Abstract
Aims
Over 75% of obese subjects fail to maintain their weight following weight loss interventions. We aimed to identify phenotypic and genetic markers associated with weight maintenance/regain following a dietary intervention.
Subjects and methods
In the 2-year Dietary Intervention Randomized Controlled Trial, we assessed potential predictors for weight changes during the ‘weight loss phase’ (0–6 months) and the ‘weight maintenance/regain phase’ (7–24 months). Genetic variation between study participants was studied using single-nucleotide polymorphisms in the leptin gene (LEP).
Results
Mean weight reduction was −5.5% after 6 months, with a mean weight regain of 1.2% of baseline weight during the subsequent 7–24 months. In a multivariate regression model, higher baseline high-molecular-weight adiponectin was the only biomarker predictor of greater success in 0- to 6-month weight loss (β = −0.222, P-value = 0.044). In a multivariate regression model adjusted for 6-month changes in weight and various biomarkers, 6-month plasma leptin reduction exhibited the strongest positive association with 6-month weight loss (β = 0.505, P-value<0.001). Conversely, 6-month plasma leptin reduction independently predicted weight regain during the following 18 months (β = −0.131, P-value<.013). Weight regain was higher among participants who had a greater (top tertiles) 6-month decrease in both weight and leptin (+ 3.4% (95% confidence interval 2.1–4.8)) as compared with those in the lowest combined tertiles (+ 0.2% (95% confidence interval −1.1 to 1.4)); P-value<0.001. Weight regain was further significantly and independently associated with genetic variations in LEP (P = 0.006 for both rs4731426 and rs2071045). Adding genetic data to the phenotypic multivariate model increased its predictive value for weight regain by 34%.
Conclusion
Although greater reduction in leptin concentrations during the initial phase of a dietary intervention is associated with greater weight loss in the short term, plasma leptin reduction, combined with the degree of initial weight loss and with genetic variations in the LEP gene, constitutes a significant predictor of subsequent long-term weight regain.
Keywords: leptin, genetic polymorphism, weight maintenance
Introduction
The global increase in the prevalence of overweight and obesity has resulted in increased mortality being the second leading cause of preventable death, primarily through effects on cardiovascular disease and type 2 diabetes.1 Weight reduction has definite beneficial effects on cardiovascular risk factors, which are sustained as long as weight loss is maintained.2–7 However, over 75% of obese subjects significantly regain body weight following non-invasive weight loss interventions, regardless of age, gender, ethnicity or weight loss program applied.8–11 Identification of individuals who are more likely to regain weight may thus be clinically helpful.
One of the suggested mechanisms for the intractable nature of obesity is that the accompanied reduction in plasma leptin concentrations following successful weight loss results in a state of relative leptin deficiency, which favors weight regain.12,13 Another may be that genetic differences in genes encoding factors controlling food consumption and energy expenditure determine the individual ability to lose and/or maintain weight.14
We have recently published the results of the 2-year Dietary Intervention Randomized Controlled Trial (DIRECT) comparing the effectiveness and safety of three nutritional protocols in weight loss: a low-fat diet; a Mediterranean diet; and a low-carbohydrate, non-restricted calorie diet.15 Adherence to the study was 95% after 1 year and 85% after 2 years. The aim of the current study was to identify phenotypic and genetic markers associated with the degree of weight maintenance/regain during the dietary intervention.
Subjects and methods
Study population and intervention
Methods of the Dietary Intervention Randomized Controlled Trial were previously reported in detail.15 The trial was conducted between July 2005 and June 2007 in a research center workplace in Dimona, Israel. Eligible participants were aged 40–65 years with body mass index (BMI) ≥27 kg m2, or had type 2 diabetes or coronary heart disease regardless of age or BMI. Pregnant or lactating women and patients with a serum creatinine level of 2 mg per 100 ml or more, liver dysfunctions (an increase of twofold or more above the upper limit of normal in alanine aminotransferase and aspartate aminotransferase), intestinal problems that would prevent consuming any of the test diets, or active cancer were excluded. Participation in another diet trial was also an exclusion criterion. Participants were randomized to low-fat, Mediterranean or low-carb diet within the strata of gender, age (below or above the median), BMI (below or above the median), history of coronary heart disease (yes/no), type 2 diabetes (yes/no) and current use of statins (none/<1 year/≥1 year) by Monte-Carlo simulations for randomization. The 322 participants were randomized as follows: low fat: n = 104, 89 men and 15 women; Mediterranean: n = 109, 89 men and 20 women; low-carbohydrate: n = 109, 99 men and 10 women. Clinic and laboratory staff members were blinded to treatment assignment. The study coordinators were blinded to all outcome data until the end of the intervention. The study was approved by the human subjects committee of Soroka Medical Center and Ben-Gurion University. The genetic substudy was approved by the institutional review board of the Hadassah-Hebrew University Medical Center. Participants provided a separate informed consent for participation in the intervention and phenotypic study and in the genetic substudy.
