Abstract
Kahalalide F is a depsipeptide under development by PharmaMar as a potential treatment for solid tumors. It is currently undergoing phase II clinical trials.
Introduction
The kahalalides consist of a series of depsipeptides that were first identified from the herbivorous marine mollusk Elysia rufescens, and later from its algal diet of Bryopsis pennata [506285]. The size and composition of this series of peptides is highly variable, ranging from a C31 tripeptide to a C75 tridecapeptide, and many show activity against cancer and AIDS-related opportunistic infections [526209]. The most active and largest peptide, kahalalide F, exhibits significant activity in vitro against various solid tumor cell lines, but significantly less activity against leukemia cells (eg, P388) [506285]. In particular, hormone-independent prostate tumors have shown sensitivity to kahalalide F both in vitro and in vivo [402301], and the drug was therefore evaluated in androgen-refractory prostate cancer in a phase I trial [543658]. Hepatoma cells have also shown sensitivity to kahalalide F [531407], and a phase II trial has been conducted in patients with liver cancer [491229]. Liver cancer globally represents one of the most common forms of cancer, with two to five cases per 100,000 people in Europe and the US. Its incidence is currently on the rise due to hepatitis C infections, and because of the aggressiveness of this type of cancer, therapeutic options have been typically limited to surgery. In cases of metastasis, conventional chemotherapy must be utilized, however, liver carcinoma has shown serious resistance to existing anticancer drugs, making the development of new lines of chemotherapy with activity against liver cancer, such as kahalalide F, a high priority in cancer treatment. In addition to liver cancer, kahalalide F is currently in phase II trials in non-small-cell lung cancer (NSCLC) and melanoma [549751], and the peptide is being investigated for a number of other solid tumors.
Synthesis and SAR
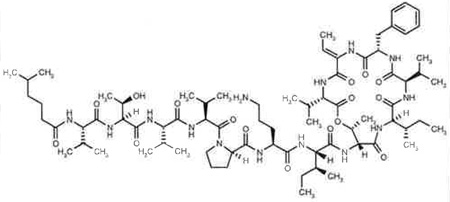
Kahalalide F was first isolated from 200 marine mollusks by fractionating ethanolic extracts by silica flash column chromatography. The eluted peptide mixtures were fractioned by repeated high performance liquid chromatography (HPLC) carried out on C18 reversed-phase columns to yield kahalalide F as a white amorphous powder (40 mg). A similar procedure was carried out to isolate kahalalide F directly from Bryopsis pennata. The molecular formula was resolved by high-resolution fast atom bombardment mass spectrometry (MS) and detailed analysis of the 13C-NMR spectrum [506285].
Data regarding the SAR for the kahalalides remains limited to data generated from isolated natural products, and only a single total synthesis has been reported for kahalalide F [506272]. The SAR data generated from isolated peptides including kahalalide F [506285], kahalalides A to G [526209], H and J [506282], K [526485] and O [526345] revealed that only the largest C75 and cyclized peptide (kahalalide F), which contains 13 amino acids and 5-methylhexanoic acid at its N-terminus, exhibited significant biological activity against solid tumors and AIDS opportunistic infections with minimum inhibitory concentration values against opportunistic infections ranging from 1.56 to 25 µg/ml [526209]. The absence of activity for the linear version of kahalalide F, known as kahalalide G, in which the ester is hydrolyzed, indicates that the cyclized system is essential for activity.
The stereochemistry of kahalalide F was evaluated several times using degradation chemistry and chiral chromatography; detailed analysis of nuclear Overhauser effects [506280], [506285], [526375] was finally firmly established through a total synthesis [506272]. The synthesis revealed that even minor modifications of the stereochemistry at valine three and four results in a product with significantly diminished biological activity, indicating that both the cyclized and linear side chains play a critical role in the biological activity of the drug. The synthesis was also an important advance for the long-term and sustainable sourcing of this drug, which would be challenging to provide using natural collections of the green alga from either Bryopsis species or Elysia rufescens. The bulk of the synthetic methodologies are not unusual since the molecule consists primarily of common amino acids. However, there were several noteworthy challenges in the synthesis of this drug, including an ester bond that is part of the cyclic system and is flanked by two β-branched and hindered amino acids, a hydrophobic sequence with a number of β-branched amino acids, a terminal fatty acid moiety, and the presence of a dehydrothreonine residue. Some of the unique methodologies used to achieve the total synthesis of this drug include the use of azabenzotriazole coupling reagents, the formation of the dehydrothreonine residue on the solid phase, protection with allyl, tert-butyl, fluorenyl and trityl-based groups, and finally, cyclization and purification in the solution phase [506272].
Preclinical Development
Kahalalide F exhibited significant activity in vitro against human lung and colon tumor cell lines (IC50 values for A549, HT29 and LoVo were 2.5, 0.25 and < 1.0 µg/ml, respectively) and significantly less activity against murine leukemic P388 and human oral epidermoid carcinoma KB cells (IC50 values of 10 and > 10 µg/ml, respectively), indicating a clear selectivity for solid tumor cell lines [506285]. Patient tumor specimens treated ex vivo at concentrations ranging from 0.01 to 1.0 µM for 14 days in a soft-agar cloning system showed growth inhibition in 100, 75, 100 and 91% of breast-, colon-, non-small-cell lung- and ovarian-derived cancers, respectively [402300]. Kahalalide F also displayed dose-dependent potent cytotoxic activity against various prostate and breast cancer cell lines after 24 h exposure, with IC50 values ranging from 0.07 (PC3) to 0.28 µM (DU145, LNCaP, SKBR3, BT474 and MCF7). However, non-tumor human cell lines derived from various origins (MCF10A, HUVEC, HMEC-1 and IMR90) were 5- to 40-fold less sensitive to kahalalide F, with IC50 values of 1.6 to 3.1 µM [515296], [526699]. In PC3 cells, the action of kahalalide F (0.5 or 1.0 µM) was rapid, with a 45-min drug exposure reducing cell survival to a similar level as a 24-h incubation, while a short exposure of 15-min duration induced 50% of the maximum achievable cytotoxicity [515296]. Preclinical in vivo models have confirmed the antitumor activity of kahalalide F in the androgen-independent prostate tumors PC3 and DU145, with tumor inhibition of 40 to 52% achieved in nude mice bearing these tumors when kahalalide F was administered intravenously at 50 to 75% of its maximum-tolerated dose (MTD); a marked profile of cytostatic rather than cytotoxic effects were observed with tumor rebound noted after treatment withdrawal [203450], [402301].
Radiosensitization to kahalalide F was evaluated in DU145, HeLa, HT29, HN30 and HOP62 cells. All cell lines displayed a dose-dependent radiosensitization with a sensitizing enhancement ratio measured at 2 Gy of 1.4, 1.87, 1.3, 2.7 and 1.6 for DU145, HeLa, HN30, HOP62 and HT29 cells, respectively, when cells were incubated at kahalalide F concentrations corresponding to IC50 concentrations [526734].
