Abstract
Objective Influenza‐associated myositis (IAM), characterized by severe lower‐extremity myalgia and reluctance to walk, is a complication of influenza among children. We investigated IAM in Nebraska during six influenza seasons, 2001–2007.
Methods During 2006–2007, we requested reports of severe influenza illness among persons aged <18 years and investigated medical records to identify and confirm IAM cases defined as severe myalgia with elevated serum creatinine kinase level in a patient aged <18 years, occurring within 7 days of laboratory confirmed influenza illness onset. Statewide hospital discharge data (HDD) were reviewed to identify retrospectively confirmed IAM cases during 2006–2007 and five previous seasons, by using surveillance data to define periods of influenza activity. Statewide IAM incidence was estimated for 2001–2002 through 2006–2007.
Results During 2006–2007, a total of 13 IAM cases were confirmed by enhanced surveillance. Median age was 6 years (range, 4–11 years). Influenza diagnosis was established by viral isolation from six patients (one influenza A and five influenza B) and rapid diagnostic tests for seven. Twelve (92%) patients, including one who died, were hospitalized for a median of 3 days (range, 1–4 days). Review of HDD identified 12 retrospectively confirmed IAM cases during 2006–2007, including four not reported through enhanced surveillance, and only one during five previous seasons (2003–2004). The HDD‐derived, retrospectively confirmed statewide IAM incidence estimates/1 00 000 population aged <18 years were 2·693 and 0·225 during 2006–2007 and 2003–2004, respectively.
Conclusion An IAM epidemic occurred in Nebraska during the 2006–2007 influenza season.
Keywords: Epidemiology, influenza, myositis, rhabdomyolysis, pediatrics
Introduction
Influenza is a common childhood disease, usually characterized by acute, self‐limited respiratory‐tract illness, with attack rates during annual seasonal epidemics that can exceed 40% among preschool and 30% among school‐age children. 1 The majority of influenza virus infections result in uncomplicated illness, with fever, cough, and upper‐respiratory symptoms. Less commonly, influenza is associated with severe, complicated illness. 2 In the United States, an estimated annual average of >2 00 000 hospitalizations are attributable to influenza among persons of all ages, and rates of hospitalization among young children are similar to those among adults at high risk for complications. 1 , 2 , 3 , 4 One study estimated that four influenza‐related excess hospitalizations per 10 000 children aged 5–14 years occur annually in the United States. 1 Although not represented in some studies of influenza‐associated hospitalizations, one severe but infrequent complication of influenza among school‐age children who might require hospitalization is influenza‐associated myositis (IAM). 5 Since first described in 1957, 6 IAM has been reported sporadically. Children are more frequently affected than adults, and IAM is often associated with influenza B. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23
In February 2007, the Nebraska Department of Health and Human Services (NDHHS) received notification of a fatal case of illness associated with laboratory‐confirmed influenza in a previously healthy girl who had experienced fever, myositis, severe myalgia, septic shock, and pneumonia. This case was published by local media, which prompted reporting of four additional patients hospitalized in Nebraska for illnesses consistent with IAM. In response, NDHHS initiated enhanced surveillance to identify severe, atypical manifestations of influenza among Nebraska residents aged <18 years, including IAM. Previously, only fatal influenza‐associated illnesses were reportable; whether IAM cases had occurred among surviving patients was unknown. Therefore, we conducted a three‐part epidemiologic investigation to identify IAM cases in Nebraska by using enhanced surveillance during the 2006–2007 influenza season and to describe clinical features (Part 1); to retrospectively compare 2006–2007 IAM incidence with the five preceding influenza seasons, 2001–2006 (Part 2); and to estimate total 2006–2007 IAM incidence by capture–recapture methodology (Part 3).
Methods
Part 1: Health department enhanced surveillance – influenza season 2006–2007
Enhanced surveillance was initiated by NDHHS to identify IAM cases. A Health Alert Network (HAN) message was sent to Nebraska physicians, hospital infection control and prevention staff, and local health department personnel on February 16, 2007, to request reports of severe and atypical manifestations of laboratory‐confirmed influenza among persons aged <18 years, occurring on or after January 1, 2007. During the 2006–2007 influenza season, routine statewide surveillance data indicated that influenza virus activity was negligible before January 2007 (NDHHS 2006–2007, unpublished data). An online form was used by hospital infection control and prevention staff or local health department personnel to report potential IAM cases to NDHHS. For reported patients, we collected additional information by review of medical records and by parent or guardian interviews.
A confirmed IAM case was defined as severe myalgia with elevated serum creatinine kinase (CK) level (>2 times upper limit of normal range) occurring within 7 days of laboratory‐confirmed influenza illness onset on or after January 1, 2007, in a patient aged <18 years who had been examined in an emergency department or who had been hospitalized. Laboratory confirmation of influenza was established by a positive influenza result on testing of upper‐respiratory‐tract clinical specimens by any of the following: rapid influenza diagnostic test, immunofluorescence, reverse transcriptase‐polymerase chain reaction, or viral culture.