Outcomes
Body weight was measured without shoes to the nearest 0.1 kg every month. Height was measured to the nearest millimeter using a wall-mounted stadiometer at baseline for BMI determination. A blood sample was drawn by venipuncture at 0800 hours, after a 12-h fast, at baseline, as well as at 6, 12 and 24 months, and stored at −80 °C until assayed for lipids, inflammatory biomarkers and insulin. Biomarkers were measured in the University of Leipzig, Leipzig, Germany: High-molecular-weight plasma adiponectin was measured by ELISA (AdipoGen, Axxora, Lörrach, Germany), with a coefficient of variation (CV) of 4.8%. Plasma leptin was assessed by ELISA (Mediagnost, Reutlingen, Germany) with a CV of 2.4%. Serum concentrations of total cholesterol, high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol and triglycerides were directly determined enzymatically (Roche, Leipzig, Germany); CV of cholesterol was 1.3% and that for triglycerides was 2.1%. Plasma insulin was measured with an enzyme immunometric assay using the IMMULITE automated analyzer (Diagnostic Products Corporation, Los Angeles, CA, USA), with a CV = 2.5%. High-sensitive C-reactive protein was measured by ELISA (DiaMed, Ottobrunn, Germany); CV = 1.9%. Genomic DNA was extracted from peripheral blood using standard methods,16 and was analyzed in the Hadassah Medical Center, Jerusalem, Israel.
Selection and analysis of single-nucleotide polymorphisms (SNPs) in the leptin (LEP) gene
The leptin gene (MIM #164160) is located on chromosome 7q31.3 and spans approximately 20 kb. It contains three exons and two introns. Exon 1 is non-coding and is separated from coding exons 2 and 3 by more than 10.5 kb. A total of 164 SNPs have been identified in this gene. To capture the common variation in the gene, we used data from the International HapMap project (http://www.hapmap.org) and from GeneCards (http://www.genecards.org) to select tag SNPs with a minimum minor allele frequency (MAF) of 0.2. Five tag SNPs were selected (their characteristics are presented in Table 1 of the supplementary material). The genotype of each individual SNP was determined using the 7300 Real Time PCR system, with fluorescent TaqMan probes (Applied Biosystems, Foster City, CA, USA). Real-time PCR was performed in a final reaction volume of 10 μl, which contained 5 μl Taqman Genotyping Master Mix, 0.5 μl Taqman SNP genotyping assay mix (Applied Biosystems) and 1 μl genomic DNA. The amplification conditions were as follows: 95 °C for 10 min, 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The samples were run together with the non-template control in a 96-well optical reaction plate. Allelic discrimination was performed on the post-PCR product. The fluorescence data of the post-PCR products were analyzed directly using allelic discrimination software of the ABI Prism 7300 instrument (Applied Biosystems).
Statistical analysis
For weight loss, the prespecified primary aim was to determine the change in weight from baseline to the 24-month point. We analyzed the biomarker data by using raw unadjusted means and without imputation for missing data. For intention-to-treat analyses, all 322 participants were included by using the most recent values for weight and blood pressure. The percentage change in weight was calculated as changes from initial weight. Weight change during 7–24 months was calculated as the difference between the 24-month weight change from initial weight (percent) and the 6-month weight change from initial weight (percent). We calculated age-adjusted Pearson's correlation to evaluate the P-value of the trend between initial quintiles of BMI and baseline characteristics. Multivariate regression models were adjusted for age, sex, diet group and selected biomarkers that were potential confounders for weight loss in the adiponectin model (leptin, insulin, thyroid-stimulaing hormone) or that were found to be correlated with 7 to 24 month weight change in univariate analyses (6-month delta of adiponectin, HDL-c and weight) in the leptin model. We cross-classified nine groups of combinations between tertiles (low, medium, high) of 6-month changes in both leptin concentrations and weight in order to calculate their corresponding subsequent weight change within 7–24 months. Insulin resistance was calculated according to the following equation: (insulin (μU ml−1) × fasting glucose (mmoll−1))/22.5 (the homeostasis model assessment). For a minimal difference of 2-kg (s.d. = 10) weight loss between groups, with 100 participants per group and 5% type I error, the power to detect significant weight loss differences is greater than 90%.
Allele and genotype frequencies were determined for each individual SNP and studied for deviation from Hardy–Weinberg equilibrium by exact tests.17 The association of each polymorphism adjusted for age, sex and diet group with the relevant outcome was tested using one-way analysis of covariance. Multivariate analysis was used to study the association of multiple genotypes with the outcome. Haplotype analysis was carried out using haplo.stats and haplo.glm, which assume that haplotypes are ambiguous because of the unknown linkage phase of genetic markers. We identified all the haplotype configurations with non-trivial probability on the basis of maximum likelihood using the expectation–maximization algorithm.18–20 The threshold for statistical significance was set at 0.05. Multiple comparisons were accounted for using the false discovery rate method.21 We used SPSS version 15, SAS Institute Inc software version 9.1 (Cary, NC, USA) and R statistical environment (http://www.r-project.org/) for statistical analyses.
Results
Baseline characteristics of the 322 participants in the Dietary Intervention Randomized Controlled Trial across initial BMI quintiles are shown in Table 1. Mean age was 51.2 years and mean BMI was 30.9 kg m−2. Of the participants, 86% were men, and all were Caucasians. Increasing values of plasma leptin, high-sensitive C-reactive protein, insulin and homeostasis model assessment of insulin resistance were significantly associated with elevated BMI (P-value<0.0001 for all). A high proportion of diabetic subjects in the lowest quintile of BMI is attributed to the inclusion criteria for the study.
