Abstract
Introduction
This review focuses on the multi-ligand receptor of the immunoglobulin superfamily, receptor for advanced glycation endproducts (RAGE). The accumulation of the multiple ligands of RAGE in cellular stress milieux links RAGE to the pathobiology of chronic disease and natural aging.
Areas Covered
In this review, we present a discussion on the ligands of RAGE and the implications of these ligand families in disease. We review the recent literature on the role of ligand-RAGE interaction in the consequences of natural aging; the macro- and microvascular complications of diabetes; obesity and insulin resistance; autoimmune disorders and chronic inflammation; tumors and Alzheimer’s disease. We discuss the mechanisms of RAGE signaling through its intracellular binding effector molecule, the formin DIAPH1. Physico-chemical evidence by which the RAGE cytoplasmic domain binds to the FH1 (formin homology 1) domain of DIAPH1, and the consequences, is also reviewed.
Expert Opinion
We discuss the modalities of RAGE antagonism currently in pre-clinical and clinical studies. Finally, we present the rationale behind potentially targeting the RAGE cytoplasmic domain-DIAPH1 interaction as a logical strategy for therapeutic intervention in the pathological settings of chronic diseases and aging in which RAGE ligands accumulate and signal.
Keywords: receptor, diabetes, obesity, inflammation, Alzheimer’s disease, neurodegeneration
1.0. Introduction: RAGE & its Diverse Ligand Families
The multi-ligand immunoglobulin superfamily molecule, receptor for advanced glycation endproducts (RAGE), was discovered on account of its ability to bind the advanced glycation endproducts (AGEs), the products of nonenzymatic glycation and oxidation of proteins and lipids that occurs at slow rates in normal health and aging, but to accelerated degrees in diabetes [1–4]. AGEs are a heterogeneous group of post-translationally modified species, including adducts such as carboxy methyl lysine (CML), carboxy ethyl lysine (CEL), pentosidine, pyralline and MOLD/GOLD, to indicate a few. Although RAGE was identified as a binding molecule for the heterogeneous AGEs, one of these AGEs, CML-AGE, is a specific AGE ligand of RAGE [5]. Beyond endogenous formation of AGEs through pre-AGEs such as glyoxal, methylglyoxal (MG) and 3-deoxyglucosone (3-DG), AGEs such as CML-AGE may also gain access to the body through the diet [6]. RAGE may contribute to the propagation of MG-derived AGE products. In diabetic kidney and diabetic retina, lower levels of the pre-AGE MG were noted in diabetic mice devoid of Ager (gene encoding RAGE) versus the wild-type diabetic controls, despite equal degrees of hyperglycemia. Further analysis uncovered that RAGE-dependent downregulation of glyoxalase1 (GLO1) contributed to these findings [7, 8].
Beyond the AGEs, RAGE is also a signaling receptor for certain members of the S100/calgranulin family and high mobility group box 1 (HMGB1), amyloid-beta (β) peptide and β-sheet fibrils, lysophosphatidic acid (LPA), Mac-1 and phosphatidylserine [9–14]. These diverse classes of ligands suggest strong links to the metabolic state; both glucose- and lipid-modified species may bind RAGE, thereby implicating their interaction with RAGE as a contributing mechanism in metabolic disorders. Table 1 provides a review of the most-commonly studied RAGE ligands.
Table 1.
Examples of RAGE ligands*
| Ligand | Reference |
|---|---|
| AGEs | [4, 5] |
| Amyloid β-peptide | [11] |
| HMGB1 | [10] |
| Lysophosphatidic acid | [12] |
| Mac-1 | [13] |
| Phosphatidylserine | [14] |
| S100/calgranulins | [9] |
Note that other putative ligands of RAGE have been described; this list represents the more commonly described ligands of the receptor
In human subjects, a rich literature has emerged on the testing of the associations of levels of soluble RAGEs in plasma or serum with the presence or absence of chronic disease states, as well as the severity of disease [15–17]. Essentially, there are two known forms of soluble RAGEs. Cell surface cleavage of full-length membrane-bound RAGE by matrix metalloproteinases (MMPs) and A-Distintegrin and Metalloprotease (ADAM)-10 results in the formation of soluble RAGE or sRAGE, which is essentially the extracellular domain [18, 19]. A distinct form of soluble RAGE may be derived from pre-mRNA alternative splicing. Endogenous secretory (es) RAGE or RAGEv1 is not identical to the aforementioned sRAGE; esRAGE contains a unique span of 16 amino acids in the C-terminal region [20]. Of measurable soluble RAGE in circulation, esRAGE represents approximately 20% of the total material.
In the sections to follow, we review the main pathological settings in which RAGE has been implicated. These conditions define the likely disease states for ultimate clinical trial testing using the multiple forms of antagonists of RAGE/RAGE signaling in pre-clinical and clinical development.
2.0. Aging – the build-up of AGEs and tissue stress
Francheschi and Campus recently reviewed the phenomenon of “inflammation” [21]. Inflammaging refers to the “sterile” non-infection associated appearance of mild indices of low-grade inflammation, such as increased expression of inflammatory biomarkers including C-reactive protein (CRP) and Interleukin-6 that accompanies the aging process. In multiple studies in the elderly, significantly higher levels of these molecules serve as biomarkers for changes in energy balance and metabolic function in aging. Despite the apparent importance of this mechanism in potentially exacerbating the impact and failure of adequate resolution of aging-associated diseases, the underlying mechanisms are not fully known. One potential mechanism is the accumulation and reduced clearance of AGEs, thereby enhancing their interaction with RAGE and its downstream consequences.
In aging tissues, increased expression and activity of the first enzyme of the polyol pathway, aldose reductase (AR) has been demonstrated [22]. Increased action of the AR pathway in aging is a key mechanism for the generation of AGEs. In aged versus young Fischer 344 rats, increased expression of AR localized to endothelium and smooth muscle cells in the aorta. Impaired endothelial-dependent relaxation (EDR) in response to acetylcholine in the aged rat aorta was accompanied by increased levels of MG, which were attenuated by inhibition of AR in the rats. Further, aging-dependent impaired EDR was prevented by treatment of the rats with recombinant sRAGE, the extracellular ligand binding domains of the receptor (V-C1-C2), which binds and sequesters RAGE ligands, thereby preventing their activation of the cell surface receptor [22].
The question of whether life style intervention in experimental models and human subjects in aging might impact the AGE-RAGE axis has been studied. First, moderate intensity exercise treatment or administration of the RAGE antagonist FPS-ZM1 in aged rats reduced levels of MG and CML-AGE, in parallel with improved EDR in the aorta, reduced pulse wave velocity (PWV) and reduced multiple markers of oxidative stress [23]. Second, the effect of nine months ingestion of control or high CML-enriched diets was tested in wild-type mice and mice devoid of Ager [24]. Diets high in CML impaired EDR and increased PWV in the wild-type but not the Ager null mice, thereby reinforcing that increased levels of AGEs may drive abnormalities in the vasculature akin to that of aging, at least in part via RAGE.
Kotani and colleagues tested the effect of exercise and increased physical activity in community-dwelling elderly human subjects on levels of soluble RAGEs, using measurement of the levels of soluble RAGEs as “surrogates” of RAGE activity in vivo. The authors reported a reduction in levels of sRAGE after six months of an interventional program designed to increase physical activity [25]. Levels of RAGE ligands were not measured in that study; it may be speculated that perhaps lower levels of sRAGE reflected lower degrees of MMP or ADAM10 activity, although this was not directly tested.
Taken together, studies in experimental models and human subjects suggest involvement of the RAGE pathway in mechanisms of vascular aging. In the section to follow, we consider the effects of this pathway on diabetic complications.
3.0. Diabetes – AGEs, oxidative and inflammatory stress and roles for RAGE
RAGE has been linked to the pathogenesis of both the macrovascular and microvascular complications of diabetes. Increased RAGE expression in the diabetic blood vessels, target cells such as cardiomyocytes, podocytes and neurons, as well as immune cells (such as monocytes/macrophages and lymphocytes) places the receptor alongside higher levels of its ligands [2]. We surmise that the co-localization of RAGE with its ligands sets the stage for increased cellular activation and stress in the diabetic milieu. Unlike the situation in acute inflammation or infection, where the offending mediating species such as lipopolysaccharide or other toll-receptor ligands are short-lived and rapidly removed with resolution of inflammation, in diabetes, ligands such as AGEs and pro-inflammatory S100/calgranulins and HMGB1 appear to persist, a situation that facilitates chronic cellular stress.
For example, in the diabetic murine and human kidney, increased expression of AGEs and S100 epitopes in early and advanced stages of kidney disease has been observed in the vascular cells, infiltrating immune cells and target cells such as the podocyte [26]. In an analogous manner, in murine and human macrovascular disease, specifically, atherosclerosis, RAGE ligands co-localize with RAGE expression in macrophages and other immune cells and smooth muscle cells in atherosclerotic plaques [27, 28]. Hence, based on these findings, experiments have been performed in animal models to probe mechanistic links between enhanced RAGE ligands and RAGE expression in the diabetic tissues.
3.1. Accelerated atherosclerosis
Definitive support for mechanistic roles for diabetes in the acceleration of atherosclerosis was published in studies in which atherosclerosis-prone mice either devoid of apolipoprotein E (APOE) or the low density lipoprotein receptor (LDLR) rendered hyperglycemic displayed increased lesion area and complexity compared to their non-diabetic cohorts. Genetic deletion of Ager in both diabetic models resulted in reduced atherosclerosis and importantly, macrophage content per lesion area was lower, in parallel with increased lesional collagen, a marker of stability (Figure 1) [29, 30]. The ligands of RAGE also accumulate in non-diabetic atherosclerosis, but to a significantly lesser degree. Even in non-diabetes, roles for RAGE in the pathogenesis of atherosclerosis in murine models was shown [31, 32].
Figure 1. Deletion of Ager suppresses diabetes-accelerated atherosclerosis and effect of diabetes and Ager deficiency on macrophage and smooth muscle cell lesional content.
(a). Male Apoe null and Apoe null/Ager null mice were rendered diabetic with streptozotocin at age 6 weeks. Mice were sacrificed and aortas were retrieved. Mean atherosclerotic lesion area at the aortic sinus is shown. (b). Immunostaining and Picrosirius Red staining of atherosclerotic lesions from the indicated Apoe null mice was performed for detection of macrophages, smooth muscle cells, T cells and collagen per lesion area (the latter using picrosirius red and polarizing microscopy) at age 24 weeks. Statistical considerations: * indicates p<0.03 vs. Apoe null/Ager null diabetic mice; ** indicates p<0.01 vs. Apoe null/Ager null diabetic mice; and ^ indicates p<0.02 vs. Apoe null/Ager null non-diabetic mice. Adapted from Circ Res 2010;106:1040–1051.
