Significance
Disclosure policies, intended to protect consumers, are a popular solution across a range of industries such as health care, financial investments, mortgages, and other services in which professional advisors may suffer from potential bias from misaligned incentives. Using field data (recorded transcripts of surgeon–patient consultations) and a randomized controlled laboratory experiment, we examine and find that disclosures of specialty bias increase patients’ trust and their likelihood of choosing a treatment in accordance with the physicians’ specialty. Professionals and policy makers should be aware of the implications on advisee trust and choice when advocating for the disclosure of advisor bias.
Keywords: disclosure, specialty bias, trust, ethics, policy
Abstract
This paper explores the impact of disclosures of bias on advisees. Disclosure—informing advisees of a potential bias—is a popular solution for managing conflicts of interest. Prior research has focused almost exclusively on disclosures of financial conflicts of interest but little is known about how disclosures of other types of biases could impact advisees. In medicine, for example, physicians often recommend the treatment they specialize in; e.g., surgeons are more likely to recommend surgery than nonsurgeons. In recognition of this bias, some physicians inform patients about their specialty bias when other similarly effective treatment options exist. Using field data (recorded transcripts of surgeon–patient consultations) from Veteran Affairs hospitals and a randomized controlled laboratory experiment, we examine and find that disclosures of specialty bias increase patients’ trust and their likelihood of choosing a treatment in accordance with the physicians’ specialty. Physicians in the field also increased the strength of their recommendation to have the specialty treatment when they disclosed their bias or discussed the opportunity for the patient to seek a consultation with a physician from another specialty. These findings have important implications for handling advisor bias, shared advisor–advisee decision-making, and disclosure policies.
Conflict of interest disclosure policies, intended to protect consumers, are ubiquitous for health care, financial investments, insurance, mortgages, and other services for which the incentives of the professional advisors and advisees are not perfectly aligned (1, 2). For example, physicians are required to disclose if they receive a referral fee for enrolling their patients into clinical trials, and registered investment advisors are required to disclose if they receive a commission in the fund they recommend to their clients. The logic for disclosure is compelling. Disclosure decreases the information gap between advisors and advisees (3) and, theoretically, allows advisees to make more informed decisions (4, 5). Prior research, however, demonstrates that conflict of interest disclosures can make it more difficult for advisees to make decisions (6, 7). Often not knowing how and whether to react to the disclosure, advisees ignore the information (8) or lose trust in their advisor (6, 9, 10), unless the information disclosed is positive; for example, the absence of any conflicts of interest (11–13). Conflict of interest disclosure can also have detrimental effects on advisors, leading them to give more biased recommendations if they disclose their conflicts of interest or if they are aware that their advisees have access to a second opinion (14, 15).
Most of the prior research on disclosure has focused on disclosures of financial conflicts of interest. Although some have discussed the importance of nonfinancial conflicts on advisor behavior and advisee choice (7, 16), little research has empirically examined how disclosures of other types of advisor biases could impact advisees. Conflict of interest disclosures often lead to a decrease in trust from advisees (6, 7), regardless of whether the disclosure is portrayed as mandatory or voluntary (7). However, some biases are unavoidable and are not created by accepting a financial conflict of interest. Acknowledging bias in those types of situations may not be viewed negatively by advisees but could instead signal that the advisor is knowledgeable or competent enough to recognize their own bias. Thus, self-disclosure of a professional bias could lead advisees to perceive their advisors as more trustworthy or competent.
One important area of professional bias, and indeed an area in which decisions have large consequences, is in medicine. Physicians overwhelmingly recommend treatments that they themselves are trained to deliver. For localized prostate cancer, for example, surgeons are more likely to recommend surgery than nonsurgeons, and radiation oncologists are more likely to recommend radiation therapy than nonradiation oncologists (17, 18). Treatment for localized prostate cancer provides an apt context to test our hypotheses for several reasons. First, the incidence of prostate cancer is high (>200,000 new cases in the United States per year) (19), and thus treatment choice for this disease is an important and common problem. Second, there is no optimal treatment; the main treatments—surgery, radiation, and active surveillance—have similar survival rates (20). Third, both surgeons and radiation oncologists are more likely to believe in the efficacy of the treatment they perform despite both specialists citing similar estimates of the treatment-specific complications due to surgery and radiation (18). In a survey, 79% of male surgeons said they would choose surgery if they were diagnosed with clinically localized prostate cancer, whereas 92% of radiation oncologists said they would choose radiation therapy (21), demonstrating the existence of specialty bias. Fourth, surgeons perform biopsies for patients who are suspected of having prostate cancer and thus tend to be the first physician to discuss the diagnosis with the patient and offer treatment advice. Some surgeons spontaneously disclose their specialty bias, most likely due to their awareness of alternative and similarly effective treatment options in an attempt to reduce the influence of their biased recommendations on the patient’s choice.