Part 2: Estimated IAM incidence by retrospective hospital discharge data review – influenza seasons 2001–2002 through 2006–2007
To retrospectively estimate and compare IAM incidence during each of the six influenza seasons during 2001–2007, we reviewed Nebraska hospital discharge data, which include deidentified inpatient and emergency department discharge records from all Nebraska acute‐care, non‐federal hospitals. These data contain up to 10 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis codes. To define periods of influenza activity, we used influenza data reported from the U.S. West North Central Region by the World Health Organization and National Respiratory and Enteric Virus Surveillance System (WHO/NREVSS) collaborating laboratories (Table 1). 24 , 25 , 26 , 27 , 28 , 29 Regional data were used because statewide influenza surveillance was initiated after influenza season 2002–2003 and Nebraska‐specific data were unavailable for influenza seasons before 2003–2004.
Table 1.
Influenza activity date ranges and corresponding number of influenza season weeks during six consecutive influenza seasons – Nebraska, 2001–2007
| Influenza season | Influenza activity date range* | No of influenza season weeks |
|---|---|---|
| 2001–2002 | Jan. 6, 2002–May 18, 2002 | 19 |
| 2002–2003 | Dec. 22, 2002–May 3, 2003 | 19 |
| 2003–2004 | Nov. 9, 2003–March 6, 2004 | 17 |
| 2004–2005 | Dec. 12, 2004–April 23, 2005 | 19 |
| 2005–2006 | Dec. 18, 2005–April 22, 2006 | 18 |
| 2006–2007 | Dec. 10, 2006–April 14, 2007 | 18 |
*Influenza activity date range includes the weeks during each influenza season when the proportion of specimens testing positive for influenza first exceeded 5%, all weeks that exceeded 5%, and the week when proportion of positives declined below 5% as reported from the U.S. West North Central Region by World Health Organization and National Respiratory and Enteric Virus Surveillance System (WHO/NREVSS) collaborating laboratories. 24 , 25 , 26 , 27 , 28 , 29
To retrospectively identify confirmed and probable IAM cases, we first selected all records with ICD‐9‐CM coding of influenza (487·0, influenza with pneumonia; 487·1, influenza with other respiratory manifestations; or 487·8, influenza with other manifestations) from Nebraska hospital discharge data records of persons aged <18 years with admission during the six previously defined 2001–2007 influenza seasons (Table 1). Among these records, we then identified records with concurrent ICD‐9‐CM coding to indicate at least one muscle disorder consistent with myositis (Box 1). Records identified by this search strategy were temporarily considered potential IAM cases and were then further defined as retrospectively confirmed or probable, or excluded. We collaborated with the Nebraska Hospital Association and member hospitals to locate and review these medical records. A retrospectively confirmed IAM case was defined as severe myalgia with elevated serum CK level (>2 times upper limit of normal range) in an inpatient or emergency department patient aged <18 years occurring within 7 days of laboratory‐confirmed influenza illness during the defined 2001–2002 through 2006–2007 influenza seasons (Table 1). A probable IAM case met the same ICD‐9‐CM coding criteria as retrospectively confirmed cases but lacked laboratory confirmation of influenza or elevated serum CK level or both.
Table Box 1.
Criteria for selection of potential influenza‐associated myositis (IAM) cases for subsequent identification as probable or retrospectively confirmed cases – Nebraska, 2001–2007
| Potential IAM case |
| Nebraska hospital discharge data record |
| Admission date during six previously defined influenza seasons (2001–2007)* |
| Aged <18 years |
| At least one ICD‐9‐CM code from both group A and group B |
| Group A |
| 487·0, Influenza with pneumonia |
| 487·1, Influenza with other respiratory manifestations |
| 487·8, Influenza |
| Group B |
| 728·88, Rhabdomyolysis |
| 728·0, Infective myositis |
| 728·9, Unspecified disorder of muscle, ligament, and fascia |
| 728·89, Other disorder of muscle, ligament, and fascia |
| 729·1, Myalgia and myositis, unspecified |
| 729·5, Pain in limb |
| 729·89, Other musculoskeletal symptoms referable to limbs |
| 729·9, Other and unspecified disorders of soft tissue |
| 710·4, Polymyositis |
| 791·3, Myoglobinuria |
ICD‐9‐CM, International Classification of Diseases, Ninth Revision, Clinical Modification.
*Refer to Table 1.
