Abstract
Objective Few prospective studies of inactivated split virion influenza vaccine have been conducted in infants and children. Our objective was to evaluate the safety, reactogenicity and immunogenicity of a thimerosal‐free inactivated influenza vaccine (Fluvax®; CSL Limited, Parkville, Australia) in children aged 6 months to <9 years.
Methods A prospective, open‐label, phase III clinical trial was conducted in 298 healthy children previously unvaccinated with influenza, commencing in the Southern Hemisphere 2005 autumn. Participants were divided into two groups (Group A: ≥6 months to <3 years; Group B: ≥3 years to <9 years), and received two doses of the 2005 vaccine, and one dose of the 2006 vaccine one year later (Group A: 0·25 ml per dose; Group B: 0·5 ml per dose). Vaccine safety and reactogenicity was evaluated for 30 days after each dose. Immunogenicity was assessed using hemagglutination inhibition and single radial hemolysis assays.
Results There were no withdrawals due to adverse events (AEs). The majority of solicited local and systemic AEs were of mild severity. A maximum intensity of severe was reported for injection site pain and fever by only 3·0% and 3·4% of participants, respectively. The vaccine was immunogenic for all antigens, with ≥95% of both younger and older children achieving seroprotection after dose 2.
Conclusions This thimerosal‐free inactivated influenza vaccine had a favorable safety profile and was immunogenic in children aged ≥6 months and <9 years. Primary and booster vaccination produced consistently immunogenic responses including in children under 3 years of age receiving 0·25 ml doses of vaccine.
Keywords: Immunogenicity, influenza vaccine, pediatrics, safety
Introduction
Influenza is a significant public health problem, 1 , 2 , 3 affecting between 20% and 43% of the pediatric population during a typical influenza season. 4 , 5 The annual hospitalization rate for laboratory‐confirmed influenza is highest in children younger than two years of age, and the mortality resulting from influenza in infancy is second only to that in very elderly patients. 6 During influenza seasons, otherwise healthy children are at increased risk for influenza‐related hospitalizations, 4 , 7 influenza‐related outpatient and emergency department visits 2 , 5 , 7 and an increased use of antibiotics and antipyretics. 2 , 5 In addition, children are major contributors to the spread of influenza infection in the community because they shed influenza virus in greater quantities and for longer durations than adults, and because of contact patterns and behavior. 3 , 8
The most effective strategy to prevent influenza and its potentially serious complications is through annual vaccination. The trivalent inactivated influenza vaccine, modified annually to reflect the predominant three strains of circulating influenza virus, has been used for decades to prevent influenza infection. The Advisory Committee on the Immunization Practices (ACIP) of the Centers for Disease Control and Prevention recommends annual trivalent inactivated influenza vaccination for all children aged 6 months to 18 years. 6 Despite this recommendation, most children do not receive an annual influenza vaccination; 9 , 10 estimated vaccine coverage remains <50% among children. 11 , 12 Concerns about vaccine safety and effectiveness, particularly in the younger age groups, are critical barriers to vaccine uptake. 11 , 13
To effectively address these concerns, evidence from prospective studies on the safety and effectiveness of the contemporary formulations of the trivalent inactivated influenza vaccine is needed. Unfortunately, such prospective studies in healthy children under the age of 9 years are limited. Therefore, the aims of our study were to evaluate the safety and immunogenicity of a trivalent inactivated influenza vaccine (Fluvax®; CSL Limited, Parkville, Victoria, Australia) in healthy children aged 6 months to <9 years.
Patients and methods
Study design
This was a prospective, multi‐center, open‐label, Phase III clinical trial (NCT00700193) conducted within Australia in a pediatric population. The study was conducted in two time periods from March 2005 to June 2006 at two sites (Murdoch Childrens Research Institute at the Royal Children’s Hospital in Melbourne and the Princess Margaret Hospital for Children in Perth). Administration of the primary vaccination was conducted in 2005 and booster vaccination in 2006.
The primary objective of this study was to evaluate the safety and reactogenicity of the trivalent inactivated influenza vaccine (Fluvax®, CSL Limited, Parkville, Victoria, Australia). The secondary objective was to evaluate the immunologic response after each dose of the vaccine. The study was conducted in accordance with the principles of the Declaration of Helsinki and the Australian regulatory requirements for Good Clinical Practice. The study protocol was approved by the Human Research Ethics Committee at each study center and written informed consent was obtained from each participant’s parent/guardian before any study‐related procedures were performed.
Participants
Healthy children were eligible to enter the study if they were aged ≥6 months and < 9 years at enrolment; they had not previously received an influenza vaccine; and were born between 36 and 42 weeks gestation.
The main exclusion criteria were as follows: an allergy to active vaccine components; a confirmed or suspected immunosuppressive condition; a known history of Guillain‐Barré Syndrome; a major congenital defect or serious illness; a history of neurologic disorders or seizures; administration of immunoglobulins or any blood products; participation in a clinical study or use of an investigational compound; immunosuppressive or immunomodulatory medication, including systemic corticosteroids; treatment with cytotoxic drugs.
Because of different dosing requirements, participants were divided into two groups according to their age at the time of the first study vaccination. Group A consisted of children who were aged at least 6 months and <3 years, and Group B consisted of children who were aged at least 3 years and <9 years.
Vaccines
The commercially available study vaccines fulfilled all the applicable regulatory requirements of the Australian Therapeutic Goods Administration. The vaccine was prepared from influenza virus propagated in the allantoic fluid of embryonated chicken eggs. Following harvest, the virus was purified in a sucrose gradient and inactivated with betapropiolactone, disrupted with detergent, purified and suspended in a phosphate‐buffered isotonic solution to produce a purified “split virion” vaccine. A 0·5 ml dose contained 15 μg of each of the three influenza hemagglutinin antigens as recommended by the WHO for the relevant Southern Hemisphere influenza season. As the antigen composition of the vaccine reflects the circulating strains of type A and B influenza, which differ from one season to another, the inactivated influenza vaccine used for the primary phase of the study (2005) was not antigenically equivalent to that used in the booster phase of the study (2006) (Table 1).
