Abstract
BACKGROUND: Identification of high-grade meningiomas in preoperative magnetic resonance imaging (MRI) is important for optimized surgical strategy and best possible resection. Numerous studies investigated subjectively determined morphological features as predictors of tumor biology in meningiomas. The aim of this study was to identify the predictive value of more reliable, quantitatively measured signal intensities in MRI for differentiation of high- and low-grade meningiomas and identification of meningiomas with high proliferation rates, respectively. PATIENTS AND METHODS: Sixty-six patients (56 World Health Organization [WHO] grade I, 9 WHO grade II, and 1 WHO grade I) were included in the study. Preoperative MRI signal intensities (fluid-attenuated inversion recovery [FLAIR], T1 precontrast, and T1 postcontrast as genuine and normalized values) were correlated with Ki-67 expression in tissue sections of resected meningiomas. Differences between the groups (analysis of variance) and Spearman rho correlation were computed using SPSS 22. RESULTS: Mean values of genuine signal intensities of meningiomas in FLAIR, T1 native, and T1 postcontrast were 323.9 ± 59, 332.8 ± 67.9, and 768.5 ± 165.3. Mean values of normalized (to the contralateral white matter) signal intensities of meningiomas in FLAIR, T1 native, and T1 postcontrast were 1.5 ± 0.3, 0.8 ± 0.1, and 1.9 ± 0.4. There was no significant correlation between MRI signal intensities and WHO grade or Ki-67 expression. Signal intensities did not differ significantly between WHO grade I and II/III meningiomas. Ki-67 expression was significantly increased in high-grade meningiomas compared with low-grade meningiomas (P < 0.01). Objectively measured values of MRI signal intensities (FLAIR, T1 precontrast, and T1 postcontrast enhancement) did not distinguish between high-grade and low-grade meningiomas. Furthermore, there was no association between MRI signal intensities and Ki-67 expression representing proliferative activity.
Meningiomas are among the most common brain tumors. Their incidence is about 1%, and they account for almost one third of all primary intracranial masses. The majority of meningiomas are very slowly growing and nonsymptomatic or minimally symptomatic entities, discovered as incidental findings on neuroimaging [1]. The World Health Organization (WHO) classification system distinguishes 3 histological grades and 15 subtypes and is a well-accepted tool for prediction of prognosis. Although most meningiomas are benign masses, certain histological subtypes reveal very high recurrence rates despite the tumors’ seemingly total removal. Grade II (atypical) and grade III (anaplastic) meningiomas are associated with an increased risk of recurrence, are more aggressive, and show invasive behavior [2]. Grade I meningiomas are generally considered as benign tumors, but recent studies indicate substantial neurological deficits and impaired long-term survival due to tumor recurrence and stroke despite their low histopathological grading in a considerable proportion of cases [3], [4]. Increased mitotic activity (more than 4 mitoses per 10 high-power fields) and elevated Ki-67 expression (Ki-67 index of more than 5% of nuclei) are reliable histopathological markers for tumor recurrence [2].
Because histopathological grading alone does not predict outcome satisfyingly, numerous studies investigated the value of preoperative magnetic resonance imaging (MRI) for prognostics. For example, Liu et al. demonstrated that hyperintensity on diffusion-weighted imaging, heterogeneous gadolinium enhancement, disruption of the arachnoid at brain tumor interface, T2 hyperintense peritumoral edema, and irregular tumor shape were independent predictors of non–grade I meningioma [5]. Other works produced comparable results, although some of these studies underline the importance of positive capsular enhancement [6], [7], whereas others emphasize the predictive value of peritumoral edema [5], [8]. All the above-cited works investigated morphological features of meningiomas summarized in subjective scoring systems, but not one of the studies objectively analyzed values of SIs in commonly used preoperative MRI sequences.
Therefore, the aim of this study was to investigate the predictive value of genuine and normalized SIs of standardized preoperative MRI (T1 pre- and postcontrast, T2, and fluid-attenuated inversion recovery [FLAIR]) as in vivo predictors of proliferative activity of meningiomas.
Material and Methods
This study was approved by the institutional review board (Martin Luther University Ethics Committee).