Cell type-specific roles for RAGE in the pathogenesis of atherosclerosis were also shown for bone marrow-derived cells and smooth muscle cells (SMCs) in diabetic mice [30, 33] and in endothelial cells, in non-diabetic mice [32]. Pharmacological approaches to suppressing RAGE-mediated effects in diabetic atherosclerosis were shown with treatment with sRAGE [28], and in uremic mice, with treatment with a neutralizing antibody against RAGE [34]. Further, data in non-diabetic mice revealed that one of the benefits of simvastatin therapy on suppression of atherosclerosis was via downregulation of RAGE and its ligand HMGB1 in the vasculature [35].
Taken together, these considerations suggest that RAGE and its oxidative and inflammatory ligands accumulate in diabetic atherosclerosis and that blockade or genetic deletion of RAGE exerts benefit, without effect on glycemia or levels of total cholesterol and triglyceride. Evidence of roles for the RAGE pathway in non-diabetic atherosclerosis underscore that RAGE and its ligands are also expressed in the inflamed and pro-oxidative environments of non-diabetic atherosclerosis as well. These finding strongly suggest that targeting RAGE might be a complementary therapeutic approach in macrovascular disease, especially in diabetes. In the sections to follow, we consider the effects of RAGE in microvascular complications of diabetes.
3.2. Nephropathy
As discussed above, RAGE is highly expressed in human and murine diabetic kidney. Experiments testing the benefits of pharmacological intervention in models of types 1 and 2 diabetes have reported that administration of sRAGE, alagebrium (AGE cross-link breaker), low molecular weight heparin, or neutralizing anti-RAGE antibodies exerted protection against the effects of diabetes on pathological and functional outcomes in the diabetic kidney [36–40].
In genetically modified models using gain- and loss-of function strategies, RAGE was linked to the pathogenesis of nephropathy [8, 37–39]. Specifically, deletion of Ager was shown to prevent mesangial sclerosis, thickening of the glomerular basement membrane, podocyte loss and reduced urinary albumin excretion [8, 37, 38]. As a key endpoint for nephropathy in clinical trials will be the prevention of loss of renal function, studies by Reiniger and colleagues specifically addressed the effects of Ager deletion on inulin clearance. Although diabetic OVE26 mice displayed a significant reduction in inulin clearance over a >6 month period, OVE26 mice devoid of Ager were protected from the loss of renal function, as measured by inulin clearance [8]. Importantly, in those studies, levels of blood glucose did not differ between OVE26 mice expressing or devoid of Ager. Of note, interesting pathogenic roles for bone marrow-derived Ager expression in diabetic mice have been shown in bone marrow transplantation studies [41]. However, although diminished podocyte loss and less loss of renal function were observed in the recipients of Ager-deficient bone marrow, the degree of glomerulosclerosis and collagen deposition was not affected, thereby suggesting that the more complete protection against indices of nephropathy in diabetes in global Ager null mice was only due, in part, to the bone marrow-derived cells.
In recent studies, the effects of pharmacological agents currently in use in diabetic subjects were tested for their effects on the expression of the ligand-RAGE axis in the diabetic kidney. Treatment of diabetic mice with agents such as gemigliptin, an inhibitor of dipeptidyl peptidase 4 (DPP4) [42], empagliflozin (a sodium glucose cotransporter 2 (SGLT2) inhibitor) [43], ramipril (angiotensin converting enzyme inhibitor) [44], and nifedipine, a calcium channel blocker [45], exerted their beneficial effects on nephropathy endpoints, in parallel with reduced expression of the ligand-RAGE axis. Such studies suggest that complementary, multi-targeted treatments, and not single target approaches, may be the most logical means to optimally treat diabetic nephropathy.
3.3. Retinopathy
Data reported from industrialized countries indicate that retinopathy is the most frequent complication of diabetes in the microvasculature and in the working age population, diabetes represents the leading cause of blindness [46]. RAGE and its ligands are expressed in multiple key cell types in the diabetic retina in experimental models, such as in retinal pigment epithelial cells, Muller ganglia and retinal endothelial cells. Exposure of these cells to RAGE ligands induces RAGE-dependent cellular stress [47–49]. Administration of sRAGE or genetic deletion of Ager in mouse models of type 1 or type 2 diabetes results in protection against multiple early retinopathic abnormalities in mouse models, including retinal permeability, loss of retinal neuronal function, endothelial and pericyte damage, and microgliosis and inflammatory perturbation [7, 50, 51]. It is acknowledged that one pitfall of rodent models is the limitation that most of the pathology does not represent the advanced stages of disease, such as microaneurysms and retinal hemorrhage.
Given the importance of diabetic retinopathy as a complication of diabetes, it is plausible that therapeutic strategies targeting RAGE might be implemented early in the course of the disease, in order to potentially prevent the earliest vascular and permeability abnormalities. Hence, a focus on local intraocular RAGE-directed therapies as a key strategy may ultimately be most logical to ensure optimal bioavailability and safety.
3.4. Distinct complications of diabetes
In distinct complications of diabetes, such as cardiac abnormalities [52], neuropathy [53] and impaired wound healing [54], as examples, roles for the RAGE signaling pathway have been demonstrated. It is important to note that particularly in full thickness excisional wound healing, topical or intraperitoneal administration of soluble RAGE exerted benefit in enhancing wound closure in type 2 diabetic db/db mice and importantly, in non-diabetic mice, topical treatment with soluble RAGE exerted no adverse effect on the wound healing process, suggesting that the receptor activation was largely triggered in pathological dermal damage and not in homeostatic responses to cellular stress [55]. Together with evidence that deletion or blockade of Ager generally does not exacerbate or mediate adverse responses to infection, but improves survival in severe sepsis in animal models [56], these considerations suggest that targeting RAGE is not likely to exert deleterious effects in chronic disease conditions in which superimposed acute illnesses may occur. In the section to follow, we review the evidence linking RAGE to obesity and the pathogenesis of insulin resistance and diabetes.
4.0. Obesity and Metabolic Dysfunction and the Growing Links to RAGE
High fat diets in human subjects and/or in animal models generate RAGE ligands such as CML-AGE, phosphoethanolamine modifications of isolevuglandins, HMGB1, and S100B, which accumulate in metabolic tissues in obesity [57–61]. RAGE is expressed to greater degrees in obese versus lean human adipose tissue, particularly in macrophages and adipocytes [59]. When mice globally devoid of Ager or their littermate controls were fed a high fat diet (HFD) (60% kcal/by fat) versus a standard low fat diet (LFD), surprisingly, despite equal degrees of food consumption, mice devoid of Ager fed HFD gained significantly less weight versus the littermate Ager-expressing controls [60]. In parallel with this observation, mice devoid of Ager displayed lower macrophage content in the visceral adipose tissue (VAT) and their adipose tissue macrophages expressed significantly higher levels of “M2”/anti-inflammatory polarized macrophages versus the wild-type controls fed the HFD. Metabolic testing revealed that mice devoid of Ager displayed higher glucose and insulin tolerance and by studies in metabolic cages, higher energy expenditure [60]. Consistent with partial yet significant roles for myeloid-derived RAGE in these phenomena, wild-type mice subjected to lethal irradiation and bone marrow transplantation with Ager null versus wild-type bone marrow displayed significantly less weight gain, metabolic dysfunction, and macrophage content with increased “M2” like inflammatory polarization compared to mice reconstituted with Ager-expressing bone marrow [60].
To ensure that these findings were not due to innate roles for RAGE in metabolic organ development, we treated adult wild-type mice with sRAGE or vehicle, beginning at the time of, or three weeks after the initiation of HFD. In both time courses, compared to vehicle-treated mice, mice treated with sRAGE displayed significantly slower weight gain despite equal degrees of food consumption [60]. Further studies are underway to identify the precise cellular depots and mechanisms that account for these roles for RAGE in obesity and high fat feeding. Therefore, this work suggests that chronic treatment with RAGE antagonists may not result in untoward weight gain and consequent metabolic dysfunction. This concept requires definitive testing in human subjects.
5.0. RAGE and Roles in Autoimmunity and Chronic Inflammation
The discovery that RAGE was a receptor for non-AGE ligands such as S100/calgranulins, HMGB1 and Mac1, as examples [9, 10], set the stage for delineating roles for this receptor in non-diabetic inflammation. The discovery of RAGE expression and function in T lymphocytes underscored that RAGE played roles in T cell activation and differentiation and first linked RAGE to the pathogenesis of autoimmune type 1 diabetes [62, 63]. Hence, analogous to the studies discussed above in obesity, this work of Herold and Clynes showed that RAGE contributed to the pathogenesis of type 1 diabetes.
In human subjects, a particular polymorphism of RAGE (“RAGEG82S”) was found to be in linkage disequilibrium with HLA-DR4, which is classically associated with rheumatoid arthritis [64, 65]. Mice vulnerable to autoimmune bone and joint tissue destruction treated with sRAGE displayed significantly less joint inflammation and bone loss compared to mice treated with the vehicle. Cultured cells expressing G82S versus the G82G wild-type displayed higher binding affinity for S100 ligands and greater degrees of matrix metalloproteinase-mediated inflammation upon treatment with the RAGE ligand S100B [64]. RAGE has been shown to be expressed in other forms of autoimmune disease, such as in the minor salivary glands of patients with Sjogrens’ syndrome [66].
It was reported that phosphatidylserine (PS) is a RAGE ligand; PS, when translocated from the inner to the outer plasma membrane, serves a signal for apoptotic cell clearance. It was shown that mice devoid of Ager subjected to inhalation of lipopolysaccharide (LPS) displayed reduced clearance of apoptotic neutrophils versus the wild-type Ager-expressing mice [14]. Impaired clearance of apoptotic cells is also a hallmark of systemic lupus erythematosus, which is modeled in B6-MRL Fas lpr/j mice; time course experiments of annexin V expression revealed that autoreactive T lymphocytes in the spleen of B6-MRL Fas lpr/j mice devoid of Ager exhibited delayed apoptosis and expressed significantly less activated caspase 3 than T lymphocytes in B6-MRL Fas lpr/j mice expressing RAGE. These studies suggested that RAGE reduces the accumulation of autoreactive CD3(+)B220(+)CD4(−)CD8(−) T lymphocytes, and that deletion of Ager exacerbates lymphoproliferative syndrome, autoimmunity, and organ injury. From the studies, we may infer that RAGE is able to rescue apoptosis of T lymphocytes when Fas/CD95 is not present or functional [67].
