Abstract
Previous studies showed that aberrant CDH1 or/and HDAC3 localization is essential for the progression of some human cancers. Here, we investigate the prognostic significance of aberrant CDH1 and HDAC3 localization in 84 pancreatic cancer patients. Our results show that increases in both membrane and cytoplasmic CDH1 correlate with lymph node metastasis (P = 0.026 and P < 0.001, respectively) and clinical stage (P = 0.020 and P < 0.001, respectively). Increased nuclear HDAC3 correlates with lymph node metastasis (P < 0.001) and advanced clinical stage (P < 0.001), but increased cytoplasmic HDAC3 does not (P > 0.05). Multivariate analysis showed that nuclear HDAC3 and cytoplasmic CDH1 (P = 0.001 and P = 0.010, respectively), as well as tumor differentiation (P = 0.009) are independent prognostic factors. Most importantly, patients with high co-expression of nuclear HDAC3 and cytoplasmic CDH1 had shorter survival times (P < 0.001), more frequent lymph node metastasis (P < 0.001), and advanced clinical stage (P < 0.001). Our studies provide convincing evidence that nuclear HDAC3 and cytoplasmic CDH1 have independent prognostic value in pancreatic cancer and provide novel targets for prognostic therapeutics.
Keywords: pancreatic cancer, histone deacetylases 3, CDH1, subcellular localization, prognosis
INTRODUCTION
Pancreatic cancer (PC) is one of the most aggressive and lethal malignancies, causing the deaths of an estimated 330,400 men and women worldwide in 2012 [1]. Total deaths due to PC are projected to increase dramatically, making it second leading cause of cancer-related deaths in the United States by 2030 [2]. Gemcitabine, the current standard first-line treatment, offers marginal symptom control and prolongation of life. Clinical trials aiming to improve the efficacy of gemcitabine have provided little improvement in survival outcomes [3]. New therapeutic strategies, including therapeutic antibodies or/and small molecule inhibitors, have been successful for a number of malignancies, but results obtained on PC treatments have so far been extremely frustrating [4]. A number of molecular mechanisms responsible for transformation and progression of PC have been identified, providing a set of potential pharmacological targets [5]. Among these is loss of adhesion between tumor cells caused by downregulation of CDH1 (also called E-cadherin) in response to genetic or epigenetic changes [6–8].
Histone acetylation is a dynamic epigenetic mechanism regulated by the histone acetyltransferases (HAT) and histone deacetylases (HDACs). HDAC3 (histone deacetylases 3), a member of class I HDACs, is overexpressed in the majority of carcinomas [9, 10], and is one of the most frequently upregulated genes in cancer [11]. Our previous study shows increased HDAC3 expression in PC [12]. HDAC3 could function as an oncogenic protein, promoting PC cell proliferation, migration, and invasion, as well as increasing drug resistance [12]. HDAC3 inversely correlates with CDH1 expression in ovarian carcinoma, and HDAC3 siRNA knock down in ovarian carcinoma cells reduced cell migration and increased CDH1 expression [13]. HDAC3 represses CDH1 through interactions with epithelial-mesenchymal transition (EMT) regulators including Snail and Twist1 [14].
This study uses high-throughput tissue microarray (TMA) and immunohistochemistry to investigate the expression and subcellular localization of CDH1 and HDAC3 in PC tissues. We analyze their association with clinicopathological factors, and address their possible value as prognostic indicators.
RESULTS
Expression of CDH1 and HDAC3 in PC tissues and adjacent normal tissues
Immunohistochemistry results are summarized in Tables 1 and 2. Strong membrane localization of CDH1 was observed in 85.7% (72/84) of normal tissues adjacent to PC (Figure 1A). In contrast, cell membrane expression of CDH1 was greatly reduced in PC tissues (Figure 1B), with high expression in 63.1% (53/84) of cases. Interestingly, higher cytoplasmic CDH1 expression was observed in PC samples (Figure 1C); 33.3% of tumor samples (28/84) but only 11.9% (10/84) of adjacent tissue samples displayed high cytoplasmic CDH1.
Table 1. Comparisons with CDH1 expression between PC and paired adjacent normal tissues (n = 84).
| Tissue sample | No.of patients | Membrane CDH1 (n, %) | P-value | Cytoplasmic CDH1 (n, %) | P-value | ||
|---|---|---|---|---|---|---|---|
| Low | High | Low | High | ||||
| Tumor | 84 | 31 (36.9) | 53 (63.1) | 0.001* | 56 (66.7) | 28 (33.3) | 0.001* |
| Adjacent normal | 84 | 12 (14.3) | 72 (85.7) | 74 (88.1) | 10 (11.9) | ||
Table 2. Comparisons with HDAC3 expression between PC and paired adjacent normal tissues (n = 84).
| Tissue sample | No.of patients | Nuclear HDAC3 (n, %) | P-value | Cytoplasmic HDAC3 (n, %) | P-value | ||
|---|---|---|---|---|---|---|---|
| Low | High | Low | High | ||||
| Tumor | 84 | 38 (45.2) | 46 (54.8) | < 0.001* | 38 (45.2) | 46 (54.8) | 0.641 |
| Adjacent normal | 84 | 68 (81.0) | 16 (19.0) | 35 (41.7) | 49 (58.3) | ||
Figure 1. Immunohistochemical expression levels and localization of CDH1 and HDAC3 in PC tissues.