Table 1.
Baseline characteristics of the DIRECT population according to quintiles of basal BMI; n = 322
|
Quintiles of baseline BMI
|
P-value of trend; age adjusted | Entire group (n = 322) | |||||
|---|---|---|---|---|---|---|---|
| Q1 (n = 64) | Q2 (n = 65) | Q3 (n = 64) | Q4 (n = 65) | Q5 (n = 64) | |||
| BMI, kg m–2 | |||||||
| Mean | 26.9 | 28.7 | 30.2 | 32.3 | 36.5 | 30.9 ± 3.6 | |
| Range | 21.6–28.0 | 28.0–29.4 | 29.4–31.1 | 31.2–33.5 | 33.5–44.3 | ||
| Age, years | 51.0 | 51.4 | 51.9 | 51.7 | 50.1 | 51.2 ± 6.4 | |
| Men, % | 88 | 91 | 84 | 95 | 72 | 86 (n = 277) | |
| Diabetic, % | 23 | 11 | 13 | 9 | 16 | 14 (n = 45) | |
| HMW adiponectin, mg per 100 ml | |||||||
| Women | 9.1 | 10.8 | 8.5 | 7.2 | 9.7 | 0.948 | 9.3 ± 3.4 |
| Men | 7.6 | 6.5 | 7.2 | 7.0 | 6.7 | 0.251 | 7.0 ± 2.6 |
| Leptin, mg per 100 ml | |||||||
| Women | 16.6 | 24.0 | 27.8 | 40.2 | 40.1 | <0.0001 | 31.0 ± 13.9 |
| Men | 5.5 | 7.5 | 9.4 | 11.4 | 15.5 | <0.0001 | 9.6 ± 6.0 |
| hs-CRP, mg per 100 ml | 3.1 | 3.4 | 4.4 | 4.3 | 6.3 | <0.0001 | 4.2 ± 3.2 |
| TG, mg per 100 ml | 163.0 | 180.0 | 175.6 | 154.7 | 181.8 | 0.818 | 170.8 ± 87.0 |
| LDL-c, mg per 100 ml | 118.2 | 122.2 | 119.8 | 111.4 | 123.9 | 0.943 | 119.0 ± 34.8 |
| HDL-c, mg per 100 ml | 40.4 | 38.0 | 38.7 | 38.1 | 37.1 | 0.108 | 38.5 ± 9.2 |
| Fasting glucose, mg per 100 ml | 96.1 | 86.1 | 86.5 | 89.3 | 98.9 | 0.42 | 91.3 ± 31.5 |
| Fasting insulin, mg per 100 ml | 9.6 | 12.6 | 13.5 | 16.6 | 18.1 | <0.0001 | 14.0 ± 8.5 |
| HOMA-IR | 2.3 | 2.7 | 3.0 | 3.8 | 4.4 | <0.0001 | 3.2 ± 2.6 |
| TSH, mIU l–1 | 1.9 | 2.1 | 2.1 | 2.0 | 2.1 | 0.896 | 2.0 ± 1.2 |
Abbreviations: BMI, body mass index; DIRECT, Dietary Intervention Randomized Controlled Trial; HDL-c, high-density lipoprotein cholesterol; HMW adiponectin, high-molecular-weight adiponectin; HOMA-IR, homeostasis model assessment of insulin resistance; hs-CRP, high-sensitivity C-reactive protein; LDL-c, low-density lipoprotein cholesterol; TG, triglyceride; TSH, thyroid-stimulating hormone.
DNA was available from 272 participants and full phenotypic and genetic data were available for 256 participants. For the five examined SNPs in the LEP gene, there was no deviation from Hardy–Weinberg equilibrium (Table 1 in the supplementary material). Overall, no significant differences in baseline BMI or plasma leptin concentrations across genotype groups were noted (Table 2) (excluding rs3282942 and plasma leptin in males).
Table 2.
