Abstract
Background
The relationship between adolescent body mass index (BMI) and future risk for end-stage renal disease (ESRD) is not fully understood, nor is it known the extent to which this association is limited to diabetic ESRD. We evaluated the association between BMI in adolescence and the risk for all-cause, diabetic, and nondiabetic ESRD.
Methods
Medical data about 1 194 704 adolescents aged 17 years who had been examined for fitness for military service between January 1, 1967, and December 31, 1997, were linked to the Israeli ESRD registry in this nation-wide population-based retrospective cohort study. Incident cases of treated ESRD between January 1, 1980, and May 31, 2010, were included. Cox proportional hazards models were used to estimate the hazard ratio (HR) for treated ESRD among study participants for their BMI at age 17 years, defined in accord with the US Centers for Disease Control and Prevention BMI for age and sex classification.
Results
During 30 478 675 follow-up person-years (mean [SD], 25.51 [8.77] person-years), 874 participants (713 male and 161 female) developed treated ESRD, for an overall incidence rate of 2.87 cases per 100 000 person-years. Compared with adolescents of normal weight, overweight adolescents (85th to 95th percentiles of BMI) and obese adolescents (≥95th percentile of BMI) had an increased future risk for treated ESRD, with incidence rates of 6.08 and 13.40 cases per 100 000 person-years, respectively. In a multivariate model adjusted for sex, country of origin, systolic blood pressure, and period of enrollment in the study, overweight was associated with an HR of 3.00 (95% CI, 2.50-3.60) and obesity with an HR of 6.89 (95% CI, 5.52-8.59) for all-cause treated ESRD. Overweight (HR, 5.96; 95% CI, 4.41-8.06) and obesity (HR, 19.37; 95% CI, 14.13-26.55) were strong and independent risk factors for diabetic ESRD. Positive associations of overweight (HR, 2.17; 95% CI, 1.71-2.74) and obesity (HR, 3.41; 95% CI, 2.42-4.79) with nondiabetic ESRD were also documented.
Conclusions
Overweight and obesity in adolescents were associated with significantly increased risk for all-cause treated ESRD during a 25-year period. Elevated BMI constitutes a substantial risk factor for diabetic and nondiabetic ESRD.
Obesity is a global health problem.1,2 The high prevalence of overweight and obesity among children, adolescents, and adults is of great concern. Since 1980, the prevalence of obesity has tripled among US school-age children and adolescents, and it has remained high, at approximately 17%, from 1999 to the present.3-6 Children and adolescents with high body mass index (BMI) often become obese adults,7,8 and obese adults are at risk for many chronic conditions such as diabetes, which confers a future risk for chronic kidney disease (CKD) and end-stage renal disease (ESRD). The relationship between obesity and CKD is complex and not yet fully understood. Few studies9-12 have examined the relationship between excess weight and risk for all-cause ESRD; although an association between BMI and ESRD in general has been documented, these studies did not determine whether such an association is limited to diabetic ESRD. In addition, previous investigations of the association between obesity and CKD or ESRD were conducted only among adults. It remains unclear whether a history of overweight and obesity during childhood or adolescence poses an additional risk.
To address these issues, we conducted a nationwide population-based retrospective cohort study evaluating the association between BMI at age 17 years among almost 1.2 million adolescents and the future risk for all-cause treated ESRD during 25 years of follow-up. We also specifically addressed the risk for diabetic vs nondiabetic ESRD.
METHODS
STUDY PARTICIPANTS
One year before their conscription into military service, all eligible Israeli adolescents undergo medical board examinations to assess their health status, including a medical history, a physical examination, a review of their medical records obtained from their primary care physician, and, where indicated, referral for further assessment (as detailed herein). All the recruits undergo a baseline measurement of weight and height, a sphygmomanometric blood pressure (BP) measurement at the right arm in the seated position,13 and a dipstick urinalysis.14 Inclusion criteria for the present study were age 17 years at the time of medical board examinations between January 1, 1967, and December 31, 1997. Because military service is not mandatory for Israeli non-Jews, the study population included only Jewish recruits, for whom military service is compulsory. Eligible individuals found to be positive for hematuria or proteinuria at enrollment dipstick screening were excluded. Hematuria was defined by a positive dipstick result, followed by sediment examination by urine microscopy demonstrating 5 or more red blood cells per high-powered field. Proteinuria was defined as a positive dipstick result, followed by a 24-hour assessment of urine quantitative protein excretion exceeding 200 mg per 24 hours.
In addition, individuals with any diagnosis suggesting a possible future risk for ESRD were excluded. These included the following diagnoses: vasculitis, hypertension, diabetes mellitus, systemic lupus erythematosus, or any known past or current kidney disease at the time of assessment, such as hematuria, proteinuria, nephrolithiasis, glomerulonephritis, cystic renal disease, urinary tract infection, acute or chronic kidney injury, and congenital or acquired anomalies of the kidneys or urinary tract (the diagnostic classification process is described herein).