In both an observational study using transcripts from recorded physician–patient interactions and in a randomized-controlled experiment conducted to test causality, we examined how surgeon admission of specialty bias influenced patient trust and likelihood to undergo surgery. By definition, specialists know more about treatments that they themselves provide; therefore, the presence of bias may be unavoidable. We therefore hypothesized that patients would consequently perceive their physicians as more trustworthy and competent due to the disclosure of a specialty bias, and disclosure would increase the likelihood of choosing a treatment in accordance with the physician’s specialty.
Our observational study data consisted of 219 transcripts (66% of eligible patients) of recorded surgeon–patient interactions in which the patients received a diagnosis of localized prostate cancer and, through chart review, had a prostate-specific antigen (PSA) level of less than 20 ng/mL and a Gleason score of 6 or 7. All of the patients were recruited from four Veterans Affairs hospitals and completed a survey before their consultation with the surgeon to capture, among other things, their treatment preferences, preferences for shared decision-making with the doctor, and demographic information.
Two research assistants independently coded the transcripts of the recordings for the presence (coded = 1) or absence (coded = 0) of a bias statement. Some statements of bias were explicit in that the word “bias” was present (n = 28), e.g., “I’m a surgeon, so I’m biased towards recommending surgery” and some statements were implicit and alluded to bias without mentioning the word bias (n = 7), e.g., “I’m a surgeon so of course I’d lean towards surgery” (intercoder reliability = 96%). The final treatment decision was determined from medical records reviewed 6 months following diagnosis (surgery = 1, other treatment = 0).
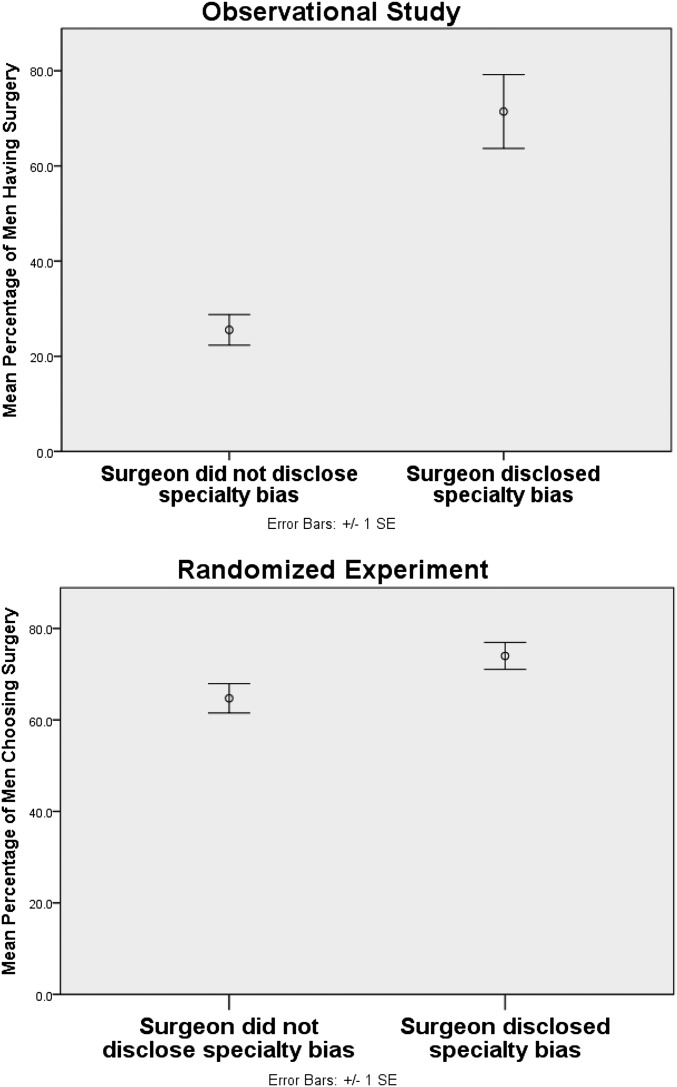
Thirty-five of 219 patients (16.0%) heard their surgeon admit to a specialty bias during their consultation. There was no difference in preference for surgery before the surgical consultation between the patients who would later hear their surgeon disclose their specialty bias (17.1%, n = 6/35) and those who would not [12.5%, n = 23/184; χ2(1, N = 219) = 0.55; P = 0.46]. After the consultation, however, patients who heard their surgeon disclose their bias were more likely to receive surgery (71.4%, n = 25/35) than those who did not hear their surgeon disclose their bias [25.5%, n = 47/184; χ2(1, N = 219) = 28.06; P < 0.001; relative risk (RR) = 2.81; 95% CI (2.06, 3.83); P < 0.001; Fig. 1 and Table 1, model 1; SI Appendix, Table S1a gives the breakdown by all treatments, as well as other patient characteristics].