An initial NCI-COMPARE analysis, which uses an algorithm to examine the differential activity of drugs against a panel of 60 human tumor cell lines [554851], produced a negative result suggesting that kahalalide F had a novel mode of action for an anticancer drug [362535], [526742]. The mechanism of action of kahalalide F has been investigated in various cell lines and, in human prostate and breast cancer cells, it appears to induce oncosis, a progression of cellular events leading to necrotic cell death [526699]. Kahalalide F has been shown to target lysosomes, an unusual mechanism of action compared with other anticancer agents [506283], [526699]. COS-1 and HeLa cells became significantly swollen and developed large vacuoles after treatment with 0.2 to 2 µM of kahalalide F for 20 min to 2 h [506283]. To identify the subcellular site of action of kahalalide F, immunofluoresence was carried out with several subcellular markers on NRK, BHK and Vero cells that had been treated with 2 µM of kahalalide F for 1 h. Kahalalide F had no effect on the morphology of the Golgi apparatus, however, labeling with Lyso Tracker Green (LTG) revealed a dramatic effect on lysosome morphology [506283]. As prostate tissue is particularly rich in lysosomes, and the androgen-independent prostate tumor cell line PC3 had shown the greatest sensitivity to kahalalide F, it was used to further investigate the lysosomal mechanism of action of kahalalide F [526699]. To examine lysosomal integrity together with cell viability, PC3 cells treated with 0.5 µM of kahalalide F for 4 h were stained with LTG and propidium iodide (PI), and then analyzed by flow cytometry. Cells with reduced LTG fluorescence also had high levels of PI incorporation, indicating dying cells with a heavily altered plasma membrane permeability. Kahalalide F also enhanced the effect of the lysomotropic agent chloroquine on organelle integrity [526699]. Electron microscopy analysis on kahalalide F-treated (1 to 3 h) prostate PC3 and breast SKBR3 cells also revealed cytoplasmic swelling with extensive vesiculation of cytoplasmic organelles, dilation of the endoplasmic reticulum elements, cytoskeletal degradation and rupture of the plasma membrane - events characteristic of oncosis. Kahalalide F exposure, however, had less effect on cell nuclei [515296], [526699].
Apoptosis does not appear to be a major mechanism of kahalalide F-induced cytotoxicity [526699], [531407], [531440]. Pretreatment of PC3 cells with a caspase inhibitor (Z-VAD-fmk, 40 µM) for 2 h before kahalalide F treatment (for an additional 22 h) did not influence kahalalide F activity. Flow cytometry did not reveal a hypodiploid peak (characteristic of apoptosis) or arrest in any phase of the cell cycle for PC3 cells incubated with kahalalide F at 0.25 or 0.5 µM for 12 to 48 h. In contrast, a hypodiploid cell population (bearing a sub-G1 DNA content), usually characteristic of apoptosis, was observed in the breast cancer cells SKBR3 and BT474 within 16 to 24 h of kahalalide F treatment (1 µM), although this cell population was not prevented by caspase inhibitors, and annexin staining was negative [531440]. DNA analysis by agarose gel electrophoresis showed that kahalalide F treatment (0.5 µM for 2 to 24 h) of PC3 cells did not induce the ladder typical of DNA degradation caused by apoptosis [526699]. Furthermore, the level of expression of Bcl-2 protein did not greatly alter sensitivity to kahalalide F; breast cancer MDA-MB-231 cells transfected with bcl-2 displayed an IC50 value of 0.74 µM after 24 h exposure to the drug, while cells transfected with empty vector showed an IC50 value of 0.39 µM [526699]. However, a low level of apoptosis was observed in HeLa and DU145 cells after 48 h of kahalalide F treatment, as assayed by annexin V labeling, although increased cytotoxicity was not observed after 24 h of drug treatment [526734]. In addition, in the human hepatoma cell line HepG2, in which kahalalide F displays an IC50 value of 0.25 µM, both apoptotic and necrotic fractions were detected in kahalalide F-treated cells (0.125 to 0.5 µM) up to 48 h after drug exposure, although processes characteristic of apoptosis (such as nuclear condensation and poly-(ATP-ribose)-polymerase cleavage) were not observed [531407]. In another human hepatoma-derived cell line, PLC/PRF/5, in which kahalalide F displays an IC50 value of 8 µM, only necrotic (and not apoptotic) cells were detected after kahalalide F treatment (4 to 8 µM) from 1 to 48 h. In fact, in a panel of human hepatoma cells, no association was detected between p53 status and IC50 values, suggesting that apoptosis is not a major mechanism of kahalalide F-induced cell death [531407].
One study reported that kahalalide F activity was independent of the level of MDR1 or HER-2/neu protein transcription, which is often upregulated in carcinogenesis [526699]. In the colon tumor cell line LoVo, kahalalide F had an IC50 value of 0.16 µM, whereas in LoVo/Dox cells, which overexpress MDR1, the IC50 value was 0.18 µM [526699]. In MCF7 cells overexpressing exogenous HER-2/neu, kahalalide F displayed an IC50 value of 0.4 µM 24 h post-treatment compared with an IC50 value of 0.28 µM in the parental cell line. Furthermore, no significant difference was reported in kahalalide F sensitivity between cell lines expressing high (SKBR3 or BT474), intermediate (LNCaP) or low (DU145, MCF7 or MDA-MB-231) levels of HER-2/neu protein. PC3 cells, which express moderate levels of HER-2/neu, had the highest sensitivity to kahalalide F [526699]. However, another study reported that cells with high HER-2/neu and/or HER-3 protein levels were particularly sensitive to kahalalide F and that the drug abrogated Akt signaling, while not affecting the phosphorylation of epidermal growth factor receptor or HER-2 [531440]. In fact, advanced COMPARE analysis, which relates gene expression profiles to cytotoxicity patterns [555675], found that kahalalide F was one of 30 compounds implicated as a transforming growth factor (TGF)α/HER-2 and epidermal growth factor (EGF) inhibitor [J Jimeno, personal communication], HER-3 and Akt status appear to be major determinants of in vitro cytotoxicity of kahalalide F, as selective downregulation of HER-3 expression and inhibition of the phosphatidylinositol-3 kinase/Akt/protein kinase B signaling pathway was observed in kahalalide F-sensitive cell lines within 4 h of exposure to the drug. Furthermore, a kahalalide F-resistant sub-line of the colon HT29 cell line expressed significantly reduced levels of HER receptors 1 to 4 compared with the parental cell line [531440], [J Jimeno, personal communication].
