Significance
CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) and ELONGATED HYPOCOTYL 5 (HY5) are two central regulators of photomorphogenesis. COP1 destabilizes HY5 to repress photomorphogenesis in darkness. On light illumination, accumulated HY5 regulates the expression of a large number of genes to optimize photomorphogenesis. Thus, COP1-HY5 defines a regulatory hub for light control of seedling development. In this work, our data indicate that BBX21 is a key component involved in the COP1-HY5 regulatory hub. BBX21 is controlled by COP1 via its E3 ubiquitin ligase activity in darkness. In the light, BBX21 promotes photomorphogenesis by binding to the promoter of HY5 and activating HY5 expression.
Keywords: Arabidopsis, photomorphogenesis, BBX21, COP1, HY5
Abstract
BBX21 (also known as SALT TOLERANCE HOMOLOG 2), a B-box (BBX)-containing protein, has been previously identified as a positive regulator of light signaling; however, the precise role of BBX21 in regulating seedling photomorphogenesis remains largely unclear. In this study, we report that CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) interacts with BBX21 in vivo and is able to ubiquitinate BBX21 in vitro. Thus, BBX21 is targeted for 26S proteasome-mediated degradation in dark-grown Arabidopsis seedlings in a COP1-dependent manner. Moreover, we show that BBX21 binds to the T/G-box in the ELONGATED HYPOCOTYL 5 (HY5) promoter and directly activates HY5 expression in the light. Transgenic seedlings overexpressing BBX21 exhibit dramatically shortened hypocotyls in the light, and this phenotype is dependent on a functional HY5. Taken together, our data suggest a molecular base underlying BBX21-mediated seedling photomorphogenesis, indicating that BBX21 is a pivotal component involved in the COP1-HY5 regulatory hub.
Plant seedlings undergo two distinct developmental processes dependent on the presence and absence of light, termed photomorphogenesis and skotomorphogenesis, respectively (1). CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) is a central repressor of seedling photomorphogenesis, and mutants impaired in COP1 display constitutively photomorphogenic phenotypes in darkness (2, 3). COP1 acts as an E3 ubiquitin ligase targeting a subset of substrates for degradation in darkness, including ELONGATED HYPOCOTYL 5 (HY5), HY5 HOMOLOG (HYH), LONG AFTER FAR-RED LIGHT 1 (LAF1), LONG HYPOCOTYL IN FAR RED (HFR1), B-BOX PROTEIN 22/SALT TOLERANCE HOMOLOG 3 (BBX22/STH3), and PHYTOCHROME INTERACTING FACTOR 3-LIKE1 (PIL1) (4–11). Of these, HY5 is considered a key signal integration point from dark to light transition (10, 12, 13). Its abundance is directly correlated with the extent of seedling photomorphogenic growth, but inversely correlated with the nuclear abundance of COP1 (4, 10, 14). As a b-ZIP type transcription factor, HY5 specifically interacts with the ACGT-containing elements (ACEs) and might directly bind to approximately one-third of the Arabidopsis genes (12, 13). Thus, HY5 ensures proper expression of a large number of downstream regulatory genes, which in turn eventually promotes photomorphogenesis in the light. Recent studies have shown that HY5 expression is regulated by HY5 itself, as well as by HYH and CALMODULIN7 (CAM7) (15, 16).
A total of 32 B-box (BBX)-containing proteins have been identified in the Arabidopsis genome (17), among which a number of BBX proteins have been shown to be involved in COP1- and HY5-mediated seedling photomorphogenesis (18). BBX4, BBX20, BBX22, BBX24, and BBX25 genetically and physically interact with COP1 and undergo COP1-mediated degradation in the dark (6, 19–24). In addition, BBX22, BBX24, and BBX25 physically interact with HY5, and, interestingly, BBX22 acts as a coactivator of HY5 action (6), whereas BBX24 and BBX25 repress the transcriptional activating activity of HY5 (22, 23). BBX21/SALT TOLERANCE HOMOLOG 2 (STH2) positively regulates anthocyanin accumulation and the inhibition of seedling hypocotyl elongation in response to light, both independently and together with HY5. Mutations in BBX21 can partially suppress the constitutively photomorphogenic phenotypes of cop1 in the dark (25), and BBX21 acts downstream of COP1 to mediate the shade avoidance response (26).
In this study, we explored the molecular roles of BBX21 in COP1- and HY5-mediated seedling photomorphogenesis. We found that BBX21 is ubiquitinated by COP1 in vitro, and that BBX21 abundance is controlled in a COP1-dependent manner in darkness in vivo. In addition, BBX21 directly binds to the T/G-box present in the HY5 promoter. Accordingly, the expression of HY5 is decreased in bbx21 but increased in transgenic plants overexpressing BBX21. Collectively, our data demonstrate that BBX21 is targeted by COP1 for 26S proteasome-mediated degradation in darkness, and that BBX21 promotes photomorphogenic development in the light by activating HY5 expression.
Results
Expression Pattern of BBX21 in Response to Light.
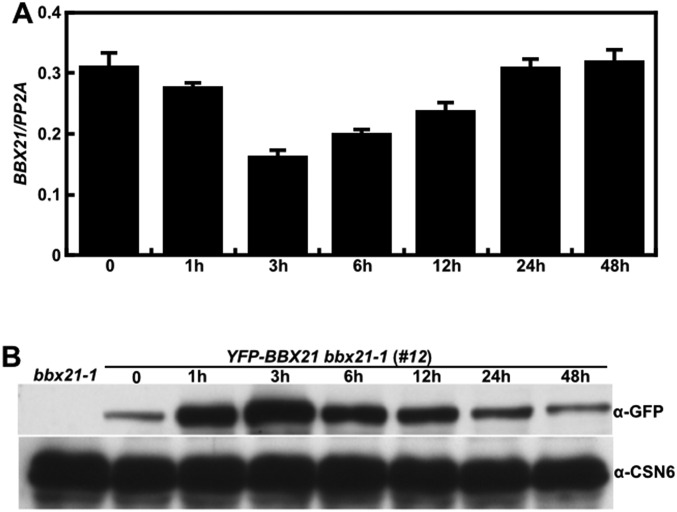
BBX21 acts as a positive regulator of light signaling (25). To further elucidate the mechanism of action of BBX21, we sought to examine the expression pattern of BBX21 during the transition from dark to light exposure. At the transcript level, BBX21 expression was slightly decreased on 1 h of light exposure, reached its lowest level after 3 h of light exposure, and then gradually increased after 6, 12, 24, and 48 h of light exposure (Fig. S1A).
Fig. S1.
Expression pattern of BBX21 during dark-to-light transition. (A) Real-time qPCR analyses of BBX21 transcript levels in Col grown in darkness for 4 d, then transferred to white light (112.3 μmol/m2/s) for 1–48 h. (B) Immunoblot analysis of YFP-BBX21 levels in YFP-BBX21 bbx21-1 (#12) overexpression seedlings grown in the dark for 4 d, then transferred to white light (112.3 μmol/m2/s) for 1–48 h. bbx21-1 served as a negative control, and anti-CSN6 served as a loading control.
We next analyzed the dynamic changes of BBX21 in YFP-BBX21 bbx21-1 (#12) (Fig. S2) seedlings grown in darkness for 4 d and then exposed to white light for 1–48 h. Interestingly, the BBX21 protein level was significantly increased after 1 h of light exposure and peaked after 3 h of light exposure, then gradually attenuated on prolonged light exposure (6, 12, and 24 h). After 48 h of light exposure, the BBX21 level declined to a level comparable to that seen in dark-grown seedlings (Fig. S1B). These findings indicate that light tightly controls BBX21 in a time-dependent manner at both the transcript and protein levels.
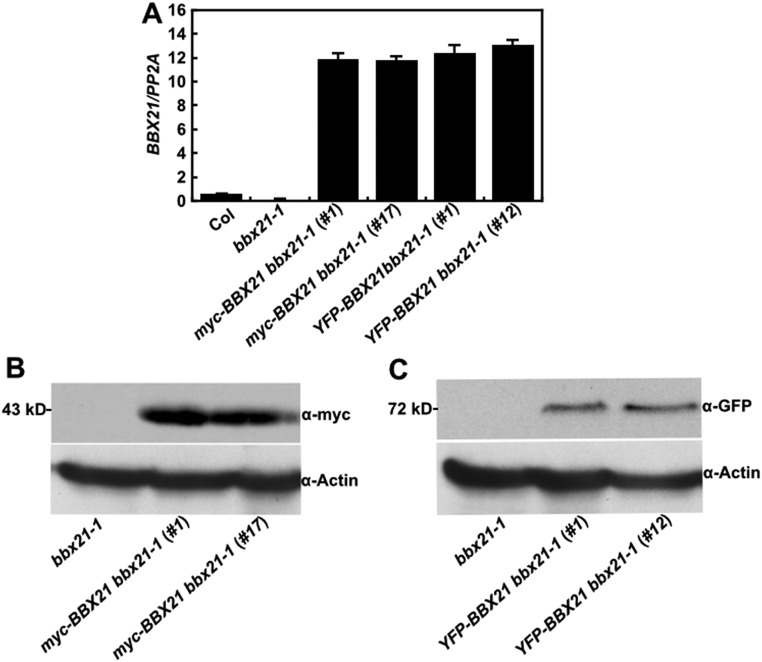
Fig. S2.