We also searched the same hospital discharge data records by using a separate and different strategy to identify other possible IAM cases, including those possibly managed conservatively in emergency departments that might have occurred among patients without laboratory confirmed or ICD‐9‐CM–coded influenza during the same study period. A suspect case was defined as a Nebraska hospital discharge data record from a patient aged <18 years with admission during the six previously defined influenza seasons (Table 1) with at least one of the following primary ICD‐9‐CM codes or code combinations: 728·88, rhabdomyolysis (without ICD‐9‐CM V‐ or E‐codes indicating injury or trauma); 079·99, unspecified viral infection plus a muscle disorder code (Group B, refer to Box 1); or 729·1, myalgia and myositis, unspecified plus 079·99, unspecified viral infection. Chart review of suspect IAM cases was not performed. We calculated the estimated incidence of retrospectively confirmed, probable, and separately identified suspect IAM cases/1 00 000 population aged <18 years per influenza season and per season week by using the 2‐year averages of U.S. Census Bureau yearly estimates of Nebraska’s population aged <18 years for the respective influenza season years as the denominators. 30 For example, for influenza season 2001–2002, we averaged 2001 and 2002 population estimates.
Part 3: Estimated IAM incidence by capture–recapture analysis – influenza season 2006–2007
To estimate the total number of confirmed IAM cases that occurred during influenza season 2006–2007, we performed capture–recapture analysis comparing all confirmed cases identified by both enhanced surveillance and retrospective hospital discharge data review. 31 , 32 , 33 With the resulting point estimate and the previously established population denominator, we calculated 2006–2007 confirmed IAM incidence/1 00 000 population aged <18 years.
Human subjects review
This investigation underwent Centers for Disease Control and Prevention human subjects review and was determined to be public health practice and not research; as such, Institutional Review Board approval was not required.
Results
Part 1: Health department enhanced surveillance – influenza season 2006–2007
Of 17 patients aged <18 years reported to NDHHS during influenza season 2006–2007 enhanced surveillance, four failed to meet the confirmed IAM case definition and were excluded, and 13 experienced illness that met the confirmed IAM case definition. Of these, six were admitted to one children’s hospital, and the other seven were reported from six different hospitals. Eight resided in Nebraska’s two largest cities (Omaha and Lincoln), and five lived in geographically dispersed locations. 10 (77%) were male, three (23%) were female, and the median age was 6 years (range, 4–11 years). Twelve (92%) of 13 patients were hospitalized; one (8%) was not admitted but required emergency department visits on four consecutive days. For the 12 inpatients, the median length of hospitalization was 3 days (range, 1–4 days). A female aged 11 years who experienced IAM, septic shock, pneumonia, and cardiopulmonary failure required intensive care and mechanical ventilation, and had rapid clinical progression to death. She had been hospitalized twice for dehydration in the prior year but had not been diagnosed with any underlying medical conditions.
Of 13 confirmed IAM patients, three without high‐risk conditions had been administered influenza vaccine during 2006–2007 at least 2 weeks before onset of influenza illness. Of 10 unvaccinated children, six did not have a high‐risk condition for which influenza vaccine is recommended, including the child who died, and four had high‐risk conditions recommended for annual influenza vaccination (three had asthma/reactive airway disease, and one was aged <5 years).
The chief complaint at admission for all 13 patients was severe myalgia. Median duration of onset from influenza illness to muscle pain was 5 days (range, 2–7 days). Illness onset ranged from February 6 to March 5, 2007. All 13 experienced acute, severe bilateral calf pain; 12 (92%) were unable to walk; and four (31%) were unable to stand. Other manifestations included crawling instead of walking (15%), wide‐based stance (15%), and standing on toes (8%). Four experienced pain in other muscle groups (three experienced neck pain, low back pain, or bilateral thigh pain, and one experienced bilateral, generalized thigh, hamstring, and foot pain). Among 12 inpatients for whom clinical data were available, five (42%) reported complete, and seven (58%) reported partial resolution of respiratory symptoms before onset of muscle pain.
Three of 13 patients experienced elevated temperatures ranging from 100·7°F to 103·0°F documented during admission or from emergency department records. Laboratory data are presented in Table 2. No neurologic deficits were identified in any patients. Rhabdomyolysis was diagnosed in five patients (42%), and two (15%) had experienced myoglobinuria, including the patient who died (peak CK = 52 999 U/l, peak myoglobin = 9020 ng/ml).
Table 2.