Metabolism and Pharmacokinetics
The degradation of kahalalide F under acid, neutral and alkaline conditions showed that the t1/2 of kahalalide F at 80°C was 1.1, 20.0 and 8.6 h at a pH of 0, 1 and 7, respectively [506275], At 26°C and a pH of 11, the t1/2 value was 1.7 h. At a pH of 7 and 11, only a single degradation product was observed, which was shown to be kahalalide G, the linear form of kahalalide F, generated after hydrolysis of the lactone. At a pH of 0 and 1, additional reaction products were observed [506275], [506282] and these were utilized in the stereochemical assignment using degradation methods. In addition, metabolism studies were completed using three different enzyme systems, including pooled human microsomes, human plasma and 5'-diphosphoglucuronyl transferase. While potential exists for hydrolysis of the lactone moiety of kahalalide F as well as hydroxylation of the fatty acid chain by mammalian enzyme systems, these products were not observed during in vitro metabolism studies [506274], [506275]. Therefore, based on human enzyme studies, kahalalide F appears to have exceptional metabolic stability.
A stable formulation of kahalalide F consists of 150 µg of drug with 3 mg of polysorbate 80, 3 mg of citric acid and 150 mg of sucrose [391191]. Lyophilized kahalalide F is considerably less stable with increasing polysorbate 80 and citric acid concentrations. The compatibility of the drug with infusion devices revealed that a significant loss of kahalalide F was associated with binding to contact surfaces of infusion containers made of low-density polyethylene [391188], [506276]. As a result, kahalalide F must be administered as a 1- to 3-h infusion in concentrations of 0.5 to 14.7 µg/ml using a glass container and silicon tubing. Lyophilized kahalalide F is reconstituted prior to administration in a solution of Cremphor EL (C) and absolute ethanol (E) in an aqueous solution (W) (CEW = 5/5/90% volume) prior to intravenous injection, and further diluted in 0.9% weight/volume sodium chloride for infusion. An in vitro biocompatibility study demonstrated that there was no significant hemolysis due to the kahalalide F formulation, nor the CE vehicle in both a static and dynamic test model [506277].
Pharmacokinetic studies in female mice were completed for both intravenous and intraperitoneal administration of the drug (280 µg/kg) [362535], [506281]. When administered intravenously to mice, plasma levels declined from a peak concentration of 1.0 µM with a t1/2 value of 35 min, and there was no accumulation of the drug after repeated intravenous administration at 24 h intervals. The total body clearance in mice was 3.8 ml/min/kg and apparent volume of distribution was 199 ml/kg. When the drug was administered intraperitoneally at the same dose, the peak concentration was 0.3 µM approximately 1 h after dosing. These data indicate that the drug is eliminated from plasma rapidly, with limited binding to extravascular tissues [506281].
An analytical method using HPLC coupled with positive ion turbo-ionspray tandem MS has been developed and validated for the analysis of kahalalide F in human plasma samples [506269], [506270], [506273]. The trifluoroacetic acid typically utilized in the reversed phase analysis of peptides was replaced with ammonium acetate to enhance sensitivity in positive ion mode. A lower limit quantitation of 1 ng/ml using a 500 µl sample volume was achieved and the drug was stable in the biomatrix for a period of 9 months at −20°C and 24 h at room temperature. The inter-assay accuracy was 15.1% at the lower limit of quantitation and between −2.68 and −9.05% for quality control solutions ranging in concentration from 2.24 to 715 ng/ml [506269].
The HPLC-MS assay method was utilized to determine pharmacokinetics and metabolism during phase I clinical trials. When kahalalide F was administered as a 1-h infusion over 5 days every 3 weeks, analysis performed after the first and second course revealed a linear relationship between dose and AUC over a dose range of 20 to 560 µg/m2/day; at doses of > 560 µg/m2/day, the AUC increased in a non-dose-proportional manner. On day 1, total plasma clearance was 11.02 ± 4.54 1/h and the terminal t1/2 value of intravenous kahalalide F in these patients was 0.54 ± 0.14 h [543658]. When kahalalide F was administered as a continuous weekly 1-h intravenous infusion, the pharmacokinetic profile was linear over a 266 to 800 µg/m2/week dose range. At 1200 µg/m2/week the median t1/2 value was 28 min, median clearance was 74 ml/min.m2 and median AUC was 533.5 ng.h/ml. In both studies, plasma levels reached concentrations predicted to be adequate for activity according to activity measured in vitro [452079], [470663].
Toxicity
A single dose of 375 µg/kg of kahalalide F was lethal in male rats. The toxicity was reversible at 150 µg/kg, but not at 300 µg/kg. Multiple daily doses (80 µg/kg for 5 days; 400 µg/kg in total) did not appear to lead to cumulative toxicity [506271].
The MTD for kahalalide F in female mice was 280 µg/kg after a single bolus intravenous injection. Doses slightly above the MTD were highly toxic, with signs of neurotoxicity followed by death; however, the drug could be administered once daily for 5 days at a dose of 280 µg/kg without any apparent toxicity [362535], [506281]. Kahalalide F exhibited little cytotoxicity to cardiac (H9 c2 (2-1)) and skeletal (L8) rat-derived cell lines with LD50 values of 5 and 0.6 mM, respectively. However, kahalalide F was significantly more toxic to liver (AML-12) and kidney (NRK-52E)-derived murine cells, with LD50 values of 0.17 and 1.6 µM, respectively. Intermediate cytotoxicity was observed against murine myelogenous stem cells (FDC-P1) with an LD50 value of 14 µM [389847]. The in vitro cell toxicity data clearly correlate well with the in vivo murine data. Molecular probes coupled with immunocytochemistry showed that at approximately 10 µM, kahalalide F was toxic to central nervous system neurons, but at this same concentration kahalalide F was not toxic to astrocytes or sensory and motor neurons in the spinal cord. As a result, kahalalide F appears to be promising for specific tumor types, and its neurotoxicity is considered to be relatively mild at or below the MTD [389847].
The toxicity of kahalalide F to mouse hematopoietic progenitor and stem cells was assessed both in cultures of committed myeloid (CFU-GM) and megakaryocytic (CFU-Meg) progenitors, and by using a competitive repopulation assay in conjunction with a mouse model [526694]. Progenitors derived from murine bone marrow (CFU-GM or CFU-Meg) were not sensitive to kahalalide F at concentrations which exceeded the plasma concentrations expected in treated patients (1 to 10 µM) after 24 h of exposure to the drug. Furthermore, kahalalide F (10 µM) did not show any toxic effect on the reconstitution of Ly-5.1+ bone marrow cells into mice at 12 months post-transplantation [526694]. This is consistent with the lack of activity of kahalalide F both in vitro and in vivo towards murine leukemic P388 cells [203450], [506285].
Clinical Development
Phase I
Since kahalalide F exhibited significant activity in vitro and in vivo against androgen-independent prostate tumor cells, it was evaluated in phase I trials for prostate cancer. Kahalalide F (20 to 930 µg/m2) was administered as a daily 1-h intravenous infusion for 5 days every 3 weeks to patients with advanced or metastatic androgen-refractory prostate cancer (n = 33). One patient was entered at each dose level of 20, 40, 80 and 160 µg/m2/day, five at 320 µg/m2/day, three at 425 µg/m2/day, seven at 560 µg/m2/day, eleven at 700 µg/m2/day, and three at 930 µg/m2/day [430063], [452079], [526702], [543658]. One patient showed a significant decrease in PSA level (> 50%) associated with clinical improvement (pain relief). Stable disease was achieved by three patients, one for 7 months and two for 2 months [543658].