Fig. 1.
Percentage of men choosing surgery in the observational setting (Upper) and randomized experiment (Lower).
Table 1.
Regression results of the likelihood to have surgery, observational study
| Predictor | Relative risk 95% CI [lower bound, upper bound] | ||
| Model 1 | Model 2 | Model 3 | |
| Presence of bias statement | 2.81*** [2.06, 3.83] | 2.07*** [1.47, 2.92] | 2.04*** [1.31, 3.15] |
| Age | 0.95*** [0.92, 0.98] | 0.97 [0.94, 1.01] | |
| Race: white | 1.37 [0.79, 2.40] | 1.12 [0.70, 1.79] | |
| Education | 0.91 [0.80, 1.04] | 0.90 [0.81, 1.00] | |
| Decision aid | 0.76 [0.47, 1.22] | 0.95 [0.49 1.85] | |
| Clinical stage T3 | 3.20*** [1.83, 5.59] | 2.69** [1.41, 5.14] | |
| Clinical stage T2 | 1.72** [1.18, 2.53] | 1.37 [0.91, 2.04] | |
| Shared decision-making | 0.82 [0.49, 1.36] | 0.92 [0.58, 1.45] | |
| Surgery preferred treatment choice before diagnosis | 1.10 [0.74, 1.63] | 1.17 [0.82, 1.68] | |
| Strength of recommendation to have surgery | 1.45* [1.08, 1.94] | ||
| Strength of recommendation to have radiation | 1.21 [0.66, 2.21] | ||
| Strength of recommendation to have active surveillance | 1.15 [0.91, 1.46] | ||
| Radiation oncologist discussed | 2.00* [1.10, 3.63] | ||
| Constant | −1.37*** | 2.76* | 0.55 |
| Corrected quasi-likelihood under independence model criterion QICC (goodness of fit) | 149.11 | 104.68 | 96.44 |
Models are clustered by doctor and have robust SEs.
P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
We found further support for our prediction when potential patient-specific confounding variables (patient demographics, clinical stage of disease, patients’ preference for shared decision-making with their physician, treatment preference before diagnosis, and decision aid received by the patient before the consultation) were added to the regression (Table 1, model 2). The surgeon’s disclosure of bias was still positively and significantly related to the patient choosing surgery [RR = 2.07; 95% CI (1.47, 2.92); P < 0.001].
The final model added controls from coding other elements of the physician–patient conversation (SI Appendix, Table S3). These controls for the strength of surgeons’ treatment recommendations for surgery, radiation, and active surveillance and whether the surgeon discussed a radiation oncologist appointment (Table 1, model 3) did not alter the significant positive relationship between bias disclosure and having surgery [RR = 2.04; 95% CI (1.31, 3.15); P = 0.001]. Further, in line with prior laboratory research using stylized experiments (14, 15), both bias disclosure and awareness that the patient might consult with a radiation oncologist were significantly and positively correlated with an increase in the strength of the physicians’ recommendation for surgery (both P < 0.01; SI Appendix, Table S2).
In sum, the observational data revealed robust results that bias disclosure was significantly positively correlated with having surgery. The results are also robust when examining just explicit disclosures of bias (SI Appendix, Table S4).