Laboratory findings for 13 patients with confirmed influenza‐associated myositis (IAM) identified by enhanced surveillance – Nebraska, 2006–07 influenza season
| Parameter | Surviving confirmed IAM cases (n = 12) | Non‐surviving case values (n = 1) | ||
|---|---|---|---|---|
| No of patients with reported values | Median value | Range | ||
| WBC count (per μl) | ||||
| Nadir/patient | 11 | 3·00 | 2·40–5·19 | 12·32 |
| Peak/patient | 11 | 3·83 | 2·40–12·30 | 21·67 |
| Peak CK (U/l) | 12 | 2963 | 442–13 426 | 52 999 |
| AST (U/l) | 5 | 105 | 50–405 | 516 |
| ALT (U/l) | 6 | 68 | 36–123 | 96 |
| Creatinine (mg/dl) | 12 | 0·5 | 0·2–0·8 | 1·0 |
| BUN (mg/dl) | 12 | 11 | 7–17 | 16 |
| Myoglobin (ng/ml) | 10 | 772 | 73–3278 | 9020 |
| Hemoglobin (g/dl) | 11 | 14 | 12·2–14·4 | 17·0 |
Of the 12 surviving patients, parents or guardians of 11 (92%) were interviewed. Of these, seven reported resolution of myositis symptoms at discharge. Four patients, including the emergency department‐discharged patient, reported incomplete improvement at discharge. For these, the median time from discharge to complete resolution was 4·5 days (range, 3–60 days). A female aged 9 years who was hospitalized for 4 days was unable to walk at discharge and experienced muscle pain for 60 days.
Viral analyses and antigenic characterization of six isolates (five from unvaccinated patients) were performed by the Centers for Disease Control and Prevention, Atlanta, Georgia (Table 3). A/New Caldonia/20/99‐like (H1N1) virus (the H1N1 vaccine strain) was isolated from the unvaccinated patient who died. B/Florida/07/2004‐like virus (distinct lineage from the vaccine strain B/Ohio/1/2005‐like) was isolated from a previously healthy male aged 6 years who had been administered live‐attenuated influenza virus vaccine 4 months earlier.
Table 3.
Diagnostic findings for detection of influenza viruses among 13 patients with confirmed influenza‐associated myositis (IAM) identified by enhanced surveillance – Nebraska, 2006–2007 influenza season
| Influenza virus | No (%) | Commercial rapid test | Viral culture | Viral antigenic characterization |
|---|---|---|---|---|
| Type B | 9 (69·2) | 4 | 5 | B/OHIO/01/2005‐LIKE (n = 4) B/FLORIDA/07/2004‐LIKE (n = 1) |
| Type A | 2 (15·4) | 1 | 1 | A/NEW CALEDONIA/20/99‐LIKE (H1N1) |
| A/B not distinguished | 2 (15·4) | 2 | 0 | NA |
NA, not applicable.
Part 2: Estimated IAM incidence by retrospective hospital discharge data review – influenza seasons 2001–2002 through 2006–2007
From Nebraska hospital discharge data, 5826 influenza cases were retrospectively identified among patients aged <18 years from the beginning of the 2001–2002 season through the end of the 2006–2007 season (Figure 1); 15·1% (878/5826) were inpatients. During 2006–2007, a total of 32 potential IAM cases were identified and subsequently further defined. Of these, 12 met the retrospectively confirmed IAM case definition – eight were previously identified by enhanced surveillance (Part 1) and the other four previously healthy patients were only identified by retrospective case finding. Of the 20 remaining potential cases, 11 were excluded for failing to meet case definitions and nine met the probable case definition after chart review. Table 4 lists clinical characteristics of four retrospectively confirmed IAM cases that were not captured by 2006–2007 enhanced surveillance and nine probable cases, of which five (55·6%) were emergency department‐discharged. During influenza season 2006–2007, estimated statewide incidence of retrospectively confirmed IAM was 2·693 cases/1 00 000 population aged <18 years.
Figure 1.
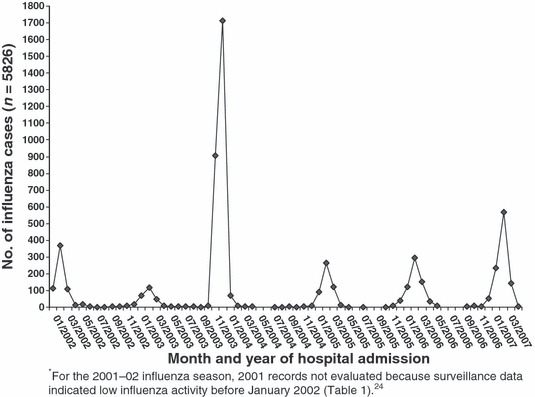
Number of influenza cases retrospectively identified from review of hospital discharge records of patients aged <18 years, by month and year of admission – Nebraska, influenza seasons 2001–2002* through 2006–2007.
Table 4.
