Abstract
Background/aims
To compare the efficacy and safety of intracameral (IC) administration at the beginning of cataract surgery, of Mydrane, a standardised ophthalmic combination of tropicamide 0.02%, phenylephrine 0.31% and lidocaine 1%, to a standard topical regimen.
Methods
In this international phase III, prospective, randomised study, the selected eye of 555 patients undergoing phacoemulsification with intraocular lens (IOL) implantation received 200 μL of Mydrane (Mydrane group) just after the first incision or a topical regimen of one drop each of tropicamide 0.5% and phenylephrine 10% repeated three times (reference group). The primary efficacy variable was achievement of capsulorhexis without additional mydriatics. The non-inferiority of Mydrane to the topical regimen was tested. The main outcome measures were pupil size, patient perception of ocular discomfort and safety.
Results
Capsulorhexis without additional mydriatics was performed in 98.9% of patients and 94.7% in the Mydrane and reference groups, respectively. Both groups achieved adequate mydriasis (>7 mm) during capsulorhexis, phacoemulsification and IOL insertion. IOL insertion was classified as ‘routine’ in a statistically greater number of eyes in the Mydrane group compared with the reference group (p=0.047). Patients in the Mydrane group reported statistically greater comfort than the reference group before IOL insertion (p=0.034). Safety data were similar between groups.
Conclusions
Mydrane is an effective and safe alternative to standard eye drops for initiating and maintaining intraoperative mydriasis and analgesia. Patients who received IC Mydrane were significantly more comfortable before IOL insertion than the reference group. Surgeons found IOL insertion less technically challenging with IC Mydrane.
Trial registration number
NCT02101359; Results.
Keywords: Clinical Trial, Conjunctiva, Cornea, Drugs, Inflammation
Introduction
The steady increase in the elderly population has resulted in a sustained increased in the volume of cataract surgery causing a significant burden on healthcare resources.1 The introduction of small-incision phacoemulsification led to substantial decreases in the duration of surgery, the postoperative course and hospitalisation. However, optimal mydriasis and anaesthesia remain fundamental for maximising the safety of the procedure and patient comfort intraoperatively. The standard topical regimen (ie, multiple drops of mydriatics and anaesthetics) presents several disadvantages. Topical drops need to be instilled repeatedly prior to surgery to ensure adequate intraoperative mydriasis, which is time consuming, can cause medication errors, ocular surface toxicity and even cardiovascular side effects.2 Maintaining adequate mydriasis during the entire procedure can be problematic, and inadequate pupil size or intraoperative pupil miosis may jeopardise the quality of phacoemulsification and intraocular lens (IOL) implantation. Hence, there is a need to optimise the preparatory steps that facilitate the technical aspects of surgery and the surgical manoeuvres.
Intracameral (IC) administration of mydriatics is an alternative to the traditional topical regimen for cataract surgery. Pilot studies have reported the safety and efficacy of various custom blended, ad hoc IC formulations, mixed on-site, containing phenylephrine and cyclopentolate or tropicamide for cataract surgery.3 4 Studies using IC anaesthetic reported increased patient comfort, especially during IOL implantation, and greater surgeon satisfaction.5 6
Thus, a combination of IC mydriatics and anaesthetics, injected after instillation of anaesthetic eye drops, could combine advantages of both techniques and result in improved mydriasis and greater surgeon and patient comfort intraoperatively. As custom preparation just prior to surgery is time consuming and prone to medication errors, a ready-to-use standardised injectable solution, prepared according to industrial quality controls, may be beneficial. Mydrane (Laboratoires Théa, Clermont-Ferrand, France) is the first standardised preservative-free ophthalmic combination of mydriatics and anaesthetic for IC administration, just prior to beginning cataract surgery. Mydrane is intended for rapid and stable mydriasis and sustained intraocular anaesthesia during surgery. In this phase III, multicentre, randomised, controlled clinical trial, we compared the efficacy and safety of IC Mydrane to a standard regimen of topical drops for phacoemulsification and IOL implantation.
Methods
Study design and patients
This multicentre, international, randomised, parallel-group study compared the efficacy and safety of IC Mydrane (Mydrane group) to a standard topical regimen (reference group) for cataract surgery. Mydrane is a proprietary injectable salt-balanced and pH-balanced solution of two mydriatics (tropicamide 0.02% and phenylephrine 0.31%) and one anaesthetic agent (lidocaine 1%) for IC delivery just prior to beginning cataract surgery. The standard topical regimen was tropicamide 0.5% (Mydriaticum 0.5%; Laboratoires Théa) and phenylephrine 10% (Neosynephrine 10% Faure; Laboratoires Europhta, Monaco). The hypothesis was that Mydrane was non-inferior to the standard topical regimen for inducing mydriasis to perform capsulorhexis without any additional mydriatics or pupil-expanding devices.
This study was conducted between September 2011 and February 2013 at 68 investigational centres in nine countries (see online supplementary appendix). Eligible patients were aged 40–88 years old, scheduled to undergo phacoemulsification with foldable IOL implantation under topical anaesthesia with clear self-sealing corneal incisions. Only one eye per patient could be included in this study. At the selection visit, a pupil diameter of at least 7 mm had to be obtained within 30 min following instillation of one drop of tropicamide 0.5% and one drop of phenylephrine 10% (maximum of three combined instillations at 10 min intervals, if necessary), otherwise, the patient was excluded. Main non-inclusion criteria were a history of intraocular surgery or combined procedures, iatrogenic, traumatic or congenital cataract, corneal, epithelial, stromal or endothelial residual or progressive corneal disease, history of ocular trauma, infection or inflammation within the previous three months, pseudoexfoliation and exfoliation syndrome.
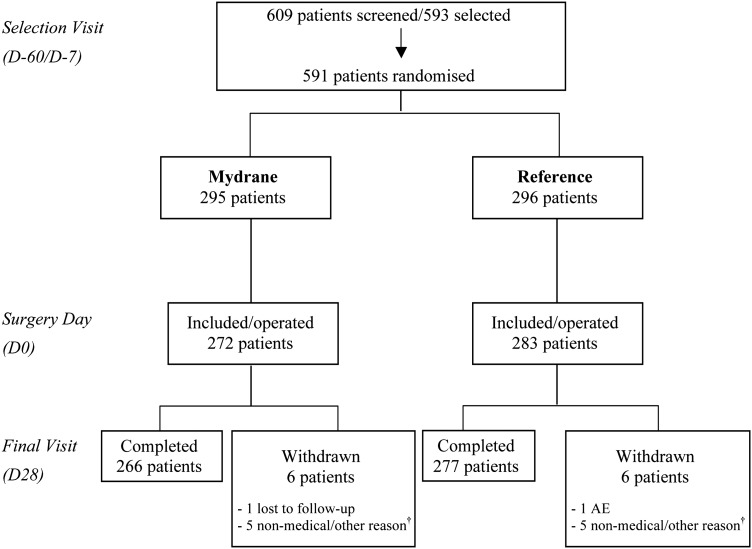
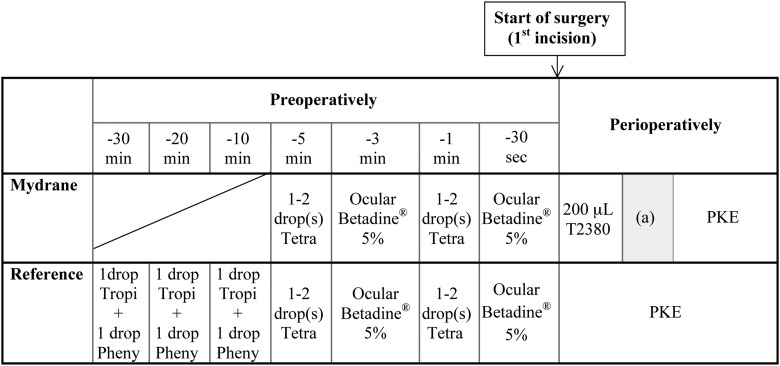
The randomisation procedure for assigning patients into the Mydrane group or reference group is presented in figure 1. At the selection visit (between 60 and 7 days preoperatively), an equal number of eligible patients were randomly assigned to one of the two study groups. The surgeon and surgical team were masked to the type of regimen and group enrolment for each eye until 30–60 min preoperatively. Figure 2 presents the preoperative and perioperative protocol for both groups. At the end of surgery, 0.1 mL of cefuroxime solution (10 mg/mL) was injected in the anterior chamber.
