Significance
Ying Yang 1 (YY1) is a ubiquitously expressed transcription factor that has been demonstrated to be essential for pro–B-cell development as well as lymphoma. It has recently been proposed that YY1 regulates the germinal center B-cell transcriptional program. We confirm this hypothesis and additionally show that YY1 is equally essential for all stages of B-cell differentiation. Through ChIP-sequencing analysis of YY1 binding, and analysis of differentially expressed genes from RNA-sequencing, our data show that, in addition to the regulation of several B-cell–specific genes, YY1 regulates many genes and pathways important in basic cellular functions, such as mitochondrial bioenergetics, transcription, ribosomal function, and cellular proliferation, thus explaining the requirement for YY1 at all stages of B-cell differentiation.
Keywords: YY1, B lymphocytes, differentiation, mitochondrial bioenergetics, transcription
Abstract
Ying Yang 1 (YY1) is a ubiquitously expressed transcription factor shown to be essential for pro–B-cell development. However, the role of YY1 in other B-cell populations has never been investigated. Recent bioinformatics analysis data have implicated YY1 in the germinal center (GC) B-cell transcriptional program. In accord with this prediction, we demonstrated that deletion of YY1 by Cγ1-Cre completely prevented differentiation of GC B cells and plasma cells. To determine if YY1 was also required for the differentiation of other B-cell populations, we deleted YY1 with CD19-Cre and found that all peripheral B-cell subsets, including B1 B cells, require YY1 for their differentiation. Transitional 1 (T1) B cells were the most dependent upon YY1, being sensitive to even a half-dosage of YY1 and also to short-term YY1 deletion by tamoxifen-induced Cre. We show that YY1 exerts its effects, in part, by promoting B-cell survival and proliferation. ChIP-sequencing shows that YY1 predominantly binds to promoters, and pathway analysis of the genes that bind YY1 show enrichment in ribosomal functions, mitochondrial functions such as bioenergetics, and functions related to transcription such as mRNA splicing. By RNA-sequencing analysis of differentially expressed genes, we demonstrated that YY1 normally activates genes involved in mitochondrial bioenergetics, whereas it normally down-regulates genes involved in transcription, mRNA splicing, NF-κB signaling pathways, the AP-1 transcription factor network, chromatin remodeling, cytokine signaling pathways, cell adhesion, and cell proliferation. Our results show the crucial role that YY1 plays in regulating broad general processes throughout all stages of B-cell differentiation.
Ying Yang 1 (YY1) is a ubiquitously expressed zinc finger-containing transcription factor that was given its name due to the ability to repress or activate transcription (1). YY1 was first identified in 1991 as being a key regulator for several viral genes, as well as a repressor of the Ig 3′κ-enhancer (1–5). YY1 can activate or repress genes by recruiting a variety of coactivators or corepressors. It can recruit several histone-modifying complexes, including those containing histone acetyltransferases or histone deacetylases, thus resulting in opposing epigenetic profiles (6, 7). It is highly conserved from Xenopus to humans, and there is even a Drosophila ortholog of YY1. YY1 has also been implicated in Polycomb-mediated repression (8), and it has strong homology to Pho, the DNA binding recruiter of Polycomb-repressive complexes in Drosophila (9, 10). Pho also plays an important role in embryonic patterning in Drosophila. In Xenopus, YY1 also is important in patterning and neural induction (11). Not surprisingly therefore, germ-line deletion of YY1 leads to embryonic lethality at the periimplantation stage (12).