Clinical and laboratory findings and demographic information for 13 patients with retrospectively confirmed* and probable influenza‐associated myositis (IAM) identified only by retrospective review of hospital discharge data – Nebraska, 2006–2007 influenza season
| Parameter | Case classification | |
|---|---|---|
| Retrospectively confirmed* | Probable | |
| Total, n | 4 | 9 |
| Median age, years | 9 | 7 |
| Range, years | 7–14 | 4–12 |
| Sex | ||
| Male, n (%) | 2 (50·0) | 7 (77·8) |
| Female, n (%) | 2 (50·0) | 2 (22·2) |
| Elevated CK, n (%) | 4 (100·0)† | 5 (55·6) |
| Range (U/l) | 869–6053 | 221–15 225 |
| Positive influenza test result, n (%) | 4 (100·0)‡ | 3 (33·3) |
| Type A, n | 1 | 0 |
| Type B, n | 3 | 2 |
| A/B not distinguished, n | 0 | 1 |
| Admission date (range) | 2/14/2007‐3/19/2007 | 2/14/2007‐3/31/2007 |
| Hospitalized, n (%) | 3 (75·0) | 4 (44·4) |
*Eight patients with retrospectively confirmed IAM not included because previously described in Part 1 of investigation.
†Elevated creatinine kinase (CK) required to meet retrospectively confirmed case definition.
‡Positive influenza test result required to meet retrospectively confirmed case definition.
During the 2001–2002 through 2005–2006 influenza seasons, nine potential IAM cases were retrospectively identified and subsequently further defined. Of these, only one met the retrospectively confirmed IAM case definition – a male aged 7 years who had been admitted on November 20, 2003, and hospitalized 2 days for bilateral calf pain and inability to walk. A rapid influenza test was positive for type A, and serum CK was 11 696 U/l. During the 2003–2004 influenza season, estimated statewide incidence of retrospectively confirmed IAM was 0·225 cases/1 00 000 population aged <18 years. Of eight remaining potential IAM cases identified and further defined during the same seasons, six were excluded and two emergency department discharges met the probable case definition after chart review. Table 5 lists the retrospectively established statewide incidence estimates of retrospectively confirmed, probable, and separately identified suspect IAM cases during 2001–2007.
Table 5.
Number of retrospectively confirmed, probable, and suspect influenza‐associated myositis (IAM) cases identified only by retrospective review of hospital discharge data and corresponding estimated incidence during six consecutive influenza seasons – Nebraska, 2001–2007
| Influenza season | Confirmed* (n) | Probable (n) | Suspect (n) | State population aged <18 years† | IAM incidence/1 00 000 population aged <18 years | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Confirmed* | Probable | Suspect | ||||||||
| Season | Season week | Season | Season week | Season | Season week | |||||
| 2001–2002 | 0 | 0 | 6 | 4 46 729 | 0 | 0 | 0 | 0 | 1·343 | 0·0707 |
| 2002–2003 | 0 | 1 | 5 | 4 45 759 | 0 | 0 | 0·224 | 0·0118 | 1·122 | 0·0590 |
| 2003–2004 | 1 | 1 | 4 | 4 45 408 | 0·225 | 0·0132 | 0·225 | 0·0132 | 0·898 | 0·0528 |
| 2004–2005 | 0 | 0 | 4 | 4 45 179 | 0 | 0 | 0 | 0 | 0·899 | 0·0473 |
| 2005–2006 | 0 | 0 | 6 | 4 45 003 | 0 | 0 | 0 | 0 | 1·348 | 0·0749 |
| 2006–2007 | 12 | 9 | 28 | 4 45 620 | 2·693 | 0·1496 | 2·020 | 0·1122 | 6·283 | 0·3491 |
*Retrospectively confirmed.
†All influenza season incidence calculations use a 2‐year population average. 30
Part 3: Estimated IAM incidence by capture–recapture analysis – influenza season 2006–2007
When capture–recapture analysis was applied to the 17 total confirmed IAM cases identified during the 2006–2007 influenza season (five only by enhanced surveillance, four only by retrospective case finding, and eight by both methods), the point estimate for the actual number of confirmed IAM cases statewide (Table 6) was calculated as 19·5 (95% confidence interval [CI] 14·7–24·3), corresponding to an estimated confirmed IAM incidence of 4·376 cases/1 00 000 population aged <18 years (95% CI 3·290–5·462).
Table 6.
Capture–recapture* estimate of overall statewide number of patients with confirmed influenza‐associated myositis (IAM) – Nebraska, 2006–2007 influenza season
| Retrospective case finding (Part 2 of investigation) | ||||
|---|---|---|---|---|
| Identified | Missed | |||
| Enhanced surveillance (Part 1 of investigation) | Identified | 8 (a = m2) | 5 (b) | 13 (n2 = a + b) |
| Missed | 4 (c) | 2·5 (z)† | ||
| 12 (n1 = a + c) | ||||
| Peterson estimate* of N = 19·5 (95% CI 14·7–24·3) = (n1 × n2)/m2 | ||||
Discussion
This is the first report of IAM that used population‐based, statewide data and capture–recapture methods to estimate incidence. All 17 confirmed IAM cases identified by either of the combined surveillance methods (Part 1, enhanced; Part 2, retrospective) occurred during an 8‐week period of the 18‐week 2006–2007 season. Approximately 20 overall 2006–2007 confirmed IAM cases were estimated by capture–recapture, and the corresponding incidence estimate was 4·376 cases/1 00 000 population aged <18 years. On the basis of hospital discharge data record review that allowed direct comparison between seasons, statewide retrospectively confirmed IAM incidence/1 00 000 population aged <18 years in 2006–2007 (2·693) was approximately 12 times higher than that estimated during 2003–2004 (0·225), the only one of five preceding influenza seasons in which a single retrospectively confirmed IAM case was identified. Additionally, 2006–2007 incidence estimates of probable cases and separately identified suspect IAM cases, likely including cases managed more conservatively, were approximately nine and five times greater, respectively, than the next highest influenza season estimates.