Having found strong evidence that surgeons’ specialty bias disclosure correlated with patients having surgery, the next step was to examine, via a randomized-controlled experiment, if a causal link existed and, if so, what drove patients who heard a bias disclosure to choose surgery. Our laboratory experiment consisted of 447 men who viewed video clips of a professional actor portraying a surgeon and who were randomized to a disclosure and nondisclosure (control) group. The men were similar in age and race to the patients in our observational study (SI Appendix, Table S1), and the actor’s statements in the video were representative of the statements made by the surgeons in our observational study. The surgeon first explained to the patient that the biopsy revealed localized prostate cancer that was slow-growing, and the patient could take his time in deciding his treatment. The surgeon went on to describe two treatment options to the patient: surgery and radiation. In the disclosure group, an extra phrase was added after the surgery option was described: “So that’s where my bias lies…Remember, I’m a surgeon so I know more about surgery than radiation.” This phrase was taken verbatim from one of the transcripts from our observational study. The control group had the same video and script, excluding this phrase.
When asked to make a decision between surgery and radiation, men were more likely to choose surgery in the disclosure group (74.0%) than the control (64.7%) [χ2(1, N = 447) = 4.51; P = 0.03; Fig. 1]. There was no significant difference between the two groups (50.7% vs. 53.1%) as to whether men indicated they would seek a consultation with a radiation oncologist before making their treatment decision [χ2(2, N = 447) = 2.32; P = 0.31].
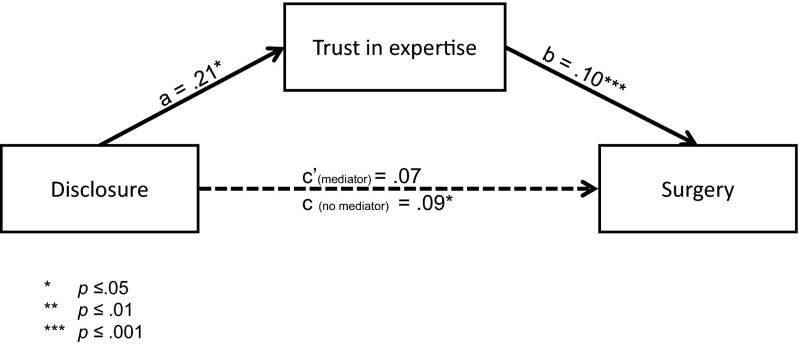
Men in the disclosure group acknowledged the bias disclosure by noting that their surgeons were significantly more likely to be biased toward recommending surgery [M = 65.73% on a 0–100% sliding scale; 95% CI (62.04, 69.41)] than in the control [M = 39.84; 95% CI (36.17, 43.51); F(1,434) = 95.80; P < 0.001; η2 = 0.18]. Despite this acknowledgment of bias, men in the disclosure group reported higher trust in the doctor’s expertise [M = 5.65 on a seven-point Likert scale; 95% CI (5.50, 5.79)] than in the nondisclosure group [M = 5.44; 95% CI (5.29, 5.59); F(1,434) = 3.7; P = 0.05; η2 = 0.009]. Trust in the doctor’s expertise also significantly explained (mediated) the relationship between surgeons’ self-disclosure of specialty bias and patients’ decisions to choose surgery (Fig. 2).
Fig. 2.
Mediation model of trust in expertise. Nonstandardized regression coefficients are shown: a refers to effect of the independent variable (IV; disclosure of bias) on the mediator (trust in expertise), b refers to the effect of the mediator on the dependent variable (DV; choosing surgery) when controlling for IV, c refers to the effect of the IV on the DV, and c′ refers to the effect of the IV on the DV when controlling for the mediator. Using Hayes PROCESS Model 4 for mediation with 5,000 bootstrap samples (29, 30), the 95% bias-corrected CIs for the size of the indirect effect for trust in the surgeon’s expertise (0.10) excluded zero [0.01, 0.22], providing evidence that the patients’ trust in the surgeon’s expertise explained (mediated) the relationship between the surgeons’ self-disclosure of a specialty bias and the patient’s decision to choose surgery.
Specialty bias is unavoidable. Physicians generally know more about the treatments they provide than about alternative treatments provided by other specialists. With both observational data and a randomized-controlled experiment, however, we find consistent evidence that, when surgeons disclose their specialty bias, patients are more likely to choose surgical treatments. Our experiment also demonstrated increased (rather than decreased) trust with disclosure of a potential bias even though men acknowledged that the surgeon was more likely to be biased toward recommending surgery.
Specialty bias does not necessarily beget poor quality or manipulative advice. Disclosure of bias can be independent of the quality of advice given. Although it would be damaging if people become untrusting of quality expert advice (22), it is often quite difficult to assess whether advice is biased or not, even in the presence of a conflict of interest (23, 24).