Patients
Overall, 66 patients with different meningiomas were included into the retrospective analysis. There were 50 women and 16 men with a mean age of 59.7 ± 15.8 years. In 56 patients (approximately 84.8%), WHO grade I tumors were diagnosed. Most frequently, meningothelial meningiomas (n = 32,) followed by transitional (n = 12), fibroblastic (n = 7), angiomatous (n = 4), and psammomatous (n = 1) subtypes were identified. Grade II tumors were identified in 9 cases (13.6%) and grade III in 1 (1.5%).
MRI
In all patients, cranial MRI was performed using a 1.5-T device (Magnetom Vision Sonata Upgrade, Siemens, Erlangen, Germany). The imaging protocol included the following sequences:
-
1.
Axial T2-weighted (T2w) fluid-attenuated inversion recovery (FLAIR) sequence (repetition time/echo time: 8000/129, slice thickness: 5 mm, acquisition matrix: 256 × 256, field of view: 230 mm);
-
2.
Axial and coronal T1-weighted (T1w) spin echo sequences (repetition time/echo time: 562/16, slice thickness: 5 mm, acquisition matrix: 256 × 256, field of view: 230 mm) before and after intravenous application of contrast medium (gadopentate dimeglumine, Magnevist, Bayer Schering Pharma, Leverkusen, Germany).
All images were available in digital form and were analyzed by one radiologist (A. S., 14 years of radiological experience) on a PACS workstation (Centricity PACS, GE Medical Systems, Milwaukee, WI).
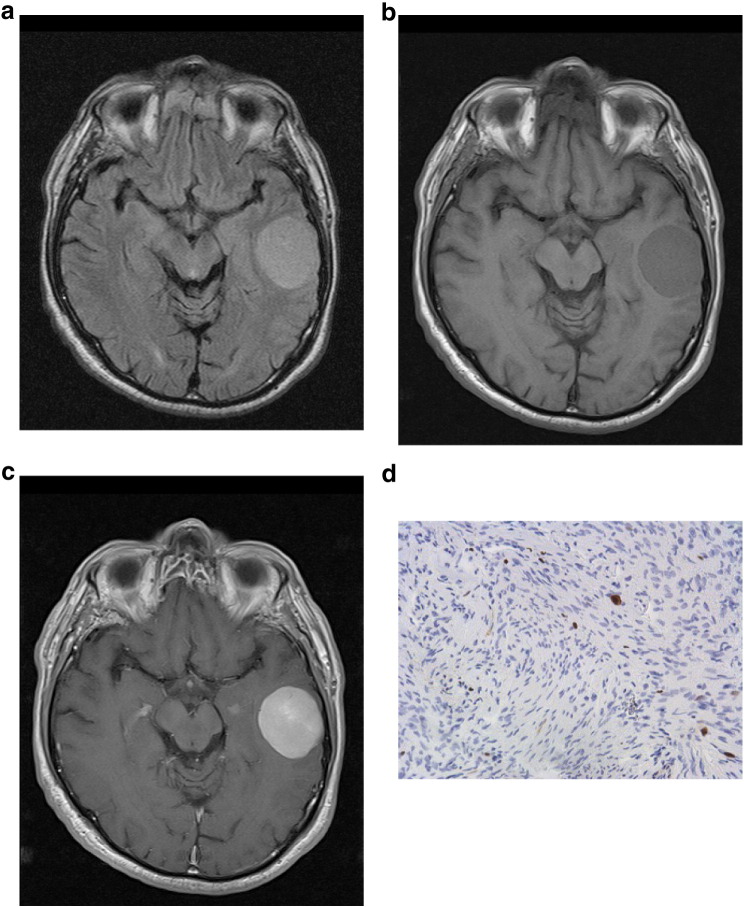
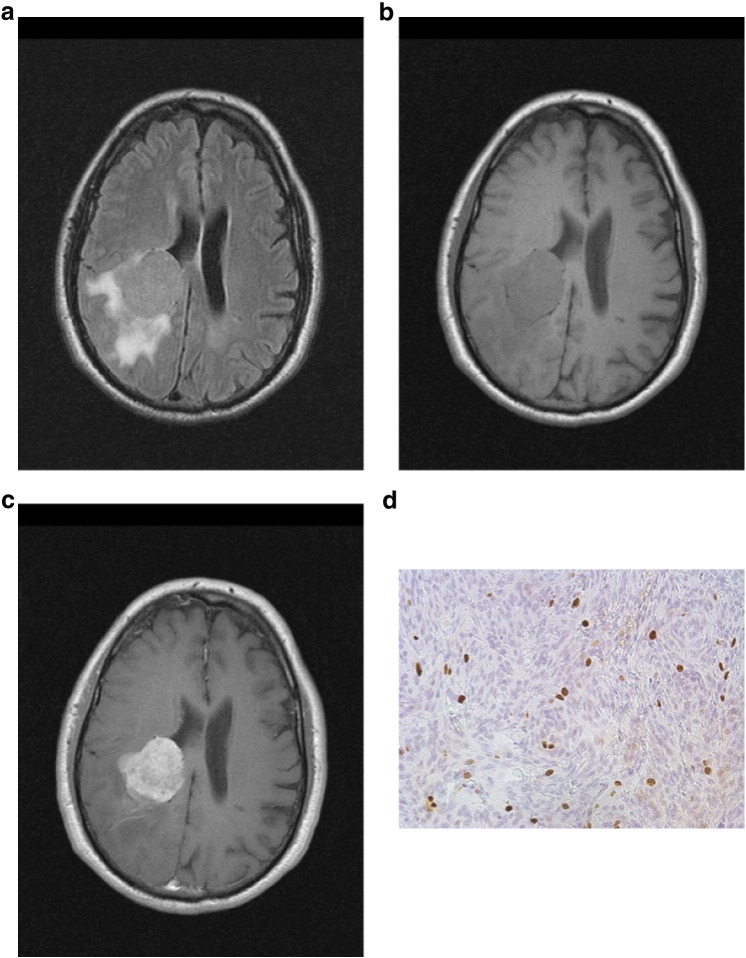
The slices with the largest diameter of each tumor were selected for signal intensity (SI) calculation. In every case, a polygonal region of interest (ROI) as large as possible was manually drawn on postcontrast T1w images. Every ROI was automatically placed also on all other images (T2w and precontrast T1w). Cystic and necrotic areas as well as large vessels of the tumors were not considered for evaluation. In all images, mean SI values were estimated. Furthermore, ROIs were drawn in the normal white matter of the