Mean baseline BMI and plasma leptin concentrations across genotype groups in the DIRECT population
| SNP | Genotype | N | BMI (kg m–2) | P-value | Plasma leptin, male (mg dl–1) | P-value | Plasma leptin, female (mg dl–1) | P-value |
|---|---|---|---|---|---|---|---|---|
| rs11760956 | GG | 139 | 31.0 ± 3.5 | 0.6343 | 8.9 ± 5.0 | 0.2967 | 28.1 ± 12.9 | 0.4284 |
| AG | 99 | 30.6 ± 3.7 | 9.7 ± 5.6 | 31.8 ± 14.7 | ||||
| AA | 24 | 30.4 ± 3.4 | 10.9 ± 10.6 | 38.2 ± 5.8 | ||||
| rs3828942 | GG | 79 | 31.2 ± 3.8 | 0.4654 | 7.6 ± 2.5 | 0.0172 | 28.1 ± 9.6 | 0.7949 |
| AG | 127 | 30.7 ± 3.5 | 8.9 ± 5.3 | 31.5 ± 15.4 | ||||
| AA | 56 | 30.4 ± 3.6 | 8.2 ± 4.0 | 30.9 ± 12.4 | ||||
| rs4731426 | CC | 87 | 30.8 ± 3.4 | 0.9511 | 8.7 ± 4.3 | 0.0834 | 30.8 ± 14.5 | 0.8641 |
| CG | 116 | 30.7 ± 3.8 | 9.1 ± 5.6 | 30.9 ± 13.5 | ||||
| GG | 59 | 30.9 ± 3.4 | 11.0 ± 2.2 | 27.3 ± 11.2 | ||||
| rs117635178 | TT | 78 | 30.6 ± 3.7 | 0.5728 | 8.4 ± 8.6 | 0.1126 | 27.9 ± 10.8 | 0.8666 |
| CT | 131 | 30.7 ± 3.4 | 9.5 ± 5.2 | 31.2 ± 14.6 | ||||
| CC | 53 | 31.2 ± 4.0 | 1.7 ± 8.5 | 29.8 ± 11.3 | ||||
| rs2071045 | TT | 144 | 30.4 ± 3.3 | 0.0918 | 9.5 ± 6.5 | 0.7644 | 29.4 ± 12.1 | 0.2388 |
| TC | 100 | 31.4 ± 4.0 | 9.3 ± 2.9 | 35.3 ± 16.4 | ||||
| CC | 18 | 30.7 ± 2.9 | 8.2 ± 3.9 | 24.3 ± 7.2 |
Abbreviations: BMI, body mass index; DIRECT, Dietary Intervention Randomized Controlled Trial; SNP, single-nucleotide polymorphism.
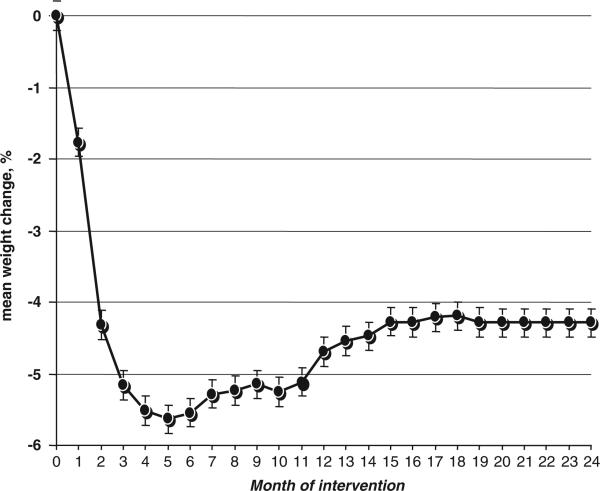
The mean weight reduction (Figure 1) among the entire study population (322 participants, intention-to-treat analysis), regardless of dietary groups, was −5.5%±5.1 of baseline weight after 6 months (weight reduction phase) and −4.3%±5.7 after 24 months, corresponding to a mean weight regain of 1.2% of baseline weight during the weight regain/maintenance phase (7–24 months). Maximal weight reduction was observed after 5 months with 5.6% of initial weight. Among the 272 participants who completed 24 months of intervention, the actual weight changes were −6.1%±5.4 after 6 months and −4.7%±5.7 after 2 years. As previously reported,15 6- and 24-month weight loss was higher in the Mediterranean and low-carbohydrate diet groups than in the low-fat group (P<0.001).
Figure 1.
Percentage of weight change over 2 years of dietary intervention; n = 322, 85% adherence after 2 years; intention-to-treat analysis.
Predictors of weight change during the weight reduction phase (months 0–6)
In a multivariate regression model (Table 3a) adjusted for age, sex, diet group, baseline weight and baseline concentrations of leptin, insulin and thyroid-stimulaing hormone, higher baseline high-molecular-weight adiponectin was significantly associated with greater success in weight loss during the first 6 months of intervention (β = −0.222, P-value = 0.044).
Table 3a.
Association between baseline characteristics and weight change during the weight-loss phase (0–6 months) of dietary intervention; multivariate regression analysisa
| Variable in the model | β-coefficient | P-value |
|---|---|---|
| Age | 0.014 | 0.762 |
| Sex | –2.287 | 0.119 |
| Diet intervention group | –0.717 | 0.049 |
| Initial weight | –0.082 | 0.003 |
| HMW adiponectin, baseline | –0.222 | 0.044 |
| Leptin concentration, baseline | 0.001 | 0.985 |
| Insulin concentration, baseline | 0.018 | 0.642 |
| TSH, baseline | –0.105 | 0.682 |
Abbreviations: HMW adiponectin, high-molecular-weight adiponectin; TSH, thyroid-stimulating hormone.
Adjusted simultaneously to all the variables in the model; the variables are in continuous form, except for sex (men vs. women) and diet intervention group (1 = low-fat, 2 = Mediterranean, 3 = low-carbohydrate); weight change is measured as percent of changes at 6 months as compared with baseline.
The addition of genetic tested parameters to this model, that is, genotypes in five different SNPs in the LEP gene, and of inferred haplotypes, did not add further predictive value for the extent of weight reduction during the first 6 months of dietary intervention (data not shown).