Figure 1.
Patient selection procedure and distribution of the study population. Only one eye of each patient underwent surgery. Patients scheduled for phacoemulsification cataract surgery were randomised at selection and included in the study on the day of surgery if they fulfilled the protocol inclusion/non-inclusion criteria. They were followed for 28 days postoperatively. †Reasons for patient withdrawal in the Mydrane group: one consent withdrawn; four final visit (D28) not performed or incomplete. Reasons for patient withdrawal in the reference group were one inclusion by error (patient included a second time in the study); one use of a concomitant medication that was prohibited; three final visit (D28) not performed. AE, adverse event; D, day.
Figure 2.
Preoperative and perioperative protocols for intracameral administration of a standardised solution of mydriatics and anaesthetic (Mydrane) or standard topical regimen (reference). In the reference group, the patients received one drop each, of tropicamide 0.5% and phenylephrine 10%, repeated three times at 10 min intervals beginning 30 min before surgery. The same regimen of tetracaine 1% (1–2 drops of Tetracaine Faure, Laboratoires Europhta; 1 and 5 min preoperatively) was used in both groups to allow povidone-iodine cleansing of the eyelids before the first incision (start of surgery) and to determine whether there was a difference in comfort between groups. Patients in the Mydrane group were injected with 200 μL of Mydrane in the anterior chamber just after the first corneal incision. Subsequently, a waiting time of 1.5 min (with the surgical microscope light switched off) was required by the protocol after injection of Mydrane and before delivery of viscoelastic, denoted by (a) in the figure. If mydriasis was considered inadequate after 1.5 min, a supplementary injection of 100 μL was permitted at the surgeon's discretion. Pheny, phenylephrine 10%; PKE, phacoemulsification; Tetra, tetracaine 1%; Tropi, tropicamide 0.5%.
All the cataract surgeries were video-recorded. Pupil diameter measurement was centralised and performed by independent and masked operators using VLC media player software (VideoLAN Non-Profit Organization, Paris, France). Pupil diameter was measured on screen captures taken at five specific stages during surgery: just before the corneal incision (T1), just before injection of viscoelastic (Duovisc; Alcon, Fort Worth, Texas, USA) (T2), just before capsulorhexis (T3), just before IOL insertion (T4) and just before cefuroxime injection (end of surgery, T5). The measurement of pupil diameters was as follows. First, the real horizontal visible iris diameter (HVID) (ie, horizontal white-to-white measurement) was measured during the selection visit with a surgical calliper (precision=0.1 mm). Then, the exact pupil size during surgery was obtained using the following formula:
 |
Corrective factor=real HVID/HVID on photograph.
All other medications used to obtain and maintain mydriasis or anaesthesia preoperatively or intraoperatively were noted. Follow-up was performed at 1 day, 1 week and 1 month postoperatively.
Primary study outcome
The primary efficacy variable was achievement of capsulorhexis without use of any additive mydriatic treatment. For both groups, an additive mydriatic treatment was defined as any supplementary medication with a mydriatic action other than stated in the study protocol and/or the use of a medical device for expanding the pupil, between first administration of the IC or topical regimen and capsulorhexis.
Secondary efficacy criteria
Anaesthesia was assessed based on the comfort reported by the patient at each stage of surgery (T1–T5). Patients were specifically asked about sensation of pressure and pain in the eye or orbit using a six-point ordinal scale: 0=no pain or pressure; 1=mild sensation of pressure but no discomfort; 2=mild discomfort due to sensation of pressure; 3=mild pain; 4=moderate pain; 5=severe pain. The evaluation at T4 (just before IOL insertion) was considered a major secondary efficacy variable as it corresponds to the time of greatest intraocular tissue manipulation. If additional anaesthetics were used, the case received the maximum score (ie, 5=severe pain).
Several surgical times were analysed: time necessary for obtaining mydriasis, time necessary to perform the main technical part of the surgical procedure, total surgical time and time spent by the patient in the operating theatre. Immediately after the surgical procedure the surgeon recorded his/her own subjective assessment of the surgery by grading each stage (first incision, second incision, capsulorhexis, phacoemulsification, cortex aspiration and IOL implantation) using the following grading: uncomplicated=routine surgery, not technically challenging; slightly complicated=slightly technically challenging surgery; complicated=technically challenging surgery.
Assessment of postoperative safety
Postoperative safety assessments included best corrected visual acuity, ocular symptoms and slitlamp examination, intraocular pressure, corneal thickness, funduscopy, corneal endothelial cell count and blood pressure and heart rate. Investigators rated the global tolerance of the study products at each follow-up visit as 0=very satisfactory; 1=satisfactory; 2=not very satisfactory; and 3=unsatisfactory. Postoperative specular microscopy was performed where equipment was available (48 centres).
Adverse events (AE) were noted. Treatment-related AEs were defined as AEs for which causal relationship with the investigational drug could not be formally excluded including AEs assessed as ‘unlikely related’ to ‘definitely related’.
Statistical analyses
The hypothesis was tested with the two-sided 95% CI of the difference (Mydrane minus reference) in responder rates between groups. Data were required for 246 evaluable patients in each treatment group in order to have 95% power for showing non-inferiority of Mydrane to the reference treatment.
The primary efficacy criterion was analysed for all patients who used the study medications and who satisfied the non-inclusion criteria concerning unauthorised previous and concomitant medications (mITT Set). Thus, the mITT Set was an intention-to-treat (ITT) Set excluding eyes that received treatment that could have influenced the quality of mydriasis. Anaesthesia assessments were primarily analysed in all randomised patients who used the study medications and who did not receive any additional anaesthetic before the start of surgery (mITT-An Set). Safety analyses were performed on all patients for whom there was evidence they received study medications (Safety Set). The per protocol (PP) Set were all patients in the mITT Set without any major protocol violation.
Descriptive statistics were calculated for the quantitative variables, and frequency distribution for the categorical variables. The quality of mydriasis was first tested for non-inferiority. If the latter test was significant, the major secondary efficacy end point was tested for superiority. As a closed testing procedure was used, no adjustment for the nominal alpha level for each test was necessary. The overall type I error was strongly controlled at the usual two-sided 0.05 level. For the primary efficacy variable, the non-inferiority was claimed at the usual 0.05 two-sided level if the lower bound of the 95% CI of the between-group difference in responder rates was not <−7.5%. Superiority tests (analysis of variance, Cochran–Mantel–Haenzel (CMH) tests based on modified ridit scores or Fisher's exact test) were used for between-group comparisons of other efficacy and safety variables. Statistical tests were two-sided at the 5% level of significance.
For the primary variable, patients with missing assessments at T3 were classified as non-responders. Missing anaesthesia assessments at T4 and T5 were replaced by the last available assessment (from T2). For other efficacy criteria, missing data were not replaced.