In a phase I study conducted in 25 patients with various solid tumors, kahalalide F was administered across six dose levels ranging from 266 µg/m2/week to 1200 µg/m2/week using a continuous 1-h weekly infusion. Clinical benefit was observed in three patients who had received previous chemotherapy: one of these was a patient with hepatocarcinoma, who received 24 infusions of 400 µg/m2/week; one with squamous carcinoma cavum, who received nine infusions at the same dose, and one patient with NSCLC who received 16 infusions at a dose of 530 µg/m2/week [470663]. Objective responses in patients with pretreated colorectal cancer, melanoma and hepatocellular carcinoma were achieved [555161]. The lack of cumulative toxicity observed with this regimen allowed the initiation of phase I studies assessing the effect of 3- and 24-h infusion schedules in patients with chemotherapy-resistant solid tumors. The aim of these longer infusion schedules, which use a starting dose equivalent to the MTD of the weekly 1-h infusion schedule, is to increase the therapeutic activity of kahalalide F [555488].
Phase II
Kahalalide F is currently being evaluated for liver cancer at a starting dose of 650 µg/m2 administered as weekly 1-h intravenous infusions [491229]. No data from this trial are yet available. Phase II, multicenter trials have also commenced for NSCLC and advanced malignant melanoma [549751]. The NSCLC trial will initially include 25 patients with relapsed or chemotherapy-refractory disease, while the melanoma trial will initially enroll 18 patients with advanced malignant disease. The regimen to be evaluated is a 650-µg/m2 dose of kahalalide F administered as a weekly 1-h intravenous infusion. The primary endpoint is response rate, while secondary objectives are the evaluation of the pharmacokinetics and further investigation of the safety profile of the product [549751].
Side Effects and Contraindications
When kahalalide F was administered as a daily 1-h intravenous infusion for 5 days every 3 weeks to patients with advanced or metastatic androgen-refractory prostate cancer, drug-related toxicities included rapidly reversible common toxicity criteria (CTC) grade 3 aspartate aminotransferase (AST) and mild myalagia at a dose of 320 µg/m2/day [430063], [452079], [526702], The dose-limiting toxicity (DLT) was reversible CTC grade 4 AST and alanine aminotransferase (ALT), which occurred at 930 µg/m2/day [526702]. The MTD was reached at 560 µg/m2/day [543658].
In the trial involving patients with various solid tumors, in which a continuous weekly 1-h intravenous infusion was administered, the MTD was 1200 µg/m2/week. The DLT was early-onset asymptomatic grade 4 elevation of transaminases, that was not reversible by day 7. Pruritus on the hands was a common toxicity at doses ranging from 266 to 1200 µg/m2/week, but bone marrow toxicity was not observed [470663]. No evidence of cumulative toxicity was observed even though a number of patients received multiple cycles of kahalalide F [555161].
A total of 60 cancer patients were assessed for adverse events across the two phase I trials in which kahalalide F was administered as a 1-h intravenous infusion. In this regimen, grade 4 aminotransferases increase (AI) was consistently the DLT, tending to coincide with lactate dehydrogenase (LDH) elevation and an ALT/alkaline phosphatase (AP) ratio greater than 5.0, indicating hepatocellular damage. Grade 4 AI with concomitant LDH elevation occurred in 20% of patients, with only one out of 13 patients with grade 4 AI displaying normal LDH. Median time to recovery from grade 3 to 4 AI to grade 1 AI was 6 days. Liver toxicity was not clinically significant. When kahalalide F was administered below a dose of 600 µg/m2, grade 4 ALT, AST and γ glutamic transpeptidase (GGT) elevation occurred in 3.8, 0 and 7.7% of patients, respectively, whereas administration of between 600 and 700 µg/m2 resulted in ALT, AST and GGT elevations in 11.1, 11.1 and 0% of patients, respectively. Above the recommended dose of 700 µg/m2, grade 4 ALT, AST and GGT occured in 50, 43.8 and 12.5% of patients, respectively. From eight patients who received the drug for over 4 months, three showed grade 4 AI and continued to receive treatment without dose reduction; no evidence of cumulative toxicity was reported [526738].
Considering the absence of cumulative toxicities and that DLTs consisted of acute transaminitis and hypersensitivity reactions without any occurrence of bone marrow toxicity, alopecia, mucositis, neurotoxicity or diarrhea, kahalalide F was well tolerated in patients with advanced pretreated cancers [555161].
Patent Commentary
The isolation of kahalalide F was described in the patent EP-00610078, which was filed by PharmaMar in August 1994; PharmaMar followed this up in May 2002 with WO-00236145, a patent describing novel formulations containing a lyophilized mixture of kahalalide F, a non-ionic surfactant, an organic acid and a bulking agent. PharmaMar also filed the patents WO-2004035613 and WO-00158934 in April 2004 and August 2001, respectively, which describe derivatives of kahalalide F for the potential treatment of solid tumors, psoriasis and viral or fungal infections.
Current Opinion
The unique mechanism of action of kahalalide F combined with its application or potential application against a number of serious forms of cancer, including melanoma, liver, prostate, pancreatic colon, breast and ovarian cancer, make it an investigational new drug with particularly significant applications. The pharmacokinetic studies indicate that the drug is rapidly eliminated from plasma with little binding to extravascular tissue. In addition, data from phase I clinical trials indicate that the drug is well tolerated without some of the serious toxicities associated with existing forms of chemotherapy. The drug is cleared rapidly and there appears to be no cumulative toxicities, making the administration safe and easy to manage. The low toxicity profile of kahalalide F also raises the possibility of combining it with other anticancer drugs that could potentially enhance its therapeutic activity. Furthermore, the activity of this drug against some forms of AIDS-related opportunistic infections in vitro broadens the potential scope of applications and utility for this investigational new drug.
Commercial Opinion
In November 2004, analysts from ING Bank predicted launch of kahalalide F in 2008, with peak sales in 2020 of €98, €182 and €909 million for liver, prostate and breast cancer, respectively [573742].
