Abstract
Introduction
Microscopic bowel inflammation is present in up to 50% of patients with spondyloarthritis (SpA) and is associated with more severe disease. Currently no reliable biomarkers exist to identify patients at risk. Calprotectin is a sensitive marker of neutrophilic inflammation, measurable in serum and stool.
Objectives
To assess whether serum and faecal calprotectin in addition to C-reactive protein (CRP) can be used to identify patients with SpA at risk of microscopic bowel inflammation.
Methods
Serum calprotectin and CRP were measured in 125 patients with SpA. In 44 of these patients, faecal samples were available for calprotectin measurement. All 125 patients underwent an ileocolonoscopy to assess the presence of microscopic bowel inflammation.
Results
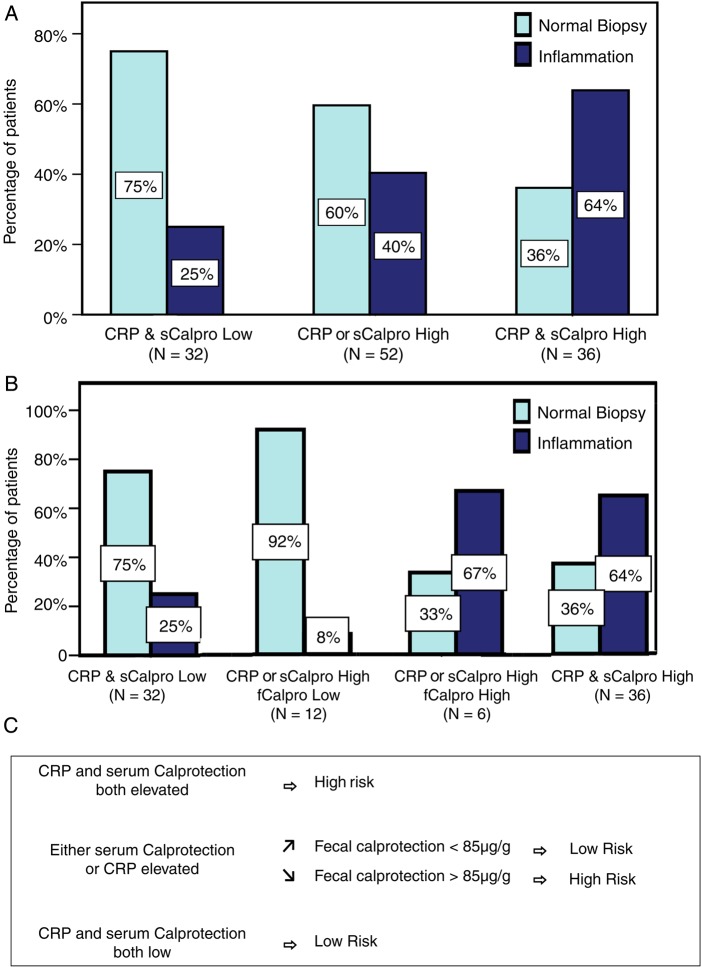
Microscopic bowel inflammation was present in 53 (42.4%) patients with SpA. Elevated serum calprotectin and CRP were independently associated with microscopic bowel inflammation. Faecal calprotectin was also significantly higher in patients with microscopic bowel inflammation. Patients with CRP and serum calprotectin elevated had a frequency of bowel inflammation of 64% vs 25% in patients with low levels of both. When either CRP or serum calprotectin was elevated, the risk was intermediate (40%) and measuring faecal calprotectin provided further differentiation. Hence we suggest a screening approach where initially serum calprotectin and CRP are assessed and, if necessary, faecal calprotectin. The model using this scenario provided an area under the ROC curve of 74.4% for detection of bowel inflammation.
Conclusions
Calprotectin measurements in stool and serum, in addition to CRP, may provide a promising strategy to identify patients with SpA at risk of bowel inflammation and could play a role in overall patient stratification.