contralateral hemisphere (SI white matter). Normalized SI was calculated in every case as the ratio SI meningioma/SI white matter. In addition, for each lesion, the ratio SI on postcontrast T1w/SI on precontrast T1w was calculated (SI contrast). Figure 1, Figure 2, a–c, exemplarily show axial images (FLAIR, T1 precontrast, T1 postcontrast) of WHO grade I (transitional) and WHO grade II (atypical) meningiomas.
Figure 1.
(a–d) Imaging findings (FLAIR, T1 precontrast, T1 postcontrast) and Ki-67 staining of a WHO grade I meningioma. Signal intensities were as follows: normalized FLAIR SI = 1.47, normalized precontrast T1 SI = 0.81, normalized postcontrast SI = 1.77, and postcontrast SI/precontrast SI = 2.24. Ki-67 index = 3%.
Figure 2.
(a–d) Imaging findings (FLAIR, T1precontrast, T1 postcontrast) and Ki-67 staining of a WHO grade II (atypical) meningioma. Signal intensities were as follows: normalized FLAIR SI = 1.45, normalized T1 precontrast SI = 0.87, normalized postcontrast SI = 1.88, and postcontrast SI/precontrast SI = 1.97. Ki-67 index = 18%.
Histopathology and Immunohistochemistry
All included meningiomas were surgically resected and histopathologically analyzed. Tumor grading was classified according to the WHO [2].
In every case, the proliferation index was estimated on Ki-67 antigen-stained specimens by using MIB-1 monoclonal antibody (DakoCytomation, Denmark) as reported previously [9]. Two high-power fields (0.16 mm2 per field, × 400) were analyzed. The area with the highest number of positive tumor nuclei was selected. Figures 1d and 2d exemplarily show Ki-67 immunostaining of WHO grade I (transitional) and WHO grade II (atypical) meningiomas.
Statistical Analysis
Statistical analysis was performed using SPSS Version 22. Differences between groups were assessed using one-way analysis of variance. Correlations between Ki-67 expression and genuine or normalized SIs, respectively, were calculated using a Spearman rho correlation. Significance level was set to .05.