Development history
PharmaMar initiated phase I trials in December 2000 [394993], with phase II trials expected to begin in 2002 [431536]. As of January 2003, kahalalide F was in phase I trials for prostate cancer and preclinical investigation for breast cancer and other solid tumors [475774]. In May 2003, kahalalide F entered phase II studies for liver carcinoma [491229]. In January 2004, PharmaMar intended to seek a development partner following the completion of phase II trials [520345]. In July 2004, PharmaMar began phase II trials in NSCLC and melanoma [549751].
| Developer | Country | Status | Indication | Date | Reference |
|---|---|---|---|---|---|
| PharmaMar SA | France | Phase II | Psoriasis | 28-OCT-04 | 567635 |
| PharmaMar SA | Spain | Phase II | Liver tumor | 29-MAY-03 | 491229 |
| PharmaMar SA | Spain | Phase II | Psoriasis | 28-OCT-04 | 567635 |
| PharmaMar SA | Western Europe | Phase II | Melanoma | 19-JUL-04 | 549751 |
| PharmaMar SA | Western Europe | Phase II | Non-small-cell lung cancer | 19-JUL-04 | 549751 |
| PharmaMar SA | Netherlands | Phase I | Prostate tumor | 01-FEB-01 | 402916 |
| PharmaMar SA | Spain | Phase I | Carcinoma | 04-JAN-01 | 394993 |
| PharmaMar SA | Spain | Phase I | Colon tumor | 04-JAN-01 | 394993 |
| PharmaMar SA | Spain | Phase I | Prostate tumor | 04-JAN-01 | 394993 |
| PharmaMar SA | Spain | Phase I | Solid tumor | 04-JAN-01 | 394993 |
| PharmaMar SA | Spain | Discovery | Breast tumor | 26-MAR-01 | 402916 |
| PharmaMar SA | Spain | Discovery | Ovary tumor | 26-MAR-01 | 402916 |
Literature classifications
Key references relating to the technology are classified according to a set of standard headings to provide a quick guide to the bibliography. These are as follows:
Chemistry: References which discuss synthesis and structure-activity relationships.
Biology: References which disclose aspects of the drug's pharmacology in animals.
Metabolism: References which discuss metabolism, pharmacokinetics and toxicity.
Clinical: Reports of clinical phase studies in volunteers providing, where available, data on the following: whether the experiment is placebo-controlled or double- or single-blind; number of patients; dosage.
Chemistry
| Study Type | Result | Reference |
|---|---|---|
| Synthesis. | Kahalalide F was first isolated from the marine mollusk (Elysia rufescens) and then directly from its algal diet (Bryopsis pennata). In both cases ethanol extracts were fractioned by silica flash column chromatography and the eluted peptide mixtures were fractioned by repeated HPLC to yield kahalalide F. |
506285 |
| SAR. | SAR data of kahalalide A to G revealed that, of all the peptides, only kahalalide F had significant biological activity that was lost when its ester bond was hydrolyzed and this cyclic compound was transformed to its linear form kahalalide G. |
526209 |
| Synthesis. | Total synthesis of kahalalide F revealed that the stereochemistry of the linear side chain, particularly of valines 3 and 4, was essential for biological activity. |
506272 |
Biology
| Study Type | Effect Studied | Experimental Model | Result | Reference |
|---|---|---|---|---|
| In vitro | Selectivity. | A549, HT29 and LoVo cells or P388 and KB cells. |
Kahalalide F displayed selectivity against solid tumor cell lines with IC50 values of 2.5, 0.25 and < 1.0 µg/ml against A549, HT29 and LoVo, respectively, while IC50 values against P388 and KB cells were 10 and > 10 µg/ml, respectively. |
506285 |
| In vitro | Efficacy. | Various prostate and breast cancer cell lines, as well as cell lines derived from normal human tissues. |
Kahalalide F displayed potent cytotoxic activity against various prostate and breast cancer cell lines (DU145, LNCaP, SKBR3, BT474 and MCF7) with IC50 values ranging from 0.07 (PC3) to 0.28 µM. However, non-tumor human cell lines derived from various origins (MCF10A, HUVEC, HMEC-1 and IMR90) were 5- to 40-fold less sensitive, with IC50 values of 1.6 to 3.1 µM. |
526699 |
| In vitro | Activity. | PC3 cells incubated with kahalalide F at 0.25 or 0.5 µM for 12 to 48 h and analyzed by flow cytometry, and PC3 cells incubated with 0.5 µM for 2 to 24 h followed by DNA analysis by agarose gel electrophoresis. |
No hypodiploid peak (characteristic of apoptosis) nor arrest in any phase of the cell cycle for PC3 cells was observed and there was no evidence of a ladder typical of the DNA degradation caused by apoptosis. |
526699 |
| In vitro | Toxicity. | Murine-derived cardiac (H9 c2 (2-1)), skeletal muscle (L8), liver (AML-12), kidney (NRK-52E), and myelogenous stem (FDC-P1) cells exposed to kahalalide F. |
LD50 values against H9 c2 (2-1), L8, AML-12, NRK-52E and FDC-P1 cells were 5 mM, 0.6 mM, 0.17 µM, 1.6 µM and 14 µM, respectively. |
389847 |
| In vitro | Toxicity. | Immunohistochemical method to determine viability of neural cells after kahalalide F exposure. |
At 10 µM, kahalalide F was toxic to CNS neurons, but spared astrocytes, sensory and motor neurons in the spinal cord. |
389847 |
Metabolism
| Metabolism | ||||
|---|---|---|---|---|
| Study Type | Effect Studied | Model Used | Result | Reference |
| In vitro | Metabolism. | HPLC was used to analyze either the influence of pH or human enzymes (either uridine 5'-diphosphoglucuronyl transferase or enzymes derived from pooled human microsomes or pooled human plasma) on the chemical degradation of kahalalide F. |
Kahalalide F was not degraded by the human-derived enzymes. It was also stable at acid pH but under basic and neutral conditions, it was metabolized to kahalalide G. |
506275 |
| In vivo | Pharmacokinetics. | Kahalalide F (280 µg/kg) was administered to mice either intravenously or intraperitoneally. |
In the intravenous study, plasma levels declined from a peak concentration of 1.0 µM with a t1/2 value of 35 min and there was no accumulation of the drug after repeated administration at 24 h intervals. Total body clearance was 3.8 ml/min/kg and apparent volume of distribution was 199 ml/kg. In the intraperitoneal study, the peak concentration was 0.3 µM approximately 1 h after dosing. |
506281 |
| In vivo | Pharmacokinetics. | A phase I study in 33 patients with advanced androgen refractory prostate cancer. Kahalalide F was administered as a daily 1-h intravenous infusion for 5 days every 3 weeks at doses ranging from 20 to 930 µg/m2. |
AUC increased in a dose-proportional manner over a dose range of 20 to 560 µg/m2/day. On day 1, total plasma clearance was 11.02 ± 4.54 1/h and the terminal t1/2 of intravenous kahalalide F was 0.54 ± 0.14 h. |
543658 |
| In vivo | Pharmacokinetics. | A phase I study in 25 patients in which kahalalide F was administered as a continuous weekly 1-h intravenous infusion at doses ranging from 266 to 1200 µg/m2. |