Transcript and protein levels of BBX21 in BBX21 overexpression seedlings. (A) Real-time qPCR analyses of BBX21 transcript levels in Col, bbx21-1, and BBX21 overexpression seedlings grown under white light for 4 d. (B) Immunoblot analysis of myc-BBX21 levels in myc-BBX21 bbx21-1 overexpression seedlings grown in the dark for 4 d. The molecular weight of myc-BBX21 is ∼43 kDa. (C) Immunoblot analysis of YFP-BBX21 levels in YFP-BBX21 bbx21-1 overexpression seedlings grown in the dark for 4 d. The molecular weight of YFP-BBX21 is ∼72 kDa. In B and C, bbx21-1 served as a negative control, and anti-actin served as a loading control.
BBX21 Interacts with COP1 and Is Ubiquitinated by COP1.
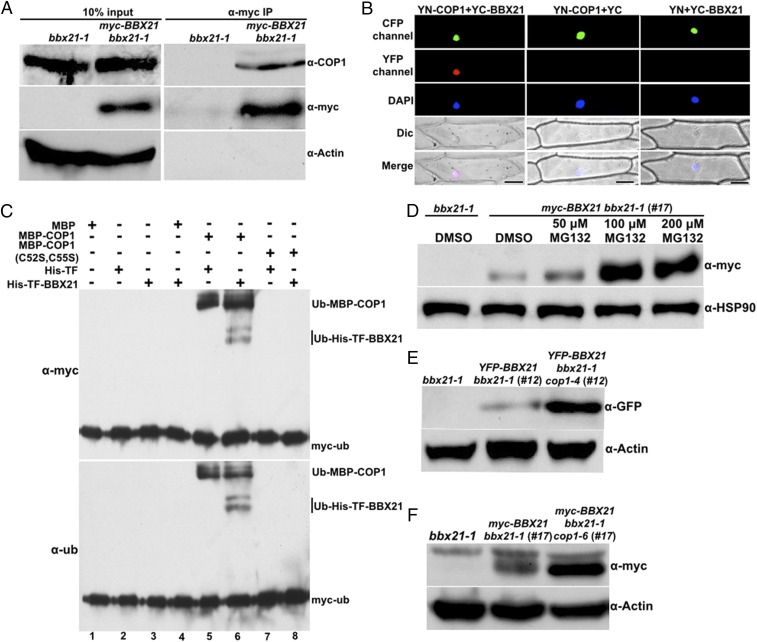
It was previously shown that BBX21 is recruited to the nuclear speckles by COP1 (25). Therefore, we asked whether BBX21 physically interacts with COP1 in Arabidopsis. To this end, we performed coimmunoprecipitation (co-IP) assays using homozygous myc-BBX21 bbx21-1 (#17) transgenic plants (Fig. S2). As shown in Fig. 1A, immunoprecipitation of myc-BBX21 pulled down COP1 in myc-BBX21 bbx21-1 transgenic seedlings, but not in bbx21-1 seedlings, demonstrating that BBX21 associates with COP1 in vivo.
Fig. 1.
BBX21 is controlled by COP1 in dark-grown seedlings. (A) Co-IP analysis showing that BBX21 interacts with COP1 in vivo. Four-day-old white light-grown bbx21-1 and myc-BBX21 bbx21-1 (#17) seedlings were transferred to darkness for 16 h and then subjected to a co-IP assay using anti-myc antibodies, with the immunoprecipitates detected using anti-COP1 and anti-myc antibodies, respectively. Actin served as a negative control. (B) BiFC assay showing the interaction of BBX21 with COP1 in onion epidemal cells. Full-length COP1 and BBX21 were fused to the split N- or C-terminal fragments of YFP (YN-COP1 or YC-BBX21). Nuclear localized CFP-CSU1 served as a marker for successful transfection. Unfused YFP N-terminal (YN) or C-terminal (YC) fragments served as negative controls, as indicated. DAPI staining marked the positions of nuclei. Dic, differential interference contrast in light microscope mode; Merge, merged images of YFP channel, DAPI, and Dic. (Scale bar: 100 μm.) (C) COP1 ubiquitinates BBX21 in vitro. Ubiquitination assays were performed in a reaction mix containing UBE1 (E1), rice 6×His-Rad6 (E2), and myc-tagged ubiquitin (myc-Ub). Ubiquitinated MBP-COP1 and 6×His-TF-BBX21 were detected by anti-ubiquitin and anti-myc monoclonal antibodies, respectively. The “+” and “−” indicate presence and absence, respectively. (D) Immunoblot analysis of myc-BBX21 protein levels in dark-grown myc-BBX21 bbx21-1 (#17) transgenic seedlings treated with DMSO or various concentrations of MG132 (50, 100, or 200 μM) for 3 h. (E) Immunoblot analysis of YFP-BBX21 protein levels in YFP-BBX21 bbx21-1 (#12) and YFP-BBX21 bbx21-1 cop1-4 (#12) transgenic seedlings grown in the dark for 4 d. (F) Immunoblot analysis of myc-BBX21 protein levels in myc-BBX21 bbx21-1 (#17) and myc-BBX21 bbx21-1 cop1-6 (#17) transgenic seedlings grown in the dark for 4 d. In D–F, bbx21-1 served as a negative control, and anti-HSP90 or anti-actin served as a loading control.
We next performed bimolecular fluorescence complementation assays (BiFCs) to further confirm the BBX21–COP1 interaction. When YN-COP1 (COP1 fused with the N-terminal of YFP) was coexpressed with YC-BBX21 (BBX21 fused with the C-terminal of YFP) in onion (Allium cepa) epidermal cells, strong YFP fluorescence signals were observed in the nucleus; however, no YFP fluorescence signals were detected when YN-COP1 was coexpressed with YC, or when YC-BBX21 was coexpressed with YN, supporting the conclusion that BBX21 physically interacts with COP1 (Fig. 1B).
To test whether COP1 is able to ubiquitinate BBX21, we performed the in vitro ubiquitination assays. MBP-COP1 itself exhibited self-ubiquitination activity (Fig. 1C, lane 5), consistent with a previous report (11), and did not ubiquitinate 6×His-tagged Trigger Factor (TF), a soluble tag (Fig. 1C, lane 5). However, when MBP-COP1 was added together with 6×His-TF-BBX21 in the reaction, both ubiquitinated MBP-COP1 and 6×His-TF-BBX21 were detected (Fig. 1C, lane 6). Moreover, mutant forms of COP1 (C52S and C55S) with impaired E3 ubiquitin ligase activity (11) could not ubiquitinate COP1 itself and 6×His-TF-BBX21 (Fig. 1C, lanes 7 and 8). Taken together, these results suggest that COP1 is able to ubiquitinate BBX21 in vitro.
BBX21 Undergoes Degradation in Darkness in a COP1-Dependent Manner.
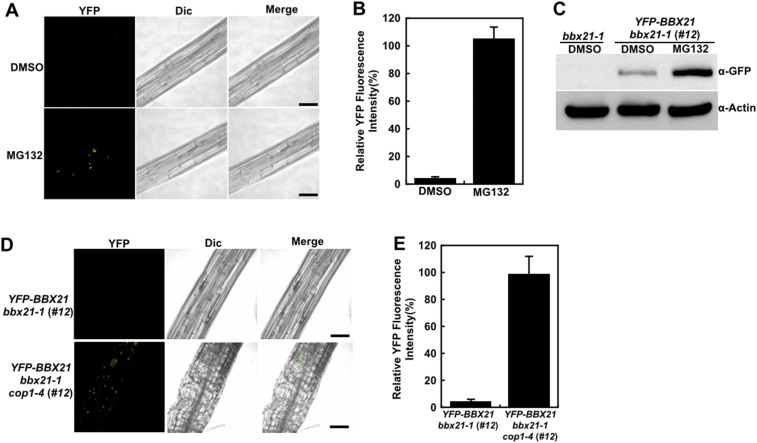
To test whether the stability of BBX21 is controlled by COP1, we first examined whether BBX21 is degraded via the 26S proteasome system. For this, myc-BBX21 bbx21-1 (#17) transgenic seedlings were first grown in darkness for 4 d and then treated with DMSO or various concentrations of MG132, a proteasome inhibitor, for 3 h. As shown in Fig. 1D, BBX21 protein levels were increased when the transgenic seedlings were treated with 50 µM MG132, and this increase became more evident when the concentration of MG132 was increased to 100 or 200 μM. Moreover, the YFP fluorescence signal increased by ∼20-fold when dark-grown YFP-BBX21 bbx21-1 (#12) seedlings were treated with 100 μM MG132 (Fig. S3 A and B). Consistently, markedly more BBX21 accumulated in YFP-BBX21 bbx21-1 (#12) transgenic seedlings after treatment with 100 μM MG132 (Fig. S3C). Taken together, these data demonstrate that MG132 treatment significantly blocked the degradation of BBX21, suggesting that BBX21 is subjected to 26S proteasome-mediated degradation in darkness.