Results
MR SIs (T1 precontrast, FLAIR, T1 postcontrast, and normalized SIs) of the investigated grade I to III meningiomas and Ki-67 expression (%) are shown in Table 1.
Table 1.
MRI SIs Displayed as Genuine and Normalized Values (SI of the Respective Meningioma‚ m’, Divided by the SI of the Unaffected Contralateral White Matter ‚wm’) and Ki-67 Expression of All Investigated Meningiomas
| Mean ± SD | Median | Range | |
|---|---|---|---|
| T1 meningioma | 332.8 ± 67.9 | 324 | 197-500 |
| T1 white matter | 407.2 ± 63.5 | 411 | 276-600 |
| T1 m/wm | 0.8 ± 0.1 | 0.8 | 0.6-1.0 |
| FLAIR meningioma | 323.9 ± 59 | 317 | 200-495 |
| FLAIR white matter | 211.5 ± 26.8 | 210 | 145-285 |
| FLAIR m/wm | 1.5 ± 0.3 | 1.5 | 1.0-2.5 |
| T1 postcontrast meningioma | 768.5 ± 165.3 | 764 | 512-1300 |
| T1 postcontrast white matter | 407.7 ± 67.6 | 413.5 | 256-630 |
| T1 pos contrast m/wm | 1.9 ± 0.4 | 1.9 | 1.1-2.9 |
| T1 postcontrast m/wm T1 native m/wm |
2.3 ± 0.4 | 2.3 | 1.7-3.5 |
| Ki-67 expression % | 5.2 ± 5.3 | 3 | 1-20 |
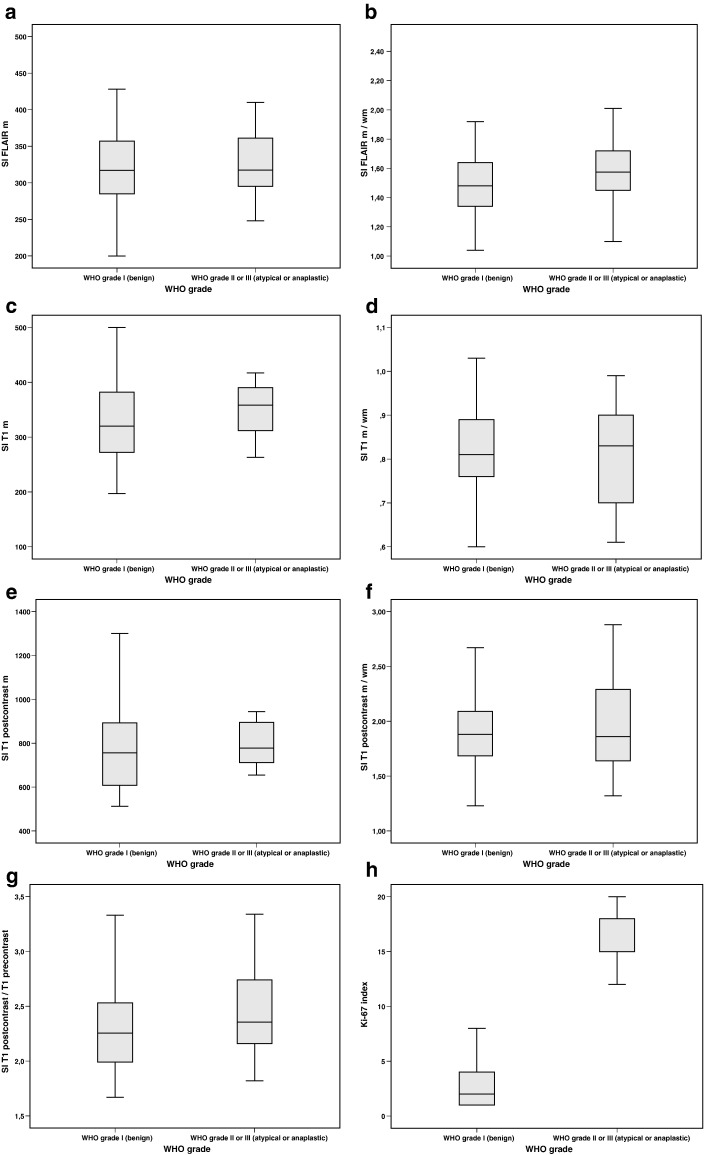
One-way analysis of variance did not show significant differences between meningiomas of WHO grade I, II, or III regarding their MRI SIs. The difference in Ki-67 expression achieved statistical significance (P < .01) between low-grade and high-grade meningiomas. Figure 3, a–h, summarize the relation of MRI SIs and Ki-67 expression between WHO grade I and WHO grade II/III meningiomas. For reasons of simplicity and clarity, high-grade meningiomas (grade II and III meningiomas) are displayed together and compared with grade I meningiomas.