The characteristics of the 17 confirmed IAM cases we identified are consistent with others reported in the medical literature. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 Pediatric myositis was first described in 1957 by Lundberg as myalgia cruris epidemica among 70 children and four adults who had experienced muscle pain associated with influenza‐like prodromal symptoms suggesting a viral etiology. 6 In 1970, Middleton et al. described 26 cases with elevated serum CK concentrations associated with influenza virus infection. 8 Influenza‐associated myositis typically occurs with a 2:1 male predominance among children aged ≤14 years and is characterized by abrupt onset of severe myalgia in calf muscles, inability to walk, and elevated serum CK levels, usually within 1 week of influenza onset, when respiratory symptoms are improving. 5 Of 17 Nebraska patients with confirmed IAM in 2006–2007, 13 were previously healthy, including the patient who died; 12 (71%) were male, with a median age of 8 years (range, 4–14 years). Fifteen (88%) required hospitalization and all experienced severe, bilateral calf pain and substantially elevated serum CK levels. Of the 13 identified by enhanced surveillance, all except one were unable to walk, and myositis occurred a median of 5 days after onset of influenza illness, when respiratory symptoms had resolved or were improving. Whereas the link between influenza and IAM is clearly established, the pathogenesis resulting in myositis is not well‐understood. Two proposed mechanisms suggest viral invasion of muscle tissue or immune‐mediated muscle damage triggered by respiratory‐tract virus infection. 5
During 2006–2007, a total of 80% (12/15) of confirmed IAM patients with available influenza type results were infected with influenza B viruses. Of six patients from whom viral isolates had been characterized antigenically, five (four influenza B and one A/H1N1) had not been vaccinated and were infected with strains similar to corresponding 2006–2007 influenza‐vaccine strains, suggesting this illness complex might have been prevented by vaccination. One child to whom vaccine was administered was infected with an influenza B virus strain that was of different lineage and antigenically distinct from the B vaccine strain. Two other vaccinated patients were infected, but viral isolates were not available for strain characterization.
The level of influenza B activity in Nebraska during the 2006–2007 season was apparently higher compared with levels in surrounding states during this same season and compared with levels during previous seasons in Nebraska. According to Nebraska surveillance data, influenza B accounted for 29·9% of positive tests during 2006–2007 compared with 16·1% and 13·7% during 2004–2005 and 2005–2006, respectively (NDHHS, unpublished data). Regional data did not demonstrate a similar trend. According to data reported from the U.S. West North Central Region by the World Health Organization and National Respiratory and Enteric Virus Surveillance System (WHO/NREVSS) collaborating laboratories, influenza B only accounted for 16·2% of positive tests during 2006–2007, a level approximately half that observed in Nebraska and substantially lower than the regional levels observed during the two preceding seasons (20·6%, 2004–2005; 26·7%, 2005–2006). 27 , 28 , 29
The number of IAM cases identified and proportion hospitalized during 2006–2007 were substantially higher, compared with the five preceding influenza seasons (2001–2006). One possible contributing factor is that awareness of a pediatric IAM case associated with fatal influenza‐associated illness occurring in February 2007 might have influenced clinical management of IAM patients. This patient’s illness occurred before that of any of the other 16 confirmed IAM cases identified through enhanced or retrospective surveillance. Media attention focusing on the fatal case might have resulted in an increase in influenza testing, thus leading to identification of more confirmed IAM cases during 2006–2007. Additionally, parents who were aware of the fatal case might have requested influenza testing and hospitalization for any children experiencing IAM‐like symptoms. For these reasons, a proportion of the hospitalized cases that we report might otherwise have been managed more conservatively, possibly in private practice settings and thus would not have been identified as confirmed cases through our surveillance methods.