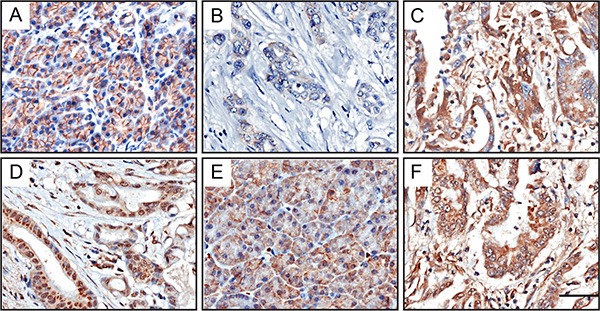
Strong membrane-associated CDH1 was observed in adjacent normal tissues (A). Low membrane CDH1 (B) and high cytoplasmic CDH1 (C) was found in tumor cells. Higher level of nuclear HDAC3 was observed in PC tissues (D), than in adjacent normal tissues (E). There was no difference in cytoplasmic HDAC3 expression between PC tissues and noncancerous samples (E, normal tissue; (F), tumor tissue). Scale bar, 50 μm.
HDAC3 was distributed in the cytoplasm and nucleus. As shown in Figure 1D, nuclear HDAC3 was highly expressed in 54.8% (46/84) of PC tissues. In contrast, HDAC3 was only seen in the nucleus of 19% (16/84) of noncancerous tissues (Figure 1E). There was no difference in cytoplasmic HDAC3 expression between PC tissues and noncancerous samples (54.8%, 46/84 vs. 58.3%, 49/84; Figure 1E, 1F).
Correlations of CDH1 and HDAC3 expression in PC tissues
An inverse correlation was identified between low membrane expression of CDH1 and high nuclear HDAC3 expression (Spearman correlation coefficient r = −0.348, P = 0.001, Supplementary Table S1). High cytoplasmic CDH1 expression positively correlated with high nuclear HDAC3 expression (Spearman correlation coefficient r = 0.440, P < 0.001, Table 3). No correlations were found between cytoplasmic HDAC3 expression and CDH1 expression location (P > 0.05, Supplementary Tables S2, S3).
Table 3. Association between nuclear HDAC3 and cytoplasmic CDH1 expression.
| Tumor tissue sample | Nuclear HDAC3 | Correlation coefficient | P-value | |
|---|---|---|---|---|
| Low | High | |||
| Cytoplasmic CDH1 Low | 34 | 22 | 0.440 | < 0.001* |
| Cytoplasmic CDH1 High | 4 | 24 | ||
Relationship of clinicopathological features with CDH1 and HDAC3 expression in PC patients
The relationships of CDH1 and HDAC3 expression levels with clinicopathological features of PC were evaluated by immunohistochemistry. As summarized in Table 4, CDH1 cell membrane expression correlated with lymph node metastasis (P = 0.026) and clinical stage (P = 0.020). High cytoplasmic CDH1 strongly correlated with lymph node metastasis (N classification, P < 0.001) and advanced clinical stage (P < 0.001). Neither cytoplasmic nor membrane CDH1 were associated with patients’ gender, age, tumor location, tumor size, tumor differentiation, invasion depth, distant metastasis, abdominal pain, jaundice or nervous invasion (P > 0.05).
Table 4. Correlation between the clinicopathologic characteristics and CDH1 expression (n = 84).
| Clinicopathological parameters | No.of patients | Membrane CDH1 (n, %) | Cytoplasmic CDH1 (n, %) | ||||
|---|---|---|---|---|---|---|---|
| Low | High | P-value | Low | High | P-value | ||
| Cases | 84 | 31 (36.9) | 53 (63.1) | 56 (66.7) | 28 (33.3) | ||
| Age (years) | |||||||
| ≤ 60 | 39 | 17 (43.6) | 22 (56.4) | 0.237a | 26 (66.7) | 13 (33.3) | 1.000a |
| > 60 | 45 | 14 (31.1) | 31 (68.9) | 30 (66.7) | 15 (33.3) | ||
| Gender | |||||||
| Male | 51 | 21 (41.2) | 30 (58.8) | 0.313a | 34 (66.7) | 17 (33.3) | 1.000a |
| Female | 33 | 10 (30.3) | 23 (69.7) | 22 (33.3) | 11 (33.3) | ||
| Tumor location | |||||||
| Head, neck | 56 | 24 (42.9) | 32 (57.1) | 0.110a | 35 (62.5) | 21 (37.5) | 0.252a |
| Body, tail | 28 | 7 (25.0) | 21 (75.0) | 21 (75.0) | 7 (25.0) | ||
| Tumor size (cm) | |||||||
| ≤ 3 | 25 | 9 (36.0) | 16 (64.0) | 0.911a | 18 (72.0) | 7 (28.0) | 0.500a |
| > 3 | 59 | 22 (37.3) | 37 (62.7) | 38 (64.4) | 21 (35.6) | ||
| Tumor differentiation | |||||||
| Well, moderate | 57 | 21 (36.8) | 36 (63.2) | 0.986a | 40 (70.2) | 17 (29.8) | 0.322a |
| Poor | 27 | 10 (37.0) | 17 (63.0) | 16 (59.3) | 11 (40.7) | ||
| Invasion depth | |||||||
| T1 + T2 | 71 | 27(38.0) | 44(62.0) | 0.618a | 49 (69.0) | 22 (31.0) | 0.286a |
| T3 + T4 | 13 | 4(30.8) | 9(69.2) | 7 (53.8) | 6 (46.2) | ||
| Lymph nodes metastasis | |||||||
| N0 (negative) | 51 | 14 (27.5) | 37 (72.5) | 0.026a* | 43 (84.3) | 8 (15.7) | < 0.001a* |
| N1 (positive) | 33 | 17 (51.5) | 16 (48.5) | 13 (39.4) | 20 (60.6) | ||
| Distant metastasis | |||||||
| Absent | 82 | 29 (35.4) | 53 (64.6) | 0.133b | 56 (68.3) | 26 (31.7) | 0.108b |
| Present | 2 | 2 (100) | 0 (0) | 0 (0) | 2 (100) | ||
| Clinical stage | |||||||
| Early stages (≤ IIa) | 49 | 13(26.5) | 36(73.5) | 0.020a* | 43(87.8) | 6(12.2) | < 0.001a* |
| Advanced stages (> IIa) | 35 | 18(51.4) | 17(48.6) | 13(37.1) | 22(62.9) | ||
| Abdominal pain | |||||||
| Absent | 38 | 13 (34.2) | 25 (65.8) | 0.642a | 22 (57.9) | 16 (42.1) | 0.121a |
| Present | 46 | 18 (39.1) | 28 (60.9) | 34 (73.9) | 12 (26.1) | ||
| Jaundice | |||||||
| Absent | 69 | 23 (33.3) | 46 (66.7) | 0.146a | 47 (68.1) | 22 (31.9) | 0.546a |
| Present | 15 | 8 (53.3) | 7 (46.7) | 9 (60.0) | 6 (40.0) | ||
| Nervous invasion | |||||||
| Negative | 51 | 20 (39.2) | 31 (60.8) | 0.585a | 33 (64.7) | 18 (35.3) | 0.636a |
| Positive | 33 | 11 (33.3) | 22 (66.7) | 23 (69.7) | 10 (30.3) | ||
Chi-square test.