Table 1.
Antigen composition of the 2005 and 2006 vaccines
| 2005 Vaccine* | 2006 Vaccine* |
|---|---|
| A/New Caledonia/20/99 (H1N1) | A/New Caledonia/20/99 (H1N1) |
| A/Wellington/1/2004 (H3N2) | A/New York/55/2004 (H3N2) |
| B/Jiangsu/10/2003 | B/Malaysia/2506/2004 |
*Each vaccine contained 15 μg of each hemagglutinin antigen from the respective influenza strains per 0·5 ml dose.
Study procedures
Participants received two doses of the 2005 vaccine (dose 1 and dose 2, primary vaccination) 30 days apart and a single dose of the 2006 vaccine 12 months later. The study was conducted in the autumn of 2005 and of 2006. The first participant was vaccinated on 7 March 2005, and the last recruited participant received dose 2 of the primary course on 1 June 2005. Booster doses were administered between 27 February 2006 and 12 June 2006.
Participants in Group A received 0·25 ml of study vaccine and participants in Group B received 0·5 ml. Group A participants turning 3 years of age after administration of dose 2 were allocated to Group B and received 0·5 ml of the booster vaccine. Each vaccine was administered by intramuscular injection into the thigh (for participants aged 12 months or younger) or into the deltoid region of the arm (for participants older than 12 months).
Participant visits were conducted on Days 0, 30 ± 3, 60 ± 3 (primary exit evaluation), 365 ± 14 and 395 ± 3 (booster exit evaluation). During the visits, blood samples were collected for the immunogenicity assessments, a medical examination was conducted, and adverse events (AEs) and serious AEs were recorded. Participants were observed for 30 minutes after administration of the vaccine.
An AE diary card was used to record solicited local and systemic AEs during the 6 days following the day of vaccination. An AE diary card was also used to record unsolicited AEs or any medications taken during the 29 days following the day of vaccination. Serious AEs were recorded (1) from the first dose of the primary vaccine to 6 months after the second primary vaccine dose and (2) for 6 months after the administration of the booster dose.
The participant’s parent graded the AEs according to severity. The first occurrence of a solicited local AE was considered related to the study vaccine. Subsequent occurrences of the same solicited local AE were assessed for causality.
Participants were also monitored for intercurrent influenza‐like illness. The criteria included an axillary temperature ≥37·5°C or an oral temperature ≥38·0°C, and at least one of the specified influenza‐like symptoms (i.e., headache, cough, sore throat, rhinitis, wheezing/shortness of breath, myalgia, ear ache, vomiting/diarrhea, reduced appetite, irritability). Participants experiencing these symptoms at any time between the day of vaccination and either of the exit evaluations were asked to attend an additional visit for medical and virologic confirmation of the influenza‐like illness.
Immunogenicity
Hemagglutination inhibition (HI) antibody titers to the A/H1N1, A/H3N2 and B antigens included in the vaccine were measured at each time point by HI assay 14 ; HI antibody titers to the B strains were also measured using the single radial hemolysis (SRH) assay. 15
The proportion of participants with a protective antibody response (≥1:40) and the geometric mean titers (GMTs) of HI antibodies were determined for each antigen after each vaccine dose. Participants were grouped by baseline antibody titer (<1:10, seronegative; ≥1:10, seropositive) and by age. Immunogenicity was assessed according to the Committee for Proprietary Medicinal Products (CPMP) criteria for adults aged 18–60 years [CPMP/BWP/214/96]. The inactivated influenza vaccine was deemed immunogenic for a given strain if at least one of the following criteria were met: (1) more than 40% of the participants in each age group seroconverted or demonstrated a significant increase in HI antibody titer by HI or SRH assay; (2) a mean geometric increase in HI antibody titer (for the HI assay) or arithmetic mean zone annulus area (AMZAA; for the SRH assay) >2·5‐fold; or (3) more than 70% of the participants in each age cohort had an HI antibody titer ≥40, or an AMZAA >25 mm2 after vaccination.
Statistical analyses
A target sample size of 300 was chosen in accordance with the Swedish Medical Products Agency specifications (related to European influenza vaccine licensure requirements, and based on sufficient power to estimate immunogenicity with reasonable precision).
Statistical analyses were performed using sas v8·2 (SAS Institute Inc., Cary, NC, USA). Safety analyses included all participants who received at least one dose of the study vaccine, consistent with the prescribed dose for their age group. Immunogenicity analyses included evaluable participants only. Participants were considered evaluable if they: (1) received at least one dose of the study vaccine, consistent with the prescribed dose for their age group; (2) had serological data for blood specimens obtained at protocol‐defined time points; and (3) had not experienced virologically confirmed influenza‐like illness for the duration of the study. While 95% confidence intervals (CI) were calculated for HI GMT values, no group comparison inferential statistics were applied.
Results
Participants
A total of 298 participants were enrolled into the study (Figure 1, Table 2). All but five participants completed the primary vaccination phase. None of these participants discontinued because of AEs; the parents of four participants withdrew consent and one was lost to follow‐up. During the interval between the primary and booster vaccinations, 10 participants in Group A and 6 in Group B discontinued. Of these 16, none discontinued because of AEs; six withdrew consent, four were lost to follow‐up, one moved away from the study area and the remaining five were cited ‘Other’ as their reason for discontinuation.
Figure 1.