Keywords: Spondyloarthritis, Inflammation, Disease Activity
Introduction
The link between bowel and joint in spondyloarthritis (SpA) has been established for several decades. A subgroup of patients with SpA develops inflammatory bowel disease (IBD). Conversely, patients with inflammatory bowel disease (IBD) can develop SpA. Furthermore, in the 1980s, it was demonstrated that up to 50% of all patients with SpA have microscopic bowel inflammation, without associated gastrointestinal (GI) symptoms. Bowel inflammation in SpA can affect the ileum as well as the colon, but is most prominent in the terminal ileum. Two types of inflammation can be distinguished based on histomorphology (not disease duration): acute inflammation whereby the epithelium is infiltrated with granulocytes but mucosal architecture is normal, and chronic inflammation (with acute inflammation or quiescent) showing disturbance of mucosal architecture and a chronic lymphoplasmacytic cellular infiltrate in the lamina propria. The mucosal changes seen in the latter type bear particular resemblance to those seen in early Crohn’s disease (CD).1 These findings were recently confirmed in the GIANT (Ghent Inflammatory Arthritis and Spondylitis) cohort.2 Bowel inflammation seems to be an important prognostic factor in SpA, as it was shown to be associated with more extensive bone marrow oedema of the sacroiliac joints, a higher risk of progression to ankylosing spondylitis (AS), and a higher risk of developing CD.3 4 However, diagnosis is made by means of endoscopy, as reliable biomarkers are lacking. Calprotectin, the heterodimer of S100A8 (MRP8) and S100A9 (MRP14), is a cytosolic protein expressed in phagocytic myeloid cells. It is released from activated monocytes and granulocytes at local sites of inflammation (eg, intestinal mucosa in IBD or synovium in inflammatory arthritis) during the early phase of the immune response. Extracellularly, it has prominent proinflammatory effects via toll-like receptor 4 dependent mechanisms. Hence it can be considered a marker of innate immune activation.5 Calprotectin can be measured in serum and stool, and elevated serum concentrations have been found in several inflammatory conditions. Moreover faecal calprotectin has been well established as a marker of disease activity in IBD.6 However, no study has yet addressed the relation between serum or faecal levels of calprotectin and bowel histology in SpA.
Methods
Patients and study parameters
One hundred and twenty-five patients with SpA from the GIANT cohort were included in this analysis. This is a prospective follow-up study including patients with newly diagnosed (expert opinion) axial and/or peripheral SpA, classified according to the Assessment of SpondyloArthritis international Society (ASAS) criteria.2 Patients were interviewed about their disease activity, drug intake and possible GI symptoms. A complete clinical examination was performed with scoring of tender and swollen joints, enthesitis and evaluation of axial mobility. Serum samples were collected from all patients at baseline. Serum samples of 39 healthy donors and 23 patients with rheumatoid arthritis (RA) were used as controls. We started collecting faecal samples in patients with SpA only later in the course of this cohort study, and this provided a greater challenge than serum sampling; hence faecal samples were available in only 44 patients. Stool samples were collected within a week before colonoscopy (but prior to bowel preparation) and were stored unprocessed at −80°C. Patients with significant GI symptoms or overt IBD were excluded from this analysis. Non-steroidal anti-inflammatory drug (NSAID) intake 3 months prior to the ileocolonoscopy was estimated by calculation of the NSAID index score, as proposed by ASAS.7
Ileocolonoscopy
All patients with SpA underwent an ileocolonoscopy to assess the presence of microscopic bowel inflammation. For each patient, 4–14 biopsy samples were taken of affected (if any) and unaffected regions of terminal ileum and colon.
Histological classification
Ileal and colonic biopsies were read by an experienced pathologist (CAC) in a blinded fashion. Biopsies were classified as normal, acutely or chronically inflamed, based on histomorphological characteristics, as previously described.1
Serum and faecal calprotectin measurement
Calprotectin in serum and stool was measured by monoclonal sandwich ELISA (Bühlmann Laboratories AG, Schoenenbuch, Switzerland). Serum: Bühlmann MRP8/14 ELISA: intra-assay precision 4.3%; inter-assay precision 5.8%; standard range 400–24 000 ng/mL. Stool: Bühlmann fCAL ELISA: intra-assay precision 4.7%; inter-assay precision <15%; standard range 10–600 µg/g (lower range ELISA procedure) and 30–1800 µg/g (extended range ELISA procedure).
Immunohistochemistry
S100A8 and S100A9 expression was detected on paraffin-embedded colon and ileum sections from 95 patients with SpA by rabbit IgG anti-S100A8 and anti-S100A9 antibodies, as previously described.8 Biotin-labelled goat anti-rabbit IgG (Linaris) was used as a secondary antibody. Staining was scored as positive or negative and semi-quantitatively into four categories.