Fisher's exact test.
P < 0.05 indicates a significant association among the variables.
As summarized in Table 5, no correlations were observed between cytoplasmic levels of HDAC3 and patients’ clinicopathologic features. Nuclear HDAC3 staining correlated with lymph node metastasis (P < 0.001) and clinical stage (P < 0.001), but did not correlate with patient's gender, age, tumor location, tumor size, tumor differentiation, invasion depth, distant metastasis, abdominal pain, jaundice, or nervous invasion (P > 0.05).
Table 5. Correlation between the clinicopathologic characteristics and HDAC3 expression (n = 84).
| Clinicopathological parameters | No.of patients | Nuclear HDAC3 (n, %) | Cytoplasmic HDAC3 (n, %) | ||||
|---|---|---|---|---|---|---|---|
| Low | High | P-value | Low | High | P-value | ||
| Cases | 84 | 38 (45.2) | 46 (54.8) | 38 (45.2) | 46 (54.8) | ||
| Age (years) | |||||||
| ≤ 60 | 39 | 19 (48.7) | 20 (51.3) | 0.551a | 18 (46.2) | 21 (53.8) | 0.875a |
| > 60 | 45 | 19 (42.2) | 26 (57.8) | 20 (44.4) | 25 (55.6) | ||
| Gender | |||||||
| Male | 51 | 20 (39.2) | 31 (60.8) | 0.168a | 25 (49.0) | 26 (51.0) | 0.387a |
| Female | 33 | 18 (54.5) | 15 (45.5) | 13 (39.4) | 20 (60.6) | ||
| Tumor location | |||||||
| Head, neck | 56 | 23 (41.1) | 33 (58.9) | 0.278a | 26 (46.4) | 30 (53.6) | 0.757a |
| Body, tail | 28 | 15 (53.6) | 13 (46.4) | 12 (42.9) | 16 (57.1) | ||
| Tumor size (cm) | |||||||
| ≤ 3 | 25 | 10 (40.0) | 15 (60.0) | 0.530a | 12 (48.0) | 13 (52.0) | 0.741a |
| > 3 | 59 | 28 (47.5) | 31 (52.5) | 26 (44.1) | 33 (55.9) | ||
| Tumor differentiation | |||||||
| Well, moderate | 57 | 27 (47.4) | 30 (52.6) | 0.569a | 26 (45.6) | 31 (54.4) | 0.920a |
| Poor | 27 | 11 (40.7) | 16 (59.3) | 12 (44.4) | 15 (55.6) | ||
| Invasion depth | |||||||
| T1 + T2 | 71 | 35 (49.3) | 36 (50.7) | 0.081a | 29 (40.8) | 42 (59.2) | 0.059a |
| T3 + T4 | 13 | 3 (23.1) | 10 (76.9) | 9 (69.2) | 4 (30.8) | ||
| Lymph nodes metastasis | |||||||
| N0 (negative) | 51 | 32 (62.7) | 19 (37.3) | < 0.001a* | 23 (45.1) | 28 (54.9) | 0.974a |
| N1 (positive) | 33 | 6 (18.2) | 27 (81.8) | 15 (45.5) | 18 (54.5) | ||
| Distant metastasis | |||||||
| Absent | 82 | 38 (46.3) | 44 (53.7) | 0.499b | 36 (43.9) | 46 (56.1) | 0.202b |
| Present | 2 | 0 (0) | 2 (100) | 2 (100) | 0 (0) | ||
| Clinical stage | |||||||
| Early stages (≤ IIa) | 49 | 31 (63.3) | 18 (36.7) | < 0.001a* | 22 (44.9) | 27 (55.1) | 0.941a |
| Advanced stages (> IIa) | 35 | 7 (20.0) | 28 (80.0) | 16 (45.7) | 19 (54.3) | ||
| Abdominal pain | |||||||
| Absent | 38 | 16 (42.1) | 22 (57.9) | 0.600a | 19 (50.0) | 19 (50.0) | 0.425a |
| Present | 46 | 22 (47.8) | 24 (52.2) | 19 (41.3) | 27 (58.7) | ||
| Jaundice | |||||||
| Absent | 69 | 33 (47.8) | 36 (52.2) | 0.307a | 31 (44.9) | 38 (55.1) | 0.902a |
| Present | 15 | 5 (33.3) | 10 (66.7) | 7 (46.7) | 8 (53.3) | ||
| Nervous invasion | |||||||
| Negative | 51 | 19 (37.3) | 32 (62.7) | 0.068a | 26 (51.0) | 25 (49.0) | 0.189a |
| Positive | 33 | 19 (57.6) | 14 (42.4) | 12 (36.4) | 21 (63.6) | ||
Chi-square test.