We next assessed the association between changes in parameters during the first 6 months of dietary intervention with weight changes. Multivariate regression models (Table 3b) adjusted for age, sex, diet group and 6-month changes (delta) of adiponectin, HDL-c, low-density lipoprotein cholesterol, triglycerides and fasting glucose revealed that 6-month changes in low-density lipoprotein cholesterol were positively and significantly associated with weight change, whereas larger increases in both high-molecular-weight adiponectin and HDL-c were significantly associated with more pronounced weight loss (P-value<0.05 for all). However, changes in leptin had the strongest positive association with 6-month changes in weight (that is, decreased more with more weight loss, β = 0.505, P-value<0.001).
Table 3b.
Associations between changes (delta) in biomarkers in the first 6 months of the weight loss dietary intervention and weight change during the weight loss phase (0–6 months) (model 1) and the weight maintenance/regain phase (7–24 months) (model 2); multivariate regression analysisa
| Model 1 to predict weight change between 0–6 months | P-value | Model 2 to predict weight change between 7–24 months | P-value | |
|---|---|---|---|---|
| Leptin, delta 0–6 months | 0.505 | <0.001 | –0.131 | 0.013 |
| Adiponectin, delta 0–6 months | –0.301 | 0.023 | 0.111 | 0.356 |
| CRP, delta 0–6 months | 0.028 | 0.743 | 0.236 | 0.002 |
| TG, delta 0–6 months | 0.006 | 0.132 | 0.003 | 0.465 |
| HDL-c, delta 0–6 months | –0.159 | 0.001 | 0.049 | 0.252 |
| LDL-c, delta 0–6 months | 0.029 | 0.002 | 0.002 | 0.838 |
| Fasting glucose, delta 0–6 months | 0.001 | 1.000 | –0.016 | 0.190 |
| Weight change in %, delta 0–6 months | — | — | –0.102 | 0.063 |
Abbreviations: CRP, C-reactive protein; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; TG, triglyceride.
Adjusted simultaneously to all the variables in the model + adjusted to the age, sex and diet group. The variables are in continuous form except for sex (men vs women) and diet intervention group (1 = low-fat, 2 = Mediterranean, 3 = low-carbohydrate diet); weight change is measured as percent of changes at 6 months as compared with baseline.
Predictors of weight change during the weight maintenance/regain phase (months 7–24)
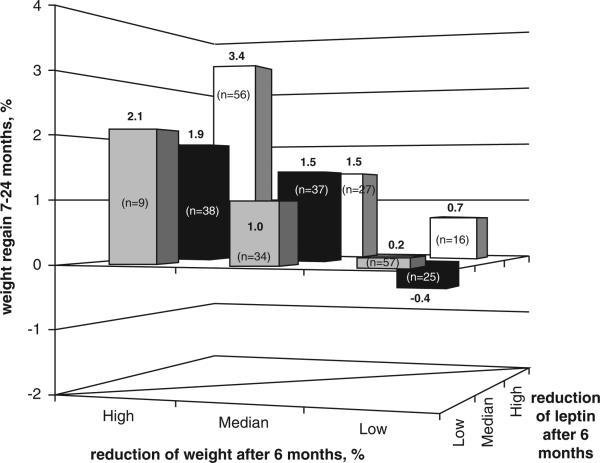
Contrary to the positive association between changes in leptin concentrations and changes in weight during the weight reduction phase, 0- to 6-month reduction in plasma leptin was negatively associated with subsequent weight loss (that is, associated with weight regain) between 7 and 24 months of intervention (β = −0.131, P-value<.013) in a multivariate regression model (Table 3b) adjusted for age, sex, diet group and 6-month delta of adiponectin, HDL-c, low-density lipoprotein cholesterol, triglycerides, fasting glucose and weight loss in the first 6 months. Figure 2 presents the weight change/regain during months 7–24 across tertiles of changes in plasma leptin concentrations and changes in weight during the first 6 months. Weight regain was higher among participants who had a higher (top tertile) 6-month decrease in both weight and leptin (+ 3.4% (95% confidence interval 2.1–4.8)) as compared with those in the lowest tertiles of both parameters (+ 0.2% (95% confidence interval −1.1 to 1.4)), P-value<0.001.
Figure 2.
Weight change/regain during 7–24 months across tertiles of changes in both plasma leptin concentrations and weight during the first 6 months of the dietary intervention.
The addition of genetic data to the model of weight regain had a substantial contribution to its predictive value. Multivariate models, each including an individual SNP assessed along with age, sex, diet group, 6-month changes in weight, leptin, adiponectin and HDL-c, showed a significant association between two SNPs and weight regain during 7–24 months (Table 4). Of note, the genotypes in those two SNPs (rs4731426 and rs2071045) did not significantly correlate with change in plasma leptin concentrations during the weight reduction phase (Table 2 in the supplementary material). A multivariate parsimonious model including three of the SNPs (rs4731426, rs2071045 and rs3828942) showed a significant association between the genotype and the outcome of weight regain (global test P-value = 0.014, Table 4). A model that included all five SNPs yielded similar results (P-value = 0.030). Addition of genetic data to the other variables in the model increased its predictive value of weight regain by 34%, jointly accounting for approximately 14.3% of the interindividual variability in weight change during 7–24 months.
Table 4.
