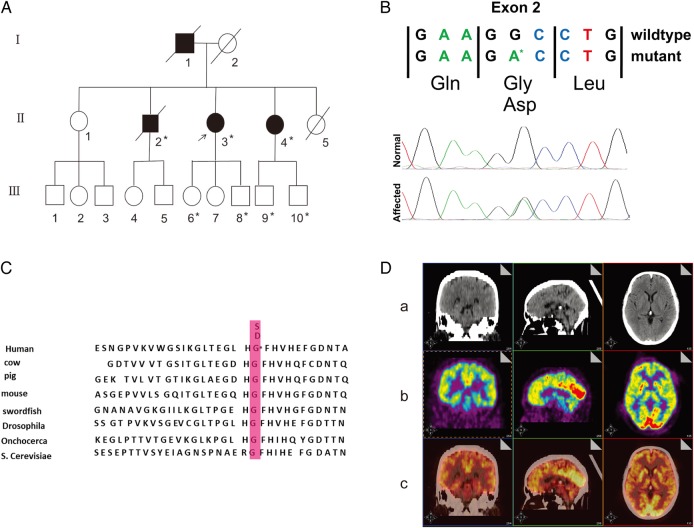
Most patients with amyotrophic lateral sclerosis (ALS) have mild cognitive impairment.1 A familial ALS (fALS) case carrying a SOD1 missense mutation with non-executive cognitive impairment was reported.2 However, cognitive impairment in fALS remains poorly understood. We report on a Chinese family with a novel SOD1 mutation, G41D, which causes slow progression of motor neuron function loss and cognitive impairment. The research was approved by the Ethics Committee of The People's Hospital of Jiangsu Province. A 62-year-old woman (the proband) was admitted to our department due to a 12-year history of progressive limb weakness and gait impairment. We obtained informed consent for the genetic study from the patient. The proband had muscle weakness in her left upper limb at 50 years of age and subsequently developed muscle weakness in both upper limbs as well as progressive upper motor neuron symptoms in her lower extremities. At 62 years of age, her muscle weakness worsened, with the appearance of atrophy in her upper limbs and diffuse cramps in her lower limbs, including gait abnormalities. She showed dysarthria and dysphonia, and no dysphagia. Neurological examination showed diffuse weakness in the distal arm muscles with less strength than antigravity, and moderate weakness in the proximal arm muscles with strength against resistance. Fasciculation was observed in these muscles. She also exhibited tongue hypotrophy with fasciculation. Additionally, there was pathological hyperreflexia. Cerebrospinal fluid analyses were normal and serum cryoglobulins were absent. MRI scans of the brain and cervical, thoracic and lumber spine were normal. The 18F-fluorodeoxyglucose cerebral positron emission tomography revealed reduced uptake in the left supermarginal gyrus and left frontopolar region (figure 1D). Electromyography revealed acute and chronic denervation changes in bulbar and all limb muscles, whereas motor and sensory nerve conductions were normal. Conduction blocks were not detected.
Figure 1.
(A) The pedigree of the described family with amyotrophic lateral sclerosis, slow motor neuron progression and mild cognitive impairment. Asterisks indicate the members who underwent genetic testing. The proband is marked by an arrow. (B) Chromatogram of the SOD1 p.Gly41Asp (c.G125>A) mutation identified by Sanger sequencing in the proband and confirmed in participants II-2, II-4, III-6, III-8, III-9 and III-10, which is not present in other unaffected family members or healthy controls. (C) The position of the mutation in exon 2 of SOD1 is highly conserved. (D) (a), CT scan image. No focal lesions were observed; (b) The 18F-fluorodeoxyglucose-positron emission tomography-scan showing reduced metabolism in the left frontal lobe (left frontal lobe mean standardised uptake values (suv)=4.9, right lobe mean suv=7.1). (c) Merged image.
Family history showed that the patient's father had developed muscle weakness and atrophy in his upper extremities at 50 years of age, and died of pneumonia at 72 years of age. However, her mother was healthy and died of chronic renal failure at 80 years of age. Her elder sister was, at the time of writing, 73 years of age and healthy. Her elder brother displayed muscle weakness and wasting in his upper limbs and was diagnosed with ALS at 46 years of age. He also had mild cognitive impairment 10 years after onset. He died from respiratory failure 15 years after symptom onset. Her younger sister developed weakness in her right hand at 50 years of age. She was diagnosed with ALS at 58 years of age. There are no affected members in the third generation of this family; however, they are all younger than 35 years of age.