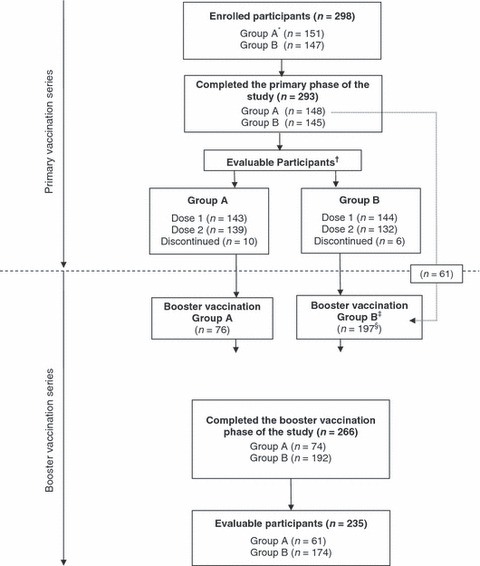
Summary of study design and participation. *Group A (infants aged ≥6 months to <3 years); Group B (children aged ≥3 years and <9 years). †Evaluable participants: Participants who received at least one dose of the study vaccine, consistent with the prescribed dose for their age group; had complete serological data for blood specimens obtained at protocol‐defined time points before and after the vaccine dose; and had not experienced confirmed influenza‐like illness for the duration of the study. ‡Group B (children aged ≥3 years and <10 years, due to 12 month interval between vaccinations). §Includes children (n = 61) who had their third birthday during the interval between the primary and booster vaccination phases.
Table 2.
Demographic and clinical characteristics of the study cohorts before administration of the primary and booster vaccinations
| Characteristic | Before primary vaccination | Before booster vaccination | ||
|---|---|---|---|---|
| Group A* (n = 151) | Group B** (n = 147) | Group A (n = 76) | Group B (n = 197) | |
| Mean age, years (SD) | 1·7 (0·43) | 5·0 (1·73) | 1·8 (0·38) | 5·1 (2·01) |
| Sex, % female (n) | 51·0 (77) | 55·1 (81) | 57·9 (44) | 50·3 (99) |
| History of influenza illness, % (n) | 12·6 (19) | 10·2 (15) | NA | NA |
| Influenza‐like illness since primary exit evaluation, % (n) | NA | NA | 2·6 (2) | 2·5 (5) |
| Influenza illness confirmed | NA | NA | 0·0 (0) | 0·0 (0) |
NA = not applicable.
*Group A (infants aged ≥6 months to <3 years).
**Group B (children aged ≥3 years and <9 years).
Sixty‐one participants from Group A who turned 3 years of age during the interval between the primary and booster vaccinations were re‐allocated to Group B for the booster vaccination. Of the 277 participants remaining in the study in 2006, 273 were eligible for booster vaccination. All but seven completed the booster vaccination. None of these children discontinued because of AEs; four withdrew consent, two were lost to follow‐up and one participant was not able to attend study visits. One additional child in Group B was given the 0·25 ml dose and was excluded from subsequent, per protocol safety and immunogenicity analyses.
Safety and reactogenicity
Solicited adverse events
Influenza vaccine was generally well‐tolerated. In both age groups, the most commonly reported local AEs were injection site erythema and pain; the most commonly reported systemic AEs were rhinitis and irritability (Table 3). Fever, irritability and loss of appetite were more commonly reported in younger (Group A) participants while injection site pain was more commonly reported in older (Group B) children (Table 3). In both groups, pain and fever were reported more frequently after the booster vaccination than after each of the individual primary vaccinations (Table 3). However, when compared with the primary vaccination phase as a whole, analysis did not indicate any notable differences. The majority of solicited AEs were of a mild severity. A maximum intensity of severe was reported for injection site pain and fever by only 3·0% and 3·4% of participants, respectively.
Table 3.
Solicited local and systemic adverse events within 6 days after administration of the vaccine
| Adverse event | Group A*, % (n) | Group B**, % (n) | ||||
|---|---|---|---|---|---|---|
| Dose 1 (n = 151) | Dose 2 (n = 151) | Booster (n = 76)*** | Dose 1 (n = 147) | Dose 2 (n = 147) | Booster (n = 196)*** | |
| Local | ||||||
| Erythema | 35·8 (54) | 37·7 (57) | 43·4 (33) | 36·7 (54) | 45·6 (67) | 43·4 (85) |
| Swelling | 15·9 (24) | 20·5 (31) | 25·0 (19) | 24·5 (36) | 27·2 (40) | 26·0 (51) |
| Pain | 36·4 (55) | 37·1 (56) | 51·3 (39) | 59·2 (87) | 61·9 (91) | 71·4 (140) |
| Systemic | ||||||
| Cough | 21·2 (32) | 31·8 (48) | 22·4 (17) | 19·0 (28) | 19·0 (28) | 16·8 (33) |
| Earache | 3·3 (5)† | 3·4 (5)†† | 1·3 (1) | 4·1 (6) | 1·4 (2) | 1·5 (3) |
| Fever | 22·5 (34) | 22·5 (34) | 39·5 (30) | 15·6 (23) | 8·2 (12) | 27·0 (53) |
| Headache | 2·0 (3)††† | 3·3 (5)† | 0·0 (0) | 13·6 (20) | 10·9 (16) | 25·0 (49) |
| Irritability | 47·7 (72) | 41·1 (62) | 38·2 (29) | 20·4 (30) | 17·0 (25) | 32·1 (63) |
| Loss of appetite | 19·2 (29) | 23·8 (36) | 21·1 (16) | 7·5 (11) | 5·4 (8) | 16·8 (33) |
| Myalgia | 0·7 (1)† | 2·7 (4)† | 6·6 (5) | 13·6 (20) | 8·2 (12) | 11·7 (23) |
| Rhinitis | 37·1 (56) | 47·7 (72) | 35·5 (27) | 21·1 (31) | 28·6 (42) | 29·6 (58) |
| Sore throat | 2·0 (3)†† | 5·3 (8)† | 6·6 (5) | 8·2 (12) | 10·9 (16) | 10·2 (20) |
| Vomiting/diarrhea | 14·6 (22) | 13·9 (21) | 17·1 (13) | 7·5 (11) | 6·8 (10) | 13·8 (27) |
| Wheezing/shortness of breath | 3·3 (5) | 8·6 (13) | 3·9 (3) | 2·7 (4) | 2·0 (3) | 4·6 (9) |
*Group A (infants aged ≥6 months to <3 years).