Within the B lineage of lymphocytes, YY1 was shown to play critical roles at the pro–B-cell stage of differentiation. Conditional deletion of YY1 with mb1-Cre resulted in a block at the pro–B-cell to pre–B-cell stage (13). In pro-B cells, the Igh locus undergoes V(D)J rearrangement. D-to-J gene rearrangement occurs first on both alleles, followed by V-to-DJ rearrangement on one allele. Because only one V-to-DJ rearrangement is allowed on each allele, all V genes should have equivalent access to the single DJ rearrangement to create a maximally diverse antibody repertoire using the potential germ-line diversity afforded by the >100 functional Vh genes. This equal access is accomplished through the process of locus contraction, in which the entire Vh portion of the large 2.8-Mb Igh locus contracts, as determined by 3D-FISH analyses (14, 15), which results in making the distal Vh genes equally as close to the DJ rearrangement as the proximal Vh genes. Unlike wild-type pro-B cells, YY1-deficient pro-B cells do not undergo locus contraction (13). They are also unable to rearrange distal Vh genes, whereas the most proximal two Vh families rearrange at almost normal levels, which may be due to defective locus contraction. When the Igh locus is poised for rearrangement, there is noncoding transcription of unrearranged V and J genes, as well as intergenic antisense transcription. All of the V region sense and antisense germ-line transcripts that we assayed in that study were found to be greatly reduced in YY1−/− pro-B cells, especially the very prominent antisense transcripts within the distal part of the Vh locus at the Pax5-activated intergenic repeat (PAIR) elements (16). We have hypothesized that this noncoding RNA in the Vh locus is at least partially responsible for locus contraction, because we showed by chromosome conformation capture (3C) that the promoters of the most prominent noncoding RNA within the distal Igh locus, PAIR elements, make direct contact with the region near DJ, presumably within a common transcription factory (16). In addition, 3D-FISH and 3C demonstrate decreased interaction of two sites in the middle and distal parts of the Igh locus with Eμ after YY1 knockdown (17). Therefore, the lack of locus contraction and lack of rearrangement of distal Vh genes in YY1-deficient pro-B cells may be due, in part, to a lack of noncoding antisense RNA in the distal part of the Vh region and to a lack of YY1-dependent long-range interactions. In addition to this role of YY1 in creating a diverse repertoire of Igh rearrangements, YY1 has been implicated in creating a diverse repertoire of Igκ rearrangements (18).
Normally, after a productive Igh rearrangement, the μ-protein signals through the pre–B-cell receptor (pre-BCR) to stop any further heavy chain rearrangement. This step is required to permit advancement to the pre–B-cell stage of differentiation. However, the defects in proper V(D)J Igh rearrangement are not the only reason why there is a block preventing progression of YY1-deficient pro-B cells into pre-B cells, because the presence of a rearranged IgH transgene is only partially able to rescue pre–B-cell differentiation (13). In these IgH transgenic YY1−/− mice, the number of pre-B, immature B, and mature B cells was still significantly lower compared with wild-type mice. The lack of robust differentiation into pre-B cells in the presence of the IgH transgene was not believed to be due to defects in expression of any known transcription factors or other regulators that were assayed by semiquantitative PCR, although the signaling component of the pre-BCR, Igα, was reduced approximately twofold (13).
B-cell progenitors in the bone marrow (BM) differentiate from pro-B to pre-B cells, and after successful rearrangement of one of the light chain loci, they become immature B cells. These cells leave the BM and further mature in the spleen into marginal zone (MZ) or follicular B cells. When a naive B cell encounters antigen, it becomes activated, enters a germinal center (GC), and becomes a GC B cell in which somatic hypermutation (SHM) and class switch recombination (CSR) occur, generating high-affinity Ig. GC B cells then differentiate into memory B cells or plasma cells (PCs), with the latter secreting large amounts of Ig. How YY1 regulates later stages of B-cell differentiation is unknown because the mb1-Cre results in a complete block in differentiation at the pro–B-cell stage. However, a recent study identified unique transcriptional signatures of genes specific for each stage of normal B-cell differentiation (19). The bioinformatic analysis of the signature genes for each stage showed that only the GC B-cell signature genes had a significant enrichment for YY1 binding motifs, and thus the authors concluded that YY1 was a regulator of the GC B-cell–specific transcriptional program. Furthermore, using an in vitro class switch culture system, it has been demonstrated that YY1 interacts with activation-induced cytidine deaminase (AID) and increases AID nuclear stability (20). AID is activated in GC B cells to carry out SHM and CSR, thus providing an additional potential role for YY1 during in vivo GC B-cell development.