Association between LEP genotype and weight regain in months 7–24
| SNP | Minor allele |
Multvariate association with individual SNPs
|
Multivariate association
|
||||||
|---|---|---|---|---|---|---|---|---|---|
|
Basic model
a
|
FDR |
Full model
b
|
FDR | General model c | Parsimonious model c | ||||
| β d | P-value | β | P-value | Adjusted r2 | Adjusted r2 | ||||
| No SNP | 0.1085 | 0.1085 | |||||||
| rs4731426 | G | 1.085 | 0.001 | 0.005 | 0.872 | 0.006 | 0.016 | ||
| rs117635178 | C | 0.477 | 0.152 | 0.152 | 0.096 | 0.776 | 0.776 | ||
| rs11760956 | A | 0.64 | 0.061 | 0.076 | 0.391 | 0.269 | 0.336 | 0.1449 | 0.1454 |
| rs2071045 | C | –0.926 | 0.016 | 0.027 | –1.026 | 0.006 | 0.016 | ||
| rs3828942 | A | –0.889 | 0.007 | 0.017 | –0.587 | 0.075 | 0.126 | ||
| P-value for addition | 0.0297 | 0.0141 | |||||||
Abbreviations: FDR, false discovery rate; SNP, single-nucleotide polymorphism.
The basic models are adjusted for age, sex and diet group only.
The full models are adjusted for age, sex, diet group and changes in weight, leptin, adiponectin and high-density lipoprotein cholesterol concentrations between 0–6 months.
The general model consists of all five SNPs. The parsimonious model consists of only three SNPs (rs4731426, rs2071045 and rs3828942).
The β value is for each additional copy of the minor allele.
Haplotypes comprising the five SNPs were inferred. Of those, six were considered ‘common’ (haplotype frequency greater than 5%). Homozygotes for the reference haplotype (C-T-G-T-A) comprised approximately 8% of the study population. A multivariate model (adjusted for age, sex, diet group and changes in weight, leptin, adiponectin and HDL-c in the weight reduction phase) revealed a significant association between LEP haplotypes and weight regain in 7–24 months (P-value = 0.031) (Table 5). A simulated model (sequential Monte-Carlo P-values) yielded similar results (P-value = 0.029).
Table 5.
Multivariate models of LEP haplotypes association with weight regain
| rs4731426 | rs117635178 | rs11760956 | rs2071045 | rs3828942 | Haplotype frequency |
Generalized linear model tests
|
Score statistics tests
|
|||
|---|---|---|---|---|---|---|---|---|---|---|
| β | P-value | Hap-Score | P-value | |||||||
| 1 | C | T | G | T | A | 0.2758 | Ref. | — | 0.3135 | 0.7539 |
| 2 | C | T | G | C | A | 0.1096 | –1.0713 | 0.0736 | –2.2448 | 0.0248 |
| 3 | C | C | G | C | G | 0.1204 | –0.8009 | 0.1585 | –1.5839 | 0.1132 |
| 4 | G | C | G | T | G | 0.0541 | 0.4235 | 0.5490 | 0.8022 | 0.4224 |
| 5 | G | T | G | T | G | 0.0961 | 0.9197 | 0.1568 | 1.9164 | 0.0553 |
| 6 | G | C | A | T | G | 0.2582 | 0.3517 | 0.4223 | 1.5868 | 0.1126 |
| — | * | * | * | * | * | 0.0858 | –0.7900 | 0.1892 | — | — |
| P-valuea | 0.0308 | |||||||||
| Simulated P-valuea | 0.0286 | |||||||||
The P-values are of asymptotic and simulated global tests.
All models are adjusted for age, sex, diet group and changes in weight, leptin, adiponectin and HDL-c concentrations between 0–6 months. The last row includes all other haplotypes. Asterisks (*) denote any of the two possible nucleotides for the individual SNP.
Discussion
This 2-year weight loss intervention study demonstrates an association between dynamics of weight change during dietary intervention and phenotypic and genetic data. Although the initial response (weight reduction during the first 6 months) was mainly positively associated with baseline high-molecular-weight adiponectin and 6-month changes in plasma leptin, the prolonged response (weight change during 7–24 months) was related to LEP gene variability and negatively associated with the degree of plasma leptin decrease during the first weight loss phase. Our results suggest that dynamics in leptin concentrations, combined with genetic variability in the LEP gene, may contribute to cycling during weight loss diets.
Circulating leptin is produced mainly by adipocytes and is primarily involved in the regulation of food intake and energy expenditure. Yet, expression of the genes encoding leptin (LEP) and leptin receptor (LEPR) has been shown to occur in many other tissues,22 which suggests a generalized homeostatic role. Circulating leptin concentrations decrease during weight reduction interventions in both rodents and humans.12,23–26 However, clinical studies in humans have so far raised conflicting results regarding the role of leptin reduction in the predisposition for weight regain.13,27–29 Our findings are consistent with the theory of relative leptin insufficiency following the weight loss phase as a determinant of weight regain, as individuals with a greater initial reduction in plasma leptin concentrations regained more weight over the full length of the intervention.