Results
Of 591 randomly selected patients, 36 (6.1%) patients were excluded preoperatively (figure 1) due to unexpected events (eg, did not fulfil exclusion criteria, patients cited personal reasons) or they withdrew consent just prior to surgery. A total of 555 patients were included and underwent cataract surgery. The mITT Set was composed of 549 patients (268 in the Mydrane group and 281 in the reference group). Six patients with at least one major deviation that could influence mydriasis (four in the Mydrane group and two in the reference group) were not included in the mITT Set. Forty-six patients in the Mydrane group and 47 patients in the reference group were excluded from the mITT-An Set because they received prohibited medications. Baseline data were similar between groups (table 1).
Table 1.
Baseline characteristics of intent-to-treat set of patients who received intracameral Mydrane or standard topical regimen for cataract surgery
| Mydrane group (N=295) |
Reference group (N=296) |
|
|---|---|---|
| Gender | ||
| Male | ||
| n (%) | 120 (40.7) | 134 (45.3) |
| Female | ||
| n (%) | 175 (59.3) | 162 (54.7) |
| Age (years) | ||
| N | 275 | 286 |
| Mean±SD | 69.2±9.4 | 70.6±9.2 |
| Min–Max | 43–87 | 34–89 |
| Time since cataract diagnosis (months) | ||
| N | 276 | 286 |
| Mean±SD | 16.9±43.9 | 11.0±16.6 |
| Median | 4.3 | 4.0 |
| Min–Max | 0–637 | 1–112 |
| Visible iris diameter at selection | ||
| N | 286 | 287 |
| Mean±SD | 11.9±0.6 | 11.9±0.5 |
| Min–Max | 10.0–14.0 | 10.0–13.5 |
Mydrane group, patients who received an intracameral injection of a standardised combination of tropicamide 0.02%, phenylephrine 0.31% and lidocaine 1% just after the first incision; n and N, number of patients; Reference group, patients who received a standard topical regimen of one drop each, of tropicamide 0.5% and phenylephrine 10% repeated three times at 10 min intervals beginning 30 min before surgery.
Efficacy of mydriasis
In the mITT Set, the capsulorhexis was successfully performed without other mydriatic treatments for 98.9% (95% CI 96.8% to 99.8%) of patients in the Mydrane group and 94.7% (95% CI 91.3% to 97.0%) in the reference group. Non-inferiority of Mydrane to the reference treatment was thus demonstrated for achieving mydriasis without any concomitant pupil-expanding treatments in the mITT, ITT and the PP Sets (table 2).
Table 2.
Mydriatic efficacy, the number of patients for whom the capsulorhexis was successfully performed without any additional mydriatic treatment
| Mydrane group | Reference group | Between-group difference* (%) | Non-inferiority testing† 95% CI |
|
|---|---|---|---|---|
| ITT | N=272 | N=283 | ||
| N (%) of patients | 267 (98.2) | 267 (94.3) | 3.8 | −4.5% to 12.1% |
| 95% CI | 95.8 to 99.4 | 91.0 to 96.7 | Non-inferiority verified | |
| mITT | N=268 | N=281 | ||
| N (%) of patients | 265 (98.9) | 266 (94.7) | 4.2 | −4.2% to 12.6% |
| 95% CI | 96.8 to 99.8 | 91.3 to 97.0 | Non-inferiority verified | |
| PP | N=254 | N=234 | ||
| N (%) of patients | 251 (98.8) | 222 (94.9) | 3.9 | −4.9% to 12.8% |
| 95% CI | 96.6 to 99.8 | 91.2 to 97.3 | Non-inferiority verified |
The non-inferiority was verified if the lower bound of this 95% CI was at least −7.5%.
*Mydrane—reference.
†Non-inferiority was based on the exact 95% CI of the between-group difference in responder rates and tested with a non-inferiority margin of −7.5%.
ITT, intent-to-treat set of patients; mITT, modified intent-to-treat set of patients; Mydrane group, patients who received an intracameral injection of a standardised combination of tropicamide 0.02%, phenylephrine 0.31% and lidocaine 1% just after the first incision; PP, per protocol; Reference group, patients who received a standard topical regimen of one drop each, of tropicamide 0.5% and phenylephrine 10% repeated three times at 10 min intervals beginning 30 min before surgery.
Additionally, in the mITT Set, the response rate defined by both the achievement of capsulorhexis without use of any additive mydriatics and a pupil size of at least 6 mm measured just before capsulorhexis (T3) was 96.8% (95% CI 93.8% to 98.6%) in the Mydrane group versus 94.3% (95% CI 90.7% to 96.7%) in the reference group.
In the Mydrane group, the mean pupil size was 7.67±0.87 mm just before capsulorhexis (T3) and remained stable at 7.71±0.91 mm at T4 and 7.46±0.96 mm at T5. In the reference group, the pupil size was 8.87±0.87 mm just before capsulorhexis (T3), 7.84±1.12 mm at T4 and 7.22±1.31 mm at T5. The pupil sizes were statistically significantly different between groups at T3 and T5 (p<0.001 and 0.02, respectively). Figure 3 presents ranges of pupil size during surgery. There were 1.2% of patients in the reference group with very small pupils (<5 mm) at T4 versus none in the Mydrane group. At T5, 5.3% of patients in the reference group had very small pupils versus 0.4% in the Mydrane group (figure 3C).
Figure 3.
Between-group comparison of the pupil size (in classes) during cataract surgery in the modified intent-to-treat set of patients. Pupil diameters were determined from photographs of the video recordings and measured by independent and masked observers at T3 (just before capsulorhexis), T4 (just before intraocular lens (IOL) implantation) and T5 (just before cefuroxime injection, end of surgery). The green arrow indicates pupil diameter to the right is ≥6 mm. Mydrane, Mydrane group; Ref, reference group.
Efficacy of anaesthetic
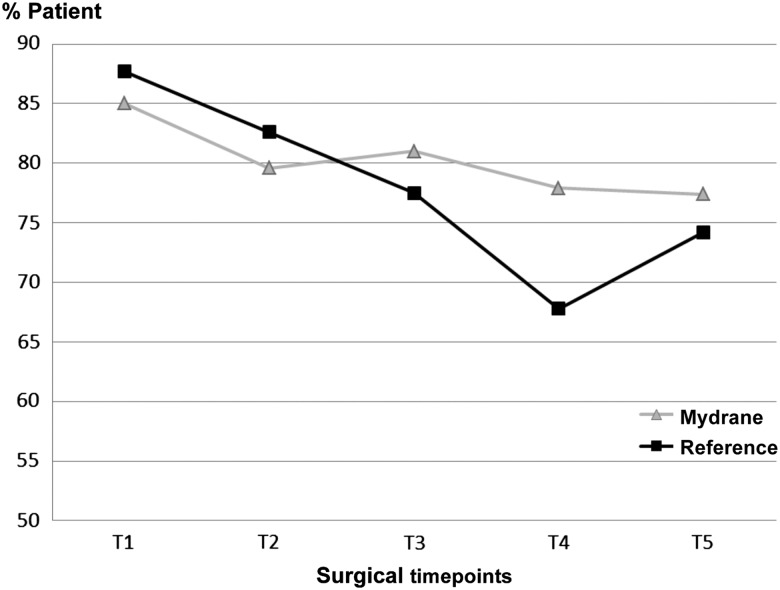
Patients in the Mydrane group experienced statistically significantly less pain or sensation of pressure compared with the reference group (p=0.034; CMH test) just before IOL insertion (T4) (figure 4). The sensation of pressure or pain between groups was comparable at the other time points (T1, T2, T3 and T5; p=0.369, 0.412, 0.409 and 0.368 respectively; CMH test). At 1 day postoperatively, 98.9% of patients in the Mydrane group and 96.8% in the reference group were very satisfied or satisfied with the entire surgery (p=0.544).