Figure 3.
(a–h) Box plots give an overview on the relation of MRI SI (a–g) and Ki-67 index between low-grade and high-grade meningiomas.
No correlation was found between MRI SIs and Ki-67 expression. Table 2 displays results of statistical analysis of the correlation between SIs (genuine and normalized) and Ki-67 expression.
Table 2.
Statistical Correlations between Genuine and Normalized SIs of Meningioma and Ki-67 Expression
| T1 m | T 1 m/wm | FLAIR m | FLAIR m/wm | T1 pc m | T1 pc m/wm | T1 pc/T1 native | |
|---|---|---|---|---|---|---|---|
| Ki-67 % |
r = − 0.9 P = − .489 |
r = − 0.186 P = .152 |
r = − 0.48 P = .723 |
r = 0.002 P = .991 |
r = − 0.99 P = .439 |
r = − 0.109 P = .396 |
r = 0.029 P = .827 |
Discussion
We investigated in meningiomas the relationship between objectively measurable SIs on standard preoperative MRI and the proliferative activity in histopathology. Expression of the proliferation associated antigen Ki-67 was significantly higher in grade II and III meningiomas, but there was neither a significant difference of MRI SIs among grade I, II, or III meningiomas nor a correlation between Ki-67 expression and MRI SIs.
Preoperative MRI is a valuable tool for identification and follow-up of meningiomas with aggressive behavior in the clinical routine. High-grade meningiomas are a significant cause of morbidity and mortality and need to be identified prior to surgery to achieve the best resection. Numerous studies investigated morphological features of meningiomas in MRI to identify in vivo attributes that predict invasiveness and increased risk of recurrence [5], [6], [7], [8], [10], [11]. Consensually, peritumoral edema, ill-defined tumor margins, heterogeneous contrast enhancement, and absence of a subarachnoidal rim surrounding the tumor are important indicators of non–grade I meningiomas with aggressive tumor growth, high recurrence risk, and a tendency of infiltration of surrounding parenchyma. Nevertheless, these are subjective criteria only describing morphological features. Interobserver and intraobserver variability significantly reduces the reliability of these prediction models [6]. Previously, few studies investigated imaging parameters semiquantitatively and their relationship to histopathological features with at least partially contradictory findings. In earlier years Maiuri and coworkers [12] analyzed T1 and T2 SIs (clinically applied as categories of hypointensity, isointensity, and hyperintensity) in meningiomas and found that T1w images may predict the presence of cysts and intratumoral blood vessels, whereas T2w images may be able to give information about histological subtype, vascularity, and consistency. More recently, Hadidy and colleagues examined the association between SI on T1w and T2w FLAIR MRI (with very approximate categories of hypointensity, isointensity, and hyperintensity) as well as contrast enhancement patterns and histopathological grading [13] and found no correlation between MRI and histopathology. Besides these studies, other authors investigated the association between cellularity, Ki-67 expression, and different apparent diffusion coefficient fractions in low-grade and high-grade meningiomas and were able to show that apparent diffusion coefficient mean is able to distinguish between grade I and grade II/III meningiomas [14]. To the best of our knowledge, no further studies exist that investigated the correlation between objectively measurable, numeric MRI SIs and histopathological features, especially Ki-67 expression representing proliferative activity.
This study clarifies that even numerically quantified SIs of meningiomas in preoperative MRI cannot distinguish between low-grade, noninvasive meningiomas and high-grade, aggressive-invasive meningiomas.
Our work suffers some limitations. Firstly, it is of retrospective nature, and secondly, the number of analyzed high-grade meningiomas is comparatively small.