A novel missense mutation (C to A codon 41 in exon 2) that resulted in an amino acid substitution of glycine to aspartate (G41D) was identified in participants II-2, II-3, II-4, III-6, III-8, III-9 and III-10 (figure 1A, B). The position of the mutation is highly conserved (figure 1C). All normal participants showed a homozygous pattern of the wild-type gene. The proband (patient II-3) and another six members of this family had a heterozygous pattern of the mutated and the wild-type gene. Three of them were confirmed to fulfil the El Escorial criteria for probable or definite ALS, exhibiting slow progression of motor neuron function loss and mild cognitive impairment. Regarding TARDBP, FUS/TLS and C9ORF72, three patients showed no pathological mutations.
Neuropsychological testing revealed moderate impairment in some cognitive domains. The proband's MMSE score was 20 (of 30). Her Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) was 41/48. We compared the neuropsychological profiles of the ALS group (three patients with ALS) and healthy group (11 healthy persons) in this family. We found that the executive domain, attention domain, language function, calculation tasks and memory were significantly impaired in the patients with ALS compared to the healthy family members. The executive domain, as assessed using the backward digit span, category verbal fluency, phonemic verbal fluency and Stroop tests, as well as the attention domain, were significantly impaired in the ALS group. The attention domain was examined by forward digit span, and the scores of the ALS and healthy groups were similar. In terms of language function measured using the Chinese version of the Boston naming test (C-BNT), the ALS group was significantly impaired compared to the healthy group. In calculation tasks and memory, the Chinese version of the Hopkins Verbal Learning Test (CVLT) delayed recall and the Rey complex figure test (RCFT) delayed recall scores were significantly lower in the ALS group (Fisher's exact test, p<0.01). These results showed that patients with ALS in this family had cognitive impairment.
SOD1-related patients with fALS have variable clinical expression of motor neuron symptoms compared to sporadic patients with ALS.3 It was reported that a missense mutation (G41S) in exon 2 of SOD1 caused rapid progressive loss of motor function, with predominantly lower motor neuron manifestations.4 However, there was significantly longer survival in patients with the G41D missense mutation in exon 2.4 To our knowledge, no cognitive impairment has previously been reported in these G41D-related patients with ALS. We hypothesise that the association between motor neuron and cognitive function might be induced by different pathological mechanisms. Alternatively, cognitive problems appear after the upper motor neuron dysfunction has been present for an extended period of time. In our study, all affected members, except the proband's father, who was unavailable for DNA analysis, showed a heterozygous mutation (c.125G>A) in exon 2 of the SOD1 gene. The only healthy sibling of the proband has wild-type SOD1, strongly supporting the notion that this SOD1 mutation is pathological. We report the unique genotype of a Chinese family with a heterozygous mutation (G41D) in the superoxide dismutase (SOD1) gene. Mild cognitive deficits occurred during the later stage of disease without significant changes in the frontal and temple regions (based on MRI scans). These clinical features postulate a possible clinical-genetic phenotype of patients with fALS with G41D mutations. However, further data are required to establish possible genotype–phenotype correlations.
Acknowledgments
The authors thank the patients and controls for having participated in this study. This work was in part supported by the National Natural Science Foundation of China (grant #30870125) and the Jiangsu Province Natural Science Foundation (grant # SBK201402229).
Footnotes
Contributors: QN, QJ were involved in the study concept and design; QN, YY, XS, TL were involved in the data acquisition; HC, XW, QX were involved in the data analyses and interpretation; QN, QJ were involved in the manuscript preparation; QN, YY, QJ were involved in the critical revision of the manuscript for important intellectual content; BZ, MS were involved in the technical and material support. All authors have approved the submitted version of the paper.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The Ethics Committee of The People's Hospital of Jiangsu Province.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Eisen A, Turner MR, Lemon R. Tools and talk: an evolutionary perspective on the functional deficits associated with amyotrophic lateral sclerosis. Muscle Nerve 2014;49:469–77. 10.1002/mus.24132 [DOI] [PubMed] [Google Scholar]
- 2. Canosa A, Calvo A, Moglia C, et al. . A familial ALS case carrying a novel p.G147C SOD1 heterozygous missense mutation with non-executive cognitive impairment. J Neurol Neurosurg Psychiatry 2014;85:1437–9. 10.1136/jnnp-2013-307552 [DOI] [PubMed] [Google Scholar]
- 3. Ajroud-Driss S, Siddique T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS). Biochim Biophys Acta 2015;1852:679–84. 10.1016/j.bbadis.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 4. Cudkowicz ME, McKenna-Yasek D, Sapp PE, et al. . Epidemiology of mutations in superoxide dismutase in amyotrophic lateral sclerosis. Ann Neurol 1997;41:210–21. 10.1002/ana.410410212 [DOI] [PubMed] [Google Scholar]