Fig. S3.
COP1 controls BBX21 abundance in dark-grown seedlings. (A) Hypocotyl cells of dark-grown YFP-BBX21 bbx21-1 (#12) seedlings treated with DMSO or 100 μM MG132 for 3 h analyzed by fluorescence microscopy. (B) Relative YFP fluorescence intensity in hypocotyls of YFP-BBX21 bbx21-1 (#12) transgenic seedlings treated with DMSO or 100 μM MG132 for 3 h. (C) Immunoblot analysis of YFP-BBX21 protein levels in dark-grown YFP-BBX21 bbx21-1 (#12) transgenic seedlings treated with DMSO or 100 μM MG132 for 3 h. bbx21-1 served as a negative control, and anti-actin served as a loading control. (D) Analysis of YFP-BBX21 in hypocotyl with fluorescence microscopy. YFP-BBX21 bbx21-1 (#12) and YFP-BBX21 bbx21 cop1-4 (#12) transgenic seedlings were grown in the dark for 4 d. (E) Relative YFP fluorescence intensity in hypocotyls of YFP-BBX21 bbx21-1 (#12) and YFP-BBX21 bbx21-1 cop1-4 (#12) transgenic seedlings grown in the dark for 4 d. In A and D, the pictures are representative images taken from the hypocotyls. YFP, YFP channel image; Dic, differential interference contrast in light microscope mode; Merge, merged images of YFP and Dic. (Scale bar: 50 μm.) In B and E, data were obtained from two independent experiments. At least 10 seedlings were measured each time. Fluorescence intensity was measured using Image J software.
We next introduced the cop1 mutation into YFP-BBX21 bbx21-1 (#12) and myc-BBX21 bbx21-1 (#17) by genetic crossing. Significantly higher YFP-BBX21 or myc-BBX21 protein levels were found in bbx21-1 cop1 compared with bbx21-1 background in darkness (Fig. 1 E and F). These findings are also consistent with the observations of YFP fluorescence in YFP-BBX21 bbx21 transgenic seedlings with or without functional COP1 (Fig. S3 D and E). Taken together, these data demonstrate that COP1 promotes the degradation of BBX21 in darkness.
Overexpression of BBX21 Leads to Hyperphotomorphogenic Growth in the Light.
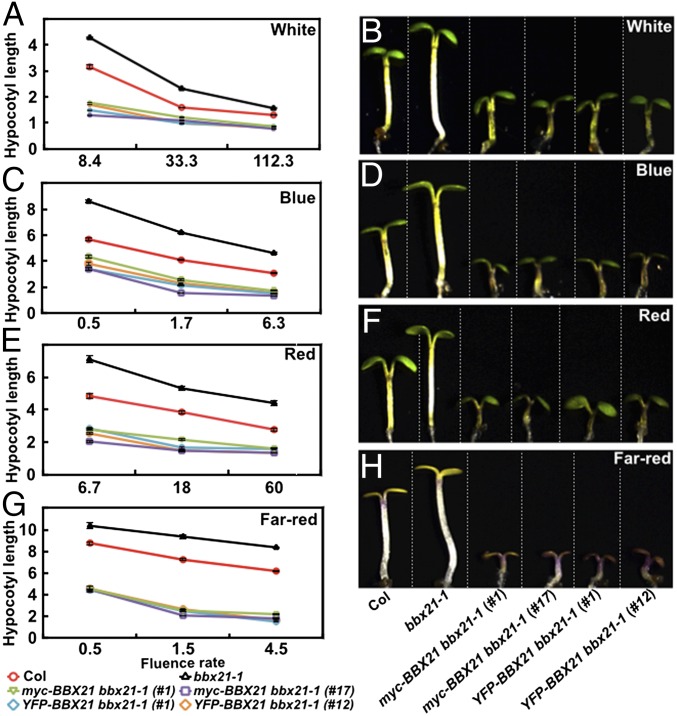
To further examine the physiological role of BBX21, we examined the light responsiveness of BBX21 overexpressors (i.e., YFP-BBX21 bbx21-1 and myc-BBX21 bbx21-1 transgenic plants in different light conditions (Fig. S2). When grown in the dark, all of the transgenic lines were indistinguishable from the wild type (WT) or bbx21-1 mutant seedlings (Fig. S4); however, both YFP-BBX21 bbx21-1 and myc-BBX21 bbx21-1 transgenic lines exhibited significantly shorter hypocotyls compared with WT when grown in various fluence rates of different light [white (W), blue (B), red (R), and far-red (FR)] conditions (Fig. 2). These data suggest that both YFP-BBX21 and myc-BBX21 transgenes are biologically functional in vivo, and that overexpression of BBX21 could confer hypersensitivity to different light, which is consistent with the positive role of BBX21 in light signaling (25).
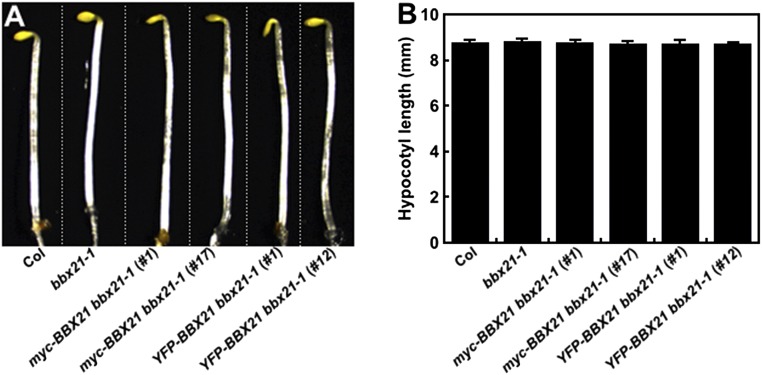
Fig. S4.
The hypocotyl lengths of BBX21 overexpression seedlings were indistinguishable from those of the WT in darkness. Hypocotyl phenotypes (A) and lengths (B) of Col, bbx21-1, and BBX21 overexpression seedlings grown in darkness for 4 d. In A, Col, bbx21-1, and various transgenic seedlings are separated by dotted lines.
Fig. 2.
BBX21 transgenic seedlings are hypersensitive to light. (A, C, E, and G) Fluence rate response curves of 4-d-old Col, bbx21-1, and BBX21 transgenic seedlings grown under white light (A), blue light (C), red light (E), and far-red light (G) conditions. Hypocotyl lengths are in millimeters. Data are mean ± SE; n ≥20. (B, D, F, and H) Hypocotyl phenotypes of 4-d-old Col, bbx21-1, and BBX21 transgenic seedlings grown under white light (33.3 μmol/m2/s) (B), blue light (6.37 μmol/m2/s) (D), red light (59.5 μmol/m2/s) (F), and far-red light (1.46 μmol/m2/s) (H) conditions. In B, D, F, and H, Col, bbx21-1, and various transgenic seedlings are separated by dotted lines. The experiments were performed three times, with similar results. The graphs depict one of these experiments.
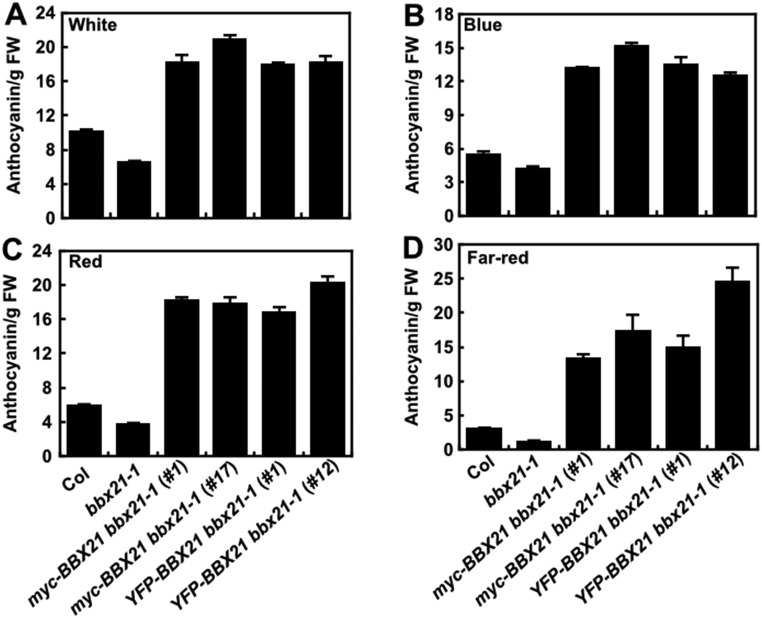
We also asked whether overexpression of BBX21 affects anthocyanin accumulation of seedlings. To this end, we measured the anthocyanin levels in WT, bbx21-1, YFP-BBX21 bbx21-1, and myc-BBX21 bbx21-1 transgenic seedlings grown in different light conditions (W, B, R, and FR) for 4 d. We observed that whereas bbx21-1 mutant seedlings accumulated less anthocyanin, consistent with a previous study (25), all of the transgenic seedlings overexpressing BBX21 accumulated dramatically more anthocyanin than WT when grown in the different light conditions (Fig. S5). These findings suggest that BBX21 positively regulates anthocyanin accumulation in the light.