Statistical analysis
Data were analysed using SPSS V.22. p<0.05 was considered statistically significant. For continuous variables the independent samples t test or ANOVA was used. In case of a skewed distribution and small sample size (faecal calprotectin), the non-parametric alternative was used (Mann–Whitney U or Kruskal–Wallis). Categorical variables were analysed using the Fisher's exact test. Cut-off values for C-reactive protein (CRP), Calprotectin in serum and stool were selected by ROC analysis with bowel inflammation as the outcome variable.
Results
Baseline characteristics
Ileocolonoscopy was performed in 125 newly diagnosed patients with SpA, all of whom provided serum samples. One hundred and four patients had axial SpA: AS (N=45 with 86.4% HLAB27+) and non-radiographic axial SpA (nr-ax SpA) (N=59 with 69.5% HLAB27+); 21 patients had peripheral SpA (55% HLAB27+). Stool samples were available for 44 patients out of this group (13 AS, 28 nr-ax SpA, 3 peripheral SpA).
Microscopic bowel inflammation in SpA and clinical characteristics
Microscopic bowel inflammation was present in 53 patients (42.4%), none of whom reported relevant GI symptoms. Macroscopic lesions were found in 31% of patients and consisted of discrete ulcerations, erosions or erythema. All but 20 patients received therapy with NSAIDs. None of the patients had prior exposure to a biological. Because we wanted to exclude possible direct interference, patients were asked to interrupt NSAID 7 days prior to colonoscopy. However, this was only introduced in the second half of the study. Fifty-one (41%) patients were able to interrupt NSAID therapy before colonoscopy. Patients on NSAIDs during colonoscopy had a higher percentage of bowel inflammation (mainly the chronic type of inflammation) than those interrupting NSAID 7 days prior to colonoscopy (p=0.016). However, this association was no longer significant when we corrected for disease activity (Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and CRP; p=0.063). No difference was seen in the NSAID index score 3 months prior to the ileocolonoscopy in patients with or without microscopic bowel inflammation (p=0.711). Age, sex, smoking status, ASAS category, HLAB27 positivity, swollen joint count, Bath Ankylosing Spondylitis Metrology Index (BASMI) and BASDAI did not differ significantly in patients with and without bowel inflammation.
Serum calprotectin in SpA, RA and healthy controls
Serum calprotectin was significantly higher in patients with SpA and RA versus healthy controls (HCs) (p<0.001 and p=0.036 resp.), but not between patients with RA and SpA (p=0.417). Smokers had significantly higher serum calprotectin levels compared with non-smokers (p=0.007), but the difference between HCs and patients with SpA or RA remained significant after correction for smoking through linear regression analysis (p<0.001 and p=0.031 respectively). Age and sex did not have a significant influence on serum calprotectin levels.
Serum calprotectin in SpA and clinical features
Serum calprotectin levels correlated moderately with CRP (r=0.386, p<0.001), but not with BASMI, BASDAI or swollen joint count. Patients with peripheral SpA had higher CRP levels (p=0.022) than those with axial SpA and tended to have higher serum calprotectin levels, although not significantly (p=0.120). There was no significant difference in serum calprotectin between patients with non-radiographic axial SpA and those with AS. NSAID use did not have a significant influence on serum calprotectin levels.
Serum markers for microscopic bowel inflammation in SpA: CRP and serum calprotectin
Serum calprotectin and CRP were significantly higher in patients with microscopic bowel inflammation versus those with normal bowel histology (p=0.033 and p=0.036 respectively) (table 1).
Table 1.
CRP, serum and faecal calprotectin according to bowel histology
| Marker | Median (range) normal biopsy |
Median (range) bowel inflammation |
p Value | Cut-off | Sens (%) | Spec (%) | Odds ratio for bowel inflammation | AUC (%) |
|---|---|---|---|---|---|---|---|---|
| CRP (mg/dl) | 0.4 (0–7.2) | 0.6 (0–10.5) | 0.036* | 0.4 | 67.3 | 54.4 | 2.46 (p=0.019) | 60.9 |
| Serum calpro (ng/mL) | 3022 (741–10 106) | 3948 (782–17 246) | 0.033* | 3340 | 60.4 | 61.1 | 2.4 (p=0.018) | 60.7 |
| Faecal calpro (µg/g) | 41 (0–1618) | 99 (14–513) | 0.036† | 85 | 64.3 | 73.3 | 4.95 (p=0.021) | 68.8 |
*Independent samples t test.
†Mann–Whitney U test.