Figure 4.
Percentage of the modified intent-to-treat-anaesthesia set of patients with ‘no sensation of pain in the eye or the orbit’ or ‘no sensation of pressure in the eye or the orbit’ at each time point during surgery (T1: before the first incision; T2: before viscoelastic injection; T3: before capsulorhexis; T4: before intraocular lens implantation; T5: before cefuroxime injection). Ocular discomfort was evaluated by the patients using a questionnaire. Mydrane, Mydrane group; %Patient, per cent of patients; Reference, reference group.
Surgeon grading
Most stages of surgery were graded as uncomplicated in both groups (table 3).
Table 3.
Surgeon satisfaction with each stage of the surgery in two groups of eyes that underwent phacoemulsification with intraocular lens implantation with intracameral Mydrane or standard topical regimen
| Mydrane group (N=268) |
Reference group (N=281) | Between-group comparison p Value |
|
|---|---|---|---|
| First incision | |||
| Uncomplicated | 266 (99.3) | 277 (98.6) | 0.448 |
| Slightly complicated | 1 (0.4) | 3 (1.1) | |
| Complicated | 1 (0.4) | 1 (0.4) | |
| Second incision | |||
| Uncomplicated | 265 (98.9) | 277 (98.6) | 0.752 |
| Slightly complicated | 2 (0.7) | 3 (1.1) | |
| Complicated | 1 (0.4) | 1 (0.4) | |
| Capsulorhexis | |||
| Uncomplicated | 246 (91.8) | 260 (92.5) | 0.769 |
| Slightly complicated | 21 (7.8) | 18 (6.4) | |
| Complicated | 1 (0.4) | 3 (1.1) | |
| Phacoemulsification | |||
| Uncomplicated | 243 (90.7) | 266 (94.7) | 0.086 |
| Slightly complicated | 22 (8.2) | 8 (2.8) | |
| Complicated | 3 (1.1) | 7 (2.5) | |
| Cortex aspiration | |||
| Uncomplicated | 244 (91.0) | 255 (90.7) | 0.865 |
| Slightly complicated | 21 (7.8) | 19 (6.8) | |
| Complicated | 3 (1.1) | 7 (2.5) | |
| IOL implantation | |||
| Uncomplicated | 259 (96.6) | 261 (92.9) | 0.047* |
| Slightly complicated | 8 (3.0) | 15 (5.3) | |
| Complicated | 1 (0.4) | 5 (1.8) | |
*Statistically significant, p<0.05, with the Cochran–Mantel–Haenzel test.
Complicated, technically challenging surgery; IOL, intraocular lens; Mydrane group, patients who received an intracameral injection of a standardised combination of tropicamide 0.02%, phenylephrine 0.31% and lidocaine 1% just after the first incision; Reference group, patients who received a standard topical regimen of one drop each, of tropicamide 0.5% and phenylephrine 10% repeated three times at 10 min intervals beginning 30 min before surgery; Slightly complicated, slightly technically challenging surgery; Uncomplicated, routine surgery, not technically challenging.
There were a statistically significantly greater number of uncomplicated cases during IOL implantation in the Mydrane group (96.6%) compared with the reference group (92.9%) (p=0.047; CMH test). Surgeon assessment of the global tolerance of the drug regimens under study was statistically significantly higher with the Mydrane regimen compared with the standard topical regimen at 1 day (p=0.011) and 1 week (p=0.035).
Duration of preoperative workup and surgery
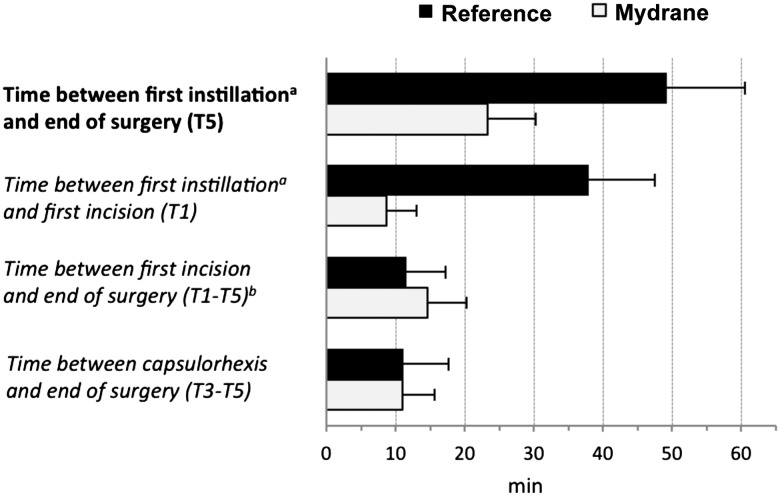
Due to the study protocol, the time between the first instillation of drops and the end of surgery was longer in the reference group compared with the Mydrane group (figure 5) (p<0.001). For the same reason, the time between the first incision (including the 1 min 30 s waiting time for the Mydrane group dictated in the protocol) and the end of surgery was statistically significantly shorter in the reference group compared with the Mydrane group (p<0.001; analysis of covariance). However, the time to perform the technical part of the cataract extraction and lens implantation (ie, between capsulorhexis and end of surgery (T3–T5)) was similar between groups (p=0.840). The Mydrane group spent 23.3±7.0 min in the preoperative and surgical rooms (combined) compared with 49.3±11.3 min for the reference group.
Figure 5.
Preoperative and surgical times (minutes) in the modified intent-to-treat set of patients. The times analysed were the time necessary for obtaining mydriasis (defined as the delay between the first instillation (eg, first injection of Mydrane for the Mydrane group or first instillation of tropicamide and phenylephrine for the reference group) and T3); time necessary to perform the main technical part of the surgical procedure, that is, from capsulorhexis to cefuroxime injection (defined as the delay between T3 and T5); total surgical time (T1–T5) and time spent by the patient in the operating theatre (from instillation of the first eye drop to cefuroxime injection). T1, before the first incision; T3, before capsulorhexis; T5, before cefuroxime injection. Analysis of covariance was used for between-group comparisons for the times T1–T5 and T3–T5 (p<0.001 and 0.840, respectively). a=Tetracaine drop 5 min before surgery in the Mydrane group; tropicamide or phenylephrine drop 30 min before surgery in the reference group. b=including the waiting time (1.5 min) required by the protocol after Mydrane injection and before viscoelastic injection for patients in the Mydrane group. Mydrane, Mydrane group; Reference, reference group.
Safety
The incidence of any ocular AE for the duration of this study was 17.7% in the Mydrane group and 19.1% in the reference group (p=0.742; Fisher's exact test). The incidence of AE considered by the investigator to be related to study treatment was 2.6% (seven patients) for the Mydrane group and 2.8% (eight patients) for the reference group (table 4).
Table 4.
Treatment-related adverse events in patients who underwent cataract surgery with intracameral Mydrane or a standard regimen of topical drops
| Mydrane group | Reference group |
|---|---|
| Complication (number of patients) | Complication (number of patients) |
| Mild ocular hyperaemia (1) Moderate macular oedema (1) Severe keratitis (1) Increased intraocular pressure (3) Posterior capsule rupture (1) |
Mild ocular hyperaemia (1) Mild ocular oedema and hyperaemia (1) Mild keratitis (2) Increased intraocular pressure (2) Moderate corneal epithelial defect (1) Moderate corneal disorder (1) |
The investigators considered posterior capsule rupture an ‘unlikely related’ adverse event with Mydrane. There were four cases of posterior capsule rupture in the reference group, none were considered related to the treatment by the investigators.