**Group B (children aged ≥3 years and <9 years).
***Sixty‐one participants turned 3 years of age during the interval between the primary and booster vaccinations and were re‐allocated from Group A to Group B for the booster vaccination phase.
†Data obtained from only a total of 150 participants.
††Data obtained from only a total of 149 participants.
†††Data obtained from only a total of 148 participants.
Unsolicited adverse events
At least one unsolicited AE was reported by 240 (80·5%) participants in the primary vaccine phase and by 113 (41·5%) in the booster vaccination phase. Of the reported events, most (88·0%) were mild to moderate in intensity and 10·9% were considered related to the study vaccine. The most commonly reported AEs were cough, influenza‐like illness, rhinitis and rhinorrhoea. No participants withdrew due to an AE.
Serious AEs were uncommon, with 15 reports collected in the study. Two reports were considered possibly related to the study vaccine; both occurred in the evening following the booster vaccination. In the first report, a 3‐year‐old female experienced vomiting and pyrexia that led to hospitalization overnight for rehydration. In the second report, a 3‐year‐old female experienced vomiting and a febrile convulsion, and was observed in the emergency department for 2 hours. Both participants fully recovered. No deaths were reported.
Intercurrent influenza‐like illness
A small number of participants in each group experienced influenza‐like illnesses (Table 4). Only one participant had a confirmed case of influenza B after the booster vaccination. However, the specimen for this child was compromised during transportation to the laboratory.
Table 4.
Reported intercurrent influenza‐like illnesses
| Variable | Between dose 1 and dose 2 | Between dose 2 and primary exit evaluation | Between primary exit evaluation and booster dose | During booster vaccination series | ||||
|---|---|---|---|---|---|---|---|---|
| Group A* (n = 151) | Group B** (n = 147) | Group A* (n = 151) | Group B** (n = 147) | Group A* (n = 76) | Group B** (n = 196) | Group A* (n = 76) | Group B** (n = 196) | |
| Reported influenza‐like signs/symptoms | 24 | 14 | 20 | 5 | 2 | 5 | 4 | 10 |
| Symptoms met criteria for influenza‐like illness | 22 | 11 | 18 | 5 | ND | ND | 4 | 9 |
| Influenza A positive specimen | 0 | 0 | 0 | 0 | ND | ND | 0 | 0 |
| Influenza B positive specimen | 0 | 0 | 0 | 0 | ND | ND | 0 | 11 |
| Influenza A and B positive specimen | 0 | 0 | 0 | 0 | ND | ND | 0 | 0 |
ND = not determined.
*Group A (infants aged ≥6 months to <3 years).
**Group B (children aged ≥3 years and <9 years).
The sample was not properly sealed and had leaked into the bag in which it was transported.
Immunogenicity
At baseline, a greater proportion of participants were seropositive (HI titer ≥10) for the A/H3N2 vaccine antigen than for either the A/H1N1 or B antigens (Figure 2). In addition, the proportion of participants at baseline with seropositive titers for vaccine antigens increased as age increased (Figure 2). Of the 34 children whose parents reported a prior year influenza‐like illness (19 in Group A, 15 in Group B), there was no difference in baseline seropositivity to any vaccine antigen compared with those who did not report previous influenza (Table 2).
Figure 2.
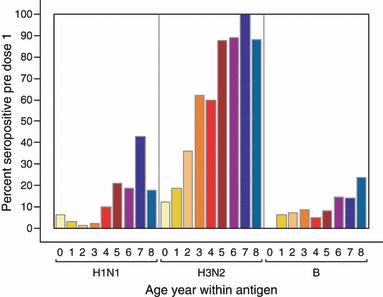
Proportion of participants with seropositive titers (≥1:10) for each influenza antigen at baseline, stratified by age.
Participants seropositive at baseline for A/H1N1 and B were more likely to have a protective antibody response to that antigen after only one vaccine dose than those who were seronegative at baseline. Of those seronegative at baseline against A/H1N1 (92·8% of participants), A/H3N2 (45·2%) and B antigens (92·8%), 97% became sero‐protected after dose 1 against A/H3N2 versus 15% for A/H1N1 and 22% for B. Of those seropositive at baseline, nearly all achieved a protective antibody titer against the A/H1N1 (95%), A/H3N2 (100%) and B antigens (100%) after dose 1. Correspondingly, when the protective antibody response was expressed as GMT and stratified by baseline serostatus, seropositive participants at baseline had high GMTs after dose 1, whereas those who were seronegative at baseline had lower GMTs after dose 1 (Table 5; 2, 3).
Table 5.