We do not believe that the surgeons in our observational study purposely disclosed their bias to effectively persuade their patients to follow their advice. Instead, we expect the surgeons were making earnest efforts to better inform patients of facts relevant to their decision. In theory, informing patients that specialists will be biased toward recommending the treatments they can deliver should lead patients to discount the specialists’ recommendations (3, 25). In practice, however, patients who are informed by their physicians of potential specialty bias are more likely to take that specialist’s treatment. Our experimental results also suggest that patients interpret such disclosures as a signal of the surgeon’s expertise or competence rather than markers of other types of trustworthiness, e.g., benevolence or integrity. Together both studies provide strong evidence of how bias disclosure influences advisee attitudes and decisions.
These findings have important implications for how we handle potential advisor bias. In all our models and with our experiment, we find a significant positive relationship between surgeons disclosing bias and patients choosing surgery. This finding is an empirical fact that physicians may want to consider when disclosing bias to patients. Based on these findings, and prior research on disclosure, it may be preferable for advisors to refrain from disclosing their bias directly to advisees (6, 7). Alternatively, patient educational materials and decision aids, designed to help patients understand their treatment alternatives, could alert patients to the existence of specialty bias. Disclosures given by third parties have been shown to reduce pressure to comply with an advisor’s recommendation (6, 7).
These findings raise additional questions. Would mandatory consultations with a radiation oncologist reduce the reliance patients have on their surgeons? Our observational study revealed that discussing a radiation oncologist appointment was correlated with stronger recommendations to have surgery. Prior experimental research has also shown that mandatory second opinions may result in greater bias from primary advisors (15), and seeing a radiation oncologist as a “second opinion” may introduce a delay that patients may find uncomfortable after a cancer diagnosis. Another option is a multidisciplinary treatment consultation where patients see the various relevant specialists at the same time, sometimes with a medical oncologist who serves as a “referee.”
Our study highlights that the current practice of surgeons spontaneously disclosing their specialty bias to patients is not only ineffective but is likely to backfire. To our knowledge, this study is the first to examine disclosure of bias on advisee choice in a real-world health care setting. We also demonstrate other consequences of disclosure on advisors that have previously only been revealed in stylized experiments: Indeed, both bias disclosure and awareness that the patient might seek a second opinion was significantly and positively correlated with an increase in the strength of the physicians’ recommendation (SI Appendix, Table S2) (9, 15). Bias disclosure can have a profound influence on advisor recommendations and advisee choice; thus, professional advisors and policy-makers should implement such disclosures with care.
Materials and Methods
Observational Study.
Participants (SI Appendix, Table S1a).
Our observational study data consisted of 219 transcripts of recorded surgeon-patient interactions from four Veterans Affairs hospitals (Ann Arbor, MI; Durham, NC; Pittsburgh, PA; and San Francisco, CA) in which the patients received a diagnosis of localized prostate cancer. All patients had PSA levels of <20 ng/mL and a Gleason score of 6 or 7. This definition corresponded to American Urological Association guidelines in which patients could consider all treatment options (surgery, radiation, and active surveillance) to be viable alternatives (26). Participants were recruited when a biopsy was scheduled or performed from the four hospitals between September 2008 and May 2012 and gave written consent. Of those diagnosed with localized prostate cancer (n = 334), 77% had their conversations with their surgeon audio recorded (n = 258). All 285 patients agreed to have their consultation with the surgeon audio recorded. Our final sample of 219 patients were those who had their recordings transcribed and in which we had access to medical records 6 months later to check the final treatment decision (66% of eligible patients). The study was approved by the institutional review board (IRB) at all participating sites: Veteran Affairs Ann Arbor Heath Care System, Durham Veteran Affairs Medical Center, San Francisco Veteran Affairs Health Care System, and Veteran Affairs Pittsburgh Healthcare System.
Procedure.
The survey before the surgeon gave the patient the diagnosis of localized prostate cancer consisted of several questions including the patients’ desire to participate in shared decision making and their current treatment preference if they were to be diagnosed with prostate cancer, as well as demographics.
Shared decision-making with the doctor was measured on a five-point scale, an adaptation of Degner and Sloan's (1992) control preference scale (27): 1 = My doctor(s) will make the decision with little input from me; 2 = My doctor(s) will make the decision but will seriously consider my opinion; 3 = My doctor(s) and I will make the decision together; 4 = I will make the decision after seriously considering my doctor(s) opinion and 5 = I will make the decision with little input from my doctor(s). Low scores, therefore, reflect patients’ preference for their physicians to make treatment decisions.