Mydrane group, patients who received an intracameral injection of a standardised combination of tropicamide 0.02%, phenylephrine 0.31% and lidocaine 1% just after the first incision; Reference group, patients who received a standard topical regimen of one drop each, of tropicamide 0.5% and phenylephrine 10%, repeated three times at 10 min intervals beginning 30 min before surgery..
The incidence of systemic AE was 4.8% in the Mydrane group and 6.0% in the reference group (p=0.577). Systemic infections were the most common systemic AE with three (1.1%) patients in the Mydrane group and five (1.8%) patients in the reference group. Nervous system disorders were the second most common systemic AE with three (1.1%) patients in each group.
At 1 week postoperatively, there were statistically significantly fewer patients who reported ongoing pain in the Mydrane group (0.7%) compared with the reference group (3.9%) (p=0.004; CMH test). At 1 month, there were statistically significantly fewer patients who reported irritation/burning/stinging in the Mydrane group (4.9%) compared with the reference group (12.3%) (p=0.005). There were no other differences in subjective symptoms between groups. Postoperatively, there were no clinically significant safety concerns in pachymetry, retinal thickness, funduscopy and IOP compared with baseline. The change in endothelial cell counts from baseline to 1 month postoperatively was −219±411 cells/mm2 (−9.1%) in the Mydrane group and −181±389 cells/mm2 (−7.6%) in the reference group (p=0.329).
Discussion
Mydrane is the first industrially manufactured and standardised mixture of mydriatics and 1% lidocaine for injection in the anterior chamber to perform phacoemulsification. The active components, concentrations and volumes in the Mydrane formulation were based on the efficacy and safety of other IC formulations used for phacoemulsification cataract surgery.3 4 This randomised clinical trial showed that IC administration of a ready-to-use, standardised combination of mydriatics and anaesthetic (Mydrane) just after the first incision produces rapid and adequate mydriasis (mean pupil size ≥7.5 mm after viscoelastic injection throughout the surgery), allowing phacoemulsification cataract surgery in conditions at least as effective as the standard topical regimen. Additionally, the presence of lidocaine 1% in Mydrane led to improved intraoperative anaesthesia, with lower patient discomfort during IOL insertion (the most active phase of surgery) compared with the standard topical regimen. Surgeons were statistically significantly more satisfied with the IOL insertion step in the Mydrane group compared with the reference group. Preoperative time was much lower in the Mydrane group as there was no requirement for topical drops preoperatively or subsequent monitoring of the patient. Immediately postoperatively the global surgeon grading was higher with Mydrane compared with the reference topical regimen.
Preoperative preparation
Interestingly, sparing patients the discomfort of intensive topical mydriatics preoperatively was considered a significant benefit by patients in previous studies of custom blended, IC formulations, prepared on-site.3 7 8 IC administration just after the first incision resulted in a considerable reduction in preoperative preparation without significantly increasing the duration of surgery. Perhaps the considerable reduction in time spent in the preoperative room and surgical suite can lead to a less stressful experience for patients in the Mydrane group. IC administration is expected to improve patient flow and surgical team efficiency. In this study, the duration of the entire procedure (from the instillation of first topical drops to the end of the surgery) decreased by about 30 min in the Mydrane group compared with the reference group. The time between the first incision and cefuroxime injection increased by 3 min in the Mydrane group compared with the reference group. This small difference was due to the study protocol that required a wait of 1.5 min after the administration of Mydrane and another 1.5 min were due instrument handling. The decrease in total presurgical and surgical time may result in a more cost-effective cataract surgery. For example, nurses and operating room technicians may spend less time administering topical drops preoperatively. Additionally, more patients could be scheduled on surgical days due to the faster turnover of patients.
In the current study, the lead investigators for each country reached the consensus that topical instillation at 30 min preoperatively was sufficient for pupil dilation. This decision concurs with the pupil dilation protocol accepted by the European Drug Agency and the US Food and Drug Administration.9 However, intraoperative miosis is a risk regardless of the duration between preoperative topical instillation of mydriatics and beginning of surgery. Intraoperative miosis has been observed in protocols dictating instillation of topical mydriatics longer than 30 min preoperatively.10–12
Efficacy of Mydrane in achieving mydriasis
Capsulorhexis is a key step for the quality of the phacoemulsification and is strongly associated to the quality of mydriasis. Therefore, we elected to choose the time T3 (ie, just before the capsulorhexis) as the most relevant surgical step (primary end point) to evaluate the efficacy of Mydrane. Hence, the non-inferiority of the Mydrane to the reference topical treatment was established based on the rate of surgeons who performed capsulorhexis without using additional mydriatics (or pupil-widening manoeuvres). Currently, in cataract surgery, a planned capsulotomy diameter of 5.0 mm requires an optimal pupil size of 6.0 mm to account for a 0.5 mm circumferential safety zone. In the Mydrane group, the rate of capsulorhexis performed without the use of additional mydriatics (or pupil-widening manoeuvres) and with a pupil size of at least 6 mm just prior to capsulorhexis was very high (96.8%) and similar to the reference group.
In this study, surgeons subjectively considered the pupil size with Mydrane was more than adequate with stable mydriasis to safely perform cataract surgery. The surgeons found that IOL implantation was less challenging in the Mydrane group compared with the reference group with more cases of IOL implantation considered ‘slightly challenging’ or ‘challenging’ in the reference group (p=0.047). Once dilated, the pupil size remained stable in the Mydrane group with a mean size of approximately 7.50 mm from viscoelastic injection until the end of surgery. These outcomes concur with a Swedish study of an IC formulation mixed on-site that reported a slightly lower pupil size at the end of surgery (approximately 6.5 mm).3 13 The differing results might be due to an additive effect of tropicamide contained in Mydrane. Notably, we found decreased mydriasis intraoperatively in the reference group. This drawback of the topical drop regimen has been previously documented.10
Efficacy of Mydrane in improving comfort and patient satisfaction
The inclusion of lidocaine in Mydrane supplements the effect of topical anaesthetic, producing better intraoperative anaesthesia. The anaesthetic agent in Mydrane is a response to a medical need suggested by the off-label use of IC anaesthetics to improve patient comfort intraoperatively. In some cases, preoperative instillation of topical anaesthesia may be insufficient during surgery, especially during IOL insertion.6 A meta-analysis of randomised controlled studies evaluating intraoperative pain and patient satisfaction with topical anaesthesia alone compared with topical anaesthesia and adjunctive IC anaesthesia for phacoemulsification found that additional IC 1% lidocaine significantly decreased patient perception of intraoperative pain, increased patient cooperation and decreased the degree to which patients were bothered by surgical manoeuvres.14 Hence, adjunctive unpreserved IC 1% lidocaine improves the effect of topical anaesthesia.5 15 16 Previous studies have shown that 1% IC lidocaine does not cause endothelial cell toxicity,17–19 induces serum concentrations of lidocaine below a minimum detectable level20 and does not diffuse into the posterior segment (even with 500 μL injection).19 An additional benefit is the mild mydriatic effect of IC lidocaine.21 22
The intraocular anaesthesia due to Mydrane likely resulted in statistically significantly less discomfort (pain or sensation of pressure) during IOL insertion in the Mydrane group compared with the reference group (p=0.034; CMH test). Patient comfort was similar between groups for the other stages of surgery. Only one patient (0.4%) in the Mydrane group received an additional anaesthetic treatment after the start of surgery compared with four patients (1.7%) in the reference group. Patient cooperation during cataract surgery is crucial for performing the optimal surgical manoeuvres during capsulorhexis to IOL implantation. The additive effect of intraocular anaesthesia with Mydrane may have enhanced patient cooperation. This observation likely explains why surgeons considered IOL implantation less difficult in the Mydrane group.