A rare coding mutation in LEP led to complete congenital deficiency of leptin and to severe obesity.30,31 However, the association between common LEP variants and body weight has not been consistent.32–36 Genetic polymorphism in the LEP gene has been shown to influence factors other than plasma leptin concentration; for example, pulse pressure and carotid intima–media thickness.37 This may suggest a non-direct effect of leptin on metabolic and health outcomes. In line with these findings, we found that rs4731426 and rs2071045 genotypes were not associated with baseline leptin concentrations or with the change in plasma leptin over the weight reduction phase, but the genotypes in those two SNPs were significantly correlated with the outcome of weight maintenance/regain, implying that their effect lies in the ‘quality’ of their product rather than in its ‘quantity’.
The SNPs examined in this study do not lead to known sequence variation in the LEP transcripts and are not known to affect splicing. Their effect, therefore, might be secondary to linkage disequilibrium with a functional genetic locus, or because of other unknown mechanisms. Although the first option is appealing, it is highly improbable, as polymorphic loci in LEP with a known functional role are extremely rare. We therefore assume that these SNPs (or other common variations in linkage disequilibrium to the ones we examined) alter gene transcription or protein function in an indirect manner, be it by influencing chromatin formation or the function of proteins involved in the transcription process, as previously suggested.38 Future functional analyses of these variants are needed to understand their mechanistic role.
Several limitations and strengths of this study warrant mentioning. First, although this is a relatively large-scale long-term dietary intervention trial, it is rather small for a genetic association study. Despite this presumed limitation, we were able to detect statistically significant differences between genetic groups. Second, because very few women were enrolled, sex-specific effects may have not been adequately probed. Third, the unique nature of the work-place that enabled a highly monitored dietary intervention over 2 years might limit the generalizability of our weight loss findings to free-living populations. Nevertheless, we believe that prediction of weight regain by phenotypic and genetic variation could be relevant elsewhere. Strengths of the study include a one-phase design in which intervention began simultaneously for all participants, as well as a relatively long duration of the study and a high adherence rate.
The combined models used herein predict approximately 15% of the interindividual variability in weight maintenance in response to the dietary intervention, allowing for additional mechanisms. Our models clearly show that the change in plasma leptin concentrations during the weight reduction phase of the dietary intervention is a significant predictor of long-term success in weight maintenance and that the genetic variability in LEP predicts another, as well as an independent, portion of this variability.
Supplementary Material
Acknowledgements
We are thankful to the 322 Dietary Intervention Randomized Controlled Trial participants for their consistent cooperation. Sources of support: (1) The Israeli Ministry of Health, Chief Scientist Office (grants received by Drs Shai, Schwarz-fuchs and Tirosh, Israel); (2) DFG grant (KFO 152, grants received by Drs Blüher and Stumvoll, Germany); (3) Sarah and Moshe Mayer Foundation for Research (grant received by Prof. Leitersdorf, Israel); and (4) The Dr Robert C and Veronica Atkins Research Foundation. This foundation was not involved in any stage of the design, conduct or analysis of the study and had no access to the study results before publication.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on International Journal of Obesity website (http://www.nature.com/ijo)
References
- 1.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 2.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction of the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood PD, Stefanick ML, Dreon DM, Frey-Hewitt B, Garay SC, Williams PT, et al. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med. 1988;319:1173–1179. doi: 10.1056/NEJM198811033191801. [DOI] [PubMed] [Google Scholar]
- 4.Elmer PJ, Grimm R, Jr, Laing B, Grandits G, Svendsen K, Van Heel N, et al. Lifestyle intervention: results of the Treatment of Mild Hypertension Study (TOMHS). Prev Med. 1995;24:378–388. doi: 10.1006/pmed.1995.1062. [DOI] [PubMed] [Google Scholar]
- 5.Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH, Jr, Kostis JB, et al. on behalf of TONE Collaborative Research Group Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). JAMA. 1998;279:839–846. doi: 10.1001/jama.279.11.839. [DOI] [PubMed] [Google Scholar]
- 6.Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, et al. Trials for the Hypertension Prevention Research Group. Long-term weight loss and changes in blood pressure: results of the trials of hypertension prevention, phase II. Ann Intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 7.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, et al. on behalf of the Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 8.Jeffery RW, Drewnowski A, Epstein LH, Stunkard AJ, Wilson GT, Wing RR, et al. Long-term maintenance of weight loss: current status. Health Psychol. 2000;19(Suppl 1):5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- 9.Tsai A, Wadden T. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med. 2005;142:56–66. doi: 10.7326/0003-4819-142-1-200501040-00012. [DOI] [PubMed] [Google Scholar]