Our findings are subject to certain limitations. We restricted 2006–2007 enhanced surveillance case finding to laboratory‐confirmed influenza cases among hospitalized patients or those presenting to emergency departments. Therefore, any IAM patients treated in hospital settings but without laboratory confirmation of influenza were not captured, including patients with false‐negative test results. Those treated in primary‐care, non‐hospital settings were also missed, even if influenza was laboratory confirmed. Furthermore, patients treated in the limited number of Nebraska hospitals that do not report hospital discharge data would not have been identified retrospectively (Part 2) and the two surveillance systems might not have been completely independent. For all these reasons, we likely under‐estimated true incidence of IAM in Nebraska during 2006–2007. Because statewide influenza surveillance data were not available for all six 2001–2007 influenza seasons, we used regional rather than Nebraska influenza surveillance data to identify weeks of influenza activity. Accordingly, we might have missed IAM cases occurring at the beginning or end of the influenza seasons when activity was low. Finally, lack of any specific ICD‐9‐CM code for IAM precluded verification or exclusion of cases defined as suspect.
These results demonstrate that a substantial increase in the number of pediatric IAM cases occurred among Nebraska residents during the 2006–2007 influenza season, an epidemic compared with five previous seasons. Pediatricians and emergency department physicians should include IAM in the differential diagnosis of illness in a child aged <18 years experiencing abrupt onset of severe, lower‐extremity myalgia, reluctance or inability to walk, and normal neurologic findings, particularly when symptoms follow influenza or influenza‐like illness. Elevated serum CK concentrations and influenza testing on upper‐respiratory specimens help establish a diagnosis of IAM. Early recognition of IAM is indicated to monitor for occurrence of rhabdomyolysis and for aggressive treatment of associated complications (e.g., metabolic abnormalities, myoglobinuria, compartment syndrome, and renal failure). 5 Most of the patients in this series, particularly those with indication for vaccination, represent missed opportunities to prevent influenza. If the recommendation for annual influenza vaccination of persons aged 6 months–18 years 34 , 35 results in high vaccine coverage among school‐age children, a reduction in influenza and associated complications such as IAM might occur in this age‐group.
Financial disclosure
The authors have no sources of financial funding regarding this article.
Conflict of interest
The authors have no financial relationships relevant to this article to disclose.
Author’s note
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Nebraska Department of Health and Human Services or the Centers for Disease Control and Prevention.
Acknowledgements
The authors thank the following for their invaluable help in making this investigation and report possible: Robin Williams, Nebraska Department of Health and Human Services; Julie M. Magri, MD, MPH, and staff of the Influenza Division Strain Surveillance Branch, Centers for Disease Control and Prevention; the Nebraska Hospital Association; and all physicians, hospital infection control and prevention staff, and local health department staff throughout Nebraska that facilitated case finding and data collection. Special thanks to all the affected children and their families for assisting our investigation by providing information regarding their illnesses.
References
- 1. Neuzil KM, Mellen BG, Wright PF, Mitchel EF Jr, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med 2000; 342:225–231. [DOI] [PubMed] [Google Scholar]
- 2. Peltola V, Zieglar T, Ruuskanen O. Influenza A and B virus infections in children. Clin Infect Dis 2003; 36:299–305. [DOI] [PubMed] [Google Scholar]
- 3. Thompson WW, Shay DK, Weintraub E et al. Influenza‐associated hospitalizations in the United States. JAMA 2004; 292:1333–1340. [DOI] [PubMed] [Google Scholar]
- 4. Izurieta HS, Thompson WW, Kramarz P et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 2000; 342:232–239. [DOI] [PubMed] [Google Scholar]
- 5. Agyeman P, Duppenthaler A, Heininger U, Aebi C. Influenza‐associated myositis in children. Infection 2004; 32:199–203. [DOI] [PubMed] [Google Scholar]
- 6. Lundberg A. Myalgia cruris epidemica. Acta Paediatr 1957; 46:18–31. [DOI] [PubMed] [Google Scholar]
- 7. Hu JJ, Kao CL, Lee PI et al. Clinical features of influenza A and B in children and association with myositis. J Microbiol Immunol Infect 2004; 37:95–98. [PubMed] [Google Scholar]
- 8. Middleton PJ, Alexander RM, Szymanski MT. Severe myositis during recovery from influenza. Lancet 1970; 2:533–535. [DOI] [PubMed] [Google Scholar]
- 9. Mejlszenkier JD, Safran AP, Healy JJ, Embree L, Ouellette EM. The myositis of influenza. Arch Neurol 1973; 29:441–443. [DOI] [PubMed] [Google Scholar]