Fisher's exact test.
P < 0.05 indicates a significant association among the variables.
Associations between CDH1 and HDAC3 expression and survival
Kaplan-Meier analysis and log-rank test were used to investigate the prognostic value of CDH1 and HDAC3 expression and classic clinicopathologic characteristics on patient survival. In univariate analysis, both membrane and cytoplasmic CDH1 expression, as well as nuclear HDAC3, were closely associated with overall survival (OS) of PC patients (P = 0.012, P < 0.001, and P < 0.001, respectively; Table 6), with Spearman correlation coefficients of 0.240, −0.435, and −0.530 (Supplementary Table S4), respectively. The log-rank test results showed that the aberrant expression levels of these proteins correlated strongly with poorer survival in PC patients (P < 0.001; Figure 2). As shown in Table 7, the cumulative 1-year survival rate was 58% in the high membrane CDH1 group (95% confidence interval [CI], 0.443–0.717), whereas it was only 32% (95% CI, 0.163–0.477) in the low expression group (Figure 2A). The cumulative 1-year survival rate was 63% (95% CI, 0.512–0.748) in the low cytoplasmic CDH1 group, whereas it was only 21% (95% CI, 0.053–0.367) in the high-expression group (Figure 2B). The 1-year survival rate was 79% in the low nuclear HDAC3 group (95% CI, 0.653–0.927), whereas it was only 24% (95% CI, 0.122–0.358) in the high staining group (Figure 2C). There was no difference in survival time associated with cytoplasmic HDAC3 expression (low vs. high, 47% (95% CI, 0.313–0.627) vs. 50% (95% CI, 0.363–0.637); Figure 2D).
Table 6. Summary of univariate and multivariate Cox regression analysis of overall survival duration in all PCs.
| Clinicopathological parameters | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Membrane CDH1 | ||||||
| Low | 1 | |||||
| High | 0.500 | 0.290–0.861 | 0.012* | |||
| Cytoplasmic CDH1 | ||||||
| Low | 1 | 1 | ||||
| High | 2.996 | 1.725–5.204 | < 0.001* | 2.204 | 1.210–4.012 | 0.010* |
| Nuclear HDAC3 | ||||||
| Low | 1 | 1 | ||||
| High | 4.020 | 2.182–7.405 | < 0.001* | 3.033 | 1.572–5.852 | 0.001* |
| Cytoplasmic HDAC3 | ||||||
| Low | 1 | |||||
| High | 0.716 | 0.418–1.227 | 0.224 | |||
| Age (years) | ||||||
| ≤ 60 | 1 | |||||
| > 60 | 0.956 | 0.558–1.639 | 0.870 | |||
| Gender | ||||||
| Male | 1 | |||||
| Female | 0.531 | 0.295–0.957 | 0.035* | |||
| Tumor location | ||||||
| Head, neck | 1 | |||||
| Body, tail | 1.189 | 0.678–2.085 | 0.546 | |||
| Tumor size(cm) | ||||||
| ≤ 3 | 1 | |||||
| > 3 | 0.797 | 0.451–1.409 | 0.436 | |||
| Tumor differentiation | ||||||
| Well, moderate | 1 | 1 | ||||
| Poor | 2.077 | 1.192–3.620 | 0.010* | 2.119 | 1.210–3.711 | 0.009* |
| Invasion depth | ||||||
| T1 + T2 | 1 | |||||
| T3 + T4 | 0.983 | 0.463–2.088 | 0.965 | |||
| Lymph nodes metastasis | ||||||
| N0(negative) | 1 | |||||
| N1(positive) | 2.060 | 1.196–3.546 | 0.009* | |||
| Distant metastasis | ||||||
| Absent | 1 | |||||
| Present | 2.372 | 0.574–9.798 | 0.233 | |||
| Clinical stage | ||||||
| Early stages (≤ IIa) | 1 | |||||
| Advanced stages (> IIa) | 2.230 | 1.294–3.845 | 0.004* | |||
| Abdominal pain | ||||||
| Absent | 1 | |||||
| Present | 0.913 | 0.531–1.569 | 0.742 | |||
| Jaundice | ||||||
| Absent | 1 | |||||
| Present | 0.976 | 0.476–2.000 | 0.947 | |||
| Nervous invasion | ||||||
| Negative | 1 | |||||
| Positive | 1.168 | 0.678–2.012 | 0.576 | |||
HR hazard ratio, 95% CI 95% confidence interval.
Figure 2. Cumulative kaplan-meier overall survival curves of 84 PC patients segmented by CDH1 (A), membrane CDH1; (B), cytoplasmic CDH1), HDAC3 (C), nuclear HDAC3; (D), cytoplasmic HDAC3), and high-risk combination group (cytoplasmic CDH1 and nuclear HDAC3 combinations) (E).