AUC, area under the ROC curve; calpro, calprotectin (S100A8/S100A9); CRP, C-reactive protein; sens, sensitivity; spec, specificity.
Serum calprotectin remained significantly linked with bowel inflammation after adjustment for other associated factors, that is, elevated CRP and NSAID use, and after correction for sample age, time between serum sampling and colonoscopy, peripheral SpA and smoking (p=0.005). Hence, serum calprotectin was a marker for bowel inflammation independent of CRP. In line with this, serum calprotectin was linked especially with inflammation of the acute type, whereas CRP was linked with chronic inflammation (not shown). Next a cut-off for serum calprotectin and CRP was chosen using receiver operating characteristic (ROC) analysis. The maximum sensitivity and specificity for detection of bowel inflammation was found at 3340 ng/mL and 0.4 mg/dL respectively. Results for both markers are summarised in table 1.
Faecal calprotectin
Stool samples were available from 44 patients with SpA, 14 of whom had microscopic bowel inflammation (acute N=8/chronic N=6). Faecal calprotectin was significantly higher in patients with microscopic bowel inflammation (p=0.036). Faecal calprotectin correlated with CRP (rS=0.481; p=0.002), but not with serum calprotectin (p=0.384). NSAID use significantly influenced faecal calprotectin levels: faecal calprotectin was higher in patients on NSAIDs at the time of colonoscopy/stool sampling (p=0.018) and was significantly correlated with the NSAID index score (rS=0.330, p=0.029). Faecal calprotectin was linked with both types of inflammation, but higher values were seen in chronic inflammation. A cut-off value for faecal calprotectin was also assessed. The optimal sensitivity (64.3%) and specificity (73.3%) for detection of bowel inflammation was seen with a cut off of 85 µg/g (table 1). Elevated faecal calprotectin remained significantly (p=0.023) associated with bowel inflammation after adjustment for the NSAID index score; however, given the low number of samples, correction for other variables could not be fully assessed.
Prediction of microscopic bowel inflammation using serum calprotectin, CRP and faecal calprotectin
The final step was to develop a prediction model for bowel inflammation using CRP, serum and faecal calprotectin. Given the low number of faecal samples and practical difficulties that faecal samples entail, we started with the two serum markers: CRP and serum calprotectin. Since serum calprotectin and CRP were independently associated with microscopic bowel inflammation, we used combinations of both markers for risk assessment: the frequency of bowel inflammation for each possible combination is depicted in figure 1A. As a second step, the added value of faecal calprotectin was assessed for each combination. When serum calprotectin and CRP were low or when both were elevated, the addition of faecal calprotectin did not add extra information. In patients with either CRP or serum calprotectin elevated, however, the frequency of bowel inflammation was significantly higher in patients who also had an elevated faecal calprotectin versus those with low faecal calprotectin (figure 1B). Figure 1C shows a suggested screening flow chart based on these results. The logistic regression model using these four scenarios generated an area under the ROC curve (predicted probabilities) of 74.4% ((0.639–0.849%) p<0.001) for bowel inflammation.
Figure 1.
(A) Frequency of microscopic bowel inflammation according to C-reactive protein (CRP) and serum calprotectin results. (B) Same as (A) with the addition of faecal calprotectin when relevant (not available in all patients). (C) Screening flow chart for microscopic bowel inflammation in SpA based on (B). fCalpro, faecal calprotectin; sCalpro, serum calprotectin.