One consequence of chronic inflammation in diseases such as SLE is accelerated atherosclerosis [68]; given the upregulation of RAGE ligands and early endothelial stress and dysfunction that marks both disorders, it is not surprising that the RAGE pathway was linked to cardiovascular diseases in autoimmune settings [69].
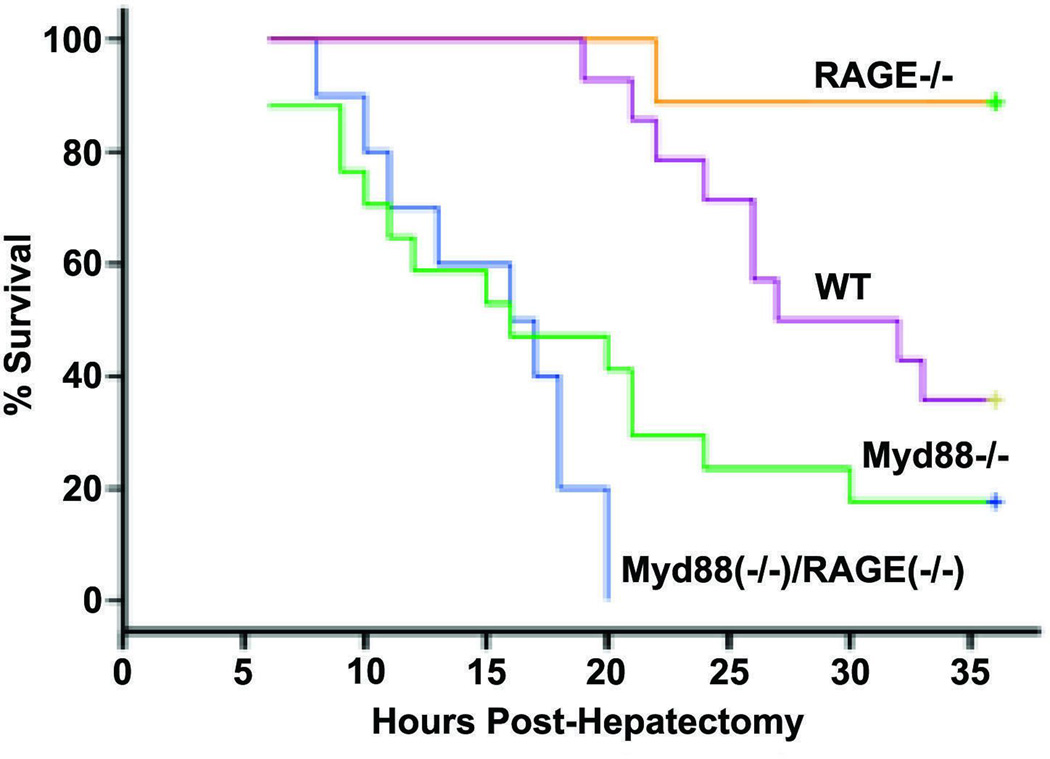
Finally, in the context of RAGE-dependent mechanisms in inflammation and autoimmunity, it is established that the proinflammatory ligand HMGB1 binds both RAGE and to toll-like receptors (TLRs); the latter are key gate keepers of innate immunity. To begin to address the inter-relationship and/or inter-dependence of RAGE versus TLR signaling, we employed extensive liver resection (85%) as an in vivo model system for testing this concept. Although Myd88, downstream of certain of the toll-like receptors, is required for survival via regulation of NF-κB and TNF-α, deletion of Ager was associated with significantly higher survival compared to wild-type, Myd88 null, or Ager/Myd88 null mice (Figure 2). Studies performed in the hepatic remnant indicate that RAGE opposes Myd88 signaling by the following mechanisms: (1) RAGE suppresses NF-kB p65 and consequent production of cyclin D1; and (2) RAGE suppresses Pim1 through a STAT3/Il6-mediated mechanism, which will both increase apoptosis and reduce the hyperplasic response [70]. From these studies, we deduced that toll-like receptor signaling was essential for activation of innate mechanisms after massive injury that were required for the animal to survive the acute stress of massive resection of the liver, and that RAGE action blunted the benefits of toll like receptor actions through inhibition of survival mechanisms in the liver, including NF-kB and Pim1. Taken together, these reports suggest that RAGE action is largely deleterious in acute stresses and therefore support the premise that extended blockade of RAGE in long-standing chronic disease may not adversely block adaptive pathways needed for acute survival in immediate stress responses.
Figure 2. Effect of Ager or/and Myd88 gene deletion on survival after extensive (85%) hepatectomy.
Kaplan-Meier curves for Wild-type (WT), Ager null, Myd88 null, or Myd88 / Ager null mice were plotted, and the statistical significance of the probability of survival of the mutants vs. WT mice was calculated. n = 10 mice/group. Statistical considerations: Ager−/− vs. WT, P = 0.01; Ager−/− vs. Myd88−/−, P < 0.001; Myd88−/− vs. WT, P = 0.013; Myd88−/− vs. Myd88−/−/Ager−/−, P = 0.144. Adapted from FASEB J 2012; 26: 882–893.
6.0. RAGE and Cancer – Many Ligands Playing Key Roles in Tumor Behavior
The discovery of HMGB1 as a RAGE ligand set the stage for uncovering if RAGE played roles in cellular migration, a key component of local tumor growth and metastases. In our first study, we showed that administration of sRAGE or antibodies to RAGE was highly protective against local tumor growth and invasion in C6 glioma-raised tumors and in distant tumor metastases in the Lewis lung carcinoma model [10]. Since that discovery, studies in human subjects have uncovered that RAGE is expressed in a broad array of human tumors, such as cancers of the breast, gastric system, colon, liver and hepatobiliary system, pancreas, prostate, oral cavity (squamous cell cancer), lung, brain and skin (melanoma) [71]. The HMGB1-RAGE axis has recently been shown through bioinformatics approaches to be a strong predictor of poor prognosis in lung cancer [72]. Beyond HMGB1, multiple other ligands of RAGE have since been implicated in tumor biology, including S100A4, S100P, S100A12, S100A7, S100A8/9, S100B, S100A14, S100A2, CML-AGE, and lysophosphatidic acid [12, 73–87].
Multiple mechanisms by which the ligand-RAGE axis exerts its effects in tumors have been described, including facilitating angiogenesis and endothelial cell proliferation; tumor cell invasion, migration and metastasis; modulation of the tumor microenvironment; promotion of autophagy; actin-polymerization; epithelial-mesenchymal transition; chemoattraction of myeloid derived macrophages; upregulation of inflammatory signaling; and protection against apoptosis [74, 75, 77, 80–82, 84–88].
RAGE is highly expressed in macrophages and emerging data suggest that RAGE regulates macrophage content and inflammatory polarization in diabetic atherosclerosis, diabetic sciatic nerve injury and in adipose tissue macrophages in high fat feeding [30, 60, 89]. Roles for RAGE in immune cells in tumors, in particular, tumor-associated macrophages (TAMs) were definitively suggested by bone marrow chimera studies in mice subjected to an inflammation-induced skin tumor; in this setting, mice globally devoid of Ager were resistant to the development of the skin tumor [90]. The site of RAGE-dependent action was identified to be the bone marrow, based on bone marrow transplantation studies in mice. In other studies, it was shown that RAGE mediates ligand S100A7-mediated breast cancer growth through recruitment of MMP9-expression in TAMs [77]. In the setting of glioma tumors, RAGE expression in TAMs contributes to the promotion of tumor-associated inflammation and angiogenesis [91], a process mediated potentially by the ligand S100B [82].
Finally, additional RAGE-dependent mechanisms in tumors may include the effect of this receptor on regulation of transcription and activation of Egr1 in hypoxia, thereby increasing conditions favorable for tumor growth and spread. In hypoxic endothelial cells, cardiomyocytes, and macrophages [92–94], deletion of Ager blocked hypoxia-mediated upregulation of Egr1; EGR1 plays major roles in regulation of inflammatory and prothrombotic genes in the hypoxic state [95]. In other studies, RAGE was linked to the pathogenesis of hypoxia induced stress in cystic fibrosis models in mice [96] and through down-regulation of Ager by silencing of hypoxia inducible factor 1 (HIF-1a) in breast tumor samples [97].
Taken together, there are multiple putative mechanisms by which RAGE may modulate tumor biology, that is, through direct effects on tumor cell proliferation, survival, migration and invasiveness; through maladaptive modulation of the tumor environment, particularly in TAMS; through enhancement of angiogenesis through effects on endothelial cells; and through upregulation of damaging proinflammatory and prothrombotic transcription factors in hypoxia, a common complication that occurs in the tumor milieu.
7.0. RAGE in the Lung: Possible Physiological Roles
In the absence of disease, low levels of RAGE are expressed in most body tissues, including vascular tissues, immune cells, neurons, cardiomyocytes, podocytes and certain epithelial cells, as examples. In contrast, the expression of RAGE is naturally high in the lung in homeostasis [98]. In the normal lung, RAGE is largely expressed in alveolar type I pneumocyte (ATI cell) [99, 100], where it is localized to the basolateral membrane of these cells, suggesting possible roles in mediating the contact between ATI cells and their natural substratum [100, 101]. Interestingly, in lung cancer, expression levels of RAGE are reported to be reduced compared to those of the non-tumor adjacent tissue [102]. Such reduction in levels of RAGE in lung cancer was suggested to support the growth of lung cancers, such as non-small cell lung tumors [103].
In mouse models of lung stress, induction of silicosis resulted in down-regulation of RAGE expression and, in mice devoid of Ager, a generally higher histological score (i.e., greater fibrotic pathology) compared to wild-type control mice [104]. In the setting of intratracheal treatment of mice with bleomycin, however, although mice devoid of Ager displayed the same early inflammatory infiltrates as wild-type mice after treatment, the mice devoid of Ager displayed higher overall survival and less lung fibrosis compared to the Ager-expressing controls [105]. Upon intratracheal administration of lipopolysaccharide, mice devoid of Ager were not protected from the ill effects when compared to the wild-type mice; however, upon intratracheal administration of E. coli, mice devoid of Ager displayed less inflammation, lower expression of inflammatory mediators, and overall less evidence of pneumonia [106]. Taken together, these considerations suggest that the function of RAGE in the lung is complex and that further experimentation is required to determine if blockade of the RAGE pathway would induce any negative outcomes in this organ.
8.0. Alzheimer’s Disease and Neurodegeneration
One of the ligands of RAGE is the amyloid-beta peptide (Aβ) and β-sheet fibrils [11]; these species accumulate in Alzheimer’s disease brain and are believed to play key roles in the pathogenesis of the disease. In addition to Aβ, other ligands of RAGE have been linked to AD, such as AGEs and HMGB1 [107–109]. It has been suggested that RAGE ligands stimulate beta secretase (BACE1), a mechanism to increase levels of Aβ [108, 109]. Further, it has been shown that diabetes exacerbates amyloid plaque deposition in AD transgenic mouse models, at least in part through AGE/RAGE-mediated activation of NF-kB [110]. In human subjects, RAGE is expressed in AD brain, especially in the hippocampus [111] and interestingly, the RAGE G82S polymorphism was suggested to be involved in increased genetic susceptibility to AD, albeit in a small preliminary study [112].