If YY1 were indeed a critical regulator of GC B-cell differentiation, then we predicted that a GC-specific deletion of YY1 should ablate the ability of naive B cells to become GC B cells. Here, we present data showing that conditional deletion of YY1 in B cells with Cγ1-Cre within 2 d after antigen activation does indeed prevent the appearance of GC B cells after antigen stimulation. To determine if YY1 was only critical for the differentiation of GC B cells, we analyzed mice in which YY1 was deleted by CD19-Cre, which deletes in the late BM stage. These mice showed that, unlike the prediction based on the bioinformatic analysis of B-signature genes, YY1 is required for every stage of B-cell maturation. Analysis of genes that bound YY1 in ChIP-sequencing (ChIP-seq) at four stages of differentiation and comparison of the RNA-sequencing (RNA-seq) gene expression profile of pro-B cells from wild-type mice vs. YY1-deficient mice provided insight into the basic common pathways regulated directly and indirectly by YY1 throughout B-cell differentiation, such as ribosomal functions, cell proliferation, transcription, and mitochondrial energetics.
Results
YY1 Is Essential for GC and PC Differentiation.
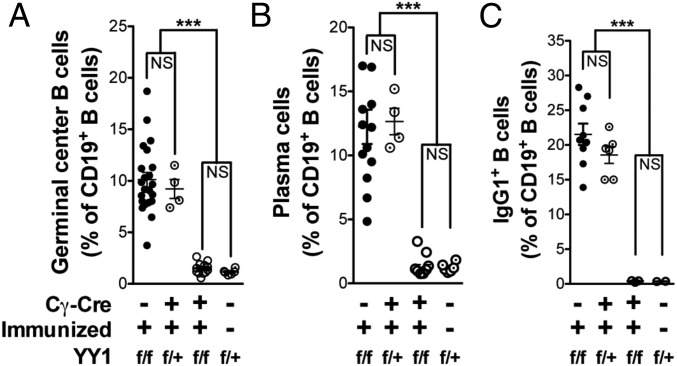
As a direct test of the prediction of the bioinformatics study by Green et al. (19), we assessed whether deletion of YY1 would preclude differentiation of naive B cells into GC B cells. We therefore crossed YY1-floxed (YY1f/f) mice with mice expressing Cre under control of the Cγ1 promoter (21). This promoter is located upstream of the Cγ1 switch region, and it only becomes active after antigen stimulation, deleting with an efficiency of 85–95% (21). Mice were immunized with ovalbumin (OVA) and LPS in alum, a protocol that generates large numbers of GC B cells, and spleens were analyzed by flow cytometry on day 8 after immunization (Fig. S1A). In control immunized Cre− littermates, 10% of the CD19+ spleen cells were GC B cells, whereas only 1.5% of B cells from YY1-deficient mice were GC B cells, similar to the percentage in unimmunized control mice (1.2%) (Fig. 1A). Absolute numbers revealed a 28-fold drop in GC B cells in immunized Cre+ versus Cre− mice (Table 1). This result demonstrates that, as predicted, YY1 is essential for the differentiation of GC B cells.
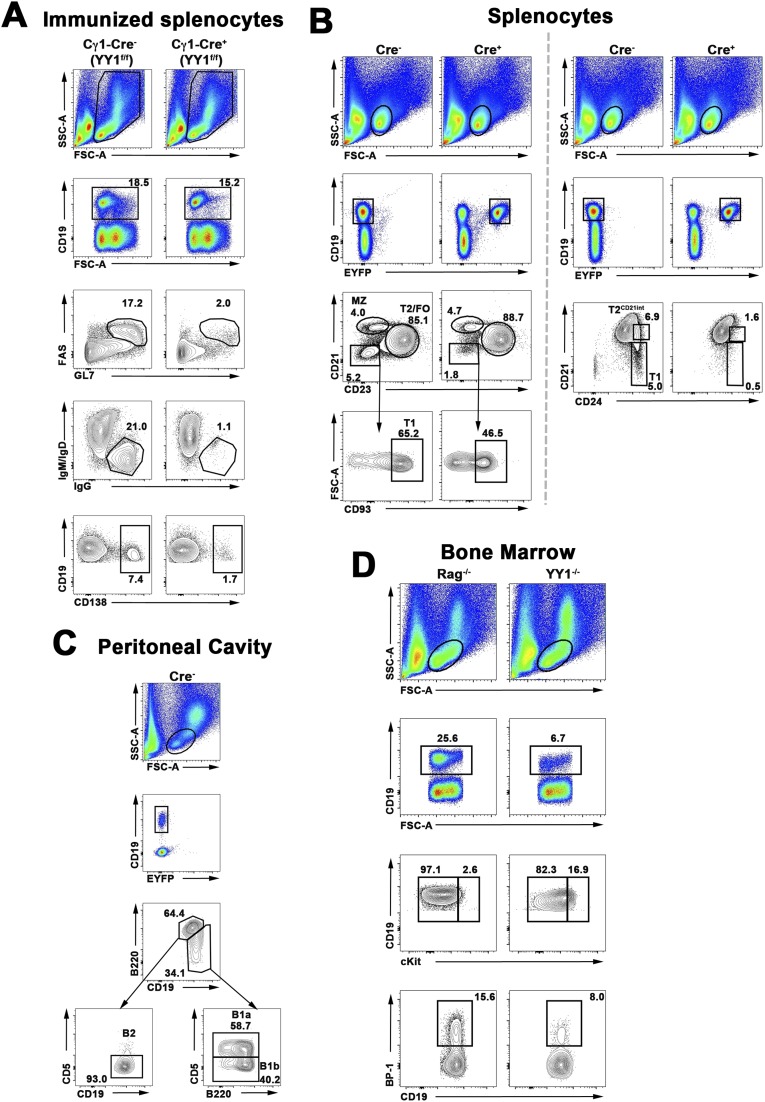
Fig. S1.