CLINICAL ASSESSMENT AND DIAGNOSTIC CLASSIFICATION
One year before conscription, individuals are asked to provide copies of all available medical files, and their family physicians are requested to submit a health history summary on a standard, structured, and comprehensive form. The summary is reviewed by the physicians conducting the primary medical board examination, who then elicit a comprehensive medical history. In addition, at the time of this medical examination, the conscript undergoes a thorough and systematic physical examination, including BP, heart rate, a dipstick urinalysis test, and anthropometric measurements. If a specific diagnosis cannot be fully verified or if its severity cannot be graded after the primary medical board evaluation, the conscript is sent for additional tests and is referred to a board-certified specialist, who may order specific medical tests that will allow more precise diagnosis and classification. Moreover, for each diagnosis, including those established by the specialist during subsequent medical evaluation, the accuracy and completeness of the medical information are assessed by a committee of 2 trained military service physicians who verify the medical information. Each diagnosis is assigned a numerical code and is recorded in a central database. This process is uniform for all participants.
PARTICIPANTS’ BMI ASSESSMENT AND CLASSIFICATION
Body mass index is calculated as weight in kilograms divided by height in meters squared. Subgroups of BMI for the study cohort were defined in accord with the 2000 US Centers for Disease Control and Prevention BMI for age and sex classification of children and adolescents15 as follows: (1) Underweight was defined as a BMI below the 5th percentile (14.00-17.70 for boys and 14.00-17.20 for girls). (2) Normal weight was defined as a BMI between the 5th and 84th percentiles (17.71-24.89 for boys and 17.21-25.19 for girls). (3) Overweight was defined as a BMI between the 85th and 94th percentiles (24.90-28.19 for boys and 25.20-29.59 for girls). (4) Obesity was defined as a BMI at or exceeding the 95th percentile (28.20-40.00 for boys and 29.60-40.00 for girls).
Secondary analyses used the Centers for Disease Control and Prevention percentile categories across the entire BMI range. These included the 5th, 10th, 25th, 50th, 75th, 85th, 90th, and 95th percentiles.
ISRAELI ESRD REGISTRY
The Israeli ESRD registry is a national administrative database maintained by the Israeli Ministry of Health.16 It contains information on patients receiving any form of renal replacement therapy (RRT) (ie, hemodialysis, peritoneal dialysis, or renal transplantation). All nephrology dialysis units in Israel report to the Israeli Ministry of Health on new patients receiving RRT and on changes in treatment modality. The database includes demographic data, a primary diagnosis, the initial type of RRT, dates of initiating dialysis, and changes in dialysis treatment modalities, as well as renal transplantation and death. Validation of the Israeli ESRD registry includes periodic linkage with the Israeli population registry to update demographic and mortality data. Reports of cadaveric donor transplantation in Israel are cross-checked with the National Laboratory for Tissue Matching, and reports on living donor renal transplantation are cross-checked with the National Transplant Center. A single primary diagnosis is recorded for each new patient in the Israeli ESRD registry.16 The present study cohort was linked to the Israeli ESRD registry using the identification numbers given to all Israeli citizens at birth or immigration.
The institutional review boards of the Israeli Defense Forces Medical Corps and Sheba Medical Center (in Tel Hashomer) approved the study. They waived the requirement for informed consent on the basis of preserving participants’ anonymity.
OUTCOME VARIABLES AND FOLLOW-UP PERIOD
The onset of ESRD was defined as the date when dialysis treatment was initiated or the date of renal transplantation, whichever came first. Incident cases of treated ESRD between January 1, 1980, and May 31, 2010, were included. The follow-up period extended from the initial medical board assessment until the initiation of RRT (incidence of ESRD), death, or May 31, 2010. Because cases of ESRD were not registered between January 1, 1967, and December 31, 1979, data from participants who were enrolled during this period were left truncated in the survival analyses before January 1, 1980.
The cause of ESRD was recorded by the responsible nephrologist at the medical center where the patient was receiving RRT; 21.2% of patients had a missing or an unknown cause of ESRD. For this analysis, the causes of ESRD were classified as diabetic or nondiabetic ESRD. The main nondiabetic ESRD causes included vasculitis, hypertension, cystic kidney disease, chronic interstitial nephritis, primary glomerular disease, and secondary glomerulonephritis.