RAGE is expressed in many of the cell types linked to the pathogenesis of AD. As illustrated in Table 2, experiments using cultured cells, primary cells, cell-type specific transgenic and genetically-modified mice implicated roles for RAGE in microglia, astrocytes, neurons and in the vasculature. Indeed, one possible mechanism by which RAGE contributes to AD is in opposing roles and alterations in Aβ transport with LRP-1 (low density lipoprotein receptor related protein 1) [113–117].
Table 2.
CNS Cell Types Implicated in AD via RAGE-dependent mechanisms*
| Ligand | Reference |
|---|---|
| Astrocytes | [141, 142] |
| Endothelial | [114, 115, 142] |
| Microglia | [113, 117] |
| Neurons | [11, 116] |
In this Table, we refer to the more commonly studied cell types implicated in AD; this does not exclude the potential involvement of other RAGE-expressing cell in the CNS in the pathogenesis of AD.
The possibilities for therapeutic opportunities targeting RAGE in AD are mounting; in addition to agents being tested against RAGE in preclinical models [118], a inhibitor of RAGE is in clinical trials after evidence of potential efficacy in prevention in cognitive decline in human subjects with Alzheimer’s Disease [119, 120].
9.0. RAGE Signaling – the Discovery of the Formin DIAPH1 as an Intracellular Signaling Effector of RAGE
The cytoplasmic domain of RAGE is short and highly charged and is essential for RAGE ligand-stimulated signaling. To discover the molecular mechanisms by which RAGE activates signaling pathways, we used a yeast-two-hybrid assay to identify binding partners of RAGE. Through this approach, we uncovered multiple copies of the formin molecule, DIAPH1 [121]. Formins are cytoplasmic actin-binding proteins that play key roles in cellular migration and actin cytoskeleton dynamics; as effectors of the Rho GTPases (RhoA, Rac1, Cdc42) involved in multiple components of intracellular signaling; and as regulators of Serum Response Factor (SRF) transcription factor and its downstream genes [122, 123].
Beyond evidence of the interaction of the RAGE cytoplasmic domain with DIAPH1 by co-localization studies in cellular models and by co-immunoprecipitation and Western blotting from cellular lysates, it was essential to test if DIAPH1 was required for RAGE signaling. To date, in primary murine SMCs, cultured BV2 microglia, human thyroid cancer cells and murine and human macrophages and THP1 cells, small interference RNA-mediated knockdown of Diaph1 or deletion of Diaph1 resulted in significantly reduced signaling in response to RAGE ligands such as S100B, CML-AGE, S100A4 and hypoxia-derived AGEs [73, 92, 124, 125], particularly manifested as reduced activation of Rac1, RhoA and cdc42. In vivo, neointimal expansion in the femoral artery consequent to guide wire induced injury was significantly lower in mice devoid of Diaph1 versus the wild-type control; key roles for RAGE/DIAPH1 signaling in SMCs accounted for these effects [125]. Of note, the response noted in mice devoid of Diaph1 was highly analogous to that observed in mice devoid of Ager, suggesting a common mechanistic link [126].
Recently, Shekhtman and colleagues used NMR spectroscopy to identify the four key amino acids in the RAGE cytoplasmic domain (Q3, R4, R5 and Q6 corresponding to Q364, R365, R366, and Q367 of the full-length RAGE) that were essential to interact with the FH1 domain of DIAPH1 (Figure 3) [127]. When R5/Q6 were mutated to alanine residues and expressed in murine SMCs, Akt signaling and cellular migration and proliferation were significantly reduced, indicating that these residues in the RAGE cytoplasmic domain were required for RAGE signaling [127].
Figure 3. Cytoplasmic tail (ct)RAGE interacts with DIAPH1 FH1 domain.
(a). Sequence alignment of the human DIAPH1 FH1 construct used in this study (NCBI accession code NP_005210) and mouse DIAPH1 FH1 (NCBI accession code NP_031884). Conserved residues are in red. (b). Overlay of 15N-edited heteronuclear single quantum coherence, HSQC, NMR spectra of free [U-15N] ctRAGE (black) and the DIAPH1 FH1-[U-15N]ctRAGE complex (red). To form the DIAPH1 FH1-ctRAGE complex, 0.5 mM unlabeled DIAPH1 FH1, in NMR buffer (10 mM potassium phosphate (pH 6.5), 100 mM NaCl, 0.02% (w/v) NaN3, in 90%/10% H2O/D2O) was added into 100 µM [U-15N]ctRAGE to yield a DIAPH1 FH1/ctRAGE molar ratio of 1:1. Due to 15N editing of the experiment, only backbone and side chain amide protons and nitrogens of ctRAGE are present in the spectrum. Most peaks do not change their positions, reflecting the fact that only a subset of ctRAGE residues interact with FH1. ctRAGE peaks that are substantially or completely broadened are labeled. Note that Q3, R4, R5, and Q6 of ctRAGE correspond to Q364, R365, R366 and Q367 of the full length RAGE (c). DIAPH1 FH1-ctRAGE interaction map. Residues broadened during the NMR titration experiment are indicated in red. J Biol Chem 2012; 287:5133–5144.
In a recent study, it was suggested that the RAGE cytoplasmic domain may interact with DOCK7 to regulate activation of cdc42 signaling [128]; it will be important to test if DOCK7 is part of a multimolecular component unit, perhaps including DIAPH1, that is required to fully activate Rho GTPase signaling initiated by RAGE ligands.
10.0. Expert Opinion
The unexpected finding that mice devoid of Ager were protected from HFD-induced obesity placed RAGE squarely in the midst of metabolic dysfunction [60]. In fact, it was surprising to discover that HFD stimulated increases in multiple families of RAGE ligands in metabolic organs, such as CML-AGEs, HMGB1 and S100/calgranulins, even in the absence of frank hyperglycemia [57–61]. These considerations thus suggested that RAGE was not only involved in the pathogenesis of diabetic complications, but also in the biochemical and metabolic perturbations that characterize the pre-diabetic or diabetic-susceptible state. These fascinating concepts set the stage for uncovering the “natural function” of RAGE and for understanding the breadth of its links to metabolic regulation. It is thus tempting to speculate that antagonism of RAGE in clinical trials might yield benefits beyond preventing or assuaging inflammation-induced exacerbation of tissue damage in diabetes, specifically by limiting weight gain and metabolic abnormalities even in subjects consuming unhealthy diet and with limited physical activity. Furthermore, as discussed above, the intriguing biology of RAGE in the lung has yet to be fully deciphered. No doubt, however, that all induced lung pathologies in the Ager null mouse are not strictly associated with negative outcomes, such as in the setting of bleomycin-induced fibrosis, in which mice devoid of Ager display protection, not greater disease. Experiments in tissue-targeted Ager-modified mice are likely to be helpful in settling this quandary.
The body of work accrued to date on the biology of RAGE also underscores that the RAGE pathway and its links to disease do not represent “one ligand-one disease.” Indeed, beyond obesity and diabetes, as illustrated above, similar considerations were discussed in this review with respect to other RAGE-associated diseases, such as autoimmunity, Alzheimer’s disease, and cancers, that is, more than one class of RAGE ligands is upregulated and accumulated in specific chronic disease settings.
Is one class of ligand more important than another in terms of disease pathogenesis? Given the expanding number of RAGE ligands and the fact that not all may be approached genetically by deletion, for example, for strategic reasons and hypothesis testing, we surmise that creative solutions to targeting RAGE will be needed. Indeed, the solution structure of the RAGE extracellular domain suggests that the ligand-binding domain is an elongated molecule containing two “patches” that mediate the interaction with ligands – a large basic patch and a large hydrophobic patch. Interestingly, both are highly conserved, suggesting the evolutionary importance and relevance, perhaps, of RAGE in chronic inflammation. Importantly, whereas S100B is largely recognized by the hydrophobic patch in the extracellular domain (residues 54–67), AGEs, in contrast, mediate their interaction with RAGE via their negative charges [129–131].
Importantly, studies have suggested that RAGE may contribute to regulation of its ligand levels. As illustrated in Figure 4, RAGE-dependent downregulation of GLO1 may limit the detoxification of the pre-AGEs, thereby increasing levels of AGEs. Further, if validated, RAGE-dependent levels of BACE1 may have implications for the sustained generation of Aβ in settings of high RAGE expression. The potential utility of BACE1 inhibitors as a viable therapeutic strategy in AD has yet to be definitively established [132]. It is also conceivable that GLO1 stimulating agents and BACE1 inhibitors might be important adjunctive strategies when coupled with RAGE antagonism.
Figure 4. RAGE & regulation of ligand levels.
Content of two of the classes of RAGE ligands, glyoxal (G) and methylglyoxal (MG)-derived AGEs, and amyloid-β-peptide (Aβ), may be regulated, in part, by RAGE, in chronic disease settings. First, in the case of AGEs, the key pre-AGE intermediates, G and MG, are detoxified by the enzyme glyoxalase 1 (GLO1). Published evidence indicates that RAGE downregulates Glo1 mRNA and activity in certain diabetic tissues. Hence, RAGE activation in high-AGE settings may stimulate AGE production and accumulation via a feed-forward loop in which RAGE suppresses the brake on pre-AGE (G & MG) detoxification, thereby favoring more conversion into AGEs. These considerations have implications for conditions such as diabetes complications. Second, in the case of Aβ, published evidence suggests that ligand-RAGE interaction might upregulate BACE1, thereby promoting further production of Aβ. These considerations have implications for conditions such as Alzheimer’s disease. Abbreviations: AGE, advanced glycation endproduct; G, glyoxal; GLO1, glyoxalase 1; and MG, methylglyoxal.
Where, then, does this leave reasonable prospects for anti-RAGE therapeutic development? The first agent tested in antagonism of the RAGE pathway was sRAGE. Administration of sRAGE to rodents was remarkably protective against the consequences of diabetes, high fat feeding, inflammation and tumors [1–4]. There are challenges to the development of sRAGE as a viable therapeutic agent, however. sRAGE, presumably acting as a ligand sink to sequester RAGE ligands and prevent their interaction with the cell surface receptor, would require long-term administration in chronic diseases. Although surmountable, the costs of production and delivery might not be inconsequential. More importantly, on a biological basis, is it possible that sRAGE might block the interaction of ligands with beneficial receptor pathways? Just as the receptor is promiscuous with respect to its diverse ligand repertoire, the ligands do not display fidelity to a single receptor, either. Although no evidence of maladaptive effects emerged from long-term sRAGE treatment in mice, it is the case that the mice were not challenged with superimposed stresses, such as infection, immunization, physical wounding or other stimuli. Would sequestering RAGE ligands from their receptor(s) exert negative consequences? We do not have the entire answer to this question at this time.