Immunohistochemical staining of bowel biopsies for S100A8 and S100A9
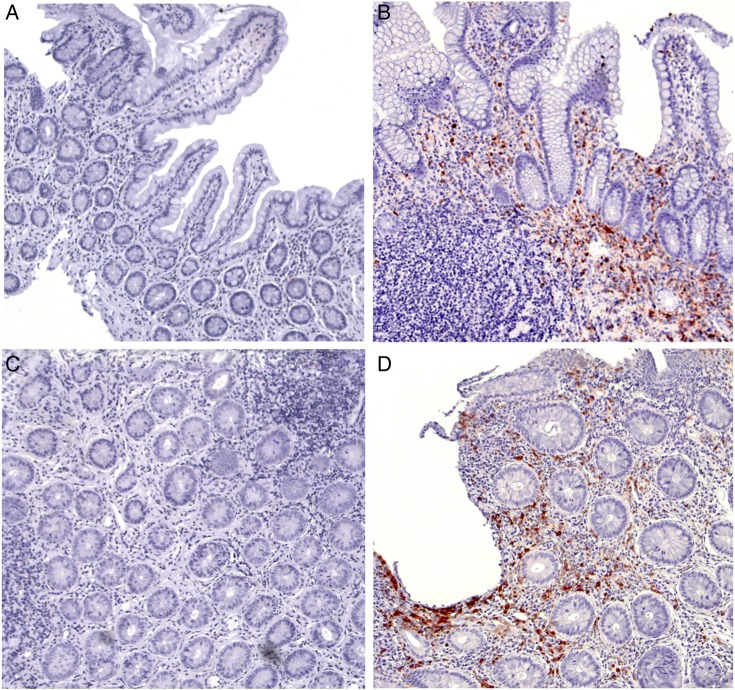
Bowel biopsies from 95 patients with SpA (44 with inflammation) were available for immunohistochemical (IHC) staining with S100A8 and S100A9 antibodies (figure 2). Biopsies with active inflammation (ie, with granulocytic infiltration) showed high expression of S100A8 and S100A9 compared with absent/minimal staining on normal biopsies or chronic inactive biopsies (figure 2). In all cases, stainings for S100A8 and S100A9 were similar. After correction for the effect of smoking, sample age and peripheral SpA on serum calprotectin levels, IHC positivity and calprotectin serum levels were significantly associated (p=0.013, data not shown).
Figure 2.
Staining for S100A8 and S100A9 shows positive staining in actively inflamed biopsies. (A) Normal ileum; (B) Acute inflammation ileum; (C) Chronic inflammation ileum: low activity; (D) Chronic inflammation ileum: high activity.
Discussion
The aim of this study was to evaluate serum and faecal calprotectin (S100A8/S100A9) as biomarkers for bowel inflammation in SpA. Patients with SpA represent a population at high risk for intestinal inflammation: they frequently have microscopic bowel inflammation, which is mainly subclinical, so that screening based on symptoms is not possible. Additionally, a smaller percentage of patients with SpA develops overt IBD over time, the risk of this being significantly greater if microscopic bowel inflammation is present at diagnosis.
Serum calprotectin was significantly higher in SpA patients with microscopic bowel inflammation, independent of CRP, elevated levels of which were also linked with bowel inflammation. Moreover, combining CRP and serum calprotectin provided added value for detection of bowel inflammation. Although much fewer stool samples were available, faecal calprotectin was also shown to be significantly higher in patients with microscopic bowel inflammation. Faecal calprotectin was linked with both types of inflammation, but higher values were seen in chronic inflammation, whereas serum calprotectin was primarily linked with inflammation of the acute type. The difference between serum and faecal calprotectin, according to the histological type of inflammation, might be explained by increased epithelial shedding occurring in chronic inflammation. This also reflected itself in the lack of correlation between serum and faecal calprotectin values (whereas there was a correlation between CRP and faecal calprotectin).
Although faecal calprotectin provided the best sensitivity and specificity for detection of bowel inflammation as a single marker, it has some disadvantages, such as reluctance on the side of the patient to collect stool samples and the fact that it is influenced by NSAID use, which is an important confounder in SpA. For this reason, we further assessed an approach where initially serum calprotectin and CRP are assessed and only in second-line faecal calprotectin. When CRP and serum calprotectin were low or when both were high, determination of faecal calprotectin did not provide added value for detection of bowel inflammation, although it did provide further differentiation when only one of these serum markers was elevated. Based on this, we propose a two-step screening approach using CRP and serum calprotectin, and only if necessary, faecal calprotectin for risk assessment of microscopic bowel inflammation in SpA. The logistic regression model using this screening approach generated an area under the ROC curve of 74.4%. However, this needs to be confirmed in an independent follow-up study.
In IBD, calprotectin is increased in inflamed bowel mucosa, serum and stool, and concentrations correlate with disease activity. Faecal calprotectin especially has been well established as a screening tool for suspected IBD as well as for evaluating disease activity.6 9–12 Importantly, faecal calprotectin was also shown to be elevated in clinically inactive patients with IBD, presumably reflecting ongoing subclinical inflammation.11 13 Accordingly, faecal calprotectin levels have been shown to correlate more closely with histological (microscopic) than macroscopic (endoscopic) scores of inflammation.11 The cut-offs defined for the faecal calprotectin ELISA we used in this study are based on distinguishing symptomatic patients with functional diseases from patients with various organic diseases: values below 50 are considered normal, values between 50 and 200 μg/g could represent mild organic disease and values >200 μg/g are considered indicative of active organic disease. The most optimal cut-off for detecting microscopic bowel inflammation in our cohort of patients with SpA was 85 µg/g.