Fig. S5.
BBX21 overexpression seedlings accumulate more anthocyanin. Anthocyanin contents of Col, bbx21-1, and BBX21 overexpression seedlings grown for 4 d under white light (112.3 μmol/m2/s) (A), blue light (0.62 μmol/m2/s) (B), red light (6.78 μmol/m2/s) (C), and far-red light (1.46 μmol/m2/s) (D) conditions. FW, fresh weight. Data are mean ± SE; n = 3. The experiments were performed three times, with similar results.
BBX21 Directly Up-Regulates HY5 Expression.
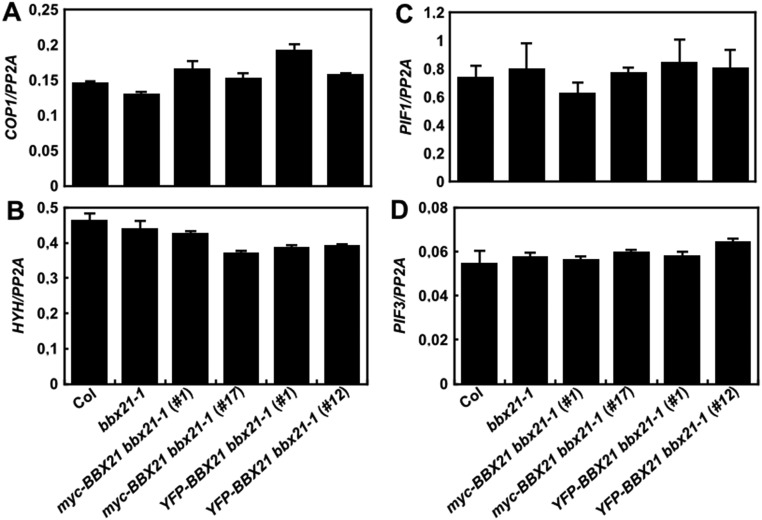
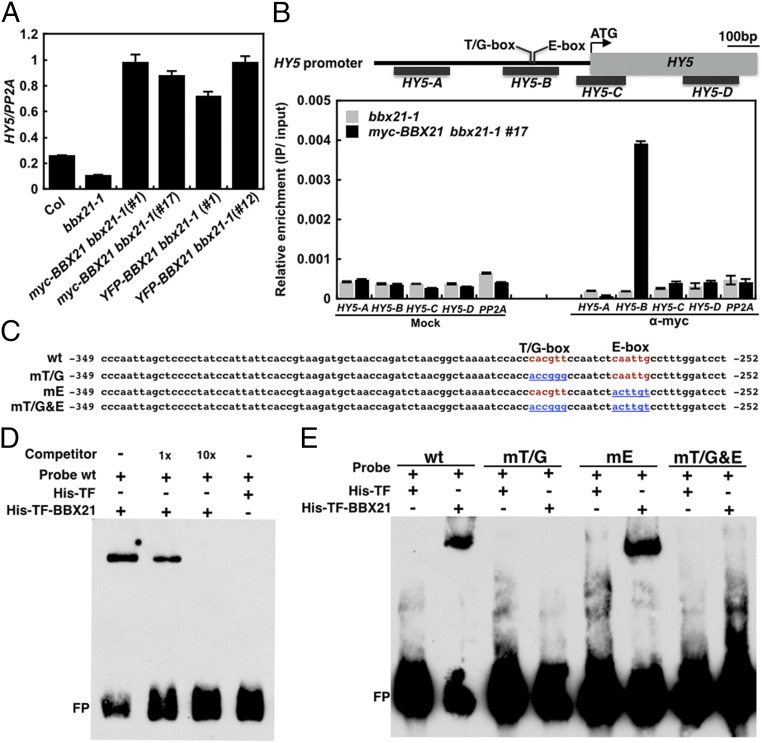
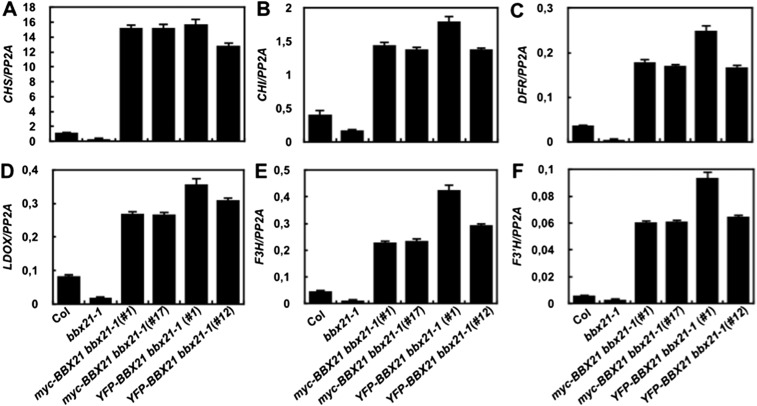
We next examined the expression of several key regulators of photomorphogenesis in the bbx21-1 mutant and BBX21 overexpression lines. Our data show that the transcript levels of COP1, HYH, PIF1, and PIF3 were not altered regardless of either a loss or gain of BBX21 function in Arabidopsis seedlings (Fig. S6); however, HY5 expression was dramatically decreased (by ∼5-fold) in bbx21-1, but increased (by ∼19- to 21-fold) in transgenic seedlings overexpressing BBX21 (Fig. 3A). In addition, the expression of six direct target genes of HY5 (i.e., CHS, CHI, F3′H, F3H, LDOX, and DOF) involved in the anthocyanin biosynthesis pathway (27) was accordingly reduced in bbx21-1 but drastically elevated in BBX21 overexpression seedlings (Fig. S7). These data suggest that BBX21 controls HY5 and HY5-regulated genes in Arabidopsis seedlings.
Fig. S6.
BBX21 does not regulate the expression of COP1, HYH, PIF1, and PIF3. Real-time qPCR analyses of COP1 (A), HYH (B), PIF1 (C), and PIF3 (D) transcript levels in Col, bbx21-1, and BBX21 overexpression seedlings grown under white light for 4 d. Error bars represent SD of three technical replicates.
Fig. 3.
BBX21 directly up-regulates the expression of HY5. (A) Real-time qPCR analyses of HY5 transcript levels in Col, bbx21-1, and BBX21 overexpression seedlings grown under white light for 4 d. (B) ChIP assays showing that BBX21 associates with the HY5 promoter in vivo. ChIP was performed with anti-myc monoclonal antibodies, and the ChIP DNA was analyzed by real-time qPCR. Error bars represent SD of three technical replicates. (C) Diagram of the WT and various mutated versions of the HY5 promoter subfragments used in the EMSA assays. The WT T/G-box and E-box elements are shown in red, and nucleotide substitutions in the mutant subfragments are shown in blue and underscored. (D and E) EMSA assays using 6×His-TF-BBX21 and the WT (D) or various mutated versions (E) of the HY5 promoter subfragments as the probes. 6×His-TF protein served as the negative control. The “+” and “−” indicate presence and absence, respectively. FP, free probe.
Fig. S7.
BBX21 controls the expression of HY5-regulated genes. Real-time qPCR analyses of CHS (A), CHI (B), DFR (C), LDOX, (D) F3H (E), and F3′H (F) transcript levels in Col, bbx21-1, and BBX21 overexpression seedlings grown under white light for 4 d.
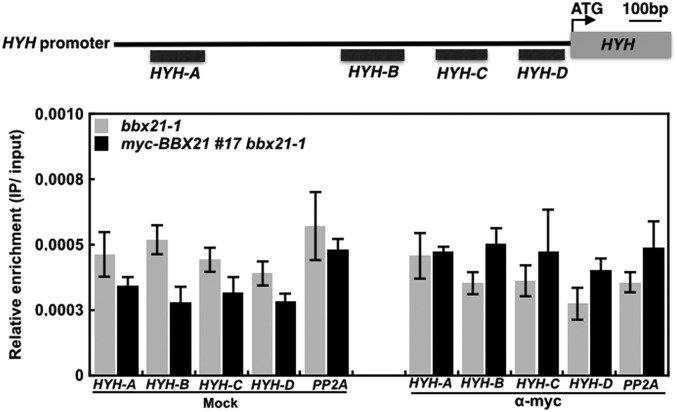
To test whether BBX21 can directly bind to the HY5 promoter and regulate the expression of HY5, we first performed chromatin immunoprecipitation (ChIP) experiments using anti-myc monoclonal antibodies and myc-BBX21 bbx21-1 (#17) transgenic plants. We found that BBX21 specifically associated with the B promoter fragment of HY5 (−414 to −215 bp) (Fig. 3B), but not with any examined promoter fragments of HYH (Fig. S8). These data indicate that BBX21 directly associates with the HY5 promoter in vivo.