Geometric mean hemagglutination inhibiting (HI) antibody response of children 6 months to 8 years receiving influenza vaccine, by baseline serostatus
| Antigen | Baseline serostatus | No. of subjects | Geometric mean HI antibody titers | ||||
|---|---|---|---|---|---|---|---|
| Baseline | After dose 1 | After dose 2 | Before booster vaccine | After booster vaccine | |||
| A/H1N1 | <10 | 261 | 5 (5, 5)* | 15 (13, 16) | 131 (117, 146) | 21 (18, 24) | 251 (222, 284) |
| ≥10 to <40 | 8 | 20 (14, 30) | 141 (26, 777) | 267 (93, 766) | 119 (12, 1197) | 357 (88, 1452) | |
| ≥40 | 18 | 95 (68, 131) | 1054 (755, 1472) | 1083 (836, 1404) | 751 (424, 1330) | 1188 (1074, 1313) | |
| A/H3N2 | <10 | 134 | 5 (5, 6)* | 109 (96, 124) | 575 (509, 650) | 27 (22, 34) | 689 (578, 820) |
| ≥10 to <40 | 10 | 15 (11, 21) | 121 (84, 173) | 454 (279, 741) | 23 (8, 71) | 460 (178, 1191) | |
| ≥40 | 143 | 283 (246, 326) | 1236 (1195, 1279) | 1250 (1220, 1280) | 870 (768, 985) | 1240 (1198, 1283) | |
| B | <10 | 261 | 5 (5, 5)* | 18 (15, 20) | 123 (110, 138) | 6 (6, 7) | 28 (23, 34) |
| ≥10 to <40 | 11 | 26 (22, 30) | 499 (266, 936) | 418 (246, 712) | 8 (5, 11) | 79 (25, 252) | |
| ≥40 | 15 | 65 (49, 87) | 558 (398, 783) | 534 (414, 689) | 6 (4, 7)* | 31 (10, 90) | |
HI, hemagglutination inhibition; CI, 95% confidence interval.
*The limit of quantitation for this HI assay was 10 and values <10 were assigned 5. Thus, there was little or no variation in these results.
Figure 3.
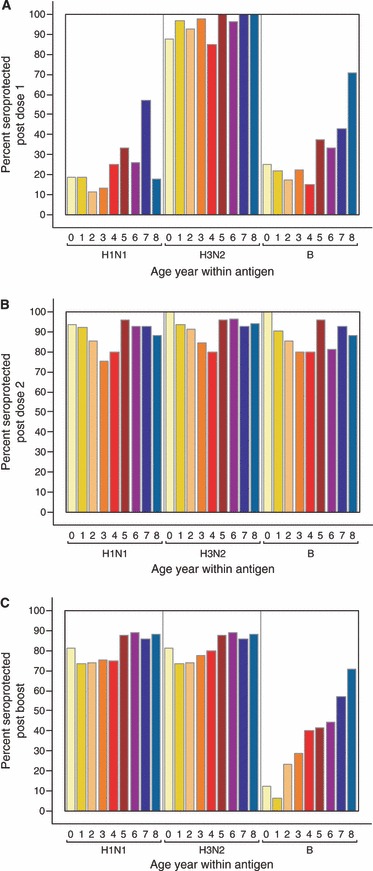
Proportion of participants with seroprotective titers (≥1:40) after dose 1 (A), after dose 2 (B) and after the booster vaccination (C), stratified by age. Note: Different B strains in the 2005 and 2006 vaccines.
The duration of protective antibody response to A/H1N1 and A/H3N2 appeared to be longer in patients with high baseline titers (HI titer ≥40) than in those participants with lower baseline HI titers, as evidenced by the preservation of HI titers after dose 2 and after 1 year (Table 5). As the influenza B strain lineage differed between 2005 and 2006 seasons, the level of protective antibody response to the B strain declined after dose 2 and before the booster vaccination.
When stratified by age, younger children appeared to achieve a similar HI response from influenza vaccination compared with older children (2, 3). As with the duration of protective antibody response, the notable exception to this finding was the seroprotection rate to the B strain antigen (which differed between the 2005 and the 2006 season) after the booster vaccination.
The study vaccine met CPMP criteria in both age groups following dose 1 and dose 2 (6, 7). In both groups, immunological responses following dose 2 were consistently higher than those observed following dose 1.
Table 6.
Summary of serological immunogenicity assay results for Group A (≥6 months to < 3 years)
| Antigen criteria | CPMP requirements | Participants | ||
|---|---|---|---|---|
| Dose 1 (n = 143) | Dose 2 (n = 139) | Booster (n = 61) | ||
| Hemagglutination inhibition assay | ||||
| H1N1: A/New Caledonia/20/99 (IVR‐116) | ||||
| 1. Seroconversion or significant increase (%) | >40 | 16·1 | 95·0 | 93·4 |
| 2. Fold increase in mean GMT | >2·5 | 3·1 | 25·6 | 15·5 |
| 3. Seroprotection (% with HI titers ≥ 40) | >70 | 16·1 | 95·7 | 100·0 |
| H3N2: A/Wellington/1/2004 (IVR‐139) | ||||
| 1. Seroconversion or significant increase (%) | >40 | 86·0 | 90·6 | na |
| 2. Fold increase in mean GMT | >2·5 | 13·7 | 49·6 | na |
| 3. Seroprotection (% with HI titer ≥40) | >70 | 97·9 | 100·0 | na |
| B Strain: B/Jiangsu/10/2003 | ||||
| 1. Seroconversion or significant increase (%) | >40 | 20·3 | 94·2 | na |
| 2. Fold increase in mean GMT | >2·5 | 3·5 | 22·3 | na |
| 3. Seroprotection (% with HI titer ≥40) | >70 | 21·0 | 95·7 | na |
| H3N2: A/New York/55/2004 (NYMC X‐157) | ||||
| 1. Seroconversion or significant increase (%) | >40 | na | na | 88·5 |
| 2. Fold increase in mean GMT | >2·5 | na | na | 20·2 |
| 3. Seroprotection (% with HI titer ≥40) | >70 | na | na | 100·0 |
| B Strain: B/Malaysia/2506/2004 | ||||
| 1. Seroconversion or significant increase (%) | >40 | na | na | 9·8 |
| 2. Fold increase in mean GMT | >2·5 | na | na | 2·3 |
| 3. Seroprotection (% with HI titer ≥40) | >70 | na | na | 11·5 |
| Single radial hemolysis assay | ||||
| B Strain: B/Jiangsu/10/2003 | ||||
| 1. Seroconversion or significant increase (%) | >40 | 44·8 | 97·1 | na |
| 2. Fold increase in AMZAA | >2·5 | 5·4 | 16·8 | na |
| 3. Seroprotection (% with ZAA >25 mm2) | >70 | 44·8 | 97·1 | na |
| B Strain: B/Malaysia/2506/2004 | ||||
| 1. Seroconversion or significant increase (%) | >40 | na | na | 75·4 |
| 2. Fold increase in AMZAA | >2·5 | na | na | 18·5 |
| 3. Seroprotection (% with ZAA >25 mm2) | >70 | na | na | 80·3 |
AMZAA, arithmetic mean zone annulus area; CPMP, Committee for Proprietary Medicinal Products (CPMP/BWP/214/96 Note for Guidance on Harmonisation of Requirements for Influenza Vaccines). GMT, geometric mean titer; HI, hemagglutination inhibition; na, not applicable.