B-cell subset gating strategy for the spleen, peritoneal cavity, and BM. (A) GC, PC, and IgG1+ B-cell gating scheme of immunized YY1f/f × Cγ1-Cre mice. YY1f/f × Cγ1-Cre− or YY1f/f × Cγ1-Cre+ mice were immunized intraperitoneally with alum/LPS/OVA. Splenocytes were analyzed 8 d after immunization. Total splenocytes (top gate) were further gated on total B cells (CD19+). From there, GC B cells are identified as Fas+GL7+ (third row). IgG1+ B cells (fourth row) are characterized as IgG1+IgMloIgDlo. PCs are identified as CD138+. Numbers inside FACS plots indicate cell percentage. (B) Gating scheme for splenic B cells. YY1f/f × hCD20-Tam-Cre+ or YY1f/f × hCD20-Tam-Cre− mice were treated with tamoxifen as described in SI Materials and Methods. Seven days after the last tamoxifen injection, splenocytes were harvested and stained. (Left) Depiction of gating strategy for MZ, T2/FO, and CD21−CD23−AA4+ T1 cells. (Right) Depiction of gating strategy for CD24hiCD21− T1 and T2CD21int cells. From the lymphocyte gate (top row), cells were gated on either total CD19+ B cells (Tam-Cre−) or YFP+CD19+ cells (Tam-Cre+). (Left) Third row shows B cells being subdivided into MZ (CD21hiCD23lo), T2/FO (CD23+CD21int), or CD21−CD23− B cells. CD21−CD23− B cells are classified as T1 cells after being gated as CD93/AA4+. (Right) Third row depicts T1 cells as CD24hiCD21− and T2CD21int cells as CD24hiCD21int. The same gating strategy was applied to the CD19-Cre mice. (C) Gating scheme for peritoneal B2 and B1 B-cell subsets. YY1f/f × hCD20-Tam-Cre− mice were treated with tamoxifen as described in SI Materials and Methods. Seven days after tamoxifen treatment, peritoneal lavage exudate cells were stained. Lymphocytes (top row) were further gated on either CD19+ cells (Cre−) or YFP+CD19+ cells (not shown). B cells were gated on either B220hiCD19+ or B220lo-intCD19hi cells. B220hiCD19+ cells lacking CD5 were B2 cells. B220lo-intCD19hi cells that were CD5+ were identified as B1a B cells. B220lo-intCD19hi cells that were low/negative for CD5 were identified as B1b B cells. The same gating strategy was applied to the CD19-Cre mice. (D) Gating scheme for c-Kithi or BP-1+ BM B cells. Rag−/− or YY1−/− (mb1-Cre) BM cells were gated by size for lymphocytes (top row), and then further gated on total B cells (CD19+). Early stage pro-B cells were c-Kithi, whereas late pro-B cells were BP-1+.
Fig. 1.