- 10. Dietzman DE, Schaller JG, Ray CG, Reed ME. Acute myositis associated with influenza B infection. Pediatrics 1976; 57:255–258. [PubMed] [Google Scholar]
- 11. McKinlay IA, Mitchell I. Transient acute myositis in childhood. Arch Dis Child 1976; 51:135–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buchta RM. Myositis and influenza. Pediatrics 1977; 60:761–762. [PubMed] [Google Scholar]
- 13. Antony JH, Procopis PG, Ouvrier RA. Benign acute childhood myositis. Neurology 1979; 29:1068–1071. [DOI] [PubMed] [Google Scholar]
- 14. Farrell MK, Partin JC, Bove KE. Epidemic influenza myopathy in Cincinnati in 1977. J Pediatr 1980; 96:545–551. [DOI] [PubMed] [Google Scholar]
- 15. Ruff RL, Secrist D. Viral studies in benign acute childhood myositis. Arch Neurol 1982; 39:261–263. [DOI] [PubMed] [Google Scholar]
- 16. O’Reilly C, Gill D, Dockeray S. Acute transient myositis of childhood. Ir J Med Sci 1983; 152:387–389. [DOI] [PubMed] [Google Scholar]
- 17. Mass A. Severe influenza myositis. Med J Aust 1985; 142:330–331. [DOI] [PubMed] [Google Scholar]
- 18. Lai PC, Leung AK. Transient childhood myositis. Med J Aust 1985; 143:222. [DOI] [PubMed] [Google Scholar]
- 19. Stang H. Acute transient myositis associated with influenza virus infection. Pediatr Infect Dis J 1989; 8:257–258. [PubMed] [Google Scholar]
- 20. Karpathios T, Kostaki M, Drakonaki S et al. An epidemic with influenza B virus causing benign acute myositis in ten boys and two girls. Eur J Pediatr 1995; 154:334–336. [DOI] [PubMed] [Google Scholar]
- 21. McIntyre PG, Doherty C. Acute benign myositis during childhood: report of five cases. Clin Infect Dis 1995; 20:722. [DOI] [PubMed] [Google Scholar]
- 22. Mackay MT, Kornberg AJ, Shield LK, Dennett X. Benign acute childhood myositis: laboratory and clinical features. Neurology 1999; 53:2127–2131. [DOI] [PubMed] [Google Scholar]
- 23. Hite LK, Glezen WP, Demmler GJ, Munoz FM. Medically attended pediatric influenza during the resurgence of the Victoria lineage of influenza B virus. J Infect Dis 2007; 11:40–47. [DOI] [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention (CDC) . WHO/NREVSS Collaborating Laboratories, West North Central Region, 2001–2002 Season, 2002. Available from http://www.cdc.gov/flu/weekly/regions2001‐2002/whoreg4.htm (accessed on 22 April 2008).
- 25. Centers for Disease Control and Prevention (CDC) . WHO/NREVSS Collaborating Laboratories, West North Central Region, 2002–2003 Season, 2003. Available from http://www.cdc.gov/flu/weekly/regions2002‐2003/whoreg4.htm (accessed on 22 April 2008).
- 26. Centers for Disease Control and Prevention (CDC) . WHO/NREVSS Collaborating Laboratories, West North Central Region, 2003–2004 Season, 2004. Available from http://www.cdc.gov/flu/weekly/regions2003‐2004/whoreg4.htm (accessed on 22 April 2008).
- 27. Centers for Disease Control and Prevention (CDC) . Influenza Isolates from the West North Central Region, Reported by WHO/NREVSS Collaborating Laboratories, 2004–2005 Season, 2005. Available from http://www.cdc.gov/flu/weekly/regions2004‐2005/whoreg4.htm (accessed on 22 April 2008).
- 28. Centers for Disease Control and Prevention (CDC) . Influenza Isolates from the West North Central Region, Reported by WHO/NREVSS Collaborating Laboratories, 2005–2006 Season, 2006. Available from http://www.cdc.gov/flu/weekly/regions2005‐2006/whoreg4.htm (accessed on 22 April 2008).
- 29. Centers for Disease Control and Prevention (CDC) . Influenza Isolates from the West North Central Region, Reported by WHO/NREVSS Collaborating Laboratories, 2006–2007 Season, 2007. Available from http://www.cdc.gov/flu/weekly/regions2006‐2007/whoreg4.htm (accessed on 22 April 2008).
- 30. U.S. Census Bureau . Population Estimates Program. Annual Estimates of the Population by Sex and Age for States and for Puerto Rico: April 1, 2000 to July 1, 2007, 2007. Available at http://www.census.gov/popest/states/asrh/SC‐EST2007‐02.html (accessed on 1 May 2008).
- 31. Grijalva CG, Craig AS, Dupont WD et al. Estimating influenza hospitalizations among children. Emerg Infect Dis 2006; 12:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grijalva CG, Weinberg GA, Bennet NM et al. Estimating the undetected burden of influenza hospitalizations in children. Epidemiol Infect 2007; 135:951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Regal RR, Hook EB. Goodness‐of‐fit based confidence intervals for estimates of the size of a closed population. Stat Med 1984; 3:287–291. [DOI] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention . Prevention and control of influenza: recommendations of the advisory committee on immunization practices (ACIP), 2008. MMWR Recomm Rep 2008; 57(No. RR‐07):1–64. [PubMed] [Google Scholar]
- 35. Finelli L, Fiore A, Dhara R et al. Influenza‐associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics 2008; 122:805–811. [DOI] [PubMed] [Google Scholar]