Although sRAGE is present in the circulation of human subjects, the extent to which it may be functional, that is, that it may possess the ability to effectively sequester maladaptive (or adaptive) ligands, is not established. Indeed, at this time, the study of sRAGE in human subjects is focused on the potential of this material to serve as a biomarker for chronic illness. Even in that setting, studies on measurement of soluble RAGEs in human subjects and their relationship to human health and disease further underscore that the biology of soluble RAGE is complex. For example, in cardiovascular disease (CVD), lower levels of sRAGE were reported to align with both worse CVD [133] and the presence of essential hypertension compared to normal age-matched control subjects [134]. However, measures of sRAGE in subjects with diabetes revealed that higher levels of sRAGE were associated with greater degrees of CVD [135].
In the setting of lung disease, high levels of sRAGE were reported to be predictors of negative outcome in lung transplantation [136]. Yet, in a distinct study, lower levels of sRAGE were associated with the degree of severity of emphysema [137].
Taken together, there is, to date, no consensus on the predictive value of sRAGE as a biomarker in disease vs. health, and disease status and extent (stage). We speculate that perhaps sRAGE measures might be of greater predictive value were each subject, prior to natural progression or disease treatment, to serve as “their own control,” such as recently published in subjects undergoing bariatric surgery [138]. In that study, the baseline level of sRAGE in the individual subject predicted the overall metabolic response, or not, to bariatric surgery.
Thus, beyond sRAGE, distinct strategies to target the extracellular domains of RAGE such as small molecule antagonists, anti-RAGE antibodies and RAGE peptide aptamers or full-length RAGE by using nanocarriers carrying RAGE siRNAs, have been tested in animals and in few cases, in human subjects to date [118–120, 139, 140]. We propose that a logical means to target the RAGE pathway may be by interfering with its signaling. As noted above, to date, distinct classes of ligands have been shown to signal through DIAPH1, including AGEs and different members of the S100/calgranulin family. Certainly, more research is required to understand the entire scope of RAGE signaling and the extent to which blocking RAGE/DIAPH1 interaction may encompass the full pathobiology of RAGE activation. Small molecule inhibitors of this pathway, under active development at this time, may serve as novel probes for dissecting RAGE signaling in response to distinct ligands, as well as templates for therapeutic development. Given the potent effects of RAGE activity in animal models and its ability to be tracked in vivo by measures of soluble RAGEs and RAGE levels in accessible peripheral blood-derived immune cells, the time appears to have come to rigorously interrogate the RAGE pathway as a logical therapeutic target for chronic disease.
Article Highlights Box.
RAGE is a multi-ligand receptor of the immunoglobulin superfamily; RAGE is expressed on multiple cell types such as vascular and immune cells, as well as target cells in diabetic complications. The expression of RAGE in most organs is low in homeostasis; a notable example is the lung, in which high levels of RAGE are observed in health.
Pharmacological modulation of, or genetic deletion of Ager, is protective in aging, in multiple animal models of diabetic complications, in obesity and metabolic dysfunction, in autoimmune and inflammatory settings, in tumors and in models of Alzheimer’s disease.
The cytoplasmic domain of RAGE binds to the formin DIAPH1. Knockdown of Diaph1 or genetic deletion of Diaph1 blocks RAGE signaling in cell types such as smooth muscle cells, macrophages, microglial cells and transformed cells.
The critical amino acids in the RAGE cytoplasmic domain have been identified that underlie the interaction between this RAGE domain and the FH1 domain of DIAPH1. Mutation of these amino acids in the RAGE cytoplasmic domain blocks the interaction with DIAPH1 and blocks RAGE ligand-induced signal transduction.
The finding that mice devoid of Ager are viable and fertile; they display protected responses to many forms of infection, sepsis and massive liver injury (resection); and they have normal life span, strongly suggests that blocking RAGE signaling is not likely to cause deleterious side effects. Hence, targeting the cytoplasmic domain of RAGE-DIAPH1 interaction with small molecule inhibitors holds great potential to safely block RAGE signaling and prevent the consequences of chronic disease. Definitive testing in human subjects is required.
Acknowledgments
The authors acknowledge the expert assistance of Ms. Latoya Woods in the preparation of this manuscript.
The authors were supported by funding for this work from the National Institutes of Health U.S.A., the Juvenile Diabetes Research Foundation (JDRF) and the American Diabetes Association (ADA). R Ramasamy and AM Schmidt are supported by NIH grants numbers P01HL60901 and 1R24DK103032, and JDRF grant 4-2011-25. AM Schmidt is also supported by NIH grant 1R01HL118565 and ADA grant 1-15-MI-14. A Shekhtman is supported by NIH grant 1R24DK103032 only.
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Daffu G, del Pozo CH, O'Shea KM, et al. Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int J Mol Sci. 2013;14:19891–19910. doi: 10.3390/ijms141019891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litwinoff EM, Hurtado Del Pozo C, Ramasamy R, et al. Emerging targets for therapeutic development in diabetes and its complications: The RAGE signaling pathway. Clin Pharmacol Ther. 2015 doi: 10.1002/cpt.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manigrasso MB, Juranek J, Ramasamy R, et al. Unlocking the biology of RAGE in diabetic microvascular complications. Trends Endocrinol Metab. 2014;25:15–22. doi: 10.1016/j.tem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramasamy R, Yan SF, Schmidt AM. The diverse ligand repertoire of the receptor for advanced glycation endproducts and pathways to the complications of diabetes. Vascul Pharmacol. 2012;57:160–167. doi: 10.1016/j.vph.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kislinger T, Fu C, Huber B, et al. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274:31740–31749. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann SM, Dong HJ, Li Z, et al. Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes. 2002;51:2082–2089. doi: 10.2337/diabetes.51.7.2082. [DOI] [PubMed] [Google Scholar]
- 7.McVicar CM, Ward M, Colhoun LM, et al. Role of the receptor for advanced glycation endproducts (RAGE) in retinal vasodegenerative pathology during diabetes in mice. Diabetologia. 2015;58:1129–1137. doi: 10.1007/s00125-015-3523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiniger N, Lau K, McCalla D, et al. Deletion of the receptor for advanced glycation end products reduces glomerulosclerosis and preserves renal function in the diabetic OVE26 mouse. Diabetes. 2010;59:2043–2054. doi: 10.2337/db09-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. ** This paper demonstrated that non-AGEs were ligands of RAGE. Specifically, this work illustrated that two members of the S100/calgranulin family (S100A12 and S100B) were ligands of RAGE. Since the publication of this work, researchers have extended the listing of S100 family members that utilize RAGE to initiate cellular signaling.
- 10. Taguchi A, Blood DC, del Toro G, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–360. doi: 10.1038/35012626. ** This paper linked "amphoterin" or more commonly known as High Mobility Group Box 1 (HMGB1) to the RAGE ligand family. HMGB1 is studied extensively in inflammation and in cancers, where its expression is upregulated. Although evidence suggests that HMGB1 may engage other cellular receptors, copious evidence indicates that RAGE is one of the key cell surface engagement sites for HMGB1.
- 11. Yan SD, Chen X, Fu J, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. ** This paper was the first to illustrate that RAGE was a cell surface receptor for amyloid beta peptide, which has been linked to the pathogenesis of Alzheimer's Disease. Since this first publication, many laboratories have reported on roles for RAGE in neurons, microglia and vascular cells in the context of Alzheimer's Disease pathology.
- 12.Rai V, Toure F, Chitayat S, et al. Lysophosphatidic acid targets vascular and oncogenic pathways via RAGE signaling. J Exp Med. 2012;209:2339–2350. doi: 10.1084/jem.20120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chavakis T, Bierhaus A, Al-Fakhri N, et al. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003;198:1507–1515. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He M, Kubo H, Morimoto K, et al. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Rep. 2011;12:358–364. doi: 10.1038/embor.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maillard-Lefebvre H, Boulanger E, Daroux M, et al. Soluble receptor for advanced glycation end products: a new biomarker in diagnosis and prognosis of chronic inflammatory diseases. Rheumatology (Oxford) 2009;48:1190–1196. doi: 10.1093/rheumatology/kep199. [DOI] [PubMed] [Google Scholar]
- 16. Vazzana N, Santilli F, Cuccurullo C, et al. Soluble forms of RAGE in internal medicine. Intern Emerg Med. 2009;4:389–401. doi: 10.1007/s11739-009-0300-1. ** This paper reviews the state of the field on the forms of soluble RAGE that are present in human subjects and provides a detailed discussion of the settings in which levels of sRAGEs have been tested in human subjects. As discussed in the text above, it remains unclear if natural sRAGEs have therapeutic and/or biomarker potential in the disease settings characterized by accumulation of RAGE ligands.
- 17.Yamagishi S, Matsui T, Nakamura K. Kinetics, role and therapeutic implications of endogenous soluble form of receptor for advanced glycation end products (sRAGE) in diabetes. Curr Drug Targets. 2007;8:1138–1143. doi: 10.2174/138945007782151298. [DOI] [PubMed] [Google Scholar]
- 18. Raucci A, Cugusi S, Antonelli A, et al. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10) FASEB J. 2008;22:3716–3727. doi: 10.1096/fj.08-109033. ** This paper describes a key molecular mechanism by which soluble RAGE may be formed via cell surface cleavage of the full-length RAGE and provides insights into the biochemical contexts in which sRAGE might be formed in vivo.