The pharmacokinetic profile was linear over a 266 to 800 (µg/m2/week dose range; at 1200 µg/m2/week, the median t1/2 value was 28 min, median clearance was 74 ml/min.m2 and median AUC was 533.5 ng.h/ml. |
470663 |
Clinical
| Clinical | |||
|---|---|---|---|
| Effect Studied | Model Used | Result | Reference |
| Efficacy. | A phase I study in patients with androgen refractory prostate cancer (n = 33) in which kahalalide F (20 to 930 µg/m2) was administered as a daily 1-h intravenous infusion for 5 days every 3 weeks. |
One patient showed a significant decrease in PSA level (> 50%) associated with clinical improvement (pain relief), while three patients exhibited stable disease for 2 (n = 2) or 7 (n = 1) months. The MTD was 560 µg/m2/day. |
543658 |
| Efficacy. | A phase I study in patients with various solid tumors (n = 25) in which kahalalide F was administered as a continuous weekly 1-h intravenous infusion at doses ranging from 266 to 1200 µg/m2. |
Three patients achieved a clinical benefit: one hepatocarcinoma patient who received 24 infusions consisting of 400 µg/m2/week, one squamous carcinoma cavum patient who received nine infusions at the same dose, and one NSCLC patient who received 16 infusions at a dose of 530 µg/m2/week. The MTD was 1200 µg/m2/week. |
470663 |
| Safety. | Two phase I trials in which 60 cancer patients were administered kahalalide F as a 1-h intravenous infusion. |
Grade 4 Al was consistently the DLT and tended to coincide with LDH elevation and an ALT/AP ratio of > 5.0, indicating hepatocellular damage; these effects were reversible and dose dependent. |
526738 |
Originator PharmaMar SA
Status Phase II Clinical
Actions Anticancer, Immunomodulator
Indications Breast tumor, Carcinoma, Colon tumor, Liver tumor, Melanoma, Non-small-cell lung cancer, Ovary tumor, Prostate tumor, Psoriasis, Solid tumor
Biotechnology Natural product, Peptide
Synonym PM-92102
CAS 1-Oxa-4,7,10,13,16-pentaazacyclononadecane, cyclic peptide derivative l-Valine, N-(5-methyl-1-oxohexyl)-d-valyl-l-threonyl-l-valyl-d-valyl-d-prolyl-l-ornithyl-d-alloisoleucyl-d-allothreonyl-d-alloisoleucyl-d-valyl-l-phenylalanyl-(2Z)-2-amino-2-butenoyl-(13→8)-lactone Registry No: 149204-42-2
Footnotes
Associated patent
Title Cytotoxic and antiviral compound.
Assignee PharmaMar SA
Publication EP-00610078 10-AUG-94
Priority GB-19930002046 03-FEB-93
Inventors Schauer PJ, Hamann MT, Gravalos D.
Associated references
• of special interest
- 203450.Faircloth G, Scheuer P, Avila J, Hendricks H, Drees M, Jimeno J. Kahalalide F (KF). A new marine depsipeptide (MADEP) with selective activity against solid tumour (ST) models. NCI EORTC SYMP NEW DRUGS CANCER THER. 1996 Abs 110. [Google Scholar]
- 362535.Faircloth G, Grant W, Smith B, Supko J, Brown A, Geldof A, Himeno J. Preclinical development of kahalalide F, a new marine compound selected for clinical studies. Abs 3823 PROC ANNU MEET AM ASSOC CANCER RES. 2000;41 [Google Scholar]
- 389847.Luber-Narod J, Smith B, Grant W, Jimeno JM, Lopez-Lazaro L, Scotto K, Shitli A, Faircloth GT. In vitro safety toxicology of kahalalide F, a marine natural product with chemotherapeutic potential against selected solid tumors. NCI EORTC SYMP NEW DRUGS CANCER THER. 2000;11 Abs 219. [Google Scholar]
- 391188.Nuijen B, Bouma M, Manada C, Jimeno J, Lopez-Lazaro L, Bult A, Schellens J, Beijnen J. Compatibility and stability of the investigational polypeptide marine anticancer agent kahalalide F in infusion devices. NCI EORTC SYMP NEW DRUGS CANCER THER. 2000;11 doi: 10.1023/a:1010641207791. Abs 449. [DOI] [PubMed] [Google Scholar]
- 391191.Nuijen B, Bouma M, Talsma H, Manada C, Jimeno J, Lopez-Lazaro L, Bult A, Beijnen J. Development of a lyophilized, parenteral pharmaceutical formulation of the investigational polypeptide marine anticancer agent kahalalide F. NCI EORTC SYMP NEW DRUGS CANCER THER. 2000;11 doi: 10.1081/ddc-100107240. Abs 450. [DOI] [PubMed] [Google Scholar]
- 394993.Zeltia On this day, 19 December 2000, PharmaMar SA has commenced phase I clinical trials for kahalalide F (KF) PRESS RELEASE. 2000 Dec 19; [Google Scholar]
- 402300.Medina LA, Gomez L, Cerna C, Faircloth G, Yochmowitz M, Weitman S. Investigation of the effects of kahalalide F (PM92102) against human tumor specimens taken directly from patients. PROC ANNU MEET AM ASSOC CANCER RES. 2001;42 Abs 1139. [Google Scholar]
- 402301.Faircloth GT, Smith B, Grant W, Jimeno J, Garcia-Gravalos L, Scotto K, Shtil A. Selective antitumor activity of kahalalide F, a marine-derived cyclic depsipeptide. PROC AM ASSOC CANCER RES. 2001;42 Abs 1140. [Google Scholar]
- 402916.Jones E. American Association of Cancer Research 92nd Annual Meeting - OVERNIGHT REPORT (Part II), Poster Sessions, New Orleans, LA, USA. IDDB MEETING REPORT. 2001 Mar 24–28; [Google Scholar]
- 430063.Schellens JH, Rademaker JL, Horenblas S, Meinhardt W, Stokvis E, De Reijke TM, Jimeno JM, Lopez-Lazaro L, Lopez-Martin JA, Beijnen JH. Phase I and pharmacokinetic study of kahalalide F in patients with advanced androgen refractory prostate cancer. AACR NCI EORTC MOL TARGETS CANCER THER. 2001;12 doi: 10.1158/1078-0432.CCR-04-1534. Abs 207. [DOI] [PubMed] [Google Scholar]
- 431536.Stratan I. Zeltia: Will the fiesta continue? BNP/PARIBAS. 2001 Nov 09; [Google Scholar]
- 452079.Schellens JH, Rademaker-Lakhai JM, Horenblas S, Mienhardt W, Stokvis E, de Reijke TM, Jimeno JM, Lopez-Lazaro L, Lopez-Martin JA, Beijnen JH. Phase I and pharmacokinetic study of kahalalide F in patients with advanced androgen resistant prostate cancer. PROC AM SOC CLIN ONCOL. 2002;21(1) doi: 10.1158/1078-0432.CCR-04-1534. Abs 451. [DOI] [PubMed] [Google Scholar]
- 470663.Ciruelos EM, Trigo T, Pardo J, Paz Ares L, Estaun N, Cuadra C, Dominguez M, Marin A, Jimeno J, Izquierdo M. A phase I clinical and pharmacokinetic (PK) study with kahalalide F (KF) in patients (pts) with advanced solid tumors (AST) with a continuous weekly (W) 1-hour iv infusion schedule. AACR NCI EORTC MOL TARGETS CANCER THER. 2002;13 Abs 95. [Google Scholar]
- 475774.Zeltia Inc. PharmaMar. COMPANY WORLD WIDE WEB SITE. 2003 Jan 09; [Google Scholar]
- 491229.PharmaMar Inc. Kahalalide F enters phase II liver carcinoma trials. PRESS RELEASE. 2003 May 29; [Google Scholar]
- 506269. Stokvis E, Rosing H, Lopez-Lazaro L, Rodriguez I, Jimeno JM, Supko JG, Schellens JHM, Beijnen JH. Quantitative analysis of the novel depsipeptide anticancer drug kahalalide F in human plasma by high-performance liquid chromatography under basic conditions coupled to electrospray ionization tandem mass spectrometry. J MASS SPECTROM. 2002;37(9):992–1000. doi: 10.1002/jms.362. • This paper describes a detailed HPLC-MS method for the evaluation of kahalalide F in human plasma samples, providing essential analytical methods for future clinical and animal studies.