STATISTICAL ANALYSIS
The study population was described by BMI percentile in accord with the Centers for Disease Control and Prevention age-specific 5th, 85th, and 95th percentiles for 17-year-old adolescents. Incidence rates of ESRD according to these BMI categories were calculated as the number of ESRD cases divided by the total number of person-years in each BMI category. Life tables were constructed and plotted to demonstrate the incidence of all-cause ESRD, as well as diabetic and nondiabetic ESRD. Cox proportional hazards models were used to estimate the hazard ratios (HRs) for ESRD, controlling for the father’s or paternal grandfather’s country of origin (grouped as Europe or Americas, Asia, NorthAfrica, or Israel) and for the period of recruitment (1967-1969, 1970-1979,1980-1989, or 1990-1997). In addition, the models were adjusted for systolic BP(<95th percentile,≥95th percentile, or unknown). These models were used for all-cause, diabetic, and nondiabetic ESRD. We conducted further analyses for diabetic and nondiabetic ESRD in which we divided the population into the Centers for Disease Control and Prevention 5th, 10th, 25th, 50th, 75th, 85th, 90th, and 95th percentiles of BMI. In these analyses, the fifth percentile was used as the reference category. In our primary analysis, unknown causes were considered nondiabetic ESRD. The proportional hazards assumption was tested graphically using log minus log graphs. We conducted sensitivity analyses in which the cases with unknown causes were excluded or considered diabetic ESRD. Additional analyses were stratified by sex, country of origin, period of enrollment in the study, and duration of the follow-up period. These analyses are summarized in the supplementary material (eTable; http://www.archinternmed.com). All statistical analyses were conducted using commercially available software (SPSS version 19; SPSS, Inc).
RESULTS
STUDY POPULATION
The cohort comprised 1 194 704 adolescents (mean [SD] age, 17.4 [0.2] years; 58.7% male). At baseline, 45 073 boys (6.4%) and 16 383 girls (3.3%) were underweight. Overweight was evident in 52 170 boys (7.4%) and in 43 963 girls (8.9%), and obesity was present in 20 296 boys (2.9%) and in 9690 girls (2.0%). Blood pressure values at enrollment for both sexes were positively related to BMI category. Detailed baseline characteristics of the entire cohort by BMI and sex are given in Table 1.
Table 1. Baseline Characteristics of 1 194 704 Participants Examined Between 1967 and 1997 According to Body Mass Index (BMI) Category at Age 17 Years.
| Male Participants (n = 701 649) |
Female Participants (n = 493 055) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Underweight | Normal Weight |
Overweight | Obese | Underweight | Normal Weight |
Overweight | Obese |
| Percentile subgroup based on the 2000 CDC sex-specific BMI forage growth charts |
<5th | 5th to 84th | 85th to 94th | ≥95th | <5th | 5th to 84th | 85th to 94th | ≥95th |
| No. (%) of participants | 45 073 (6.4) | 584 110 (83.2) | 52 170 (7.4) | 20 296 (2.9) | 16 383 (3.3) | 423 019 (85.8) | 43 963 (8.9) | 9690 (2.0) |
| Age, mean (SD), y | 17.4 (0.2) | 17.4 (0.2) | 17.4 (0.2) | 17.4 (0.2) | 17.4 (0.2) | 17.4 (0.2) | 17.4 (0.2) | 17.4 (0.3) |
| BMIa | ||||||||
| Mean (SD) | 16.97 (0.59) | 20.90 (1.78) | 26.20 (0.93) | 30.57 (2.04) | 16.59 (0.51) | 20.92 (1.95) | 26.76 (1.17) | 31.86 (2.01) |
| Range | 14.00-17.70 | 17.71-24.89 | 24.90-28.19 | 28.20-40.00 | 14.00-17.20 | 17.21-25.19 | 25.20-29.59 | 29.60-40.00 |