- 19.Zhang L, Bukulin M, Kojro E, et al. Receptor for advanced glycation end products is subjected to protein ectodomain shedding by metalloproteinases. J Biol Chem. 2008;283:35507–35516. doi: 10.1074/jbc.M806948200. [DOI] [PubMed] [Google Scholar]
- 20.Yonekura H, Yamamoto Y, Sakurai S, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370:1097–1109. doi: 10.1042/BJ20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 22.Hallam KM, Li Q, Ananthakrishnan R, et al. Aldose reductase and AGE-RAGE pathways: central roles in the pathogenesis of vascular dysfunction in aging rats. Aging Cell. 2010;9:776–784. doi: 10.1111/j.1474-9726.2010.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu Q, Wang B, Zhang XF, et al. Contribution of receptor for advanced glycation end products to vasculature-protecting effects of exercise training in aged rats. Eur J Pharmacol. 2014;741:186–194. doi: 10.1016/j.ejphar.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Grossin N, Auger F, Niquet-Leridon C, et al. Dietary CML-enriched protein induces functional arterial aging in a RAGE-dependent manner in mice. Mol Nutr Food Res. 2015;59:927–938. doi: 10.1002/mnfr.201400643. [DOI] [PubMed] [Google Scholar]
- 25.Kotani K, Caccavello R, Sakane N, et al. Influence of Physical Activity Intervention on Circulating Soluble Receptor for Advanced Glycation end Products in Elderly Subjects. J Clin Med Res. 2011;3:252–257. doi: 10.4021/jocmr704w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanji N, Markowitz GS, Fu C, et al. Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J Am Soc Nephrol. 2000;11:1656–1666. doi: 10.1681/ASN.V1191656. [DOI] [PubMed] [Google Scholar]
- 27.Burke AP, Kolodgie FD, Zieske A, et al. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- 28. Park L, Raman KG, Lee KJ, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–1031. doi: 10.1038/2012. * This paper demonstrated that administration of soluble RAGE to mice with diabetes and atherosclerosis attentuated the progression of atherosclerosis and lesion complexity. This work set the stage for the testing of distinct complications of diabetes in multiple animal models and in multiple species.
- 29.Soro-Paavonen A, Watson AM, Li J, et al. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57:2461–2469. doi: 10.2337/db07-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bu DX, Rai V, Shen X, et al. Activation of the ROCK1 branch of the transforming growth factor-beta pathway contributes to RAGE-dependent acceleration of atherosclerosis in diabetic ApoE-null mice. Circ Res. 2010;106:1040–1051. doi: 10.1161/CIRCRESAHA.109.201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun L, Ishida T, Yasuda T, et al. RAGE mediates oxidized LDL-induced pro-inflammatory effects and atherosclerosis in non-diabetic LDL receptor-deficient mice. Cardiovasc Res. 2009;82:371–381. doi: 10.1093/cvr/cvp036. [DOI] [PubMed] [Google Scholar]
- 32.Harja E, Bu DX, Hudson BI, et al. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest. 2008;118:183–194. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koulis C, Kanellakis P, Pickering RJ, et al. Role of bone-marrow- and non-bone-marrow-derived receptor for advanced glycation end-products (RAGE) in a mouse model of diabetes-associated atherosclerosis. Clin Sci (Lond) 2014;127:485–497. doi: 10.1042/CS20140045. [DOI] [PubMed] [Google Scholar]
- 34.Bro S, Flyvbjerg A, Binder CJ, et al. A neutralizing antibody against receptor for advanced glycation end products (RAGE) reduces atherosclerosis in uremic mice. Atherosclerosis. 2008;201:274–280. doi: 10.1016/j.atherosclerosis.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Liu M, Yu Y, Jiang H, et al. Simvastatin suppresses vascular inflammation and atherosclerosis in ApoE(−/−) mice by downregulating the HMGB1-RAGE axis. Acta Pharmacol Sin. 2013;34:830–836. doi: 10.1038/aps.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson AM, Gray SP, Jiaze L, et al. Alagebrium reduces glomerular fibrogenesis and inflammation beyond preventing RAGE activation in diabetic apolipoprotein E knockout mice. Diabetes. 2012;61:2105–2113. doi: 10.2337/db11-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myint KM, Yamamoto Y, Doi T, et al. RAGE control of diabetic nephropathy in a mouse model: effects of RAGE gene disruption and administration of low-molecular weight heparin. Diabetes. 2006;55:2510–2522. doi: 10.2337/db06-0221. [DOI] [PubMed] [Google Scholar]
- 38.Wendt TM, Tanji N, Guo J, et al. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol. 2003;162:1123–1137. doi: 10.1016/S0002-9440(10)63909-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto Y, Kato I, Doi T, et al. Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J Clin Invest. 2001;108:261–268. doi: 10.1172/JCI11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flyvbjerg A, Denner L, Schrijvers BF, et al. Long-term renal effects of a neutralizing RAGE antibody in obese type 2 diabetic mice. Diabetes. 2004;53:166–172. doi: 10.2337/diabetes.53.1.166. [DOI] [PubMed] [Google Scholar]
- 41.Tesch G, Sourris KC, Summers SA, et al. Deletion of bone-marrow-derived receptor for AGEs (RAGE) improves renal function in an experimental mouse model of diabetes. Diabetologia. 2014;57:1977–1985. doi: 10.1007/s00125-014-3291-z. [DOI] [PubMed] [Google Scholar]
- 42.Jung E, Kim J, Ho Kim S, et al. Gemigliptin improves renal function and attenuates podocyte injury in mice with diabetic nephropathy. Eur J Pharmacol. 2015;761:116–124. doi: 10.1016/j.ejphar.2015.04.055. [DOI] [PubMed] [Google Scholar]
- 43.Ojima A, Matsui T, Nishino Y, et al. Empagliflozin, an Inhibitor of Sodium-Glucose Cotransporter 2 Exerts Anti-Inflammatory and Antifibrotic Effects on Experimental Diabetic Nephropathy Partly by Suppressing AGEs-Receptor Axis. Horm Metab Res. 2015 doi: 10.1055/s-0034-1395609. [DOI] [PubMed] [Google Scholar]
- 44.Fukami K, Yamagishi S, Coughlan MT, et al. Ramipril inhibits AGE-RAGE-induced matrix metalloproteinase-2 activation in experimental diabetic nephropathy. Diabetol Metab Syndr. 2014;6:86. doi: 10.1186/1758-5996-6-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsui T, Yamagishi S, Takeuchi M, et al. Nifedipine inhibits advanced glycation end products (AGEs) and their receptor (RAGE) interaction-mediated proximal tubular cell injury via peroxisome proliferator-activated receptor-gamma activation. Biochem Biophys Res Commun. 2010;398:326–330. doi: 10.1016/j.bbrc.2010.06.093. [DOI] [PubMed] [Google Scholar]
- 46.Nentwich MM, Ulbig MW. Diabetic retinopathy - ocular complications of diabetes mellitus. World journal of diabetes. 2015;6:489–499. doi: 10.4239/wjd.v6.i3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dahrouj M, Desjardins DM, Liu Y, et al. Receptor mediated disruption of retinal pigment epithelium function in acute glycated-albumin exposure. Exp Eye Res. 2015;137:50–56. doi: 10.1016/j.exer.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zong H, Ward M, Madden A, et al. Hyperglycaemia-induced pro-inflammatory responses by retinal Muller glia are regulated by the receptor for advanced glycation end-products (RAGE) Diabetologia. 2010;53:2656–2666. doi: 10.1007/s00125-010-1900-z. [DOI] [PubMed] [Google Scholar]
- 49.Warboys CM, Toh HB, Fraser PA. Role of NADPH oxidase in retinal microvascular permeability increase by RAGE activation. Invest Ophthalmol Vis Sci. 2009;50:1319–1328. doi: 10.1167/iovs.08-2730. [DOI] [PubMed] [Google Scholar]
- 50.Kaji Y, Usui T, Ishida S, et al. Inhibition of diabetic leukostasis and blood-retinal barrier breakdown with a soluble form of a receptor for advanced glycation end products. Invest Ophthalmol Vis Sci. 2007;48:858–865. doi: 10.1167/iovs.06-0495. [DOI] [PubMed] [Google Scholar]
- 51.Barile GR, Pachydaki SI, Tari SR, et al. The RAGE axis in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005;46:2916–2924. doi: 10.1167/iovs.04-1409. [DOI] [PubMed] [Google Scholar]
- 52.Ramasamy R, Schmidt AM. Receptor for advanced glycation end products (RAGE) and implications for the pathophysiology of heart failure. Curr Heart Fail Rep. 2012;9:107–116. doi: 10.1007/s11897-012-0089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sugimoto K, Yasujima M, Yagihashi S. Role of advanced glycation end products in diabetic neuropathy. Curr Pharm Des. 2008;14:953–961. doi: 10.2174/138161208784139774. [DOI] [PubMed] [Google Scholar]
- 54.Sorci G, Riuzzi F, Giambanco I, et al. RAGE in tissue homeostasis, repair and regeneration. Biochim Biophys Acta. 2013;1833:101–109. doi: 10.1016/j.bbamcr.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 55.Goova MT, Li J, Kislinger T, et al. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am J Pathol. 2001;159:513–525. doi: 10.1016/S0002-9440(10)61723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Christaki E, Lazaridis N, Opal SM. Receptor for advanced glycation end products in bacterial infection: is there a role for immune modulation of receptor for advanced glycation end products in the treatment of sepsis? Curr Opin Infect Dis. 2012;25:304–311. doi: 10.1097/QCO.0b013e3283519b82. [DOI] [PubMed] [Google Scholar]
- 57.Guo L, Chen Z, Amarnath V, et al. Isolevuglandin-type lipid aldehydes induce the inflammatory response of macrophages by modifying phosphatidylethanolamines and activating the receptor for advanced glycation endproducts. Antioxidants & redox signaling. 2015;22:1633–1645. doi: 10.1089/ars.2014.6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uribarri J, Cai W, Woodward M, et al. Elevated serum advanced glycation endproducts in obese indicate risk for the metabolic syndrome: a link between healthy and unhealthy obesity? J Clin Endocrinol Metab. 2015;100:1957–1966. doi: 10.1210/jc.2014-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gaens KH, Goossens GH, Niessen PM, et al. Nepsilon-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler Thromb Vasc Biol. 2014;34:1199–1208. doi: 10.1161/ATVBAHA.113.302281. [DOI] [PubMed] [Google Scholar]
- 60. Song F, Hurtado del Pozo C, Rosario R, et al. RAGE regulates the metabolic and inflammatory response to high-fat feeding in mice. Diabetes. 2014;63:1948–1965. doi: 10.2337/db13-1636. ** This paper demonstrated that mice devoid of Ager were protected from high fat diet-induced obesity and the metabolic consequences. This work illustrated that upon induction of high fat feeding, although mice devoid of Ager consume the high fat diet food, they increase their energy expenditure, and thereby are at least partially protected from obesity. This work sets the stage for the determination of the key RAGE-expressing cell types in high fat feeding that lead to obesity and metabolic dysfunction.