- 506270.Stokvis E, Rosing H, Lopez-Lazaro L, Jimeno JM, Supko JG, Schellens JHM, Beijnen JH. Bioanalysis of the novel peptide anticancer drug kahalalide F in human plasma by H.P.L.C under basic conditions coupled with positive turbo-ionspray tandem mass spectrometry. BR J CLIN PHARMACOL. 2002;53(5):543P. doi: 10.1002/jms.362. [DOI] [PubMed] [Google Scholar]
- 506271. Brown AP, Morrissey RL, Faircloth GT, Levine BS. Preclinical toxicity studies of kahalalide F, a new anticancer agent: Single and multiple dosing regimens in the rat. CANCER CHEMOTHER PHARMACOL. 2002;50(4):333–340. doi: 10.1007/s00280-002-0499-2. • This is the most comprehensive preclinical toxicology study completed to date, which concludes that the minimal lethal dose in rats after a single intravenous injection is 375 µg/kg (2250 µg/m2) and that the nervous system is potentially the site where kahalalide F action leads to lethal effects The toxic effects at 150 µg/kg are reversible and the drug can safely be administered daily without cumulative toxicity.
- 506272. Lopez-Macia A, Jimenez JC, Royo M, Giralt E, Albericio F. Synthesis and structure determination of kahalalide F (1,2) JAM CHEM SOC. 2001;123(46):11398–11401. doi: 10.1021/ja0116728. • This report describes the first synthesis, the complete stereochemical assignment and the synthetic methods utilized to produce the drug for clinical trials.
- 506273.Nuijen B, Bouma M, Floriano P, Manada C, Rosing H, Stokvis E, Kettenes Van den Bosch JJ, Bult A, Beijnen JH. Development of an HPLC method with UV detection for the pharmaceutical quality control of the novel marine anticancer agent kahalalide F. J LIQ CHROMATOGR RELAT TECHNOL. 2001;24(20):3141–3155. [Google Scholar]
- 506274.Nuijen B, Bouma M, Talsma H, Manada C, Jimeno JM, Lopez-Lazaro L, Bult A, Beijnen JH. Development of a lyophilized parenteral pharmaceutical formulation of the investigational polypeptide marine anticancer agent kahalalide F. DRUG DEVIND PHARM. 2001;27(8):767–780. doi: 10.1081/ddc-100107240. [DOI] [PubMed] [Google Scholar]
- 506275. Sparidans RW, Stokvis E, Jimeno JM, Lopez-Lazaro L, Schellens JH, Beijnen JH. Chemical and enzymatic stability of a cyclic depsipeptide, the novel, marine-derived, anti-cancer agent kahalalide F. ANTICANCER DRUGS. 2001;12(7):575–582. doi: 10.1097/00001813-200108000-00003. • This paper is the first to present the chemical and enzymatic stability of kahalalide F under acidic and basic conditions as well as enzymatic stability based on pooled human microsomes, pooled human plasma and 5’-dlphosphoglvcutonyl transferase. The results of this study demonstrate that kahalalide F is highly resistant to metabolism and hydrolysis under acidic conditions, however the drug is less stable at neutral and basic conditions, resulting in the ester hydrolysis product kahalalide G.
- 506276.Nuijen B, Bouma M, Manada C, Jimeno JM, Lopez-Lazaro L, Bult A, Beijnen JH. Compatibility and stability of the investigational polypeptide marine anticancer agent kahalalide F in infusion devices. INVEST NEW DRUGS. 2001;19(4):273–281. doi: 10.1023/a:1010641207791. [DOI] [PubMed] [Google Scholar]
- 506277.Nuijen B, Bouma M, Manada C, Jimeno JM, Bult A, Beijnen In vitro hemolysis and buffer capacity studies with the novel marine anticancer agent kahalalide F and its reconstitution vehicle cremophor EL/ethanol. JH PDA J PHARM SCI TECH. 2001;55(4):223–229. [PubMed] [Google Scholar]
- 506280.Goetz G, Yoshida WY, Scheuer PJ. The absolute stereochemistry of kahalalide F. TETRAHEDRON. 1999;55(25):7739–7746. [Google Scholar]
- 506281.Supko JG, Lu H, Jimeno JM, Grant W, Faircloth GT, Glynn T. Preclinical pharmacology studies with the marine natural product kahalalide F. CLIN CANCER RES. 1999;5(11):3792S. [Google Scholar]
- 506282.Goetz G, Nakao Y, Scheuer PJ. Two acyclic kahalalides from the sacoglossan mollusk Elysia rufescens. J NAT PROD. 1997;60(6):562–567. [Google Scholar]
- 506283.Garcia RM, Bonay P, Avila J. The antitumoral compound kahalalide F acts on cell lysosomes. CANCER LETT. 1996;99(1):43–50. doi: 10.1016/0304-3835(95)04036-6. [DOI] [PubMed] [Google Scholar]
- 506285.Hamann MT, Scheuer PJ. Kahalalide F: A bioactive depsipeptide from the sacoglossan mollusk Elysia rufescens and the green alga Bryopsis sp. J AM CHEM SOC. 1993;115(13):5825–5826. [Google Scholar]
- 515296.Suarez Y, Gonzalez L, Cuadrado A, Berciano M, Lafarga M, Munoz A. Kahalalide F induces oncosis in human prostate and breast cancer cells. CLIN CANCER RES. 2003;9(16 Suppl 1) Abs C10. [PubMed] [Google Scholar]
- 520345.PharmaMar SA. PharmaMar business review day meeting with analysts and investors. PRESS RELEASE. 2004 Jan 28; [Google Scholar]
- 526209. Hamann MT, Otto CS, Scheuer PJ, Dunbar DC. Kahalalides: Bioactive peptides from a marine mollusk Elysia rufescens and its algal diet Bryopsis sp(1) J ORG CHEM. 1996;61(19):6594–6600. doi: 10.1021/jo960877+. • This paper describes the first SAR study based on the naturally occurring peptides ranging from a C31 tripeptide to the most active and largest peptide, kahalalide F, and kahalalide G, a C75 tridecapeptide.