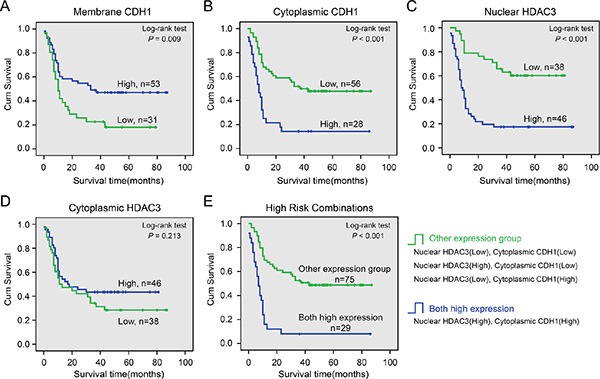
P-values were calculated by the log-rank test.
Table 7. Comparisons with cumulative 1-year survival rate between different groups.
| Variables | Cumulative 1-year survival rate | 95% CI |
|---|---|---|
| Membrane CDH1 | ||
| Low | 32% | 0.163–0.477 |
| High | 58% | 0.443–0.717 |
| Cytoplasmic CDH1 | ||
| Low | 63% | 0.512–0.748 |
| High | 21% | 0.053–0.367 |
| Nuclear HDAC3 | ||
| Low | 79% | 0.653–0.927 |
| High | 24% | 0.122–0.358 |
| Cytoplasmic HDAC3 | ||
| Low | 47% | 0.313–0.627 |
| High | 50% | 0.363–0.637 |
| High risk combinations | ||
| Both high expression | 12% | 0.002–0.238 |
| Other expression group | 64% | 0.522–0.758 |
95% CI, 95% confidence interval.
Univariate analysis also indicated that gender, tumor differentiation, lymph node metastasis, and clinical stage correlated with patient survival (P = 0.035, P = 0.010, P = 0.009, and P = 0.004, respectively). Multivariate analysis shows that cytoplasmic CDH1 expression, nuclear HDAC3 expression, and tumor differentiation were independent prognostic factors for PC patients (Table 6). Membrane CDH1 expression, gender, lymph node metastasis, and clinical stage were not associated with survival (Table 6). To further investigate the association of survival time with cytoplasmic CDH1 and nuclear HDAC3 expression, a final concomitant model was constructed. As shown in Figure 2E, the log-rank test showed that high co-expression of these two proteins correlated with shorter survival time of PC patients (P < 0.001). The cumulative proportion of 1-year survival was only 12% (95% CI, 0.002–0.238) in the high co-expression group and 64% (95% CI, 0.522–0.758) in other combination groups (Table 7). Moreover, Spearman correlation analysis revealed a positive correlation between the high co-expression group and lymph nodes metastasis, clinical stage (r = 0.436 and r = 0.506, respectively, Supplementary Table S5).
DISCUSSION
Cellular functions are dictated by protein activity and content. There are numerous strategies to regulate proteins varying from modulating gene expression to post-translational modifications to control of protein localization [15]. Numerous studies demonstrate functionally relevant subcellular translocation of specific individual proteins [16]. For example, β-catenin is found at multiple subcellular localizations, including at cell junctions, where it stabilizes cell-cell contacts; in the cytoplasm, where β-catenin levels are controlled by protein stability regulating processes; and in the nucleus, where β-catenin is involved in transcriptional regulation and chromatin interactions [17, 18]. Moreover, β-catenin nuclear import and accumulation drives tumor formation and correlates with clinical tumor grade [19]. Another example is BRCA1, whose prognostic significance varies with its subcellular distribution. Nuclear detection of the protein is associated with a worse prognosis, while cytoplasmic localization predicts lower probability of recurrence due to fewer lymph node metastases [20].
Dysfunction of the CDH1-mediated cell adhesion system plays an important role in pancreatic tumor progression to invasive, metastatic carcinoma [21, 22]. Epigenetic modifications contribute to loss of CDH1 expression [23, 24]. Yao R et al. [25] found that HDAC3 binds the CDH1 promoter, resulting in reduced local histone acetylation and CDH1 transcriptional repression [25]. We previously revealed that HDAC3 is overexpressed in PC tissue, and increased HDAC3 can promote malignant tumor phenotypes [12]. Moreover, Hayashi A et al. [13] found that HDAC3 was inversely correlated with CDH1 expression in ovarian carcinoma. In this study, we determined the expression pattern of CDH1 and HDAC3 proteins in PC tissues, and the clinicopathological and prognostic value of those subcellular localizations.
High-throughput TMA was employed to perform our research. First, we found that CDH1 was predominantly found on the cell membrane and in the cytoplasm, while HDAC3 localized to cell nucleus and cytoplasm. Further analysis revealed that the cell membrane CDH1 was greatly reduced in PC tissues compared to noncancerous epithelia, whereas nuclear HDAC3 was abnormally upregulated. Furthermore, there was an inverse association between these two proteins in PC tissues, consistent with recent reports on ovarian carcinoma [13].
It is worth noting that abnormal cytoplasmic CDH1 in PC tissues, and higher cytoplasmic CDH1 expression were associated with more aggressive tumor-associated variables, including lymph node metastasis and advanced clinical stage. Moreover, PC patients with high cytoplasmic CDH1 expression had shorter OS than the low-expression group. In contrast, reduced membrane CDH1 correlated with lymph node metastasis, advanced clinical stage, and shorter survival time. Multivariate analyses demonstrate that cytoplasmic but not membrane CDH1 expression was an independent prognostic factor for PC. Previously, Deeb G et al. [26] found that cytoplasmic staining of CDH1 in lung cancer tissues correlates with shorter patient survival. Ito K et al. [27] revealed that CDH1 cytoplasmic staining may be due to CDH1 proteolytic cleavage by a membrane-bound metalloprotease, yielding a soluble form. Although nuclear staining of CDH1 protein has been associated with skin Merkel cell carcinomas [28], we did not observe nuclear CDH1 in our PC patient cohort. Taken together, cytoplasmic CDH1 expression appears to represent altered protein localization related to PC tumorigenicity.