| Weight, mean (SD), kg | 50.8 (4.5) | 62.9 (7.4) | 79.2 (6.9) | 92.2 (9.6) | 44.3 (3.5) | 55.1 (6.4) | 70.2 (6.2) | 83.9 (8.2) |
| Height, mean (SD), cm | 172.8 (7.0) | 173.3 (6.7) | 173.7 (6.8) | 173.6 (7.4) | 163.4 (6.2) | 162.2 (6.0) | 162.9 (6.1) | 162.2 (6.3) |
| Israeli born, No. (%) | 39 911 (88.5) | 509 913 (87.3) | 45 239 (86.7) | 17 801 (87.7) | 14 975 (91.4) | 381 783 (90.3) | 39 244 (89.3) | 8686 (89.6) |
| Country of origin, No. (%) | ||||||||
| Europe or Americas | 14 923 (33.1) | 234 266 (40.1) | 25 155 (48.2) | 9940 (49.0) | 6842 (41.8) | 197 421 (46.7) | 20 894 (47.5) | 4652 (48.0) |
| Asia | 16 556 (36.7) | 159 522 (27.3) | 11 800 (22.6) | 4383 (21.6) | 5463 (33.3) | 104 618 (24.7) | 9498 (21.6) | 2012 (20.8) |
| North Africa | 10 781 (23.9) | 155 210 (26.6) | 11 958 (22.9) | 4547 (22.4) | 2993 (18.3) | 90 031 (21.3) | 10 602 (24.1) | 2376 (24.5) |
| Israel | 2087 (4.6) | 27 213 (4.7) | 2509 (4.8) | 1106 (5.4) | 851 (5.2) | 22 020 (5.2) | 2177 (5.0) | 496 (5.1) |
| Unknown | 726 (1.6) | 7899 (1.4) | 748 (1.4) | 320 (1.6) | 234 (1.4) | 8929 (2.1) | 792 (1.8) | 154 (1.6) |
| Blood pressure, mean (SD), mm Hg | ||||||||
| Systolic | 116.1 (11.9) | 119.6 (11.6) | 123.8 (11.5) | 126.3 (11.6) | 111.0 (12.0) | 113.5 (11.7) | 117.7 (11.7) | 121.4 (12.1) |
| Diastolic | 71.8 (8.4) | 73.2 (8.2) | 75.2 (8.0) | 76.8 (8.0) | 70.1 (8.3) | 71.3 (8.2) | 73.7 (8.1) | 76.0 (8.2) |
| Unknown, No. (%) | 9322 (20.7) | 156 875 (26.9) | 13 602 (26.1) | 4126 (20.3) | 2548 (15.6) | 86 140 (20.4) | 7745 (17.6) | 1117 (11.5) |
| Follow-up person-years, mean (SD) | 25.12 (8.40) | 26.25 (8.95) | 25.41 (9.17) | 23.79 (8.78) | 23.56 (7.95) | 24.94 (8.49) | 24.06 (8.35) | 21.75 (7.59) |
| Death, No. (%) | 965 (2.1) | 14 916 (2.6) | 1592 (3.1) | 671 (3.3) | 102 (0.6) | 2631 (0.6) | 341 (0.8) | 82 (0.8) |
Abbreviation: CDC, US Centers for Disease Control and Prevention.
Calculated as weight in kilograms divided by height in meters squared.
BMI AND RISK FOR ALL-CAUSE ESRD
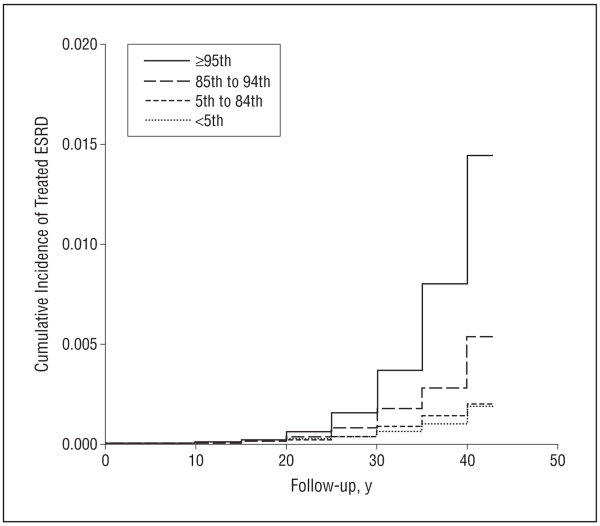
During 30 478 675 follow-up person-years (mean [SD], 25.51 [8.77] person-years), 874 participants (713 male and 161 female) developed treated ESRD, for an overall incidence rate of 2.87 cases per 100 000 person-years. Figure 1 shows the cumulative incidence of ESRD by BMI category at age 17 years. While the incidence rates for all-cause treated ESRD among the underweight and normal-weight groups were similar, overweight adolescents and obese adolescents had an increased future risk for treated ESRD, with incidence rates of 6.08 and 13.40 cases per 100 000 person-years, respectively (Table 2). In a multivariate model adjusted for sex, country of origin, systolic BP, and period of enrollment in the study, overweight in adolescence was associated with an HR of 3.00 (95% CI, 2.50-3.60) and obesity with an HR of 6.89 (95% CI, 5.52-8.59) for all-cause treated ESRD (Table 2, model 2). The associations for overweight and obesity were similar between male vs female participants; for boys the adjusted HRs were 2.89 (95% CI, 2.34-3.56) and 6.99 (95% CI, 5.52-8.85), respectively, and for girls the adjusted HRs were 3.41 (95% CI, 2.34-4.98) and 6.14 (95% CI, 3.28-11.5), respectively. Restricting the study population to participants who had at least 10 years of follow-up data did not change the associations (HR, 3.14; 95% CI, 2.61-3.78 for overweight; and HR, 7.11; 95% CI, 5.67-8.91 for obesity) (eTable).
Figure 1.
Cumulative incidence of treated end-stage renal disease (ESRD) among participants according to body mass index percentile subgroup. Log-rank P < .001.