Calprotectin in serum and stool has been evaluated in other SpA studies: Elevated serum levels of calprotectin in SpA were also described by De Rycke et al14 and Oktayoglu et al.15 Similar to our analysis, a correlation was found with CRP, but not with BASDAI, Ankylosing Spondylitis Disease Activity Score (ASDAS) or swollen joint count. Recently serum calprotectin was shown to be an independent marker of radiographic spinal progression in axial SpA.16 Interestingly, bowel inflammation in SpA was previously shown to be linked with evolution to AS.3
Klingberg et al17 found increased faecal levels of calprotectin in two-thirds of patients with AS. However, in this study, only eight patients received a colonoscopy. Bjarnason et al18 also found increased faecal calprotectin in first-degree relatives of patients with AS, but no endoscopy was performed. Hence, our study is the first in which measurements of calprotectin in SpA are directly linked to bowel histology. We also confirmed high expression of S100A8 and S100A9 in actively inflamed intestinal mucosa, and positive immunostaining was linked with higher serum calprotectin levels, suggesting that serum levels originate at least partly from inflamed bowel mucosa.
As calprotectin reflects phagocyte activation due to any cause, a large number of inflammatory conditions may lead to moderately increased serum levels (whereas faecal calprotectin selectively reflects intestinal inflammation).19 Accordingly, we found no significant difference between patients with RA and those with SpA, and observed that patients with peripheral SpA tended to have higher serum calprotectin levels. However, serum calprotectin remained significantly associated with bowel inflammation after correction for the presence of peripheral arthritis.
Strengths of this study are the inclusion of a well defined cohort of newly diagnosed patients with SpA and the availability of histological diagnosis of bowel inflammation in all patients, something that was absent in previous trials investigating calprotectin in SpA. The main weakness is the low number of faecal samples; on the one hand due to a lapse in planning, but also due to patient reluctance to provide faecal samples, which, on the other hand, might also reflect the situation in daily practice.
Conclusion
Serum and faecal calprotectin represent surrogate markers for microscopic bowel inflammation in SpA. Our results suggest that serum and faecal calprotectin measurements in addition to CRP may be useful in identifying patients with SpA at higher risk of subclinical bowel inflammation who might benefit from further invasive checkup. Hence, these markers could play a role in overall patient stratification.
Acknowledgments
The investigators would like to thank Claudia Solé and Lynn Supply for excellent technical assistance.
Footnotes
Contributors: HC, FVdB and DE: study concept and design. HC, GV, TV, JR, MBD, ML, DF, CAC, MDV, JD, FVdB and DE: data acquisition. HC and SB: analysis and interpretation of data. HC, FVdB and DE: manuscript preparation. GV, SB, KD, TV, JR, DF, CAC and JD: manuscript revision.
Funding: DE is supported by a fund of Scientific Research–Flanders (FWO) and the Research Council of Ghent University. DE is also a member of a multidisciplinary research platform (MRP) of Ghent University and is supported by Interuniversity Attraction Pole (IUAP) grant Devrepair from the Belspo Agency (project P7/07). This project received funding from the EU’s seventh framework programme under EC-GA no. 305266 ‘MIAMI’.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The study protocol was approved by the Ethical Committee of Ghent University Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cuvelier C, Barbatis C, Mielants H, et al. . Histopathology of intestinal inflammation related to reactive arthritis. Gut 1987;28:394–401. 10.1136/gut.28.4.394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Praet L, Van den Bosch FE, Jacques P, et al. . Microscopic gut inflammation in axial spondyloarthritis: a multiparametric predictive model. Ann Rheum Dis 2013;72:414–17. 10.1136/annrheumdis-2012-202135 [DOI] [PubMed] [Google Scholar]