HDAC3 is the only class I HDAC found in the nucleus, cytoplasm, and plasma membrane [29, 30]. Previous studies focused on its function as an epigenetic modifier, repressing transcription through histone deacetylation [10, 31, 32]. Few studies have investigated the prognostic role of altered HDAC3 localization in PC. In this study, we found HDAC3 in the cytoplasm and nucleus of tumor cells, but not on the plasma membrane. Higher nuclear HDAC3 expression was observed in PC relative to adjacent normal tissues, while cytoplasmic expression of HDAC3 was indistinguishable. Cytoplasmic staining of HDAC3 was not associated with any clinicopathologic features or survival in PC patients. In contrast, increased nuclear HDAC3 expression was strongly associated with N classification and advanced clinical stage. For example, nuclear HDAC3 expression was detected in 80.0% of patients with high tumor grade (> IIa), but only 36.7% in the low tumor grade group (≤ IIa), suggesting that nuclear HDAC3 plays an important role in tumor progression in PC patients. Univariate analysis showed that nuclear HDAC3 in PC was associated with patients’ OS. Higher nuclear HDAC3 correlates with worse prognosis. Furthermore, according to multivariate analysis, overexpression of nuclear HDAC3 has independent prognostic significance for PC. It is of particular note that high nuclear HDAC3 expression was positively associated with increased cytoplasmic CDH1. High co-expression of these two proteins correlated with shorter patient survival, with a cumulative 1-year survival of 12% (95% CI, 0.002–0.238) compared to that of 64% (95% CI, 0.522–0.758) in other expression levels group. Escaffit F et al. [33] reported that nuclear localization of HDAC3 decreases the efficiency of apoptosis induction, and HDAC3 cytoplasmic relocalization is important for the apoptotic process.
We speculate that first, pancreatic tumor cells may have escaped apoptosis, at least in part, through HDAC3 overexpression in cell nucleus. Secondly, high concentrations of nuclear HDAC3 may directly inhibit CDH1 promoters, leading to reduced CDH1 cell membrane expression. Additionally, nuclear HDAC3 expression may upregulate membrane-bound metalloprotease expression through epigenetic modification of the associated target gene, leading to increased cytoplasmic CDH1. Together, our findings strongly indicate that nuclear HDAC3 upregulation is crucial for the aggressive behaviors and worse prognosis of PC patients, which suggest that HDAC3 may be an effective therapeutic target. Unfortunately, clinical data for HDAC inhibitors (HDACIs) are inadequate, because few studies have included patients with PC and few PC patients entered the HDACIs phase II/III trials that did [34]. More high quality clinical trials recruiting candidates with PC are required to determine the efficacy of these therapies. Selective HDACIs, potentially targeting HDAC3, may yield more potent efficacy and fewer side effects than pan-HDACIs.
In summary, these data strongly suggest the importance of nuclear HDAC3 and cytoplasmic CDH1 in the progression and clinical outcome of human PC. These markers provide strong candidates for targeted therapy of PC patients. Larger prospective studies could further validate these findings.
MATERIALS AND METHODS
Patients and tissue samples
This study was approved by the Ethics and Research Committees of Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, and was conducted in accordance with the Declaration of Helsinki Principles. TMAs containing 90 PC tissues and corresponding non-tumor tissues were purchased from ShGnghGi Outdo Biotech Company (China). The TMAs contained well-documented clinicopathological information, including patients’ age, sex, tumor size and location, tumor differentiation, invasion depth, lymph node metastasis, distant metastasis, clinical stage, abdominal pain, jaundice, nervous invasion, and follow-up data (ended in December, 2011). Six patients were excluded due to lack of completed clinical and follow-up data. In total, 84 patients were included, 51 males and 33 females, with a median age of 62 years old (ranging from 38 to 85 years old). The overall survival time ranged from 0 to 87 months, with a median of 15 months. Detailed information can be found in Table 8.
Table 8. Detailed clinical information of patients with PC.
| Characteristics | Categories | Number |
|---|---|---|
| Overall survival median (range, months) | 15 (0–87) | |
| Age median (range, years) | 62 (38–85) | |
| Tumor location | Head, neck | 56 |
| Body, tail | 28 | |
| Tumor size (cm) | ≤ 3 | 25 |
| > 3 | 59 | |
| Tumor differentiation | Well, moderate | 57 |
| Poor | 27 | |
| Invasion depth | T1 + T2 | 71 |
| T3 + T4 | 13 | |
| Lymph nodes metastasis | N0 (negative) | 51 |
| N1 (positive) | 33 | |
| Distant metastasis | Absent | 82 |
| Present | 2 | |
| Clinical stage | Early stages (≤ IIa) | 49 |
| Advanced stages (> IIa) | 35 | |
| Abdominal pain | Absent | 38 |
| Present | 46 | |
| Jaundice | Absent | 69 |
| Present | 15 | |
| Nervous invasion | Negative | 51 |
| Positive | 33 | |
Immunohistochemistry
Immunohistochemistry was performed based on the standard streptavidin-peroxidase (S-P) method (Zymed, San Francisco, CA). After deparaffinization and rehydration, TMA sections were subjected to high pressure for antigen retrieval for 5 minutes. Endogenous peroxidase activity was blocked using 100 μL of peroxidase block for 10 min. The slides were subsequently incubated overnight at 4°C with primary antibodies as follows: CDH1 (dilution 1:300, BD Biosciences), HDAC3 (dilution 1:500, Abcam). After washing in 1× phosphate buffered saline (PBS), the sections were incubated with biotinylated secondary antibodies (Zymed, San Francisco, CA) for 30 min at room temperature, followed by incubation with streptavidin horseradish peroxidase complex. Finally, sections were incubated with DAB for 2 min. Positive controls were used in each experiment following supplier's instructions. Negative controls applying appropriate IgG to replace primary antibody were also run in each experiment (Supplementary Figure 1A, 1B).