Patient pain at 1 week and ocular irritation/burning/stinging at 4 weeks were statistically lower in the Mydrane group compared with the reference group. Possibly, IC administration reduces the need for preoperative eye drops, mitigating corneal toxicity and ocular surface damage. However, the reference group received multiple eye drops preoperatively and intraoperatively, which may increase the risk of developing corneal damage in the early postoperative period. This greater propensity towards ocular surface damage could make patients in the reference group less resistant to AEs related to topical steroids and non-steroidal anti-inflammatory drugs commonly prescribed to prevent postoperative inflammation.
Safety of the constituents of IC Mydrane
Mydrane contains two mydriatics, a parasympatholytic (tropicamide 0.02%) and a sympathomimetic (phenylephrine 0.31%). Tropicamide was included due to the reduced cardiovascular risk23 and faster recovery (6 h vs 24 h) compared with other parasympatholytic agents, including cyclopentolate. The concentration of the constituents in Mydrane is very low compared with topical mydriatics, which should ensure greater safety and lower side effects. Notably, we found the changes in heart rate and blood pressure were similar between groups for the duration of this study (data not included). Similar cardiovascular outcomes have been reported in a comparison of cataract surgery with IC or topical mydriatics.20 However, a previous comparison reported a statistically significant decrease in pulse rate in patients who received topical mydriatics compared with IC mydriatics for cataract surgery.3 The relatively low concentration (0.31%) of phenylephrine in the Mydrane formulation likely mitigated any cardiac or systemic events.
An advantage of IC mydriatics or anaesthetics is the substantially increased bioavailability resulting in decreased systemic absorption.12 Additionally, washout of the IC Mydrane was performed with a viscoelastic injection once maximal mydriasis was achieved, further mitigating the risk of systemic side effects. The outcomes of this study indicate that IC administration of Mydrane was safe intraoperatively and postoperatively out to 1 month. The incidence of ocular AE related to the treatment (or with an unknown relationship) was below 3% in both groups. Our results with Mydrane are consistent with studies of IC lidocaine alone. Endothelial cell loss observed in the Mydrane group was similar to the reference group and was also consistent with the range of loss (4.3–16.8%) reported after routine phacoemulsification cataract surgery.24 25 The effect of IC lidocaine 17 18tropicamide or phenylephrine4 on endothelial cells remains unclear.
Clinically meaningful macular oedema was persistent in only one patient in the Mydrane group. The incidence of clinical macular oedema in our study is similar to recent studies of routine cataract surgery. A previous study26 reported 3.8% of 106 eyes developed macular oedema after cataract surgery. There were no serious AEs requiring hospitalisation or resulting in permanent visual loss in both groups in our study. This is especially important as this is the first large-scale study of a unique IC combination (Mydrane) that has been produced via standardised manufacturing practices. This outcome concurs with previous studies of IC lidocaine, IC mydriatics and on-site formulations blended for IC use that reported no difference in AEs between IC and topical administration.8 22
Study limitations
A drawback of the present study was that a double-blind comparison was not feasible because the test treatment differed from the reference treatment in several aspects (dose regimen, packaging and administration route). To overcome this limitation, screen captures from the video recording were centrally reviewed by two independent observers masked to the route of administration or the type of medications. We believe the rigorous conservative methods used for pupil size assessments in this study increase the overall strength of the conclusions. This initial clinical trial enrolled a relatively homogeneous population of patients with cataract who responded well to mydriatics preoperatively. This inclusion represents the ‘real world’ clinical experience in the vast majority of patients undergoing cataract surgery. However, our results cannot be extrapolated to the minority of patients with cataract with poor pupil dilation such as those with long-standing diabetes, exfoliation syndrome, posterior iris synechiae or a history of treatments potentially inducing intraoperative floppy iris syndrome. In this study, the ITT analysis mitigated overoptimistic estimates of the efficacy mydriasis and anaesthesia induced by Mydrane due to the removal of non-compliers. Some consider this analysis too cautious,27 and hence, our outcomes may be considered understated. However, this was an international, multicentre trial including surgical centres and hospital-based clinics. The various worldwide locations and the types of centres and varying surgeon experience mean the results of this study are directly applicable to—and more indicative of—actual clinical practice.
Additionally, due to ethical concerns, the inclusion/exclusion criteria dictated that only patients with a normal response to topical tropicamide plus phenylephrine were selected. This excluded patients who may have difficulty achieving mydriasis and more technically challenging surgical cases. However, this is not a limitation in daily practice as Mydrane is applicable to patients with a good mydriatic response, which comprise the majority of patients in clinical practice. Additionally, the mydriatic response can be easily assessed during the dilated retinal exam at the screening visit. Hence, the incorporation of Mydrane into the surgical routine will not add an extra step for patients.
A final drawback of the present study is that 1-month outcomes may not necessarily be indicative of longer-term outcomes. However, the aim of this study was to evaluate the mydriatic and anaesthetic properties of Mydrane to determine its suitability for cataract surgery. Based on this objective, we believe the 1-month period is appropriate for this evaluation because (i) postoperative follow-up by most cataract surgeons is 1 month; (ii) the potentially severe AEs of Mydrane (mostly toxicity on endothelial cells and macula, and bacterial contamination) were typically expected within days to weeks postoperatively; (iii) the patients included in the study and presenting with an AE had to be regularly followed following good clinical practice requirements and (iv) the long-term AEs of cataract surgery (ie, retinal breaks and detachments) are related to the surgical procedure rather than to the products used preoperatively or intraoperatively.
In conclusion, this evaluation of Mydrane indicates that it is efficacious and safe for IC injection just prior to beginning cataract surgery in patients with satisfactory pupil dilation as checked during the preoperative visit. Mydrane may have potential advantages in terms of rapidly achieving mydriasis, maintaining a stable pupil size during surgery and satisfactory patient comfort during intraocular manipulation. This ready-to-use combination of mydriatics and anaesthetics appears to be a simple approach. In addition to shortening the length of time the patient spends in the clinic on the surgical day, Mydrane may allow better patient flow in the operating room.
Supplementary Material
Acknowledgments
Our sincerest appreciation to Professor Joseph Colin, MD, chairman of the Department of Ophthalmology at Bordeaux University Medical School for having guided this project with expert professional advice and great kindness as the leading investigator of this clinical study. We miss his support and friendship and are deeply saddened by his premature departure. Sandrine Guyon for managing the study. Leonard Kaufman, Marie-Paule Derde and Sylvie Nisslé for statistical analyses. The named authors prepared the manuscript and received writing and formatting assistance from Thierry Radeau from Radeau Consulting.
Correction notice: This article has been corrected since it was published Online First. The Collaborators have been added to the article.