Table 2. Risk for All-Cause Treated End-Stage Renal Disease (ESRD) in Adulthood According to Body Mass Index (BMI) Category at Age 17 Yearsa.
| Variable | Underweight | Normal Weight | Overweight | Obese |
|---|---|---|---|---|
| Percentile subgroup based on the 2000 CDC sex-specific BMI for age growth charts |
<5th | 5th to 84th | 85th to 94th | ≥95th |
| No. of participants | 61 456 | 1 007 129 | 96 133 | 29 986 |
| Follow-up person-years | 1 517 963 | 25 883 215 | 2 384 098 | 693 576 |
| Incident cases of ESRD, No. (%) | 35 (0.1) | 601 (0.1) | 145 (0.2) | 93 (0.3) |
| Incidence rate per 100 000 person-years | 2.30 | 2.32 | 6.08 | 13.40 |
| Crude HR (95% CI) | 1.12 (0.80-1.58) | 1 [Reference] | 2.74 (2.28-3.28) | 6.92 (5.56-8.61) |
| Model 1 adjusted for sex, county of origin, and period of enrollment in the study, HR (95% CI) |
0.96 (0.68-1.35) | 1 [Reference] | 3.01 (2.51-3.61) | 6.92 (5.55-8.62) |
| Model 2 adjusted for sex, country of origin, period of enrollment in the study, and systolic blood pressure, HR (95% CI)b |
0.96 (0.68-1.35) | 1 [Reference] | 3.00 (2.50-3.60) | 6.89 (5.52-8.59) |
Abbreviations: CDC, US Centers for Disease Control and Prevention; HR, hazard ratio.
Normal weight is the reference category for all models. All models yielded P < .001.
Systolic blood pressure above or below the 95th age-specific and sex-specific percentiles.
BMI AND RISK FOR DIABETIC AND NONDIABETIC ESRD
We estimated the association between BMI and treated diabetic ESRD. Compared with normal weight adolescents, overweight adolescents at age 17 years had 6 times the risk for diabetic ESRD (HR, 5.96; 95% CI, 4.41-8.06), and obese adolescents at age 17 years had 19 times the risk for diabetic ESRD (HR, 19.37; 95% CI, 14.13-26.55) (Table 3). The associations of overweight (HR, 2.17; 95% CI, 1.71-2.74) and obesity (HR, 3.41; 95% CI, 2.42-4.79) at age 17 years with nondiabetic ESRD were also significant.
Table 3. Hazard Ratios for Diabetic and Nondiabetic End-Stage Renal Disease (ESRD) in Adulthood According to Body Mass Index (BMI) Category at Age 17 Yearsa.
| Variable | Percentile Subgroup Based on the 2000 CDC Sex-Specific BMI for Age Growth Charts |
Incident Cases of ESRD, No. |
Incidence Rate per 100 000 Person-Years |
Crude HR (95% CI) |
Model 1 Adjusted for Sex, Country of Origin, and Period of Enrollment in the Study, HR (95% CI) |
Model 2 Adjusted for Sex, Country of Origin, Period of Enrollment in the Study, and Systolic Blood Pressure, HR (95% CI)b |
|---|---|---|---|---|---|---|
| Diabetic ESRD (n = 258) | ||||||
| Underweight | <5th | 4 | 0.26 | 0.62 (0.23-1.69) | 0.49 (0.18-1.33) | 0.49 (0.18-1.34) |
| Normal weight | 5th to 84th | 134 | 0.51 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Overweight | 85th to 94th | 63 | 2.72 | 5.39 (3.99-7.27) | 6.01 (4.45-8.12) | 5.96 (4.41-8.06) |
| Obese | ≥95th | 57 | 8.21 | 20.58 (15.09-28.06) | 19.81 (14.48-27.10) | 19.37 (14.13-26.55) |
|
| ||||||
| Nondiabetic ESRD (n = 616) | ||||||
| Underweight | <5th | 31 | 2.04 | 1.23 (0.86-1.78) | 1.09 (0.76-1.57) | 1.09 (0.76-1.57) |
| Normal weight | 5th to 84th | 467 | 1.8 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Overweight | 85th to 94th | 82 | 3.43 | 1.99 (1.57-2.51) | 2.17 (1.71-2.75) | 2.17 (1.71-2.74) |
| Obese | ≥95th | 36 | 5.19 | 3.35 (2.39-4.71) | 3.41 (2.42-4.79) | 3.41 (2.42-4.79) |
Abbreviations: CDC, US Centers for Disease Control and Prevention; HR, hazard ratio.
Normal weight is the reference category for all models. All models yielded P < .001.
Systolic blood pressure above or below the 95th age-specific and sex-specific percentiles.