Fig. S8.
ChIP assays showing that BBX21 is not associated with the HYH promoter in vivo. ChIP was performed with anti-myc antibodies, and ChIP DNA was analyzed by real-time qPCR. Error bars represent SD of three technical replicates.
Interestingly, the B promoter fragment of HY5 contains a T/G-box and an E-box, which are known to be bound by HY5/HYH and CAM7, respectively (15, 16). To investigate whether BBX21 could bind to the HY5 promoter directly, we performed electrophoretic mobility shift assays (EMSAs) using recombinant 6×His-TF-BBX21 proteins and a 98-bp HY5 promoter subfragment (−349 to −252 bp) containing the T/G-box and E-box (Fig. 3C). As shown in Fig. 3D, 6×His-TF alone showed no binding activity, whereas 6×His-TF-BBX21 was able to bind to the HY5 promoter subfragment with high affinity. Moreover, increasing amounts of unlabeled subfragments obviously decreased BBX21 binding to the biotin-labeled probes (Fig. 3D), indicating that BBX21 directly binds to the HY5 promoter subfragment in vitro.
We next performed EMSAs to delineate the exact binding cis-element of BBX21 in the HY5 promoter. We used the 98-bp WT and mutant probes in which T/G- and E-boxes were mutated individually or together (Fig. 3C). We found that BBX21 could bind to the WT and mE subfragments that contain the WT T/G-box; however, mutations of the T/G-box (in either mT/G or mT/G&E) efficiently abolished BBX21 binding to the promoter subfragment (Fig. 3E), indicating that the T/G-box is the binding site for BBX21 in the HY5 promoter. Taken together, our data demonstrate that BBX21 up-regulates HY5 expression by directly binding to the T/G-box present in the HY5 promoter.
Mutation of HY5 Restores the Hyperphotomorphogenic Phenotypes of BBX21 Overexpressors in the Light.
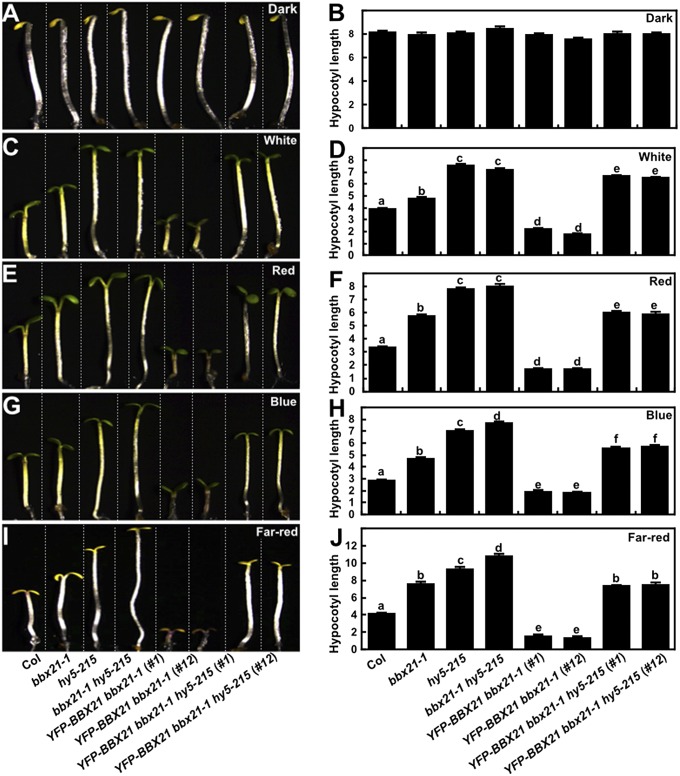
To obtain genetic evidence supporting the role of HY5 as a pivotal target of BBX21, we introduced the hy5-215 mutation into the YFP-BBX21 bbx21-1 transgenic plants by genetic crossing. We observed that the YFP-BBX21 bbx21-1 hy5-215 seedlings were indistinguishable from the WT and YFP-BBX21 bbx21-1 seedlings when grown in the dark (Fig. 4 A and B); however, when grown in the light (W, R, B, and FR), the YFP-BBX21 bbx21-1 hy5-215 seedlings exhibited significantly longer hypocotyls than the WT and YFP-BBX21 bbx21-1 seedlings (Fig. 4 C–J). Notably, the hypocotyl lengths of YFP-BBX21 bbx21-1 hy5-215 seedlings were similar to those of bbx21-1 seedlings in FR light; however, in W, R, and B light conditions, they were longer than those of bbx21-1 but shorter than those of hy5-215 and bbx21-1 hy5-215 (Fig. 4 C–J). These data indicate that disruption of HY5 in BBX21 overexpressors efficiently restored their hyperphotomorphogenic phenotypes, supporting the notion that BBX21 functions in photomorphogenesis by directly activating HY5 expression.
Fig. 4.
Mutation of HY5 restores the hyperphotomorphogenic phenotypes of BBX21 overexpressors in the light. Hypocotyl phenotypes and lengths of 4-d-old Col, various mutants, and transgenic seedlings grown in darkness (A and B), white light (33.3 μmol/m2/s) (C and D), red light (59.5 μmol/m2/s) (E and F), blue light (6.37 μmol/m2/s) (G and H), and far-red light (1.46 μmol/m2/s) (I and J) conditions. Hypocotyl lengths are in millimeters. Data are means ± SE; n ≥20. Letters above the bars indicate significant differences (P < 0.05) as determined by one-way ANOVA with Tukey’s post hoc analysis. In A, C, E, G, and I, Col, various mutant and transgenic seedlings are separated by dotted lines. The experiments were performed three times, with similar results. The graphs depict one of these experiments.
Discussion
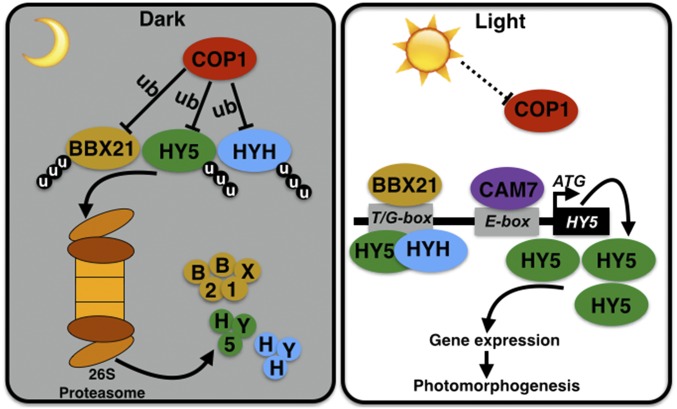
As an E3 ubiquitin ligase, COP1 is able to target multiple substrates for ubiquitination and promote their protein turnover via the 26S proteasome system. A series of COP1 interacting proteins have been identified and characterized as COP1 direct targets, including several BBX proteins (28, 29). CO/BBX1, BBX20, BBX22, BBX24, and BBX25 interact with COP1 and are regulated in a COP1-dependent manner (5, 6, 20–22, 30, 31). Previouslypublished results show that BBX21 colocalizes with COP1 in the nuclear speckles, and that bbx21 partially suppresses the constitutively photomorphogenic phenotypes of cop1 in darkness, implying that BBX21 is a potential downstream target of COP1 (25, 26). In this study, we provide evidence for this hypothesis based on several lines of evidence: BBX21-COP1 interaction in vivo, ubiquitination of BBX21 by COP1 in vitro, stabilization of BBX21 by MG132 treatment, and COP1-mediated degradation of BBX21 in vivo. Our data suggest that BBX21 acts downstream of COP1 and is targeted by COP1 via its E3 ubiquitin ligase activity in darkness (Fig. 5).
Fig. 5.
A working model depicting how BBX21 is involved in the COP1-HY5 signaling hub. In darkness, activated COP1 directly targets downstream substrates including BBX21, HY5, and HYH for ubiquitination, which subsequently triggers their proteolysis via the 26S proteasome system. On light illumination, the activity of COP1 is inhibited, resulting in the accumulation of BBX21, HY5, and HYH. Accumulated BBX21, HY5, and HYH directly bind to the T/G-box, and CAM7 associates with the E-box in the HY5 promoter. Together, these factors induce HY5 expression, leading to a high abundance of HY5, which regulates a large number of genes involved in promoting photomorphogenesis. ub, ubiquitination; u, ubiquitin.