Scoring of immunohistochemistry
A double-blind method, carried out independently by two investigators without access to the patients’ clinical and pathological features, was used to analyze immunohistochemistry results. Five visual fields from different areas of each specimen were chosen at random for the immunohistochemistry evaluation. HDAC3 and CDH1 expression was scored according to staining intensity and the percentage of positive cells as previously described [35]. The percentage of positive cells was scored as follows: 0% (0), 1%–10% (1), 11%–50% (2) and 51%–100% (3). Staining intensity was scored as follows: no staining (0), week (1), moderate (2), and strong (3). Comprehensive score = staining percentage × intensity. CDH1 or HDAC3 expression was classified as follows: < 6 low expression, ≥ 6 high expression.
Statistical analysis
All statistical analyses were carried out using the SPSS 13.0 software. The χ2 test and Fisher's exact test were used to analyze the correlation between the clinicopathologic characteristics and CDH1 and HDAC3 expression as appropriate. Overall survival (OS) was defined as the interval from date of diagnosis until death from any cause. Data were censored for living patients and patients lost between follow-ups. The OS was estimated using the Kaplan-Meier method and compared using the log-rank test. Significant variables were further analyzed by multivariate analysis to test for independent prognosis. Bivariate correlations between variable factors were calculated by Spearman rank correlation coefficients. P-values < 0.05 were considered statistically significant.
SUPPLEMENTARY MATERIALS FIGURE AND TABLES
ACKNOWLEDGMENTS AND FUNDING
This study was supported in part by the National Natural Science Foundation of China (grant NO. 81502017, 81502018, 81572315, 81101846, 81171887 and 91229117), by Research Grant from Shanghai Hospital Development Center (SHDC12014128), by National Key Clinical Discipline-Oncology, and by Songjiang Liandong Program(0702N14002).
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interests.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 4.Chames P, Kerfelec B, Baty D. Therapeutic antibodies for the treatment of pancreatic cancer. ScientificWorldJournal. 2010;10:1107–1120. doi: 10.1100/tsw.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le N, Sund M, Vinci A. Prognostic and predictive markers in pancreatic adenocarcinoma. Dig Liver Dis. 2015 doi: 10.1016/j.dld.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Hofler H. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994;54:3845–3852. [PubMed] [Google Scholar]
- 7.Grady WM, Willis J, Guilford PJ, Dunbier AK, Toro TT, Lynch H, Wiesner G, Ferguson K, Eng C, Park JG, Kim SJ, Markowitz S. Methylation of the CDH1 promoter as the second genetic hit in hereditary diffuse gastric cancer. Nat Genet. 2000;26:16–17. doi: 10.1038/79120. [DOI] [PubMed] [Google Scholar]
- 8.Shibata T, Hirohashi S. [E-cadherin cell adhesion system in human cancer. Seikagaku. 2006;78:647–656. [PubMed] [Google Scholar]
- 9.Liu X, Wang JH, Li S, Li LL, Huang M, Zhang YH, Liu Y, Yang YT, Ding R, Ke YQ. Histone deacetylase 3 expression correlates with vasculogenic mimicry through the phosphoinositide3-kinase / ERK-MMP-laminin5gamma2 signaling pathway. Cancer Sci. 2015;106:857–866. doi: 10.1111/cas.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Y, Yue Y, Pan M, Sun J, Chu J, Lin X, Xu W, Feng L, Chen Y, Chen D, Shin VY, Wang X, Jin H. Histone deacetylase 3 inhibits new tumor suppressor gene DTWD1 in gastric cancer. Am J Cancer Res. 2015;5:663–673. [PMC free article] [PubMed] [Google Scholar]
- 11.Pilarsky C, Wenzig M, Specht T, Saeger HD, Grutzmann R. Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data. Neoplasia. 2004;6:744–750. doi: 10.1593/neo.04277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiao F, Hu H, Yuan C, Jin Z, Guo Z, Wang L, Wang L. Histone deacetylase 3 promotes pancreatic cancer cell proliferation, invasion and increases drug-resistance through histone modification of P27, P53 and Bax. Int J Oncol. 2014;45:1523–1530. doi: 10.3892/ijo.2014.2568. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi A, Horiuchi A, Kikuchi N, Hayashi T, Fuseya C, Suzuki A, Konishi I, Shiozawa T. Type-specific roles of histone deacetylase (HDAC) overexpression in ovarian carcinoma: HDAC1 enhances cell proliferation and HDAC3 stimulates cell migration with downregulation of E-cadherin. Int J Cancer. 2010;127:1332–1346. doi: 10.1002/ijc.25151. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi A, Horiuchi A, Kikuchi N, Hayashi T, Fuseya C, Suzuki A, Konishi I, Shiozawa T. Type-specific roles of histone deacetylase (HDAC) overexpression in ovarian carcinoma: HDAC1 enhances cell proliferation and HDAC3 stimulates cell migration with downregulation of E-cadherin. Int J Cancer. 2010;127:1332–1346. doi: 10.1002/ijc.25151. [DOI] [PubMed] [Google Scholar]
- 15.Bauer NC, Doetsch PW, Corbett AH. Mechanisms Regulating Protein Localization. Traffic. 2015;16:1039–1061. doi: 10.1111/tra.12310. [DOI] [PubMed] [Google Scholar]