Treatment preference was measured with the following question: “Although you may not have cancer, we would like to know what treatment you think you might have if you were to have prostate cancer.” Patients answered from a treatment list consisting of surgery, external beam radiation, brachytherapy, watchful waiting/active surveillance, and other (e.g., hormone or experimental therapies).
All patients agreed to have their consultation with the surgeon audio recorded. Everyone in the examination room during the appointment provided consent to be audio recorded; this included physicians and any significant others accompanying the patient. A research associate set up an unobtrusive audio recorder in the examination room before the consultation and then left the room before the patient entered.
Statistical analysis.
We conducted generalized estimating equations with a Poisson distribution and log link function with robust SEs and exchangeable correlation structure to account for our clustered data (multiple patients seeing the same doctor) and to report the RR of having surgery when patients heard a bias statement from their surgeon (28). This analysis accounts for nonindependent observations and thus also accounts for surgeon individual differences (e.g., persuasiveness). Our first model examined the relationship between having surgery and the presence of bias statements. Our second regression model examined if the relationship between choosing surgery and the presence of bias statements held when controlling for patient-specific variables (e.g., age, race, education, and clinical stage of disease) that might contribute to changing the likelihood of choosing surgery. Our third regression model included variables from the surgeon–patient interaction (e.g., strength of treatment recommendations). The control variables are described in more detail in SI Appendix, Materials and Methods.
Randomized Experiment.
Participants (SI Appendix, Table S1b).
Our experiment consisted of 447 US male citizens above the age of 50 y recruited from GMI Lightspeed, a survey company, to participate in the 10-min study. The study was approved by Georgetown University and Duke University IRBs. To detect a 10% difference in treatment choice with a power of 80% required a sample of ∼440 participants.
Procedure.
Men were asked to imagine that they were meeting with a surgeon to learn the results of a recent prostate biopsy. They viewed video clips of a professional actor portraying an urologist. The urologist’s statements were representative of statements made by the urologists in our observational study. Men gave consent to participate and clicked a link that randomly assigned them to one of two groups: those who heard their surgeon self-disclose their specialty bias in the video (disclosure group) and those who did not (control/nondisclosure group).
After watching the video, the men responded to various questions (see SI Appendix, Fig. S1 and SI Appendix, Materials and Methods for more details).
Supplementary Material
Acknowledgments
The observational study was funded by an Investigator-Initiated Research (IIR) Award Merit Award from the US Department of Veterans Affairs (IIR 05-283) (to A.F.). The experiment was funded by Georgetown University departmental funds (to S.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1604908113/-/DCSupplemental.
References
- 1.Moore DA, Tetlock PE, Tanlu L, Bazerman MH. Conflicts of interest and the case of auditor independence: Moral seduction and strategic issue cycling. Acad Manage Rev. 2006;31(1):10–29. [Google Scholar]
- 2.Fung A, Graham M, Weil D. Full Disclosure: The Perils and Promise of Transparency. Cambridge Univ Press; New York: 2007. [Google Scholar]
- 3.Healy PM, Palepu KG. Information asymmetry, corporate disclosure, and the capital markets: A review of the empirical disclosure literature. J Account Econ. 2001;31(1–3):405–440. [Google Scholar]
- 4.Barry MJ, Edgman-Levitan S. Shared decision making: Pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780–781. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 5.Beauchamp TL, Childress JF. Principles of Biomedical Ethics. Oxford Univ Press; Oxford, UK: 2001. [Google Scholar]
- 6.Sah S, Loewenstein G, Cain DM. The burden of disclosure: Increased compliance with distrusted advice. J Pers Soc Psychol. 2013;104(2):289–304. doi: 10.1037/a0030527. [DOI] [PubMed] [Google Scholar]
- 7.Sah S, Loewenstein G, Cain DM. 2014. Insinuation anxiety: Fear of signaling distrust after conflict of interest disclosures. Available at m.law.uchicago.edu/files/files/sah-ssrn-id1970961.pdf. Accessed March 15, 2016.