HY5 is a key regulator not only in seedling photomorphogenesis, but also in root development, various hormone signaling pathways, and other processes (32–34). In the dark, HY5 is ubiquitinated by COP1 and subsequently degraded via the 26S proteasome system (28, 29). In the light, HY5 is required for proper expression of approximately one-third of Arabidopsis genes to promote various developmental processes (12, 13). At the posttranslational level, light triggers the inactivation of COP1, which ultimately allows the accumulation of HY5; however, at the transcriptional level, a complicated regulatory network converges on the HY5 promoter to precisely modulate its expression in response to light. It has been shown that HY5, HYH, and CAM7 can directly bind to the HY5 promoter to activate its expression. Specifically, HY5 and HYH associate with the T/G-box, and CAM7 binds to the E-box in the HY5 promoter (15, 16). In this study, we show that BBX21 directly binds to the T/G-box in the HY5 promoter as well (Fig. 3E). These findings suggest that multiple transcription factors converge on the HY5 promoter to ensure fine-tuned control of HY5 expression (Fig. 5); however, how BBX21, HY5, HYH, and CAM7 work in concert to precisely control HY5 expression in response to dynamic light changes awaits further investigation.
Given that BBX21, HY5, HYH, and CAM7 individually play positive roles in light signaling (7, 25, 34, 35), it is possible that these factors may modulate a common set of downstream target genes, and that they have partially overlapping functions in light-mediated development. In addition, BBX21 interacts with both HY5 and HYH in yeast and living plant cells (25), and HY5 associates and forms heterodimers with HYH to bind to the G-box cis-element (7). Thus, BBX21, HY5, and HYH may form heterodimers to bind to the T/G-box in the HY5 promoter to mediate its expression. It has been proposed that a potential as-yet unknown transcription factor binding to ACG-box in the HY5 promoter may be responsible for repression of HY5 in response to light (16). Therefore, it appears that BBX21, HY5, HYH, and CAM7, together with additional unidentified factors, are coordinately involved in the transcriptional regulation of HY5 in the light.
Collectively, our findings support a model in which BBX21 acts as a direct downstream target of COP1 and a direct activator of HY5 (Fig. 5). Our elucidation of the molecular roles of BBX21 in the COP1-HY5 signaling hub provides previously unidentified insight into the complicated but delicate regulatory network that controls plants’ responses to their dynamic light environment.
Materials and Methods
Plant Materials and Growth Conditions.
The bbx21-1 (25), hy5-215 (34), cop1-4, and cop1-6 (36) mutants are in the Columbia-0 (Col-0) ecotype. Seeds were surface-sterilized with 30% (vol/vol) commercial Clorox bleach and 0.02% Triton X-100 for 10 min, washed three times with sterile water, and then sown on 1× Murashige and Skoog (MS) medium supplemented with 0.4% Difco Bacto agar (BD Diagnostic Systems) and 1% (wt/wt) sucrose. The seeds were stratified in darkness for 3 d at 4 °C and then transferred to light chambers maintained at 22 °C. For generating transgenic plants overexpressing BBX21, the full-length BBX21 ORF was first cloned into the pDONR-221 vector (Invitrogen), and then introduced into the plant binary vector pEarley Gateway 104 or pEarley Gateway 203 (37) using Gateway LR Clonase Enzyme Mix (Invitrogen). These constructs were then introduced into Arabidopsis bbx21-1 mutant plants via the floral dip method (38).
In Vitro Ubiquitination Assays.
The pMAL-c2X-MBP-COP1 and pMAL-c2X-MBP-COP1 (C52S and C55S) vectors (11) were described previously. To generate pCold-6×His-TF-BBX21, the full-length BBX21 coding sequence was amplified by PCR and then cloned into the NdeI/XbaI sites of the pCold-6×His-TF vector (Takara). In vitro ubiquitination assays were performed as described previously (39) with minor modifications. Ubiquitination reaction mixtures (60 μL) contained 30 ng of UBE1 (E1; Boston Biochem) and rice His-Rad6 E2 (6), and 500 ng of myc-tagged ubiquitin (myc-Ub; Boston Biochem) in a reaction buffer containing 50 mM Tris pH 7.5, 10 mM MgCl2, 2 mM ATP, and 0.5 mM DTT. In addition, 500 ng of MBP or MBP-COP1 (previously incubated with 20 μM zinc cloride), and 500 ng of 6×His-TF-BBX21 or 6×His-TF were applied in the reactions as indicated. After a 2-h incubation at 30 °C, the reactions were stopped by adding 10 μL of 5× sample loading buffer. One-half of each mixture (∼35 μL) was then separated onto 8% SDS/PAGE gels. Ubiquitinated MBP-COP1 and TF-BBX21 were detected using monoclonal anti-ubiquitin (Santa Cruz Biotechnology) and anti-myc (Sigma-Aldrich) antibodies, respectively.
ChIP.
The ChIP assays were performed as described previously (12). Chromatin isolation was performed using bbx21-1 or myc-BBX21 bbx21-1 transgenic seedlings grown under constant white light for 4 d. The resuspended chromatin was sonicated at 4 °C to ∼250- to 500-bp fragments. The sheared chromatin was immunoprecipitated, washed, reverse cross-linked, and finally amplified. Approximately 10% of sonicated but nonimmunoprecipitated chromatin was reverse cross-linked and used as an input DNA control. Both immunoprecipitated DNA and input DNA were analyzed by real-time quantitative PCR (qPCR; Applied Biosystems). Monoclonal anti-myc antibody (Sigma-Aldrich) was used. The primers used for this assay are listed in Table S1.
Table S1.
Primers used in this study
| Primer name | Primer sequences (5′-3′) | |
| Real-time qPCR | ||
| BBX21(F) | GCACGGCCGACGAAGCATCT | |
| BBX21(R) | TGTGTTCGTTCGCAGCGTGGA | |
| HY5(F) | CCATCAAGCAGCGAGAGGTCATCAA | |
| HY5(R) | CGCCGATCCAGATTCTCTACCGGAA | |
| CHS(F) | AGCTGATGGACCTGCAGGCATCTTGGC | |
| CHS(R) | TGCATGTGACGTTTCCGAATTGTCGAC | |
| CHI(F) | ATGTCTTCATCCAACGCCTGCGCC | |
| CHI(R) | GACGGTGAAGATCACGAATTTACC | |
| DFR(F) | ATGGTTAGTCAGAAAGAGACCGTGTGTG | |
| DFR(R) | CGTTTCGCAAATCAAGAAGATGTTGTAC | |
| LDOX(F) | TGGGTCACTGCAAAATGTGT | |
| LDOX(R) | CGGAGACTCAACACTCACCA | |
| F3H (F) | ATGGCTCCAGGAACTTTGACTGAGCTA | |
| F3H (R) | GATCTGACGGCAGATCTCTCCTCTTTT | |
| F3′H (F) | GTTGAGAAAGATTAGTTCTGTTCATCT | |
| F3′H (R) | GTTGACTACACACATGTTCACCAACTG | |
| COP1 (F) | AAGAGTGTAGTACGGAGGGAAGG | |
| COP1 (R) | TAGACGACTGTAGCGAGTGAAGG | |
| HYH (F) | TGTCTCTCCAACGACCCAAT | |
| HYH (R) | GCTTCCATGTCAGGAACCAT | |
| PIF1(F) | CGTACCCAAGTTCGAGCAGGGTGA | |
| PIF1(R) | CCTGTTGTGTGGTTTCCGTGATCC | |
| PIF3(F) | ATTTTCCCACACCAGCTCCACAAC | |
| PIF3(R) | GCTCAAGACAGGAACCCTTCTCCA | |
| PP2A(F) | TATCGGATGACGATTCTTCGTGCAG | |
| PP2A(R) | GCTTGGTCGACTATCGGAATGAGAG | |
| ChIP real-time qPCR | ||
| HY5-A(F) | CAATGGTGTGGCTGGATATG | |
| HY5-A(R) | GGGCAGTGCTTTCAAAAGTG | |
| HY5-B(F) | GGCCATGTGACAGAATGAAAG | |
| HY5-B(R) | AGGATCCAAAGGCAATTGAG | |
| HY5-C(F) | GCTCTTTTCCTCTTTATCCTTTTCAC | |
| HY5-C(R) | TGTTCCTGCATTTTTCTTACTCTTTG | |
| HY5-D(F) | GAAAGAGAACAAGCGGCTGAA | |
| HY5-D(R) | CCCATCACGCAACCGTTATT | |
| HYH-A(F) | CATGGTTTGCAGCTCGATTA | |