Hemagglutination inhibition assay: seroconversion: % of participants with an antibody titer increase from <10 pre‐vaccination to ≥40 post‐vaccination. Significant increase: % of participants with antibody titer ≥10 pre‐vaccination and at least a fourfold increase post‐vaccination.
Single radial hemolysis assay: seroconversion: % of participants with zone annulus area increase from 4 mm2 pre‐vaccination to >25 mm2 post‐vaccination. Significant increase: % of participants with zone annulus area >4 mm2 pre‐vaccination and at least a 50% increase post‐vaccination.
Table 7.
Summary of serological immunogenicity assay results for Group B (≥3 years to < 9 years)
| Antigen criteria | CPMP requirements | Participants | ||
|---|---|---|---|---|
| Dose 1 (n = 144) | Dose 2 (n = 132) | Booster (n = 174) | ||
| Hemagglutination inhibition assay | ||||
| H1N1: A/New Caledonia/20/99 (IVR‐116) | ||||
| 1. Seroconversion or significant increase (%) | >40 | 24·3 | 93·9 | 72·4 |
| 2. Fold increase in mean GMT | >2·5 | 3·4 | 22·3 | 9·0 |
| 3. Seroprotection (% with HI titer ≥40) | >70 | 25·7 | 95·5 | 98·3 |
| H3N2: A/Wellington/1/2004 (IVR‐139) | ||||
| 1. Seroconversion or significant increase (%) | >40 | 68·1 | 70·5 | na |
| 2. Fold increase in mean GMT | >2·5 | 6·1 | 8·8 | na |
| 3. Seroprotection (% with HI titer ≥40) | >70 | 98·6 | 100 | na |
| B Strain: B/Jiangsu/10/2003 | ||||
| 1. Seroconversion or significant increase (%) | >40 | 32·6 | 93·2 | na |
| 2. Fold increase in mean GMT | >2·5 | 4·3 | 22·2 | na |
| 3. Seroprotection (% with HI titer ≥40) | >70 | 34·0 | 94·7 | na |
| H3N2: A/New York/55/2004 (NYMC X‐157) | ||||
| 1. Seroconversion or significant increase (%) | >40 | na | na | 35·6 |
| 2. Fold increase in mean GMT | >2·5 | na | na | 3·7 |
| 3. Seroprotection (% with HI titer ≥40) | >70 | na | na | 99·4 |
| B Strain: B/Malaysia/2506/2004 | ||||
| 1. Seroconversion or significant increase (%) | >40 | na | na | 43·1 |
| 2. Fold increase in mean GMT | >2·5 | na | na | 6·0 |
| 3. Seroprotection (% with HI titer ≥40) | >70 | na | na | 44·8 |
| Single radial hemolysis assay | ||||
| B Strain: B/Jiangsu/10/2003 | ||||
| 1. Seroconversion or significant increase (%) | >40 | 47·2 | 96·2 | na |
| 2. Fold increase in AMZAA | >2·5 | 6·4 | 15·4 | na |
| 3. Seroprotection (% with ZAA >25 mm2) | >70 | 47·9 | 97·0 | na |
| B Strain: B/Malaysia/2506/2004 | ||||
| 1. Seroconversion or significant increase (%) | >40 | na | na | 68·4 |
| 2. Fold increase in AMZAA | >2·5 | na | na | 16·0 |
| 3. Seroprotection (% with ZAA >25 mm2) | >70 | na | na | 89·7 |
AMZAA, arithmetic mean zone annulus area; CPMP, Committee for Proprietary Medicinal Products (CPMP/BWP/214/96 Note for Guidance on Harmonisation of Requirements for Influenza Vaccines); GMT, geometric mean titer; HI, hemagglutination inhibition; na, not applicable.
Hemagglutination inhibition assay: seroconversion: % of participants with an antibody titer increase from <10 pre‐vaccination to ≥40 post‐vaccination. Significant increase: % of participants with antibody titer ≥10 pre‐vaccination and at least a fourfold increase post‐vaccination.
Single radial hemolysis assay: seroconversion: % of participants with zone annulus area increase from 4 mm2 pre‐vaccination to >25 mm2 post‐vaccination. Significant increase: % of participants with zone annulus area >4 mm2 pre‐vaccination and at least a 50% increase post‐vaccination.
In Group A, the A strains passed the CPMP criteria following the booster dose and the B strain passed the CPMP criteria based on the SRH assay but not the HI assay (Table 6). In Group B, all strains passed the CPMP criteria following the booster dose (Table 7).