Conclusion
To optimize surgical therapy, it is necessary to identify aggressive and invasive meningiomas on preoperative MRI. Numerous subjective scoring systems have been developed on the basis of morphological features of meningiomas in MRI to separate high-grade from low-grade meningiomas with varying sensitivities. This is the first study that investigated the value of objectively measured, quantified SIs as predictors of aggressive tumor behavior. Counterintuitively, quantitatively measured SIs of standardized preoperative MRI were not able to differentiate low -from high-grade meningiomas or to identify meningiomas with a high proliferative activity.
There are no conflicts of interest.
References
- 1.Vernooij MW, Ikram MA, Tanghe HL, Vincent AJPE, Hofman A, Krestin GP, Niessen W.J., Breteler M.M.B., van der Lugt A. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357:1821–1828. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- 2.Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006;5:1045–1054. doi: 10.1016/S1474-4422(06)70625-1. [DOI] [PubMed] [Google Scholar]
- 3.van Alkemade H, de Leau M, Dieleman EMT, Kardaun JWPF, van Os R, Vandertop WP, van Furth W.R., Stalpers L.J.A. Impaired survival and long-term neurological problems in benign meningioma. Neuro-Oncology. 2012;14:658–666. doi: 10.1093/neuonc/nos013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM. Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol. 1997;21:1455–1465. doi: 10.1097/00000478-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Liu Yi, Chotai S., Chen M., Jin S., Qi S.-T., Pan J. 2015. SCMCSJS-TQJP. Preoperative radiologic classification of convexity meningioma to predict the survival and aggressive meningioma behavior; pp. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin B-J, Chou K-N, Kao H-W, Lin C, Tsai W-C, Feng S-W, Lee M.-S., Hueng D.-Y. Correlation between magnetic resonance imaging grading and pathological grading in meningioma. J Neurosurg. 2014;121:1201–1208. doi: 10.3171/2014.7.JNS132359. [DOI] [PubMed] [Google Scholar]
- 7.Kawahara Y, Nakada M, Hayashi Y, Kai Y, Uchiyama N, Nakamura H., Kuratsu J.-I., Hamada J.-I. Prediction of high-grade meningioma by preoperative MRI assessment. J Neurooncol. 2012;108:147–152. doi: 10.1007/s11060-012-0809-4. [DOI] [PubMed] [Google Scholar]
- 8.Hashiba T, Hashimoto N, Maruno M, Izumoto S, Suzuki T, Kagawa N, Yoshimine T. Scoring radiologic characteristics to predict proliferative potential in meningiomas. Brain Tumor Pathol. 2006;23:49–54. doi: 10.1007/s10014-006-0199-4. [DOI] [PubMed] [Google Scholar]
- 9.Surov A, Caysa H, Wienke A, Spielmann RP, Fiedler E. Correlation between different ADC fractions, cell count, Ki-67, total nucleic areas and average nucleic areas in meningothelial meningiomas. Anticancer Res. 2015;35:6841–6846. [PubMed] [Google Scholar]
- 10.Yang S-Y, Park C-K, Park S-H, Kim DG, Chung YS, Jung H-W. Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatry. 2008;79:574–580. doi: 10.1136/jnnp.2007.121582. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda D, Chiocca EA. Editorial: dural tail sign. J Neurosurg. 2012;117:643–644. doi: 10.3171/2012.2.JNS12266. [DOI] [PubMed] [Google Scholar]
- 12.Maiuri F, Iaconetta G, de Divitiis O, Cirillo S, Di Salle F, De Caro ML. Intracranial meningiomas: correlations between MR imaging and histology. Eur J Radiol. 1999;31:69–75. doi: 10.1016/s0720-048x(98)00083-7. [DOI] [PubMed] [Google Scholar]
- 13.Hadidy AM, Nadi MM, Ahmad TM, Al-Hussaini MA, Al-Abaddi AA, Musharbash AF, Maani W.S. Descriptive epidemiological analysis, MRI signals intensity and histopathological correlations of meningiomas. Neurosciences (Riyadh) 2010;15:11–14. [PubMed] [Google Scholar]
- 14.Surov A, Gottschling S, Mawrin C, Prell J, Spielmann RP, Wienke A, Fiedler E. Diffusion-weighted imaging in meningioma: prediction of tumor grade and association with histopathological parameters. TRANON The Authors. 2015;8:517–523. doi: 10.1016/j.tranon.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]