| HYH-A(R) | GCTGTATGCAAACGTGGAGA | |
| HYH-B(F) | GCGCGCATAAGTCCACTATC | |
| HYH-C(F) | GCCTATTACGCCATGCAAAT | |
| HYH-C(R) | GCGTGTGGGTTTAGGACAAT | |
| HYH-D(F) | TTGAAACAGTTGCATTTGTGG | |
| HYH-D(R) | CTTTCTTCTTTGGGGCCACT | |
| Plasmid constructs | ||
| BBX21attB1 | GGGGACAAGTTTGTACAAAAAAGCAGGCTTC ATGAAGATCAGGTGCGACGTCTG | pDONR221-BBX21 |
| BBX21attB2 | GGGGACCACTTTGTACAAGAAAGCTGGGTCC CAGAAAGATCTAAACTTTTTATTAG | |
| COP1-NcoI(F) | CATGCCATGGAGGAAGAGATTTCGACGGATCCGG | pSY728-YFPn-COP1 |
| COP1-NotI(R) | AAGGAAAAAAGCGGCCGGTCGCAGCGAGTACCA GAACTTTG | |
| BBX21-NcoI(F) | CATGCCATGGAGATGAAGATCAGGTGCGACGTCTG | pSY738-YFPc-BBX21 |
| BBX21-NotI(R) | AAGGAAAAAAGCGGCCGGTCCAGAAAGATCTA AACTTTTTAT | |
| BBX21-NdeI(F) | GGAATTCCATATGATGAAGATCAGGTGCGACGTCTG | pCold-His-TF-BBX21 |
| BBX21-XbaI(R) | CTAGTCTAGATTACCAGAAAGATCTAAACTTTTTAT | |
| proHY5wt-KpnI(F) | CGGGGTACCCCCAATTAGCTCCCCTATCC | pLacZ-2u-proHY5wt |
| proHY5wt-XhoI (R) | CCGCTCGAGAGGATCCAAAGGCAATTGAG | |
| proHY5wt-KpnI(F) | CGGGGTACCCCCAATTAGCTCCCCTATCC | pLacZ-2u-proHY5mT |
| proHY5muT-XhoI(R) | CCGCTCGAGAGGATCCAAAGGCAATTGAGATTGGCCCGGTGG | |
| proHY5wt-KpnI(F) | CGGGGTACCCCCAATTAGCTCCCCTATCC | pLacZ-2u-proHY5mE |
| proHY5muE-XhoI (R) | CCGCTCGAGAGGATCCAAAGGACAAGTAGATTGGAACGTGGG | |
| proHY5wt-KpnI(F) | CGGGGTACCCCCAATTAGCTCCCCTATCC | pLacZ-2u-proHY5mT&E |
| proHY5muT&E-XhoI(R) | CCGCTCGAGAGGATCCAAAGGACAAGTAGATTGGCCCGGTGG | |
Underscored nucleotides indicate restriction sites for cloning.
EMSA.
EMSAs were performed using biotin-labeled probes and the LightShift Chemiluminescent EMSA Kit (Thermo Fisher Scientific) as described previously (40). The 98-bp promoter subfragment of HY5 upstream of ATG (-349 to -252 bp) was cloned into the pLacZi2µ vector. Various mutated versions of HY5 promoter subfragments were produced by primer-based site-directed mutagenesis using the WT promoter subfragment (in the pLacZi2µ vector) as the template. The WT and various mutated versions of HY5 promoter subfragments were PCR-amplified, mixed with biotin, and kept in UV light for 30 min for biotin labeling. Then 500 ng of purified 6×His-TF-BBX21 or 6×His-TF protein was incubated together with 40 fmol of biotin-labeled probes in a 20-μL reaction mixture containing 10 mM Tris⋅HCl pH 7.5, 0.05% Nonidet P-40, 10 mM MgCl2, 5% (vol/vol) glycerol, and 0.1 μg/mL poly (dI∙dC).
Reactions were incubated at 25 °C for 20 min and separated on 6% (vol/vol) native polyacrylamide gels in 0.5×TBE buffer. The gels were electroblotted to Hybond N+ nylon membranes (Millipore) in 0.5×TBE for 40 min, after which the labeled probes were detected according to the manufacturer’s protocol provided with the EMSA kit.
Detailed descriptions of co-IP analysis, immunoblot analysis, BiFC assays, anthocyanin measurements, hypocotyl length measurements, and real-time qPCR assays are provided in SI Materials and Methods.
SI Materials and Methods
Co-IP Assays and Immunoblot Analyses.
For Co-IP assays, 1 mg of total proteins was extracted from 4-d-old Arabidopsis seedlings in protein extraction buffer containing 50 mM Tris·HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 10% (vol/vol) glycerol, 0.1% Tween 20, 1 mM PMSF, and 1× cOmplete Protease Inhibitor Mixture (Roche). The extracts were incubated with 4 μL of anti-myc antibodies (Sigma-Aldrich) coupled with 25 μL of Protein-A Sepharose (GE Healthcare) for 3 h at 4 °C. The Sepharose was then washed three times with protein extraction buffer. The precipitates were eluted into 100 mM glycine (pH 2.5) and 100 mM NaCl, immediately neutralized by 2 M Tris⋅HCl pH 9.0 and 100 mM NaCl, and finally concentrated using StrataClean Resin (Stratagene) before immunoblot analyses.
For immunoblots, Arabidopsis WT or mutant seedlings were homogenized in a protein extraction buffer containing 50 mM Tris pH 7.5, 200 mM NaCl, 1 mM EDTA, 10% (vol/vol) glycerol, 1 mM DTT, 1 mM PMSF, and 1× cOmplete Protease Inhibitor Mixture. For MG132 treatment, 4-d-old dark-grown seedlings were incubated in liquid MS medium containing various concentrations of MG132 (Sigma-Aldrich) for 3 h. MG132 was dissolved in DMSO. The primary antibodies used in this study were anti-COP1 (36), anti-myc (Sigma-Aldrich), anti-GFP (Abmart), and anti-actin (Sigma-Aldrich).
BiFC Assays.
The full-length coding sequences of COP1 and BBX21 were cloned into NcoI/NotI sites of the pSY728 and pSY738 vectors (41), respectively, to generate constructs expressing YFPn-COP1 or YFPc-BBX21 fusion proteins. Each pair of recombinant constructs encoding nYFP and cYFP fusions was cobombarded into onion epidermal cells and then incubated in 1× MS solid medium containing 4% (wt/wt) sucrose for 24 h at 22 °C in darkness, followed by imaging analyses by confocal microscopy. Nuclear localized CFP-CSU1 (pAM-PAT-35SS-CFP-CSU1), under the control of the 35S promoter, served as a marker for successful transfection (42).
Measurement of Hypocotyl Length.
To measure the hypocotyl length of seedlings, seeds were cold-treated at 4 °C for 3 d and then transferred to continuous white light for 8 h to induce uniform germination. The seeds then were transferred to white or monochromatic light (W, B, R, or FR) conditions and incubated at 22 °C for 4 d. The hypocotyl lengths of seedlings were measured using ImageJ software.
Anthocyanin Measurements.
Anthocyanin levels were measured as described previously (7). In brief, 4-d-old seedlings grown in various light conditions were collected, weighed, frozen in liquid nitrogen, and ground to a fine powder. Total plant pigments were extracted overnight in 0.6 mL of 1% (vol/vol) HCl in methanol. After the addition of 0.2 mL of water, anthocyanin was extracted with 0.65 mL of chloroform. The quantity of anthocyanins was determined by spectrophotometric measurements of the aqueous phase (A530–A657) and normalized to the total fresh weight of tissue used in each sample.
Real-Time qPCR.
Total RNAs were extracted from 4-d-old Arabidopsis seedlings grown under white light using the RNeasy Plant Mini Kit (Qiagen). cDNAs were synthesized from 2 mg of total RNAs using the SuperScript II First-Strand Synthesis System (Thermo Fisher Scientific) according to the manufacturer’s instructions. The cDNAs were then subjected to real-time qPCR using the CFX96 Touch Real-Time PCR Detection System (Applied Biosystems) and SYBR Green PCR Master Mix (Takara). PCR was performed in triplicate for each sample, and the transcript levels were normalized to that of a PP2A gene. All primers used for this assay are listed in Table S1.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (31330048), the National Basic Research Program of China 973 Program (2012CB910900), the Peking-Tsinghua Center for Life Sciences (to X.W.D.), and the National Institutes of Health (GM-47850). D.X. was supported in part by a Postdoctoral Fellowship from the Peking-Tsinghua Center for Life Sciences.
Footnotes
The authors declare no conflict of interest.
2Deceased August 29, 2012.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607687113/-/DCSupplemental.