- 3.De Vos M, Mielants H, Cuvelier C, et al. . Long-term evolution of gut inflammation in patients with spondyloarthropathy. Gastroenterology 1996;110:1696–703. 10.1053/gast.1996.v110.pm8964393 [DOI] [PubMed] [Google Scholar]
- 4.Van Praet L, Jans L, Carron P, et al. . Degree of bone marrow oedema in sacroiliac joints of patients with axial spondyloarthritis is linked to gut inflammation and male sex: results from the GIANT cohort. Ann Rheum Dis 2014;73:1186–9. 10.1136/annrheumdis-2013-203854 [DOI] [PubMed] [Google Scholar]
- 5.Vogl T, Tenbrock K, Ludwig S, et al. . Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med 2007;13:1042–9. 10.1038/nm1638 [DOI] [PubMed] [Google Scholar]
- 6.Foell D, Wittkowski H, Roth J. Monitoring disease activity by stool analyses: from occult blood to molecular markers of intestinal inflammation and damage. Gut 2009;58:859–68. 10.1136/gut.2008.170019 [DOI] [PubMed] [Google Scholar]
- 7.Dougados M, Simon P, Braun J, et al. . ASAS recommendations for collecting, analysing and reporting NSAID intake in clinical trials/epidemiological studies in axial spondyloarthritis. Ann Rheum Dis 2011;70:249–51. 10.1136/ard.2010.133488 [DOI] [PubMed] [Google Scholar]
- 8.Seeliger S, Vogl T, Engels IH, et al. . Expression of calcium-binding proteins MRP8 and MRP14 in inflammatory muscle diseases. Am J Pathol 2003;163:947–56. 10.1016/S0002-9440(10)63454-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meuwis MA, Vernier-Massouille G, Grimaud JC, et al. . Serum calprotectin as a biomarker for Crohn's disease. J Crohns Colitis 2013;7:e678–683. 10.1016/j.crohns.2013.06.008 [DOI] [PubMed] [Google Scholar]
- 10.Schmid KW, Lugering N, Stoll R, et al. . Immunohistochemical demonstration of the calcium-binding proteins MRP8 and MRP14 and their heterodimer (27E10 antigen) in Crohns-disease. Hum Pathol 1995;26:334–7. 10.1016/0046-8177(95)90067-5 [DOI] [PubMed] [Google Scholar]
- 11.Fagerberg UL, Lööf L, Lindholm J, et al. . Fecal calprotectin: a quantitative marker of colonic inflammation in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2007;45:414–20. 10.1097/MPG.0b013e31810e75a9 [DOI] [PubMed] [Google Scholar]
- 12.Van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ 2010;341:c3369 10.1136/bmj.c3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiang JY, Ouyang Q, Li GD, et al. . Clinical value of fecal calprotectin in determining disease activity of ulcerative colitis. World J Gastroenterol 2008;14:53–7. 10.3748/wjg.14.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Rycke L, Baeten D, Foell D, et al. . Differential expression and response to anti-TNF alpha treatment of infiltrating versus resident tissue macrophage subsets in autoimmune arthritis. J Pathol 2005;206:17–27. 10.1002/path.1758 [DOI] [PubMed] [Google Scholar]
- 15.Oktayoglu P, Bozkurt M, Mete N, et al. . Elevated serum levels of calprotectin (myeloid-related protein 8/14) in patients with ankylosing spondylitis and its association with disease activity and quality of life. J Investig Med 2014;62:880–4. 10.1097/JIM.0000000000000095 [DOI] [PubMed] [Google Scholar]
- 16.Turina MC, Sieper J, Yeremenko N, et al. . Calprotectin serum level is an independent marker for radiographic spinal progression in axial spondyloarthritis. Ann Rheum Dis 2014;73:1746–8. 10.1136/annrheumdis-2014-205506 [DOI] [PubMed] [Google Scholar]
- 17.Klingberg E, Carlsten H, Hilme E, et al. . Calprotectin in ankylosing spondylitis—frequently elevated in feces, but normal in serum. Scand J Gastroenterol 2012;47:435–44. 10.3109/00365521.2011.648953 [DOI] [PubMed] [Google Scholar]
- 18.Bjarnason I, Helgason KO, Geirsson AJ, et al. . Subclinical intestinal inflammation and sacroiliac changes in relatives of patients with ankylosing spondylitis. Gastroenterology 2003;125:1598–605. 10.1053/j.gastro.2003.08.035 [DOI] [PubMed] [Google Scholar]
- 19.Frosch M, Ahlmann M, Vogl T, et al. . The myeloid-related proteins 8 and 14 complex, a novel ligand of toll-like receptor 4, and interleukin-1beta form a positive feedback mechanism in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum 2009;60:883–91. 10.1002/art.24349 [DOI] [PubMed] [Google Scholar]