- 61.Fujiya A, Nagasaki H, Seino Y, et al. The role of S100B in the interaction between adipocytes and macrophages. Obesity (Silver Spring, Md) 2014;22:371–379. doi: 10.1002/oby.20532. [DOI] [PubMed] [Google Scholar]
- 62.Chen Y, Akirav EM, Chen W, et al. RAGE ligation affects T cell activation and controls T cell differentiation. J Immunol. 2008;181:4272–4278. doi: 10.4049/jimmunol.181.6.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Y, Yan SS, Colgan J, et al. Blockade of late stages of autoimmune diabetes by inhibition of the receptor for advanced glycation end products. J Immunol. 2004;173:1399–1405. doi: 10.4049/jimmunol.173.2.1399. [DOI] [PubMed] [Google Scholar]
- 64.Hofmann MA, Drury S, Hudson BI, et al. RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes Immun. 2002;3:123–135. doi: 10.1038/sj.gene.6363861. [DOI] [PubMed] [Google Scholar]
- 65.Alghasham A, Rasheed Z. Therapeutic targets for rheumatoid arthritis: Progress and promises. Autoimmunity. 2014;47:77–94. doi: 10.3109/08916934.2013.873413. [DOI] [PubMed] [Google Scholar]
- 66.Katz J, Stavropoulos F, Bhattacharyya I, et al. Receptor of advanced glycation end product (RAGE) expression in the minor salivary glands of patients with Sjogren's syndrome: a preliminary study. Scand J Rheumatol. 2004;33:174–178. doi: 10.1080/03009740310004775. [DOI] [PubMed] [Google Scholar]
- 67.Goury A, Meghraoui-Kheddar A, Belmokhtar K, et al. Deletion of receptor for advanced glycation end products exacerbates lymphoproliferative syndrome and lupus nephritis in B6-MRL Fas lpr/j mice. J Immunol. 2015;194:3612–3622. doi: 10.4049/jimmunol.1402342. [DOI] [PubMed] [Google Scholar]
- 68.Matsuura E, Kobayashi K, Lopez LR. Atherosclerosis in autoimmune diseases. Current rheumatology reports. 2009;11:61–69. doi: 10.1007/s11926-009-0009-1. [DOI] [PubMed] [Google Scholar]
- 69.Nienhuis HL, Westra J, Smit AJ, et al. AGE and their receptor RAGE in systemic autoimmune diseases: an inflammation propagating factor contributing to accelerated atherosclerosis. Autoimmunity. 2009;42:302–304. doi: 10.1080/08916930902831746. [DOI] [PubMed] [Google Scholar]
- 70.Zeng S, Zhang QY, Huang J, et al. Opposing roles of RAGE and Myd88 signaling in extensive liver resection. FASEB J. 2012;26:882–893. doi: 10.1096/fj.11-192997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Malik P, Chaudhry N, Mittal R, et al. Role of receptor for advanced glycation end products in the complication and progression of various types of cancers. Biochim Biophys Acta. 2015;1850:1898–1904. doi: 10.1016/j.bbagen.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 72.Chang YH, Chen CM, Chen HY, et al. Pathway-based gene signatures predicting clinical outcome of lung adenocarcinoma. Sci Rep. 2015;5:10979. doi: 10.1038/srep10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Medapati MR, Dahlmann M, Ghavami S, et al. RAGE Mediates the Pro-Migratory Response of Extracellular S100A4 in Human Thyroid Cancer Cells. Thyroid. 2015;25:514–527. doi: 10.1089/thy.2014.0257. [DOI] [PubMed] [Google Scholar]
- 74.Zhu L, Ren L, Chen Y, et al. Redox status of high-mobility group box 1 performs a dual role in angiogenesis of colorectal carcinoma. J Cell Mol Med. 2015 doi: 10.1111/jcmm.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shen Z, Deng H, Fang Y, et al. Identification of the interplay between SOX9 and S100P in the metastasis and invasion of colon carcinoma. Oncotarget. 2015 doi: 10.18632/oncotarget.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khorramdelazad H, Bagheri V, Hassanshahi G, et al. S100A12 and RAGE expression in human bladder transitional cell carcinoma: a role for the ligand/RAGE axis in tumor progression? Asian Pac J Cancer Prev. 2015;16:2725–2729. doi: 10.7314/apjcp.2015.16.7.2725. [DOI] [PubMed] [Google Scholar]
- 77.Nasser MW, Wani NA, Ahirwar DK, et al. RAGE mediates S100A7-induced breast cancer growth and metastasis by modulating the tumor microenvironment. Cancer Res. 2015;75:974–985. doi: 10.1158/0008-5472.CAN-14-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reeb AN, Li W, Sewell W, et al. S100A8 is a novel therapeutic target for anaplastic thyroid carcinoma. J Clin Endocrinol Metab. 2015;100:E232–E242. doi: 10.1210/jc.2014-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duan Z, Chen G, Chen L, et al. Determinants of concentrations of N(epsilon)-carboxymethyl-lysine and soluble receptor for advanced glycation end products and their associations with risk of pancreatic cancer. International journal of molecular epidemiology and genetics. 2014;5:152–163. [PMC free article] [PubMed] [Google Scholar]
- 80.Yang M, Zeng P, Kang R, et al. S100A8 contributes to drug resistance by promoting autophagy in leukemia cells. PLoS One. 2014;9:e97242. doi: 10.1371/journal.pone.0097242. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Yin C, Li H, Zhang B, et al. RAGE-binding S100A8/A9 promotes the migration and invasion of human breast cancer cells through actin polymerization and epithelial-mesenchymal transition. Breast Cancer Res Treat. 2013;142:297–309. doi: 10.1007/s10549-013-2737-1. [DOI] [PubMed] [Google Scholar]
- 82.Wang H, Zhang L, Zhang IY, et al. S100B promotes glioma growth through chemoattraction of myeloid-derived macrophages. Clin Cancer Res. 2013;19:3764–3775. doi: 10.1158/1078-0432.CCR-12-3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yan W, Chang Y, Liang X, et al. High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases. Hepatology. 2012;55:1863–1875. doi: 10.1002/hep.25572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shubbar E, Vegfors J, Carlstrom M, et al. Psoriasin (S100A7) increases the expression of ROS and VEGF and acts through RAGE to promote endothelial cell proliferation. Breast Cancer Res Treat. 2012;134:71–80. doi: 10.1007/s10549-011-1920-5. [DOI] [PubMed] [Google Scholar]
- 85.Jin Q, Chen H, Luo A, et al. S100A14 stimulates cell proliferation and induces cell apoptosis at different concentrations via receptor for advanced glycation end products (RAGE) PLoS One. 2011;6:e19375. doi: 10.1371/journal.pone.0019375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nasser MW, Qamri Z, Deol YS, et al. S100A7 enhances mammary tumorigenesis through upregulation of inflammatory pathways. Cancer Res. 2012;72:604–615. doi: 10.1158/0008-5472.CAN-11-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nagy N, Brenner C, Markadieu N, et al. S100A2, a putative tumor suppressor gene, regulates in vitro squamous cell carcinoma migration. Lab Invest. 2001;81:599–612. doi: 10.1038/labinvest.3780269. [DOI] [PubMed] [Google Scholar]
- 88.Elangovan I, Thirugnanam S, Chen A, et al. Targeting receptor for advanced glycation end products (RAGE) expression induces apoptosis and inhibits prostate tumor growth. Biochem Biophys Res Commun. 2012;417:1133–1138. doi: 10.1016/j.bbrc.2011.12.060. [DOI] [PubMed] [Google Scholar]
- 89.Juranek JK, Geddis MS, Song F, et al. RAGE deficiency improves postinjury sciatic nerve regeneration in type 1 diabetic mice. Diabetes. 2013;62:931–943. doi: 10.2337/db12-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gebhardt C, Riehl A, Durchdewald M, et al. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med. 2008;205:275–285. doi: 10.1084/jem.20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen X, Zhang L, Zhang IY, et al. RAGE expression in tumor-associated macrophages promotes angiogenesis in glioma. Cancer Res. 2014;74:7285–7297. doi: 10.1158/0008-5472.CAN-14-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu Y, Toure F, Qu W, et al. Advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling and up-regulation of Egr-1 in hypoxic macrophages. J Biol Chem. 2010;285:23233–23240. doi: 10.1074/jbc.M110.117457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shang L, Ananthakrishnan R, Li Q, et al. RAGE modulates hypoxia/reoxygenation injury in adult murine cardiomyocytes via JNK and GSK-3beta signaling pathways. PLoS One. 2010;5:e10092. doi: 10.1371/journal.pone.0010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chang JS, Wendt T, Qu W, et al. Oxygen deprivation triggers upregulation of early growth response-1 by the receptor for advanced glycation end products. Circ Res. 2008;102:905–913. doi: 10.1161/CIRCRESAHA.107.165308. [DOI] [PubMed] [Google Scholar]
- 95.Yan SF, Fujita T, Lu J, et al. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat Med. 2000;6:1355–1361. doi: 10.1038/82168. [DOI] [PubMed] [Google Scholar]
- 96.Iannitti RG, Casagrande A, De Luca A, et al. Hypoxia promotes danger-mediated inflammation via receptor for advanced glycation end products in cystic fibrosis. Am J Respir Crit Care Med. 2013;188:1338–1350. doi: 10.1164/rccm.201305-0986OC. [DOI] [PubMed] [Google Scholar]
- 97.Tafani M, Schito L, Pellegrini L, et al. Hypoxia-increased RAGE and P2X7R expression regulates tumor cell invasion through phosphorylation of Erk1/2 and Akt and nuclear translocation of NF-{kappa}B. Carcinogenesis. 2011;32:1167–1175. doi: 10.1093/carcin/bgr101. [DOI] [PubMed] [Google Scholar]
- 98. Brett J, Schmidt AM, Yan SD, et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143:1699–1712. * This paper demonstrated that RAGE was expressed in most tissues in health at very low levels, except for the lung. The highest levels of RAGE in the basal and unperturbed state were shown to be in the lung tissue.
- 99.Marinakis E, Bagkos G, Piperi C, et al. Critical role of RAGE in lung physiology and tumorigenesis: a potential target of therapeutic intervention? Clin Chem Lab Med. 2014;52:189–200. doi: 10.1515/cclm-2013-0578. [DOI] [PubMed] [Google Scholar]
- 100. Shirasawa M, Fujiwara N, Hirabayashi S, et al. Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes Cells. 2004;9:165–174. doi: 10.1111/j.1356-9597.2004.00712.x. ** This work demonstrated that the principal site of RAGE expression in the lung is the type 1 alveolar pneumocyte and provided insight into possible physiological roles for RAGE in this tissue.
- 101.Fehrenbach H, Kasper M, Tschernig T, et al. Receptor for advanced glycation endproducts (RAGE) exhibits highly differential cellular and subcellular localisation in rat and human lung. Cell Mol Biol (Noisy-le-grand) 1998;44:1147–1157. [PubMed] [Google Scholar]
- 102. Schraml P, Bendik I, Ludwig CU. Differential messenger RNA and protein expression of the receptor for advanced glycosylated end products in normal lung and non-small cell lung carcinoma. Cancer Res. 1997;57:3669–3671. ** This paper demonstrated that in lung cancers, the expression of RAGE was downregulated compared to the normal lung tissue. This work provided important insights into possible physiological roles for RAGE in the lung tissue.