Discussion
This study is the first to our knowledge to examine the safety and immunogenicity of the inactivated influenza vaccine, Fluvax® in children. The results demonstrate that it has a favorable safety profile, and is well‐tolerated and immunogenic when administered to children aged between 6 months and <9 years. Further, the vaccine was consistently immunogenic in children under 3 years of age, who are at greatest risk of influenza‐related complications. 5
The ACIP and the American Academy of Pediatrics now recommend immunization against influenza for healthy children aged between 6 months and 18 years. 6 Both in Europe and Australia, influenza vaccination is not yet universally recommended and publically funded for children, although it is under consideration. Our findings provide further evidence of both safety and immunogenicity in the pediatric population consistent with effectiveness of inactivated influenza vaccine. 16
These findings are consistent with the few previous reports in young children with other inactivated influenza vaccines 17 , 18 , 19 , 20 , although differences in sample size, age groups and the methods of reporting make direct comparisons with those reports difficult. Nevertheless, minor degrees of pain and erythema at the injection site, 18 , 20 and fever, 20 appear to be relatively common following influenza vaccination.
At the time of study commencement, Australian national dosing recommendations for influenza vaccination in children under 2 years of age was 0·125 ml. 21 Since 2007, the Australian national dosing recommendation for influenza vaccination in children aged 6 months to 3 years is 0·25 ml. 22 Our study confirms that the higher dose was well‐tolerated and did not lead to an excess of AEs in the younger age group of our study.
In terms of immunogenicity, the seroconversion rates in our study were generally similar to those observed in children in other studies. 17 , 18 , 19 The greater immune response following dose 2 in the primary vaccination phase reinforces the importance of an initial two‐dose schedule for an optimal immune response in children previously unvaccinated. 12 , 23
An unexpected finding in this study was the discrepancy between the HI and SRH assay results for the influenza B strain following the booster dose in Group A. CPMP requirements were met according to the SRH assay result but not according to the HI assay result. The SRH assay has been reported to be more sensitive than the HI assay for the detection of antibodies against influenza B strain. 24 , 25 Therefore, the booster vaccine is considered to have demonstrated adequate immunogenicity for influenza B strain when measured by SRH.
Two distinct lineages of influenza B virus, B/Victoria and B/Yamagata, circulated during the 2005 and 2006 influenza seasons with very little cross‐reactivity between the B strains (Dr. Robert Newman, National Institute of Biological Standards and Control, UK, personal communication). The B/Jiangsu/10/2003 strain (primary vaccine) is in the B/Yamagata lineage while B/Malaysia/2506/2004 strain (booster vaccine) is in the B/Victoria lineage. Therefore, given that the strains were so different, few participants were in effect primed for the booster dose leading to lower seroresponse rates. Optimal protection against influenza B infection particularly in young children may require inclusion of influenza B viruses of both the B/Victoria and B/Yamagata lineages in future inactivated influenza vaccines.
The main scientific limitation of this study was the lack of a placebo control group. Arbitrarily, all first occurrences of solicited local AEs were deemed related to the study vaccine. In terms of immunogenicity, each participant in effect acted as their own control as assessments were based on blood specimens taken before and after vaccination. There was no circulating influenza at the time of vaccination, as indicated by the scarcity of virologically confirmed cases of influenza‐like illness in our study participants and in community‐based surveillance systems. Therefore, we attribute the observed immune responses in this study to vaccination.
In conclusion, this study has shown that the thimerosal‐free inactivated influenza vaccine Fluvax® has a favorable safety profile and is immunogenic in children aged between 6 months and <9 years. The primary vaccine and the booster vaccine were consistently immunogenic, even in children under 3 years of age suggesting that younger children should also benefit from influenza vaccination as much as older children.
Conflict of Interest
Terry Nolan received an honorarium for providing advice on clinical trials of influenza vaccine in the elderly, and travel assistance for presentation of research findings at international scientific meetings. The MCRI received research funding from CSL Ltd to conduct clinical trials of influenza vaccines.
Acknowledgements
The authors would like to acknowledge the participants and their families for their involvement in this study, and study staff from Melbourne (Dr. K. Alexander, L. Baker, M. Boglis, J. Briggs, C. Brophy, Dr. J. Buttery, A. Cheung, Dr. J. Davey, W‐L. Fah, S. Gabriel, D. Gercovich, J. Gibson, E. Hill, Dr. L. Horng, M. Kefford, R. Lawrence, S. Lenko, B. Lim, E. Macken, A. McEvoy, L. McGrath, P. Nathan, Dr. S. O’Dea, K‐A. O’Grady, J. O’Sullivan, Dr. N. Rose, J. Ryrie, C. Sandhu, D. Saunders, K. Scott, B. Sherry, P. Staig, S. Simms, J. Sonego, J. Spotswood, Dr. L. Thorn, E. Urban, S. Walker, M. West, and T. Yap) and Perth (J. Adams, S. Bilic, S. Curtis, J. Kent, J. Macbride, L. Montgomery, S. Nadall, Dr. K. Prosser, Dr. T. Stoney, and M. Trainor). This study was sponsored by CSL Limited (Parkville, Victoria, Australia). In compliance with the Uniform Requirements for Manuscripts, established by the International Committee of Medical Journal Editors, the sponsor of this study did not impose any impediment, directly or indirectly, on the publication of the study’s results. The authors acknowledge the independent medical writing assistance for an early draft provided by ProScribe Medical Communications (http://www.proscribe.com.au), funded from an unrestricted financial grant from CSL Limited. ProScribe’s services complied with international guidelines for Good Publication Practice.