Collaborators: The investigators of the ICMA Study 2 Group are: FRANCE: J. Colin, Hôpital Pellegrin Tripode, Bordeaux, France; P. Bonicel, Centre Hospitalier Régional, Orléans, France; J.M. Bosc, Clinique Sourdille, Nantes, France; P. Bouchut, Clinique Ophtalmologique Thiers, Bordeaux, France; C. Boureau-Andrieux, Clinique Geoffroy Saint-Hilaire, Paris, France; J.-M. Buffet, Polyclinique Saint-François, Desertine, France; F. Chiambaretta, Hôpital Gabriel Montpied, CHU de Clermont-Ferrand, Clermont-Ferrand, France; B. Cochener, CHU Morvan, Brest, France; I. Cochereau, Fondation A. de Rothschild, Paris, France; A. Muselier, Hôpital Général, CHU de Dijon, Dijon, France; B. Delemazure, Centre Hospitalier de Belfort-Montbéliard, Belfort, France; N. Duquesne, Clinique Saint Charles, Lyon, France; N. Francoz, Polyclinique La Pergola, Vichy, France; P. Gain, Centre Hospitalier Bellevue, CHU Saint Etienne, Saint Etienne, France; S. Jaulerry, Centre Hospitalier de Bigorre, Tarbes, France; F. L'Herron, Clinique Saint-Michel et Sainte-Anne, Quimper, France; M. Labetoulle, Hôpital Bicêtre, Université Paris sud, Paris, France; L. Laroche, CHNO des XV-XX, Paris, France; P. Lenoble, Hôpital Emile Muller, Centre Hospitalier de Mulhouse, Mulhouse, France; F. Malacaze, Hôpital Purpan, CHU de Toulouse, Toulouse, France; P. Gohier, CHRU Hôtel Dieu, Angers, France; D. Milea, CHRU Hôtel Dieu, Angers, France; M. Muraine, Hôpital Charles Nicolle, CHU de Rouen, Rouen, France; F. Normand, Centre Hospitalier Saint Joseph Saint Luc, Lyon, France; J.-M. Perone, CHR Bon Secours, Metz, France; C. Pey, Clinique Bon Secours, Le Puy en Velay, France; P.-J. Pisella, Hôpital Bretonneau, CHRU de Tours, Tours, France; P.-Y. Robert, Hôpital Dupuytren, CHU de Limoges, Limoges, France; P. Rozot, Clinique Monticelli, Marseille, France; M. Weber, Clinique Ophtalmologique, Nantes, France; W. Williamson, Centre Hospitalier F. Mitterand, Pau, France; M. Mercie, Hôpital Jean Bernard, Poitiers, France; T. Bourcier, Nouvel Hôpital Civil, Strasbourg, France; A. Berard, Hôpital privé Les Franciscaines, Nîmes, France; S. Allieu, Clinique Beau Soleil, Montpellier, France; J. Uzzan, Clinique Mathilde, Rouen, France; B. Le Bot, Clinique de la côte d'Emeraude, Saint Malo, France; C. Mazit, Clinique de l'Anjou, Angers, France; T. Lebrun, Clinique du Landy, Saint-Ouen, France; N. Salaun, Hôpital d'Instruction des Armées Legouest, Metz, France GERMANY: A. Kampik, AugenKlinik der Ludwig-Maximilians, Universität München, Munich, Germany; A. Liekfeld, Ernst von Bergman Klinikum, Potsdam, Germany; I. Lanzl, Chiemsee Augen Tagesklinik, Prien, Germany BELGIUM: M.-J. Tassignon, Universitair Ziekenhuis Antwerpen, Edegem, Belgium; E. Mertens, Medipolis, Antwerpen, Belgium; S. Pourjavan, Cliniques universitaires Saint-Luc, Bruxelles, Belgium; G. Sallet, Ooginstituut, Aalst, Belgium PORTUGAL: M. Lobo, Association for Innovation and Biomedical Research on Light and Image (AIBILI), Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal; C. Aguiar, Hospital de Santo Antonio, Porto, Portugal; A. Limao, Instituto de Microcirugia Ocular (IMO), Lisboa, Portugal; J. M. Trigo, Centro Hospitalar de Lisboa Central, Hospital de Santo Antonio dos Capuchos, Lisboa, Portugal ITALY: G. Beltram, U.O. di Oculistica, Azienda Ospedaliera, Pordenone, Italy; F. Fasce, U.O. C. di Oculistica, Fondazione Centro S. Raffaele, Milano, Italy; U. Menchini, A.O.U. Careggi, Clinica Oculistica, Firenze, Italy SPAIN: J. Alió, Instituto Oftalmologico de Alicante, Alicante, Spain; J. Costa Vila, Clínicas Corachán, Barcelona, Spain; J. Fernandez, Hospital Torrecárdenas, Almeria, Spain; J. Torras, Hospital Casa De Maternitat, Barcelona, Spain ALGERIA: D. Hartani, CHU Mustapha, Alger, Algeria; S. Mohabeddine, Clinique Diar Saada, Alger, Algeria; S. Lazreg, Private Practice, Dar El Beida, Blida, Algeria; M. Daghbouche, Clinique Ophtalmologique Daghbouche, Constantine, Algeria; S. E. Benmoussa, Clinique d'Ophtalmologie Benmoussa, Constantine, Algeria; A. Smaili, Clinique D'Ophtalmologie En-Nour, Batna, Algeria; M. Meziane, Clinique D'Ophtalmologie Nour, Oran, Algeria AUSTRIA: O. Findl, Hanush Krankenhaus Wien, Wien, Austria; G. Grabner, Universitätsklinik Für Augenheilkunde Und Optometrie, Der Paracelsus, Austria SWEDEN: A. Behndig, Umeå University Hospital, Umeå, Sweden; C. G. Laurell, St. Erik Eye Hospital, Stockholm, Sweden
Contributors: All authors have given final approval of this version to be published. ML, OF, JA, JC and AB participated in drafting the manuscript, study design, data collection and screening, data analysis and evidence synthesis and revising the manuscript. FM, BC, CL, SL, DH and MJT participated in literature search, data collection, data analysis and evidence synthesis and drafting the manuscript. ML and AB participated in hypothesis generation, evidence synthesis and revising the manuscript. ML, OF, FM, JA, BC, CL, SL, DH, JC MJT and AB participated in study design, data collection, screening and revising the manuscript.
Funding: This clinical study was sponsored by Laboratoires THEA, Clermont-Ferrand, France. The sponsor participated in the design of the study, conducting the study, data collection, data management, data analysis, interpretation of the data, preparation of the manuscript.
Competing interests: ML has served as a consultant for Allergan, Alcon, Bausch and Lomb, MSD, Santen/Novagali and Théa. OFis a scientific advisor to Abbott Medical Optics, Carl Zeiss Meditec AG and Croma-Pharma. M-JT is consultant for Théa, Ellex Medical Lasers and Morcher GmbH. BC has financial interests with Abbott Medical Optics, Alcon and Bausch & Lomb.
Patient consent: Obtained.
Ethics approval: Ethics committee approvals were obtained in each country prior to enrolling any patient. This study was conducted in accordance with the Good Clinical Practice guidelines, the Declaration of Helsinki and local health regulations.
Provenance and peer review: Not commissioned; internally peer reviewed.
