brisslert M, et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40(17):e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Momen-Heravi F, Bala S, Bukong T, Szabo G. Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomedicine. 2014;10(7):1517–1527. doi: 10.1016/j.nano.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian Y, Li S, Song J, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 27.Toffoli G, Hadla M, Corona G, et al. Exosomal doxorubicin reduces the cardiac toxicity of doxorubicin. Nanomedicine (Lond.) 2015 doi: 10.2217/nnm.15.118. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Banizs AB, Huang T, Dryden K, et al. In vitro evaluation of endothelial exosomes as carriers for small interfering ribonucleic acid delivery. Int. J. Nanomedicine. 2014;9:4223–4230. doi: 10.2147/IJN.S64267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu L, Wickline SA, Hood JL. Magnetic resonance imaging of melanoma exosomes in lymph nodes. Magn. Reson. Med. 2015;74:266–271. doi: 10.1002/mrm.25376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oude Blenke E, Klaasse G, Merten H, Pluckthun A, Mastrobattista E, Martin NI. Liposome functionalization with copper-free “click chemistry”. J. Control. Release. 2015;202:14–20. doi: 10.1016/j.jconrel.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 31.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 32.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94(11):3791–3799. [PubMed] [Google Scholar]
- 33.Witwer KW, Buzas EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles. 2013;2:20360. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ekstrom EJ, Bergenfelz C, Von Bulow V, et al. WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells. Mol. Cancer. 2014;13:88. doi: 10.1186/1476-4598-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolfers J, Lozier A, Raposo G, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001;7(3):297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 36.Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin. Cell Dev. Biol. 2015;40:82–88. doi: 10.1016/j.semcdb.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Van Den Boorn JG, Schlee M, Coch C, Hartmann G. SiRNA delivery with exosome nanoparticles. Nat. Biotechnol. 2011;29(4):325–326. doi: 10.1038/nbt.1830. [DOI] [PubMed] [Google Scholar]
- 38.Bell BM, Kirk ID, Hiltbrunner S, Gabrielsson S, Bultema JJ. Designer exosomes as next-generation cancer immunotherapy. Nanomedicine. 2015;12(1):163–169. doi: 10.1016/j.nano.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Petersen KE, Manangon E, Hood JL, et al. A review of exosome separation techniques and characterization of B16-F10 mouse melanoma exosomes with AF4-UV-MALS-DLS-TEM. Anal. Bioanal. Chem. 2014;406(30):7855–7866. doi: 10.1007/s00216-014-8040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371(1):48–61. doi: 10.1016/j.canlet.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delcayre A, Estelles A, Sperinde J, et al. Exosome display technology: applications to the development of new diagnostics and therapeutics. Blood Cells Mol. Dis. 2005;35(2):158–168. doi: 10.1016/j.bcmd.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Estelles A, Sperinde J, Roulon T, et al. Exosome nanovesicles displaying G protein-coupled receptors for drug discovery. Int. J. Nanomedicine. 2007;2(4):751–760. [PMC free article] [PubMed] [Google Scholar]
- 43.Shen B, Wu N, Yang JM, Gould SJ. Protein targeting to exosomes/microvesicles by plasma membrane anchors. J. Biol. Chem. 2011;286(16):14383–14395. doi: 10.1074/jbc.M110.208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kooijmans SA, Stremersch S, Braeckmans K, et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control. Release. 2013;172(1):229–238. doi: 10.1016/j.jconrel.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin. Immunopathol. 2011;33(5):441–454. doi: 10.1007/s00281-010-0234-8. [DOI] [PubMed] [Google Scholar]