- 103.Bartling B, Hofmann HS, Weigle B, et al. Down-regulation of the receptor for advanced glycation end-products (RAGE) supports non-small cell lung carcinoma. Carcinogenesis. 2005;26:293–301. doi: 10.1093/carcin/bgh333. [DOI] [PubMed] [Google Scholar]
- 104.Ramsgaard L, Englert JM, Tobolewski J, et al. The role of the receptor for advanced glycation end-products in a murine model of silicosis. PLoS One. 2010;5:e9604. doi: 10.1371/journal.pone.0009604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.He M, Kubo H, Ishizawa K, et al. The role of the receptor for advanced glycation end-products in lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1427–L1436. doi: 10.1152/ajplung.00075.2007. [DOI] [PubMed] [Google Scholar]
- 106.Ramsgaard L, Englert JM, Manni ML, et al. Lack of the receptor for advanced glycation end-products attenuates E. coli pneumonia in mice. PLoS One. 2011;6:e20132. doi: 10.1371/journal.pone.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mazarati A, Maroso M, Iori V, et al. High-mobility group box-1 impairs memory in mice through both toll-like receptor 4 and Receptor for Advanced Glycation End Products. Exp Neurol. 2011;232:143–148. doi: 10.1016/j.expneurol.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guglielmotto M, Aragno M, Tamagno E, et al. AGEs/RAGE complex upregulates BACE1 via NF-kappaB pathway activation. Neurobiol Aging. 2012;33:196 e13–196 e27. doi: 10.1016/j.neurobiolaging.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 109.Cho HJ, Son SM, Jin SM, et al. RAGE regulates BACE1 and Abeta generation via NFAT1 activation in Alzheimer's disease animal model. FASEB J. 2009;23:2639–2649. doi: 10.1096/fj.08-126383. [DOI] [PubMed] [Google Scholar]
- 110.Wang X, Yu S, Hu JP, et al. Streptozotocin-induced diabetes increases amyloid plaque deposition in AD transgenic mice through modulating AGEs/RAGE/NF-kappaB pathway. Int J Neurosci. 2014;124:601–608. doi: 10.3109/00207454.2013.866110. [DOI] [PubMed] [Google Scholar]
- 111.Miller MC, Tavares R, Johanson CE, et al. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer's disease. Brain Res. 2008;1230:273–280. doi: 10.1016/j.brainres.2008.06.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li K, Dai D, Zhao B, et al. Association between the RAGE G82S polymorphism and Alzheimer's disease. J Neural Transm. 2010;117:97–104. doi: 10.1007/s00702-009-0334-6. [DOI] [PubMed] [Google Scholar]
- 113.Fang F, Lue LF, Yan S, et al. RAGE-dependent signaling in microglia contributes to neuroinflammation, Abeta accumulation, and impaired learning/memory in a mouse model of Alzheimer's disease. FASEB J. 2010;24:1043–1055. doi: 10.1096/fj.09-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Deane R, Sagare A, Zlokovic BV. The role of the cell surface LRP and soluble LRP in blood-brain barrier Abeta clearance in Alzheimer's disease. Curr Pharm Des. 2008;14:1601–1605. doi: 10.2174/138161208784705487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Donahue JE, Flaherty SL, Johanson CE, et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer's disease. Acta Neuropathol. 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- 116.Arancio O, Zhang HP, Chen X, et al. RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. EMBO J. 2004;23:4096–4105. doi: 10.1038/sj.emboj.7600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lue LF, Walker DG, Brachova L, et al. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer's disease: identification of a cellular activation mechanism. Exp Neurol. 2001;171:29–45. doi: 10.1006/exnr.2001.7732. [DOI] [PubMed] [Google Scholar]
- 118.Deane R, Singh I, Sagare AP, et al. A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122:1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Galasko D, Bell J, Mancuso JY, et al. Clinical trial of an inhibitor of RAGE-Abeta interactions in Alzheimer disease. Neurology. 2014;82:1536–1542. doi: 10.1212/WNL.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Walker D, Lue LF, Paul G, et al. Receptor for advanced glycation endproduct modulators: a new therapeutic target in Alzheimer's disease. Expert opinion on investigational drugs. 2015;24:393–399. doi: 10.1517/13543784.2015.1001490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hudson BI, Kalea AZ, Del Mar Arriero M, et al. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem. 2008;283:34457–34468. doi: 10.1074/jbc.M801465200. ** This paper was the first report that the cytoplasmic domain of RAGE bound to the formin, DIAPH1 and showed that knock-down of DIAPH1 protected cells from RAGE ligand-induced signal transduction and cellular migration. This work sets the stage for the study of this novel signaling axis in the pathogenesis of RAGE ligand-induced pathobiologies in vivo.
- 122.Kühn S, Geyer M. Formins as effector proteins of Rho GTPases. Small GTPases. 2014;5:e29513. doi: 10.4161/sgtp.29513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Young KG, Copeland JW. Formins in cell signaling. Biochim Biophys Acta. 2010;1803:183–190. doi: 10.1016/j.bbamcr.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 124.Bianchi R, Kastrisianaki E, Giambanco I, et al. S100B protein stimulates microglia migration via RAGE-dependent up-regulation of chemokine expression and release. J Biol Chem. 2011;286:7214–7226. doi: 10.1074/jbc.M110.169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Toure F, Fritz G, Li Q, et al. Formin mDia1 mediates vascular remodeling via integration of oxidative and signal transduction pathways. Circ Res. 2012;110:1279–1293. doi: 10.1161/CIRCRESAHA.111.262519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sakaguchi T, Yan SF, Yan SD, et al. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest. 2003;111:959–972. doi: 10.1172/JCI17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rai V, Maldonado AY, Burz DS, et al. Signal transduction in receptor for advanced glycation end products (RAGE): solution structure of C-terminal rage (ctRAGE) and its binding to mDia1. J Biol Chem. 2012;287:5133–5144. doi: 10.1074/jbc.M111.277731. ** This paper was the first to demonstrate the specific amino acids in the cytoplasmic domain of RAGE that were required to bind to the formin, DIAPH1. Mutations of two of the key amino acids in the RAGE tail (R5/Q6) to alanines blocked the interaction of the RAGE cytoplasmic domain with DIAPH1 and suppressed RAGE ligand-mediated signaling in cultured cells. This work critically sets the stage for defining means to suppress the interaction of the RAGE cytoplasmic domain with DIAPH1. Indeed, small molecule library screening, now aggressively underway, has greatly benefited from the key insights derived from this work.
- 128.Yamamoto K, Murata H, Putranto EW, et al. DOCK7 is a critical regulator of the RAGE-Cdc42 signaling axis that induces formation of dendritic pseudopodia in human cancer cells. Oncol Rep. 2013;29:1073–1079. doi: 10.3892/or.2012.2191. [DOI] [PubMed] [Google Scholar]
- 129.Koch M, Chitayat S, Dattilo BM, et al. Structural basis for ligand recognition and activation of RAGE. Structure. 2010;18:1342–1352. doi: 10.1016/j.str.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Park H, Adsit FG, Boyington JC. The 1.5 Å crystal structure of human receptor for advanced glycation endproducts (RAGE) ectodomains reveals unique features determining ligand binding. J Biol Chem. 2010;285:40762–40770. doi: 10.1074/jbc.M110.169276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xue J, Rai V, Singer D, et al. Advanced glycation end product recognition by the receptor for AGEs. Structure. 2011;19:722–732. doi: 10.1016/j.str.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vassar R. BACE1 inhibitor drugs in clinical trials for Alzheimer's disease. Alzheimers Res Ther. 2014;6:89. doi: 10.1186/s13195-014-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Falcone C, Emanuele E, D'Angelo A, et al. Plasma levels of soluble receptor for advanced glycation end products and coronary artery disease in nondiabetic men. Arterioscler Thromb Vasc Biol. 2005;25:1032–1037. doi: 10.1161/01.ATV.0000160342.20342.00. [DOI] [PubMed] [Google Scholar]
- 134.Geroldi D, Falcone C, Emanuele E, et al. Decreased plasma levels of soluble receptor for advanced glycation end-products in patients with essential hypertension. J Hypertens. 2005;23:1725–1729. doi: 10.1097/01.hjh.0000177535.45785.64. [DOI] [PubMed] [Google Scholar]
- 135.Colhoun HM, Betteridge DJ, Durrington P, et al. Total soluble and endogenous secretory receptor for advanced glycation end products as predictive biomarkers of coronary heart disease risk in patients with type 2 diabetes: an analysis from the CARDS trial. Diabetes. 2011;60:2379–2385. doi: 10.2337/db11-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Christie JD, Shah CV, Kawut SM, et al. Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. Am J Respir Crit Care Med. 2009;180:1010–1015. doi: 10.1164/rccm.200901-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cheng DT, Kim DK, Cockayne DA, et al. Systemic soluble receptor for advanced glycation endproducts is a biomarker of emphysema and associated with AGER genetic variants in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:948–957. doi: 10.1164/rccm.201302-0247OC. [DOI] [PubMed] [Google Scholar]
- 138.Parikh M, Chung M, Sheth S, et al. Randomized pilot trial of bariatric surgery versus intensive medical weight management on diabetes remission in type 2 diabetic patients who do NOT meet NIH criteria for surgery and the role of soluble RAGE as a novel biomarker of success. Ann Surg. 2014;260:617–622. doi: 10.1097/SLA.0000000000000919. discussion 22-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reverdatto S, Rai V, Xue J, et al. Combinatorial library of improved peptide aptamers, CLIPs to inhibit RAGE signal transduction in mammalian cells. PLoS One. 2013;8:e65180. doi: 10.1371/journal.pone.0065180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ku SH, Hong J, Moon HH, et al. Deoxycholic acid-modified polyethylenimine based nanocarriers for RAGE siRNA therapy in acute myocardial infarction. Arch Pharm Res. 2015 doi: 10.1007/s12272-014-0527-x. [DOI] [PubMed] [Google Scholar]
- 141.Choi BR, Cho WH, Kim J, et al. Increased expression of the receptor for advanced glycation end products in neurons and astrocytes in a triple transgenic mouse model of Alzheimer's disease. Exp Mol Med. 2014;46:e75. doi: 10.1038/emm.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Askarova S, Yang X, Sheng W, et al. Role of Abeta-receptor for advanced glycation endproducts interaction in oxidative stress and cytosolic phospholipase A(2) activation in astrocytes and cerebral endothelial cells. Neuroscience. 2011;199:375–385. doi: 10.1016/j.neuroscience.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]