Contributor Information
Collaborators: P. Bonicel, J. M. Bosc, P. Bouchut, C. Boureau-Andrieux, J.-M. Buffet, F. Chiambaretta, I. Cochereau, A. Muselier, B. Delemazure, N. Duquesne, N. Francoz, P. Gain, S. Jaulerry, F. L'Herron, L. Laroche, P. Lenoble, F. Malacaze, P. Gohier, D. Milea, M. Muraine, F. Normand, J.-M. Perone, C. Pey, P.-J. Pisella, P.-Y. Robert, P. Rozot, M. Weber, W. Williamson, M. Mercie, T. Bourcier, A. Berard, S. Allieu, J. Uzzan, B. Le Bot, C. Mazit, T. Lebrun, N. Salaun, A. Kampik, A. Liekfeld, I. Lanzl, E. Mertens, S. Pourjavan, G. Sallet, M. Lobo, C. Aguiar, A. Limao, J. M. Trigo, G. Beltram, F. Fasce, U. Menchini, J. Costa Vila, J. Fernandez, J. Torras, S. Mohabeddine, M. Daghbouche, S. E. Benmoussa, A. Smaili, M. Meziane, G. Grabner, and C. G. Laurell
References
- 1.Hodge W, Horsley T, Albiani D, et al. . The consequences of waiting for cataract surgery: a systematic review. CMAJ 2007;176:1285–90. 10.1503/cmaj.060962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraunfelder FT, Scafidi AF. Possible adverse effects from topical ocular 10% phenylephrine. Am J Ophthalmol 1978;85:447–53. 10.1016/S0002-9394(14)75239-0 [DOI] [PubMed] [Google Scholar]
- 3.Lundberg B, Behndig A. Intracameral mydriatics in phacoemulsification cataract surgery. J Cataract Refract Surg 2003;29:2366–71. 10.1016/S0886-3350(03)00522-4 [DOI] [PubMed] [Google Scholar]
- 4.Mori Y, Miyai T, Kagaya F, et al. . Intraoperative mydriasis by intracameral injection of mydriatic eye drops: in vivo efficacy and in vitro safety studies. Clin Experiment Ophthalmol 2011;39:456–61. 10.1111/j.1442-9071.2010.02456.x [DOI] [PubMed] [Google Scholar]
- 5.Carino NS, Slomovic AR, Chung F, et al. . Topical tetracaine versus topical tetracaine plus intracameral lidocaine for cataract surgery. J Cataract Refract Surg 1998;24:1602–8. 10.1016/S0886-3350(98)80350-7 [DOI] [PubMed] [Google Scholar]
- 6.Crandall AS, Zabriskie NA, Patel BC, et al. . A comparison of patient comfort during cataract surgery with topical anesthesia versus topical anesthesia and intracameral lidocaine. Ophthalmology 1999;106:60–6. 10.1016/S0161-6420(99)90007-6 [DOI] [PubMed] [Google Scholar]
- 7.Soong T, Soultanidis M, Claoué C, et al. . Safety of intracameral mydriasis in phacoemulsification cataract surgery. J Cataract Refract Surg 2006;32: 375–6. 10.1016/j.jcrs.2005.12.088 [DOI] [PubMed] [Google Scholar]
- 8.Behndig A, Eriksson A. Evaluation of surgical performance with intracameral mydriatics in phacoemulsification surgery. Acta Ophthalmol Scand 2004;82:144–7. 10.1111/j.1600-0420.2004.00241.x [DOI] [PubMed] [Google Scholar]
- 9.Lawuyi LE, Gurbaxani A. The clinical utility of new combination phenylephrine/ketorolac injection in cataract surgery. Clin Ophthalmol 2015;9:1249–54. 10.2147/OPTH.S72321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bäckström G, Behndig A. Redilation with intracameral mydriatics in phacoemulsification surgery. Acta Ophthalmol Scand 2006;84:100–4. 10.1111/j.1600-0420.2005.00546.x [DOI] [PubMed] [Google Scholar]
- 11.Lundqvist O, Koskela T, Behndig A. A paired comparison of intracameral mydriatics in refractive lens exchange surgery. Acta Ophthalmol 2014;92:482–5. 10.1111/aos.12256 [DOI] [PubMed] [Google Scholar]
- 12.Behndig A, Lundberg B, Bäckström G. Intracameral mydriatics in cataract surgery, cataract surgery. In: Farhan Z, (ed. Vol. 12 InTech, 2013:149–72. http://www.intechopen.com/books/cataract-surgery/intracameral-mydriatics-in-cataract-surgery (accessed 6 May 2013). [Google Scholar]
- 13.Lundberg B, Behndig A. Intracameral mydriatics in phacoemulsification surgery obviate the need for epinephrine irrigation. Acta Ophthalmol Scand 2007;85:546–50. 10.1111/j.1600-0420.2007.00892.x [DOI] [PubMed] [Google Scholar]
- 14.Ezra DG, Nambiar A, Allan BD. Supplementary intracameral lidocaine for phacoemulsification under topical anesthesia. A meta-analysis of randomized controlled trials. Ophthalmology 2008;115:455–87. 10.1016/j.ophtha.2007.09.021 [DOI] [PubMed] [Google Scholar]
- 15.Gills JP, Cherchio M, Raanan MG. Unpreserved lidocaine to control discomfort during cataract surgery using topical anesthesia. J Cataract Refract Surg 1997; 23:545–50. 10.1016/S0886-3350(97)80211-8 [DOI] [PubMed] [Google Scholar]
- 16.Tan CS, Fam HB, Heng WJ, et al. . Analgesic effect of supplemental intracameral lidocaine during phacoemulsification under topical anesthesia: a randomised controlled trial. Br J Ophthalmol 2011;95:837–41. 10.1136/bjo.2010.188003 [DOI] [PubMed] [Google Scholar]
- 17.Eggeling P, Pleyer U, Hartmann C, et al. . Corneal endothelial toxicity of different lidocaine concentrations. J Cataract Refract Surg 2000;26:1403–8. 10.1016/S0886-3350(00)00379-5 [DOI] [PubMed] [Google Scholar]
- 18.Poyales-Galan F, Pirazzoli G. Clinical evaluation of endothelial cell decrease with VisThesia in phacoemulsification surgery. J Cataract Refract Surg 2005;31: 2157–61. 10.1016/j.jcrs.2005.07.016 [DOI] [PubMed] [Google Scholar]
- 19.Rigal-Sastourne JC, Huart B, Pariselle G, et al. . Diffusion de la lidocaïne après injection intracamérulaire. J Fr Ophtalmol 1999;22:21–4. [PubMed] [Google Scholar]
- 20.Wirbelauer C, Iven H, Bastian C, et al. . Systemic levels of lidocaine after intracameral injection during cataract surgery. J Cataract Refract Surg 1999;25:648–51. 10.1016/S0886-3350(99)00005-X [DOI] [PubMed] [Google Scholar]
- 21.Lincoff H, Zweifach P, Brodie S, et al. . Intraocular injection of lidocaine. Ophthalmology 1985;92:1587–91. 10.1016/S0161-6420(85)33820-4 [DOI] [PubMed] [Google Scholar]
- 22.Morgado G, Barros P, Martins J, et al. . Comparative study of mydriasis in cataract surgery: topical versus Mydriasert versus intracameral mydriasis in cataract surgery. Eur J Ophthalmol 2010;20:989–93. [DOI] [PubMed] [Google Scholar]
- 23.Rengstorff RH, Doughty CB. Mydriatic and cycloplegic drugs: a review of ocular and systemic complications. Am J Optom Physiol Opt 1982;59:162–77. [PubMed] [Google Scholar]
- 24.Bourne RR, Minassian DC, Dart JK, et al. . Effect of cataract surgery on the corneal endothelium: modern phacoemulsification compared with extracapsular cataract surgery. Ophthalmology 2004;111:679–85. 10.1016/j.ophtha.2003.07.015 [DOI] [PubMed] [Google Scholar]
- 25.O'Brien PD, Fitzpatrick P, Kilmartin DJ, et al. . Risk factors for endothelial cell loss after phacoemulsification surgery by a junior resident. J Cataract Refract Surg 2004;30:839–43. 10.1016/S0886-3350(03)00648-5 [DOI] [PubMed] [Google Scholar]
- 26.Anastasilakis K, Mourgela A, Symeonidis C, et al. . Macular edema after uncomplicated cataract surgery: a role for phacoemulsification energy and vitreoretinal interface status? Eur J Ophthalmol 2015;25:192–7. 10.5301/ejo.5000536 [DOI] [PubMed] [Google Scholar]
- 27.Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res 2011;2: 109–12. 10.4103/2229-3485.83221 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.