- 10.Dansinger ML, Tatsioni A, Wong JB, Chung M, Balk EA. Meta-analysis: the effect of dietary counseling for weight loss. Ann Intern Med. 2007;147:41–50. doi: 10.7326/0003-4819-147-1-200707030-00007. [DOI] [PubMed] [Google Scholar]
- 11.Serdula MK, Mokdad AH, Williamson DF, Galuska DA, Mendlein JM, Heath GW. Prevalence of attempting weight loss and strategies for controlling weight. JAMA. 1999;282:1353–1358. doi: 10.1001/jama.282.14.1353. [DOI] [PubMed] [Google Scholar]
- 12.Leibel R, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uusitupa M. Gene-diet interaction in relation to the prevention of obesity and type 2 diabetes: evidence from the Finnish Diabetes Prevention Study. Nutr Metab Cardiovasc Dis. 2005;15:225–233. doi: 10.1016/j.numecd.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. for the Dietary Intervention Randomized Controlled Trial (DIRECT) Group Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 16.Hobbs HH, Brown MS, Goldstein JL, Russell DW. Deletion of exon encoding cysteine-rich repeat of LDL receptor alters its binding specificity in a subject with familial hypercholesterolemia. J Biol Chem. 1986;261:13114–13120. [PubMed] [Google Scholar]
- 17.Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet. 2005;76:887–893. doi: 10.1086/429864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lake SL, Lyon H, Tantisira K, Silverman EK, Weiss ST, Laird NM, et al. Estimation and tests of haplotype-environment interaction when linkage phase is ambiguous. Hum Hered. 2003;55:56–65. doi: 10.1159/000071811. [DOI] [PubMed] [Google Scholar]
- 19.Sinnwell JP, Schaid DJ. Haplo.stats: Statistical analysis of haplotypes with traits and covariates when linkage phase is ambiguous. R package version 1.1.1. 2004 Apr; [Google Scholar]
- 20.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 21.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300. [Google Scholar]
- 22.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52:1201–1210. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacLean PS, Higgins JA, Jackman MR, Johnson GC, Felming-Elder BK, Wyatt HR, et al. Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1577–R1588. doi: 10.1152/ajpregu.00810.2005. [DOI] [PubMed] [Google Scholar]
- 24.Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau JA, Heymsfield S, Joanisse DR, et al. Effects of weight change on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol. 2003;285:R183–R192. doi: 10.1152/ajpregu.00474.2002. [DOI] [PubMed] [Google Scholar]
- 25.Scarpace PJ, Matheny M, Zhang Y, Shek EW, Prima V, Zolotukhin S, et al. Leptin-induced leptin resistance reveals separate roles for the anorexic and thermogenic responses in weight maintenance. Endocrinology. 2002;143:3026–3035. doi: 10.1210/endo.143.8.8966. [DOI] [PubMed] [Google Scholar]
- 26.Filozof CM, Murúa C, Sanchez MP, Brailovsky C, Perman M, Gonzalez CD, et al. Low plasma leptin concentration and low rates of fat oxidation in weight-stable post-obese subjects. Obes Res. 2000;8:205–210. doi: 10.1038/oby.2000.23. [DOI] [PubMed] [Google Scholar]
- 27.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118:2583–2591. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogels N, Westerterp-Plantenga MS. Successful long-term weight maintenance: a 2-year follow-up. Obesity. 2007;15:1258–1566. doi: 10.1038/oby.2007.147. [DOI] [PubMed] [Google Scholar]
- 29.Mavri A, Stegnar M, Sabovic M. Do baseline serum leptin levels predict weight regain after dieting in obese women? Diabetes Obes Metab. 2001;3:293–296. doi: 10.1046/j.1463-1326.2001.00134.x. [DOI] [PubMed] [Google Scholar]
- 30.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 31.Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet. 1998;18:213–215. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- 32.Le Stunff C, Le Bihan C, Schork NJ, Bougneres P. A common promoter variant of the leptin gene is associated with changes in the relationship between serum leptin and fat mass in obese girls. Diabetes. 2000;49:2196–2200. doi: 10.2337/diabetes.49.12.2196. [DOI] [PubMed] [Google Scholar]
- 33.Li WD, Reed DR, Lee JH, Xu W, Kilker RL, Sodam BR, et al. Sequence variants in the 5′ flanking region of the leptin gene are associated with obesity in women. Ann Hum Genet. 1999;63:227–234. doi: 10.1046/j.1469-1809.1999.6330227.x. [DOI] [PubMed] [Google Scholar]
- 34.Mammes O, Betoulle D, Aubert R, Herbeth B, Siest G, Fumeron F. Association of the G-2548A polymorphism in the 5′ region of the LEP gene with overweight. Ann Hum Genet. 2000;64:391–394. doi: 10.1017/s0003480000008277. [DOI] [PubMed] [Google Scholar]
- 35.Jiang Y, Wilk JB, Borecki I, Williamson S, DeStefano AL, Xu G, et al. Common variants in the 5′ region of the leptin gene are associated with body mass index in men from the National Heart, Lung, and Blood Institute Family Heart Study. Am J Hum Genet. 2004;75:220–230. doi: 10.1086/422699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karvonen MK, Pesonen U, Heinonen P, Laakso M, Rissanen A, Naukkarinen H, et al. Identification of new sequence variants in the leptin gene. J Clin Endocr Metab. 1998;83:3239–3242. doi: 10.1210/jcem.83.9.5135. [DOI] [PubMed] [Google Scholar]
- 37.Gaukrodger N, Mayosi BM, Imrie H, Avery P, Baker M, Connell JM, et al. A rare variant of the leptin gene has large effects on blood pressure and carotid intima-medial thickness: a study of 1428 individuals in 248 families. J Med Genet. 2005;42:474–478. doi: 10.1136/jmg.2004.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peltonen L, McKusick VA. Genomics and medicine: dissecting human disease in the postgenomic era. Science. 2001;291:1224–1229. doi: 10.1126/science.291.5507.1224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.