References
- 1. Lambert S, O’ Grady KA, Gabriel S, Carter R, Nolan T. The cost of seasonal respiratory illnesses in Australian children: the dominance of patient and family costs and implications for vaccine use. Commun Dis Intell 2004; 28:510–516. [PubMed] [Google Scholar]
- 2. Lambert SB, O’ Grady KF, Gabriel SH, Nolan TM. Respiratory illness during winter: a cohort study of urban children from temperate Australia. J Paediatr Child Health 2005; 41:125–129. [DOI] [PubMed] [Google Scholar]
- 3. Principi N, Esposito S, Marchisio P, Gasparini R, Crovari P. Socioeconomic impact of influenza on healthy children and their families. Pediatr Infect Dis J 2003; 22(10 Suppl):S207–S210. [DOI] [PubMed] [Google Scholar]
- 4. Izurieta HS, Thompson WW, Kramarz P, Shay DK, Davis RL, DeStefano F et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 2000; 342:232–239. [DOI] [PubMed] [Google Scholar]
- 5. Neuzil KM, Mellen BG, Wright PF, Mitchel EF Jr, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med 2000; 342:225–231. [DOI] [PubMed] [Google Scholar]
- 6. Fiore AE, Shay DK, Broader K, Iskander JK, Uyeki TM, Mootrey G et al. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep 2008; 57 (RR‐7):1–59. [PubMed] [Google Scholar]
- 7. O’Brien MA, Uyeki TM, Shay DK, Thompson WW, Kleinman K, McAdam A et al. Incidence of outpatient visits and hospitalizations related to influenza in infants and young children. Pediatrics 2004; 113(3 Pt 1):585–593. [DOI] [PubMed] [Google Scholar]
- 8. Glezen WP. Influenza control. N Engl J Med 2006; 355:79–81. [DOI] [PubMed] [Google Scholar]
- 9. Dominguez SR, Daum RS. Physician knowledge and perspectives regarding influenza and influenza vaccination. Hum Vaccin. 2005; 1:74–79. [DOI] [PubMed] [Google Scholar]
- 10. Principi N, Esposito S. Pediatric influenza prevention and control. Emerg Infect Dis 2004; 10:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grant VJ, Le Saux N, Plint AC, Correll R, Gaboury I, Ellis E et al. Factors influencing childhood influenza immunization. CMAJ 2003; 168:39–41. [PMC free article] [PubMed] [Google Scholar]
- 12. Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, Strikas RA. Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55(RR‐10):1–42. [PubMed] [Google Scholar]
- 13. Humiston SG, Lerner EB, Hepworth E, Blythe T, Goepp JG. Parent opinions about universal influenza vaccination for infants and toddlers. Arch Pediatr Adolesc Med 2005; 159:108–112. [DOI] [PubMed] [Google Scholar]
- 14. Palmer DF, Dowie WR, Coleman MT, Schild GC. Advanced Laboratory Techniques for Immunological Diagnosis. Immunology Series No. 6, Procedural Guide Part 2, 25–62: Haemagglutination‐Inhibition Test. P.H.S. Atlanta: United States Department of Health, Education and Welfare, 1975. [Google Scholar]
- 15. Schild GC, Pereira MS, Chakraverty P. Single radial haemolysis: a new method for the assay of antibody to influenza haemagglutinin. Bull WHO 1975; 52:43–50. [PMC free article] [PubMed] [Google Scholar]
- 16. Hambidge SJ, Glanz JM, France EK, McClure D, Xu S, Yamasaki K et al. Safety of trivalent inactivated influenza vaccine in children 6 to 23 months old. JAMA 2006; 296:1990–1997. [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez M, Pirez MC, Ward E, Dibarboure H, Garcia A, Picolet H. Safety and immunogenicity of a paediatric presentation of an influenza vaccine. Arch Dis Child 2000; 83:488–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanra G, Marchisio P, Feiterna‐Sperling C, Gaedicke G, Lazar H, Durrer P et al. Comparison of immunogenicity and tolerability of a virosome‐adjuvanted and a split influenza vaccine in children. Pediatr Infect Dis J 2004; 23:300–306. [DOI] [PubMed] [Google Scholar]
- 19. Mitchell DK, Ruben FL, Gravenstein S. Immunogenicity and safety of inactivated influenza virus vaccine in young children in 2003–2004. Pediatr Infect Dis J 2005; 24:925–927. [DOI] [PubMed] [Google Scholar]
- 20. Neuzil KM, Dupont WD, Wright PF, Edwards KM. Efficacy of inactivated and cold‐adapted vaccines against influenza A infection, 1985 to 1990: the pediatric experience. Pediatr Infect Dis J 2001; 20:733–740. [DOI] [PubMed] [Google Scholar]
- 21. National Health and Medical Research Council . The Australian Immunisation Handbook. 8th edn Canberra: National Health and Medical Research Council, 2003. p. 141–142. [Google Scholar]
- 22. National Health and Medical Research Council . The Australian Immunisation Handbook. 9th edn Canberra: National Health and Medical Research Council, 2008. [Google Scholar]
- 23. Ritzwoller DP, Bridges CB, Shetterly S, Yamasaki K, Kolczak M, France EK. Effectiveness of the 2003–2004 influenza vaccine among children 6 months to 8 years of age, with 1 vs 2 doses. Pediatrics 2005; 116:153–159. [DOI] [PubMed] [Google Scholar]
- 24. Chakraverty P. Comparison of haemagglutination‐inhibition and single‐radial‐haemolysis techniques for detection of antibodies to influenza B virus. Arch Virol 1980; 63:285–289. [DOI] [PubMed] [Google Scholar]
- 25. Mancini G, Donatelli I, Arangio‐Ruiz G, Rozera C, Macchia T. Comparison of haemagglutination‐inhibition and single radial haemolysis techniques for detecting antibodies to influenza A and B viruses. J Hyg (Lond) 1983; 91:157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]