YY1 deletion prevents the differentiation of naive B cells into GC B cells, PCs, and IgG1+ isotype-switched cells. YY1f/f × Cγ1-Cre mice were immunized with OVA/LPS/alum. Splenic B-cell populations from immunized mice and unimmunized controls were analyzed by flow cytometry 8 d after immunization. YY1 deletion prevented the differentiation of naive B cells into GC B cells (A), PCs (B), and IgG1+ B cells (C). Values from individual mice are displayed, and they are derived from a total of four experiments. ***P < 0.0001. NS, not significant.
Table 1.
Absolute number of GC, PC, and IgG1+ B cells
| Cre | Immunized | YY1 | GC | PC | IgG1+ |
| Cγ1-Cre− | Yes | f/f | 7.66 ± 0.29† | 5.16 ± 0.24 | 10.64 ± 0.44 |
| Cγ1-Cre+ | Yes | f/f | 0.27 ± 0.08*** | 0.37 ± 0.02*** | 0.49 ± 0.10*** |
| Cγ1-Cre− | No | f/f | 0.17 ± 0.01*** | 0.26 ± 0.03*** | 0.17 ± 0.08† |
×106 ± SEM.
P < 0.001.
We also analyzed these mice for the production of PCs and for IgG1+ isotype-switched cells because these cells predominantly derive from GC B cells. Cre− littermates had nearly 12% PCs, whereas YY1-deficient mice had 1.3% PCs, similar to unimmunized control mice (Fig. 1B). The immunization protocol that we used with alum as the adjuvant promotes CSR to IgG1. Cre−-immunized control mice had 21.5% IgG1+ B cells, similar to the level in heterozygous YY1+/− mice (18.6%), but the percentage in YY1−/− mice