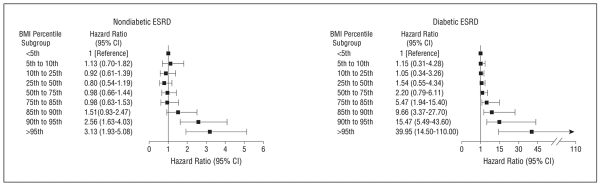
Among the causes of nondiabetic ESRD, we specifically addressed cystic kidney disease because of its strong genetic basis. Strikingly, elevated BMI at age 17 years was a risk factor for ESRD related to cystic kidney disease, with an HR of 2.57 (95% CI, 1.42-4.67) for BMI above the 85th percentile. We further characterized the association of BMI with cause-specific ESRD across the entire BMI scale (Figure 2). A significant association for nondiabetic ESRD is shown starting at the 90th to 95th percentiles (equivalent to a BMI of 26.11-28.2 for boys and a BMI of 26.7-29.59 for girls), whereas the association for diabetic ESRD is stronger and already evident within the normal weight range corresponding to the 75th to 85th percentiles (equivalent to a BMI of 23.41-24.9 for boys and a BMI of 23.41-25.18 for girls). In a sensitivity analysis that assumed all missing causes of ESRD were attributable to diabetes, the risks for overweight-associated (HR, 2.03; 95% CI, 1.52-2.71) and obesity-associated (HR, 3.80; 95% CI, 2.58-5.61) nondiabetic ESRD were not materially altered. The corresponding HRs were 4.19 (95% CI, 3.30-5.32) for overweight-associated diabetic ESRD and 10.66 (95% CI, 8.10-14.00) for obesity-associated diabetic ESRD.
Figure 2.
Hazard ratios for diabetic and nondiabetic end-stage renal disease (ESRD) by body mass index (BMI) percentile subgroup. Model 2 is adjusted for sex, country of origin, period of enrollment in the study, and systolic blood pressure (above or below the 95th age-specific and sex-specific percentiles). Black boxes indicate significant results (P < .001).
COMMENT
In this long-term nationwide population-based study, overweight and obesity at age 17 years were strongly and positively associated with the incidence of future treated ESRD, although the absolute risk for ESRD remains low. Although the results for diabetic ESRD were remarkable, with risks increasing 6-fold and 19-fold among overweight and obese adolescents, respectively, our results also indicate a substantial association between elevated BMI and nondiabetic ESRD. Our findings were independent of potential ESRD risk confounders such as age, sex, country of origin, systolic BP, and period of enrollment in the study.
Several limitations of this study warrant consideration. First, body weight and height were measured only once. Therefore, the effects of weight loss or weight gain on risk for ESRD during the follow-up period could not be determined. Nevertheless, adolescent BMI status has been previously shown to be strongly related to adult BMI status.17,18 Second, participants’ glomerular filtration rates at enrollment were unavailable. Consequently, some participants who subsequently developed treated ESRD may have had asymptomatic or undetected early-stage CKD. However, CKD is rare at age 17 years.19 Moreover, because at enrollment participants underwent a baseline medical evaluation that followed standardized and thorough protocols, including physical examination, measurement of BP, and urine dipstick test, and because high-risk participants were excluded from the study, it is unlikely that our results would be biased by undetected early CKD. Third, additional measures of adiposity such as waist circumference and waist to hip ratio were unavailable to us. Fourth, our study was restricted to Jewish recruits, so its generalizability to other populations may be limited.
The strengths of our study include the use of a large nationwide cohort that included both sexes and detailed clinical assessment parameters (including urinalysis), together with a long follow-up period and comprehensive documentation of ESRD. All the study enrollees had similar medical assessment protocols at the same age during adolescence, which included measured rather than reported weight, height, and BP values. Therefore, we were able to adhere to consistent exclusion criteria, particularly the exclusion of participants with proteinuria.
Previous studies20-27 have shown an association between elevated BMI in adulthood and risk for CKD or ESRD. In contrast to some earlier studies,10,20 we found no significant sex-based differences in the association between BMI and ESRD, nor did we find any increased11,21 or decreased9 risk among underweight participants. In a large health maintenance organization–based cohort study, Hsu et al9 reported that, compared with normal weight, overweight (25.00-29.99 BMI) was associated with approximately double the risk for ESRD, and obesity was associated with relative risks of 3.57, 6.12, and 7.07 for BMI categories of 30.00 to 34.99, 35.00 to 39.99, and 40.00 or higher, respectively. Our study demonstrated stronger associations for adolescents at the lower range of BMI (our highest BMI did not exceed 40.00). Yet, the associations shown by Hsu et al among adults without baseline kidney disease and among adults younger than 40 years at enrollment were similar to our results, supporting our study findings. Demonstrating the association of ESRD with elevated BMI in adolescence may allow early detection during childhood. While this association does not prove causation, the finding highlights another possible benefit in the urgent need to address childhood and adolescent obesity as a possible modifiable risk factor. Although the absolute risk for ESRD is low, the interpretation of our findings should consider that only 2% of all patients with CKD have ESRD. Therefore, the absolute risk for antecedent stages of CKD may be even greater.