- 526345.Horgen FD, delos Santos DB, Goetz G, Sakamoto B, Kan Y, Nagai H, Scheuer PJ. A new depsipeptide from the Sacolgossan mollusk Elysia ornata and the green alga Bryopsis sp. J NAT PROD. 2000;63(1):152–154. doi: 10.1021/np990402o. [DOI] [PubMed] [Google Scholar]
- 526375.Bonnard I, Manzanares I, Rinehart KL. Stereochemistry of kahalalide F. J NA T PROD. 2003;66(11):1466–1470. doi: 10.1021/np030334c. [DOI] [PubMed] [Google Scholar]
- 526485.Kan Y, Fujita T, Sakamoto B, Hokama Y, Nagai H. Kahalalide K: A new cyclic depsipeptide from the hawaiian green alga Bryopsis species. J NAT PROD. 1999;62(8):1168–1172. doi: 10.1021/np990053y. [DOI] [PubMed] [Google Scholar]
- 526694.Gomez SG, Bueren JA, Faircloth GT, Jimeno J, Albella B. In vitro toxicity of three new antitumoral drugs (trabectedin, aplidin and kahalalide F) on hematopoietic progenitors and stem cells. EXP HEMATOL. 2003;31(11):1104–1111. [PubMed] [Google Scholar]
- 526699. Suarez Y, Gonzalez L, Cuadrado A, Berciano M, Lafarga M, Munoz A. Kahalalide F, a new marine-derived compound, induces oncosis in human prostate and breast cancer cells. MOL CANCER THER. 2003;2(9):863–872. • This is an in vitro study demonstrating that the androgen-independent prostate tumor line PC3 was the most sensitive to kahalalide F among a panel of breast and prostate tumor cell lines. A series of experiments in this paper also demonstrated that the cytotoxic action of kahalalide F was mediated largely via oncosis rather than apoptosis with the lysosome specifically targeted.
- 526702.Rademaker-Lakhar JM, Horenblasi S, Meinhardti W, Stokvisi E, De Reijke TM, Jimeno JM, Lopez-Lazaro L, Lopez-Martin JA, Beijnen JH, Schellens JH. Phase I and pharmacokinetic study of kahalalide F in patients with advanced androgen refractory prostate cancer. BR J CLIN PHARMACOL. 2003;56(4):469. doi: 10.1158/1078-0432.CCR-04-1534. [DOI] [PubMed] [Google Scholar]
- 526734.Cordoba S, Zapata I, Romero J, Jimeno JA, Lopez-Martin JA, de la Torre A, Vargas JA, Sanchez-Prieto R, Regueiro CA, Magallon R. Kahalalide F (KF), a new marine compound, in vitro radiosensitizes human tumoral cell lines. EUR J CANCER SUPPL; 5; 2003. Abs 572. [Google Scholar]
- 526738.Schellens HM, Paz-Ares L, Trigo JM, Pardo B, Ruiz-Casado A, Ciruelos E, Garcia M, Beijnen J, Izquierdo MA. Liver toxicity: A predictable and manageable toxicity for kahalalide F (KF) EUR J CANCER SUPPL. 2003;1(5) Abs 549. [Google Scholar]
- 526742.Garcia-Fernandez LF, Reyes F, Sanchez-Puelles JM. The marine pharmacy: New antitumoral compounds from the sea. PHARM NEWS. 2002;9(6):495–501. [Google Scholar]
- 531407.Sewell JM, Langdon SP, Smyth JF, Jodrell DI, Guichard S. Kahalalide F appears to promote necrotic cell death in hepatoma cell lines. PROC ANNU MEET AM ASSOC CANCER RES. 2004;45 Abs 1509. [Google Scholar]
- 531440.Janmaat ML, Kruyt FA, Jimeno JM, Rodriguez JA, Giaccone G. Kahalalide F (KF) induces caspase-independent cytotoxicity that correlates with HER2/neu and/or HER3 expression levels and is accompanied by down-regulation of Akt signaling. PROC ANNU MEET AM ASSOC CANCER RES. 2004;45 Abs 5328. [Google Scholar]
- 543658. Beijnen JH. Phase I and pharmacokinetic study of kahalalide F in patients with advanced androgen refractory prostate cancer. PROC AM SOC CLIN ONCOL. 2004;23 doi: 10.1158/1078-0432.CCR-04-1534. Abs 2076. • This phase I study in 33 prostate cancer patients established that the MTD of kahalalide F was 560 µg/m2/day when delivered as a daily 1-h intravenous infusion for 5 days every 3 weeks.
- 549751.PharmaMar Inc. PharmaMar begins phase II NSCLC and melanoma trials. PRESS RELEASE. 2004 Jul 19; [Google Scholar]
- 554851.Paull KD, Shoemaker RH, Hodes L, Monks A, Scudiero DA, Rubinstein L, Plowman J, Boyd MR. Display and analysis of patterns of differential activity of drugs against human tumor cell lines: Development of mean graph and COMPARE algorithm. J NATL CANCER INST. 1989;81(14):1088–1092. doi: 10.1093/jnci/81.14.1088. [DOI] [PubMed] [Google Scholar]
- 555161.Jimeno J, Lopez-Martin JA, Ruiz-Casado AR, Izquierdo MA, Scheuer PJ, Rinehart K. Progress in the clinical development of new marine-derived anticancer compounds. ANTICANCER DRUGS. 2004;15(4):321–329. doi: 10.1097/00001813-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 555488.PharmaMar SA. Drug development pipeline: Kahalalide. COMPANY COMMUNICATION. 2004 Aug 19; [Google Scholar]
- 555675.Wosikowski K, Schuurhuis D, Johnson K, Paull KD, Myers TG, Weinstein JN, Bates SE. Identification of epidermal growth factor receptor and c-ErbB2 pathway inhibitors by correlation with gene expression patterns. JNATL CANCER INST. 1997;69(20):1505–1515. doi: 10.1093/jnci/89.20.1505. [DOI] [PubMed] [Google Scholar]
- 567635.PharmaMar SA. PharmaMar's kahalalide F, phase II trials for the treatment of severe psoriasis. PRESS RELEASE. 2004 Oct 28; [Google Scholar]
- 573742.Bennett S, Parkes R, Herrmann M, Rivela J. European Biotech in focus. ING BANK NV. 2004 Nov 05;18 [Google Scholar]