References
- 1.Sullivan JA, Deng XW. From seed to seed: The role of photoreceptors in Arabidopsis development. Dev Biol. 2003;260(2):289–297. doi: 10.1016/s0012-1606(03)00212-4. [DOI] [PubMed] [Google Scholar]
- 2.Deng XW, Caspar T, Quail PH. cop1: A regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev. 1991;5(7):1172–1182. doi: 10.1101/gad.5.7.1172. [DOI] [PubMed] [Google Scholar]
- 3.Deng XW, et al. COP1, an Arabidopsis regulatory gene, encodes a protein with both a zinc-binding motif and a G beta homologous domain. Cell. 1992;71(5):791–801. doi: 10.1016/0092-8674(92)90555-q. [DOI] [PubMed] [Google Scholar]
- 4.Ang LH, et al. Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol Cell. 1998;1(2):213–222. doi: 10.1016/s1097-2765(00)80022-2. [DOI] [PubMed] [Google Scholar]
- 5.Chang CS, Maloof JN, Wu SH. COP1-mediated degradation of BBX22/LZF1 optimizes seedling development in Arabidopsis. Plant Physiol. 2011;156(1):228–239. doi: 10.1104/pp.111.175042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datta S, et al. LZF1/SALT TOLERANCE HOMOLOG3, an Arabidopsis B-box protein involved in light-dependent development and gene expression, undergoes COP1-mediated ubiquitination. Plant Cell. 2008;20(9):2324–2338. doi: 10.1105/tpc.108.061747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holm M, Ma LG, Qu LJ, Deng XW. Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev. 2002;16(10):1247–1259. doi: 10.1101/gad.969702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang IC, Yang JY, Seo HS, Chua NH. HFR1 is targeted by COP1 E3 ligase for post-translational proteolysis during phytochrome A signaling. Genes Dev. 2005;19(5):593–602. doi: 10.1101/gad.1247205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo Q, et al. COP1 and phyB physically interact with PIL1 to regulate its stability and photomorphogenic development in Arabidopsis. Plant Cell. 2014;26(6):2441–2456. doi: 10.1105/tpc.113.121657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osterlund MT, Hardtke CS, Wei N, Deng XW. Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature. 2000;405(6785):462–466. doi: 10.1038/35013076. [DOI] [PubMed] [Google Scholar]
- 11.Seo HS, et al. LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature. 2003;423(6943):995–999. doi: 10.1038/nature01696. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, et al. Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell. 2007;19(3):731–749. doi: 10.1105/tpc.106.047688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, et al. Genome-wide mapping of the HY5-mediated gene networks in Arabidopsis that involve both transcriptional and post-transcriptional regulation. Plant J. 2011;65(3):346–358. doi: 10.1111/j.1365-313X.2010.04426.x. [DOI] [PubMed] [Google Scholar]
- 14.Pacín M, Legris M, Casal JJ. Rapid decline in nuclear costitutive photomorphogenesis1 abundance anticipates the stabilization of its target elongated hypocotyl5 in the light. Plant Physiol. 2014;164(3):1134–1138. doi: 10.1104/pp.113.234245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbas N, Maurya JP, Senapati D, Gangappa SN, Chattopadhyay S. Arabidopsis CAM7 and HY5 physically interact and directly bind to the HY5 promoter to regulate its expression and thereby promote photomorphogenesis. Plant Cell. 2014;26(3):1036–1052. doi: 10.1105/tpc.113.122515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Binkert M, et al. UV-B–responsive association of the Arabidopsis bZIP transcription factor ELONGATED HYPOCOTYL5 with target genes, including its own promoter. Plant Cell. 2014;26(10):4200–4213. doi: 10.1105/tpc.114.130716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khanna R, et al. The Arabidopsis B-box zinc finger family. Plant Cell. 2009;21(11):3416–3420. doi: 10.1105/tpc.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gangappa SN, Botto JF. The BBX family of plant transcription factors. Trends Plant Sci. 2014;19(7):460–470. doi: 10.1016/j.tplants.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Datta S, Hettiarachchi GH, Deng XW, Holm M. Arabidopsis CONSTANS-LIKE3 is a positive regulator of red light signaling and root growth. Plant Cell. 2006;18(1):70–84. doi: 10.1105/tpc.105.038182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan XY, et al. BZS1, a B-box protein, promotes photomorphogenesis downstream of both brassinosteroid and light signaling pathways. Mol Plant. 2012;5(3):591–600. doi: 10.1093/mp/sss041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holm M, Hardtke CS, Gaudet R, Deng XW. Identification of a structural motif that confers specific interaction with the WD40 repeat domain of Arabidopsis COP1. EMBO J. 2001;20(1-2):118–127. doi: 10.1093/emboj/20.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gangappa SN, et al. The Arabidopsis B-BOX protein BBX25 interacts with HY5, negatively regulating BBX22 expression to suppress seedling photomorphogenesis. Plant Cell. 2013;25(4):1243–1257. doi: 10.1105/tpc.113.109751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang L, et al. Arabidopsis STO/BBX24 negatively regulates UV-B signaling by interacting with COP1 and repressing HY5 transcriptional activity. Cell Res. 2012;22(6):1046–1057. doi: 10.1038/cr.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan H, et al. Nuclear localization and interaction with COP1 are required for STO/BBX24 function during photomorphogenesis. Plant Physiol. 2011;156(4):1772–1782. doi: 10.1104/pp.111.180208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Datta S, Hettiarachchi C, Johansson H, Holm M. SALT TOLERANCE HOMOLOG2, a B-box protein in Arabidopsis that activates transcription and positively regulates light-mediated development. Plant Cell. 2007;19(10):3242–3255. doi: 10.1105/tpc.107.054791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crocco CD, Holm M, Yanovsky MJ, Botto JF. AtBBX21 and COP1 genetically interact in the regulation of shade avoidance. Plant J. 2010;64(4):551–562. doi: 10.1111/j.1365-313X.2010.04360.x. [DOI] [PubMed] [Google Scholar]
- 27.Shin J, Park E, Choi G. PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner, with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J. 2007;49(6):981–994. doi: 10.1111/j.1365-313X.2006.03021.x. [DOI] [PubMed] [Google Scholar]
- 28.Lau OS, Deng XW. The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci. 2012;17(10):584–593. doi: 10.1016/j.tplants.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Huang X, Ouyang X, Deng XW. Beyond repression of photomorphogenesis: Role switching of COP/DET/FUS in light signaling. Curr Opin Plant Biol. 2014;21:96–103. doi: 10.1016/j.pbi.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Jang S, et al. Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. EMBO J. 2008;27(8):1277–1288. doi: 10.1038/emboj.2008.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu LJ, et al. COP1-mediated ubiquitination of CONSTANS is implicated in cryptochrome regulation of flowering in Arabidopsis. Plant Cell. 2008;20(2):292–306. doi: 10.1105/tpc.107.057281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen H, et al. Integration of light and abscisic acid signaling during seed germination and early seedling development. Proc Natl Acad Sci USA. 2008;105(11):4495–4500. doi: 10.1073/pnas.0710778105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sibout R, et al. Opposite root growth phenotypes of hy5 versus hy5 hyh mutants correlate with increased constitutive auxin signaling. PLoS Genet. 2006;2(11):e202. doi: 10.1371/journal.pgen.0020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oyama T, Shimura Y, Okada K. The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev. 1997;11(22):2983–2995. doi: 10.1101/gad.11.22.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kushwaha R, Singh A, Chattopadhyay S. Calmodulin7 plays an important role as transcriptional regulator in Arabidopsis seedling development. Plant Cell. 2008;20(7):1747–1759. doi: 10.1105/tpc.107.057612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNellis TW, et al. Genetic and molecular analysis of an allelic series of cop1 mutants suggests functional roles for the multiple protein domains. Plant Cell. 1994;6(4):487–500. doi: 10.1105/tpc.6.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Earley KW, et al. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 2006;45(4):616–629. doi: 10.1111/j.1365-313X.2005.02617.x. [DOI] [PubMed] [Google Scholar]
- 38.Clough SJ, Bent AF. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 39.Xu D, et al. Arabidopsis COP1 SUPPRESSOR 2 represses COP1 E3 ubiquitin ligase activity through their coiled-coil domains association. PLoS Genet. 2015;11(12):e1005747. doi: 10.1371/journal.pgen.1005747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu D, et al. Convergence of Light and ABA signaling on the ABI5 promoter. PLoS Genet. 2014;10(2):e1004197. doi: 10.1371/journal.pgen.1004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bracha-Drori K, et al. Detection of protein–protein interactions in plants using bimolecular fluorescence complementation. Plant J. 2004;40(3):419–427. doi: 10.1111/j.1365-313X.2004.02206.x. [DOI] [PubMed] [Google Scholar]
- 42.Xu D, et al. The RING-finger E3 ubiquitin ligase COP1 SUPPRESSOR1 negatively regulates COP1 abundance in maintaining COP1 homeostasis in dark-grown Arabidopsis seedlings. Plant Cell. 2014;26(5):1981–1991. doi: 10.1105/tpc.114.124024. [DOI] [PMC free article] [PubMed] [Google Scholar]