In the present study, we attempted to quantify the extent to which this association is limited to diabetic ESRD. The well-known association between increased BMI and diabetes28 was previously suspected as the main link between elevated BMI and future risk for CKD and ESRD. Indeed, we found that already within the normal BMI range, the risk for diabetic ESRD increases with increasing BMI, a finding supported by the results of a previous study18 that suggested an association between high normal BMI and future incidence of diabetes. Most important, we found that overweight and obesity have significant associations with nondiabetic ESRD. In nondiabetic ESRD, the risk increased only among overweight and obese adolescents and not among normal-weight individuals. Moreover, while the absence of diabetic nephropathy securely excluded a diagnosis of diabetic ESRD, some cases of diabetic ESRD may represent misclassification of ESRD causes other than diabetic nephropathy.29,30 Such a misclassification, if present, would only underestimate the association between obesity and non-diabetic ESRD. On the other hand, because we did not have follow-up data on the development of diabetes for the entire cohort, we could not determine the extent to which the association of increased BMI with ESRD is mediated by diabetes. It was previously shown that diabetes independently predicts not only diabetic ESRD but also nondiabetic ESRD, although to a much lesser extent.30 However, in the present study, elevated BMI preceded any possible development of diabetes because we excluded individuals with diabetes at enrollment. Moreover, in a case-control study,12 increased BMI at age 20 years was associated with CKD in individuals without diabetes or hypertension, suggesting additional causal pathways that may be accelerated by elevated BMI, even in the absence of diabetic nephropathy and with other underlying causes. This is further supported by the fact that for ESRD secondary to cystic kidney disease, a condition with a well-defined monogenetic cause that theoretically should not be significantly influenced by BMI status, elevated BMI was also a risk factor.
Several possible diabetes-independent processes can be invoked as possibly contributing to the pathogenesis of excess weight–related CKD and ESRD. These include leptin-related renal fibrosis,31,32 elevated plasma renin and aldosterone levels,33-35 and presumed preceding underlying obesity-associated focal segmental glomerulosclerosis, renal hyperperfusion, and hyperfiltration.36-39 Future understanding of the mechanisms underlying the relationship between childhood obesity and the development of CKD may help prevent ESRD, especially in an era of prevalent childhood and adolescent obesity.
Supplementary Material
Acknowledgments
Funding/Support: Access to anonymized databases was provided by the Israeli Defense Forces Medical Corps and the Israeli Ministry of Health.
Role of the Sponsor: Neither the Israeli Defense Forces Medical Corps nor the Israeli Ministry of Health was involved in the design or conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Drs Vivante and Calderon-Margalit had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Vivante, Golan, Leiba, and Calderon-Margalit. Acquisition of data: Vivante and Tzur. Analysis and interpretation of data: Vivante, Golan, Tirosh, Skorecki, and Calderon-Margalit. Drafting of the manuscript: Vivante, Tzur, Leiba, and Calderon-Margalit. Critical revision of the manuscript for important intellectual content: Golan, Leiba, Tirosh, Skorecki, and Calderon-Margalit. Statistical analysis: Tirosh, Skorecki, and Calderon-Margalit. Administrative, technical, and material support: Vivante and Tzur. Study supervision: Golan.
Conflict of Interest Disclosures: None reported.
Online-Only Material: An eTable is available at http://www.archinternmed.com.
REFERENCES
- 1.Abelson P, Kennedy D. The obesity epidemic. Science. 2004;304(5676):1413. doi: 10.1126/science.304.5676.1413. [DOI] [PubMed] [Google Scholar]
- 2.Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in over-weight among US children and adolescents, 1999-2000. JAMA. 2002;288(14):1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003-2006. JAMA. 2008;299(20):2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 7.Deshmukh-Taskar P, Nicklas TA, Morales M, Yang SJ, Zakeri I, Berenson GS. Tracking of overweight status from childhood to young adulthood: the Bogalusa Heart Study. Eur J Clin Nutr. 2006;60(1):48–57. doi: 10.1038/sj.ejcn.1602266. [DOI] [PubMed] [Google Scholar]
- 8.Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. Do obese children become obese adults? a review of the literature. Prev Med. 1993;22(2):167–177. doi: 10.1006/pmed.1993.1014. [DOI] [PubMed] [Google Scholar]
- 9.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144(1):21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 10.Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int. 2004;65(5):1870–1876. doi: 10.1111/j.1523-1755.2004.00582.x. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds K, Gu D, Muntner P, et al. Body mass index and risk of ESRD in China. Am J Kidney Dis. 2007;50(5):754–764. doi: 10.1053/j.ajkd.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17(6):1695–1702. doi: 10.1681/ASN.2005060638. [DOI] [PubMed] [Google Scholar]