- 8.Hampson LA, et al. Patients’ views on financial conflicts of interest in cancer research trials. N Engl J Med. 2006;355(22):2330–2337. doi: 10.1056/NEJMsa064160. [DOI] [PubMed] [Google Scholar]
- 9.Loewenstein G, Cain DM, Sah S. The limits of transparency: Pitfalls and potential of disclosing conflicts of interest. Am Econ Rev. 2011;101(3):423–428. [Google Scholar]
- 10.Licurse A, Barber E, Joffe S, Gross C. The impact of disclosing financial ties in research and clinical care: A systematic review. Arch Intern Med. 2010;170(8):675–682. doi: 10.1001/archinternmed.2010.39. [DOI] [PubMed] [Google Scholar]
- 11.Pearson SD, Kleinman K, Rusinak D, Levinson W. A trial of disclosing physicians’ financial incentives to patients. Arch Intern Med. 2006;166(6):623–628. doi: 10.1001/archinte.166.6.623. [DOI] [PubMed] [Google Scholar]
- 12.Sah S, Loewenstein G. Nothing to declare: Mandatory and voluntary disclosure leads advisors to avoid conflicts of interest. Psychol Sci. 2014;25(2):575–584. doi: 10.1177/0956797613511824. [DOI] [PubMed] [Google Scholar]
- 13.Hall MA, Dugan E, Balkrishnan R, Bradley D. How disclosing HMO physician incentives affects trust. Health Aff (Millwood) 2002;21(2):197–206. doi: 10.1377/hlthaff.21.2.197. [DOI] [PubMed] [Google Scholar]
- 14.Cain DM, Loewenstein G, Moore DA. The dirt on coming clean: Perverse effects of disclosing conflicts of interest. J Legal Stud. 2005;34(1):1–25. [Google Scholar]
- 15.Sah S, Loewenstein G. Conflicted advice and second opinions: Benefits, but unintended consequences. Organ Behav Hum Decis Process. 2015;130:89–107. [Google Scholar]
- 16.Shaw DM. A piece of my mind. Beyond conflicts of interest: Disclosing medical biases. JAMA. 2014;312(7):697–698. doi: 10.1001/jama.2014.8035. [DOI] [PubMed] [Google Scholar]
- 17.Zeliadt SB, et al. Why do men choose one treatment over another?: A review of patient decision making for localized prostate cancer. Cancer. 2006;106(9):1865–1874. doi: 10.1002/cncr.21822. [DOI] [PubMed] [Google Scholar]
- 18.Fowler FJ, Jr, et al. Comparison of recommendations by urologists and radiation oncologists for treatment of clinically localized prostate cancer. JAMA. 2000;283(24):3217–3222. doi: 10.1001/jama.283.24.3217. [DOI] [PubMed] [Google Scholar]
- 19.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 20.Sidana A, et al. Treatment decision-making for localized prostate cancer: What younger men choose and why. Prostate. 2012;72(1):58–64. doi: 10.1002/pros.21406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore MJ, O’Sullivan B, Tannock IF. How expert physicians would wish to be treated if they had genitourinary cancer. J Clin Oncol. 1988;6(11):1736–1745. doi: 10.1200/JCO.1988.6.11.1736. [DOI] [PubMed] [Google Scholar]
- 22.Kesselheim AS, et al. A randomized study of how physicians interpret research funding disclosures. N Engl J Med. 2012;367(12):1119–1127. doi: 10.1056/NEJMsa1202397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sah S, Loewenstein G. Effect of reminders of personal sacrifice and suggested rationalizations on residents’ self-reported willingness to accept gifts: A randomized trial. JAMA. 2010;304(11):1204–1211. doi: 10.1001/jama.2010.1310. [DOI] [PubMed] [Google Scholar]
- 24.Lo B, Ott C. What is the enemy in CME, conflicts of interest or bias? JAMA. 2013;310(10):1019–1020. doi: 10.1001/jama.2013.221227. [DOI] [PubMed] [Google Scholar]
- 25.Crawford VP, Sobel J. Strategic information transmission. Econom J Econom Soc. 1982;50(6):1431–1451. [Google Scholar]
- 26.Thompson I, et al. 2007. Guideline for the management of clinically localized prostate cancer: 2007 update. Available at https://www.auanet.org/common/pdf/education/clinical-guidance/Prostate-Cancer.pdf. Accessed March 15, 2016.
- 27.Degner LF, Sloan JA. Decision making during serious illness: What role do patients really want to play? J Clin Epidemiol. 1992;45(9):941–950. doi: 10.1016/0895-4356(92)90110-9. [DOI] [PubMed] [Google Scholar]
- 28.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 29.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 30.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Guilford Press; New York: 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.