- 16.He H, Lee MC, Zheng LL, Zheng L, Luo Y. Integration of the metabolic/redox state, histone gene switching, DNA replication and S-phase progression by moonlighting metabolic enzymes. Biosci Rep. 2013;33:e18. doi: 10.1042/BSR20120059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voronkov A, Krauss S. Wnt/beta-catenin signaling and small molecule inhibitors. Curr Pharm Des. 2013;19:634–664. doi: 10.2174/138161213804581837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prakash S, Swaminathan U. beta catenin in health: A review. J Oral Maxillofac Pathol. 2015;19:230–238. doi: 10.4103/0973-029X.164537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamieson C, Sharma M, Henderson BR. Targeting the beta-catenin nuclear transport pathway in cancer. Semin Cancer Biol. 2014;27:20–29. doi: 10.1016/j.semcancer.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Mylona E, Melissaris S, Nomikos A, Theohari I, Giannopoulou I, Tzelepis K, Nakopoulou L. Effect of BRCA1 immunohistochemical localizations on prognosis of patients with sporadic breast carcinomas. Pathol Res Pract. 2014;210:533–540. doi: 10.1016/j.prp.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Beuran M, Negoi I, Paun S, Ion AD, Bleotu C, Negoi RI, Hostiuc S. The epithelial to mesenchymal transition in pancreatic cancer: A systematic review. Pancreatology. 2015;15:217–225. doi: 10.1016/j.pan.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Nagathihalli NS, Merchant NB. Src-mediated regulation of E-cadherin and EMT in pancreatic cancer. Front Biosci (Landmark Ed) 2012;17:2059–2069. doi: 10.2741/4037. [DOI] [PubMed] [Google Scholar]
- 23.Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong C, Wu Y, Yao J, Wang Y, Yu Y, Rychahou PG, Evers BM, Zhou BP. G9a interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer. J Clin Invest. 2012;122:1469–1486. doi: 10.1172/JCI57349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao R, Jiang H, Ma Y, Wang L, Wang L, Du J, Hou P, Gao Y, Zhao L, Wang G, Zhang Y, Liu DX, Huang B, et al. PRMT7 induces epithelial-to-mesenchymal transition and promotes metastasis in breast cancer. Cancer Res. 2014;74:5656–5667. doi: 10.1158/0008-5472.CAN-14-0800. [DOI] [PubMed] [Google Scholar]
- 26.Deeb G, Wang J, Ramnath N, Slocum HK, Wiseman S, Beck A, Tan D. Altered E-cadherin and epidermal growth factor receptor expressions are associated with patient survival in lung cancer: a study utilizing high-density tissue microarray and immunohistochemistry. Mod Pathol. 2004;17:430–439. doi: 10.1038/modpathol.3800041. [DOI] [PubMed] [Google Scholar]
- 27.Ito K, Okamoto I, Araki N, Kawano Y, Nakao M, Fujiyama S, Tomita K, Mimori T, Saya H. Calcium influx triggers the sequential proteolysis of extracellular and cytoplasmic domains of E-cadherin, leading to loss of beta-catenin from cell-cell contacts. Oncogene. 1999;18:7080–7090. doi: 10.1038/sj.onc.1203191. [DOI] [PubMed] [Google Scholar]
- 28.Han AC, Soler AP, Tang CK, Knudsen KA, Salazar H. Nuclear localization of E-cadherin expression in Merkel cell carcinoma. Arch Pathol Lab Med. 2000;124:1147–1151. doi: 10.5858/2000-124-1147-NLOECE. [DOI] [PubMed] [Google Scholar]
- 29.Gao Z, He Q, Peng B, Chiao PJ, Ye J. Regulation of nuclear translocation of HDAC3 by IkappaBalpha is required for tumor necrosis factor inhibition of peroxisome proliferator-activated receptor gamma function. J Biol Chem. 2006;281:4540–4547. doi: 10.1074/jbc.M507784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Longworth MS, Laimins LA. Histone deacetylase 3 localizes to the plasma membrane and is a substrate of Src. Oncogene. 2006;25:4495–4500. doi: 10.1038/sj.onc.1209473. [DOI] [PubMed] [Google Scholar]
- 31.Barneda-Zahonero B, Parra M. Histone deacetylases and cancer. Mol Oncol. 2012;6:579–589. doi: 10.1016/j.molonc.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Hage P, Petitalot A, Monsoro-Burq AH, Maczkowiak F, Driouch K, Formstecher E, Camonis J, Sabbah M, Bieche I, Lidereau R, Lallemand F. The Tumor-Suppressor WWOX and HDAC3 Inhibit the Transcriptional Activity of the beta-Catenin Coactivator BCL9-2 in Breast Cancer Cells. Mol Cancer Res. 2015;13:902–912. doi: 10.1158/1541-7786.MCR-14-0180. [DOI] [PubMed] [Google Scholar]
- 33.Escaffit F, Vaute O, Chevillard-Briet M, Segui B, Takami Y, Nakayama T, Trouche D. Cleavage and cytoplasmic relocalization of histone deacetylase 3 are important for apoptosis progression. Mol Cell Biol. 2007;27:554–567. doi: 10.1128/MCB.00869-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koutsounas I, Giaginis C, Theocharis S. Histone deacetylase inhibitors and pancreatic cancer: are there any promising clinical trials? World J Gastroenterol. 2013;19:1173–1181. doi: 10.3748/wjg.v19.i8.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo W, Fang W, Li S, Yao K. Aberrant expression of nuclear vimentin and related epithelial-mesenchymal transition markers in nasopharyngeal carcinoma. Int J Cancer. 2012;131:1863–1873. doi: 10.1002/ijc.27467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