- 13.Israeli E, Schochat T, Korzets Z, Tekes-Manova D, Bernheim J, Golan E. Prehypertension and obesity in adolescents: a population study. Am J Hypertens. 2006;19(7):708–712. doi: 10.1016/j.amjhyper.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Vivante A, Afek A, Frenkel-Nir Y, et al. Persistent asymptomatic isolated microscopic hematuria in Israeli adolescents and young adults and risk for end-stage renal disease. JAMA. 2011;306(7):729–736. doi: 10.1001/jama.2011.1141. [DOI] [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1–190. [PubMed] [Google Scholar]
- 16.Calderon-Margalit R, Gordon ES, Hoshen M, Kark JD, Rotem A, Haklai Z. Dialysis in Israel, 1989-2005—time trends and international comparisons. Nephrol Dial Transplant. 2008;23(2):659–664. doi: 10.1093/ndt/gfm597. [DOI] [PubMed] [Google Scholar]
- 17.The NS, Suchindran C, North KE, Popkin BM, Gordon-Larsen P. Association of adolescent obesity with risk of severe obesity in adulthood. JAMA. 2010;304(18):2042–2047. doi: 10.1001/jama.2010.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tirosh A, Shai I, Afek A, et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med. 2011;364(14):1315–1325. doi: 10.1056/NEJMoa1006992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 20.Iseki K, Ikemiya Y, Fukiyama K. Predictors of end-stage renal disease and body mass index in a screened cohort. Kidney Int Suppl. 1997;63:S169–S170. [PubMed] [Google Scholar]
- 21.Ramirez SP, McClellan W, Port FK, Hsu SI. Risk factors for proteinuria in a large, multiracial, Southeast Asian population. J Am Soc Nephrol. 2002;13(7):1907–1917. doi: 10.1097/01.asn.0000018406.20282.c8. [DOI] [PubMed] [Google Scholar]
- 22.Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169(4):342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291(7):844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 24.Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis. 2005;46(4):587–594. doi: 10.1053/j.ajkd.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Gelber RP, Kurth T, Kausz AT, et al. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis. 2005;46(5):871–880. doi: 10.1053/j.ajkd.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Perry HM, J, Miller JP, Fornoff JR, et al. Early predictors of 15-year end-stage renal disease in hypertensive patients. Hypertension. 1995;25(4, pt 1):587–594. doi: 10.1161/01.hyp.25.4.587. [DOI] [PubMed] [Google Scholar]
- 27.Munkhaugen J, Lydersen S, Widerøe TE, Hallan S. Prehypertension, obesity, and risk of kidney disease: 20-year follow-up of the HUNT I study in Norway. Am J Kidney Dis. 2009;54(4):638–646. doi: 10.1053/j.ajkd.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Kopelman PG. Obesity as a medical problem. Nature. 2000;404(6778):635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 29.Gambara V, Mecca G, Remuzzi G, Bertani T. Heterogeneous nature of renal lesions in type II diabetes. J Am Soc Nephrol. 1993;3(8):1458–1466. doi: 10.1681/ASN.V381458. [DOI] [PubMed] [Google Scholar]
- 30.Brancati FL, Whelton PK, Randall BL, Neaton JD, Stamler J, Klag MJ. Multiple Risk Factor Intervention Trial. Risk of end-stage renal disease in diabetes mellitus: a prospective cohort study of men screened for MRFIT. JAMA. 1997;278(23):2069–2074. [PubMed] [Google Scholar]
- 31.Wolf G, Chen S, Han DC, Ziyadeh FN. Leptin and renal disease. Am J Kidney Dis. 2002;39(1):1–11. doi: 10.1053/ajkd.2002.29865. [DOI] [PubMed] [Google Scholar]
- 32.Stenvinkel P, Lönnqvist F, Schalling M. Molecular studies of leptin: implications for renal disease. Nephrol Dial Transplant. 1999;14(5):1103–1112. doi: 10.1093/ndt/14.5.1103. [DOI] [PubMed] [Google Scholar]
- 33.Giacchetti G, Faloia E, Mariniello B, et al. Overexpression of the renin-angiotensin system in human visceral adipose tissue in normal and overweight subjects. Am J Hypertens. 2002;15(5):381–388. doi: 10.1016/s0895-7061(02)02257-4. [DOI] [PubMed] [Google Scholar]
- 34.Mallamaci F, Ruggenenti P, Perna A, et al. REIN Study Group ACE inhibition is renoprotective among obese patients with proteinuria. J Am Soc Nephrol. 2011;22(6):1122–1128. doi: 10.1681/ASN.2010090969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med. 1981;304(16):930–933. doi: 10.1056/NEJM198104163041602. [DOI] [PubMed] [Google Scholar]
- 36.Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59(4):1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 37.Chen HM, Li SJ, Chen HP, Wang QW, Li LS, Liu ZH. Obesity-related glomerulopathy in China: a case series of 90 patients. Am J Kidney Dis. 2008;52(1):58–65. doi: 10.1053/j.ajkd.2008.02.303. [DOI] [PubMed] [Google Scholar]
- 38.Chagnac AWT, Weinstein T, Korzets A, Ramadan E, Hirsch J, Gafter U. Glomerular hemodynamics in severe obesity. Am J Physiol Renal Physiol. 2000;278(5):F817–F822. doi: 10.1152/ajprenal.2000.278.5.F817. [DOI] [PubMed] [Google Scholar]
- 39.Weisinger JR, Kempson RL, Eldridge FL, Swenson RS. The nephrotic syndrome: a complication of massive obesity. Ann Intern Med. 1974;81(4):440–447. doi: 10.7326/0003-4819-81-4-440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.