was only 0.4% (Fig. 1C). A half-dosage of YY1 also appeared to be sufficient for fairly normal GC and PC B-cell development as assayed in YY1f/+ heterozygotes. Together, these data clearly demonstrate that YY1 is essential for the differentiation of naive B cells into GC B cells, isotype-switched B cells, and PCs in vivo.
YY1 Is Required for Peripheral B-Cell Differentiation.
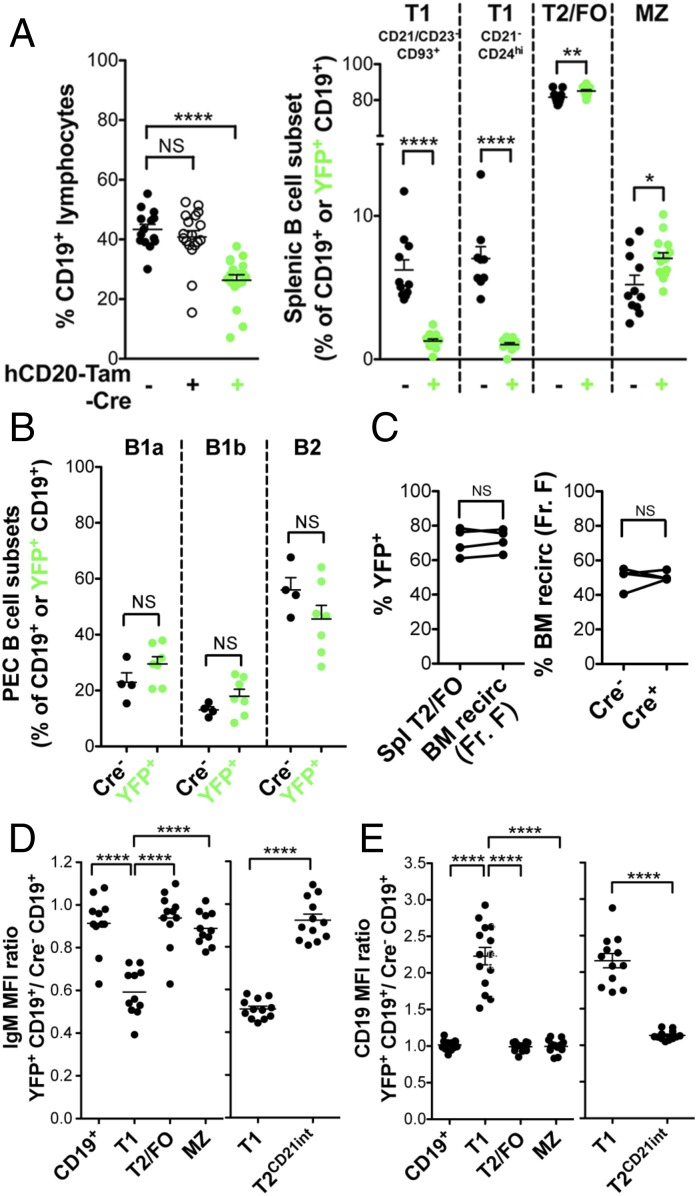
The study of Green et al. (19) suggested that YY1 was predominantly enriched in the signature genes of GC B cells and not of other B-cell subsets. Therefore, to determine if YY1 played a role in the differentiation of peripheral B cells, we crossed the YY1f/f mice with mice expressing Cre under control of the CD19 promoter (22). Although CD19 is expressed in B cells from the pro–B-cell stage onward, CD19-Cre does not delete efficiently in the BM (23). Because the CD19 promoter is still leaky in peripheral cells, we also crossed these mice to ROSA26-EYFP reporter mice to identify the cells in which the CD19-Cre is active (24). A transcriptional stop in front of the EYFP gene is flanked with loxP sites, and will be deleted in cells that express Cre. Thus, EYFP expression indicates cells in which Cre is active. Approximately 80% of spleen cells are YFP+ when CD19-Cre is used, whereas only ∼33% of BM cells are YFP+ (23).
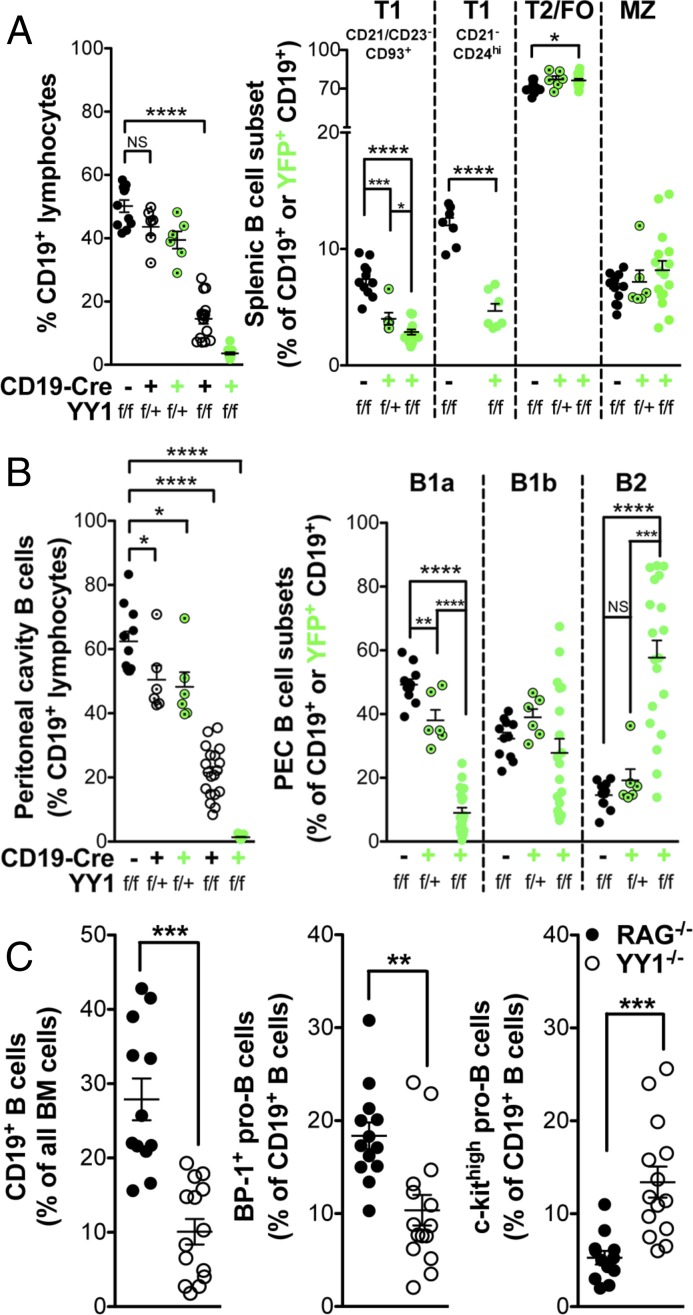
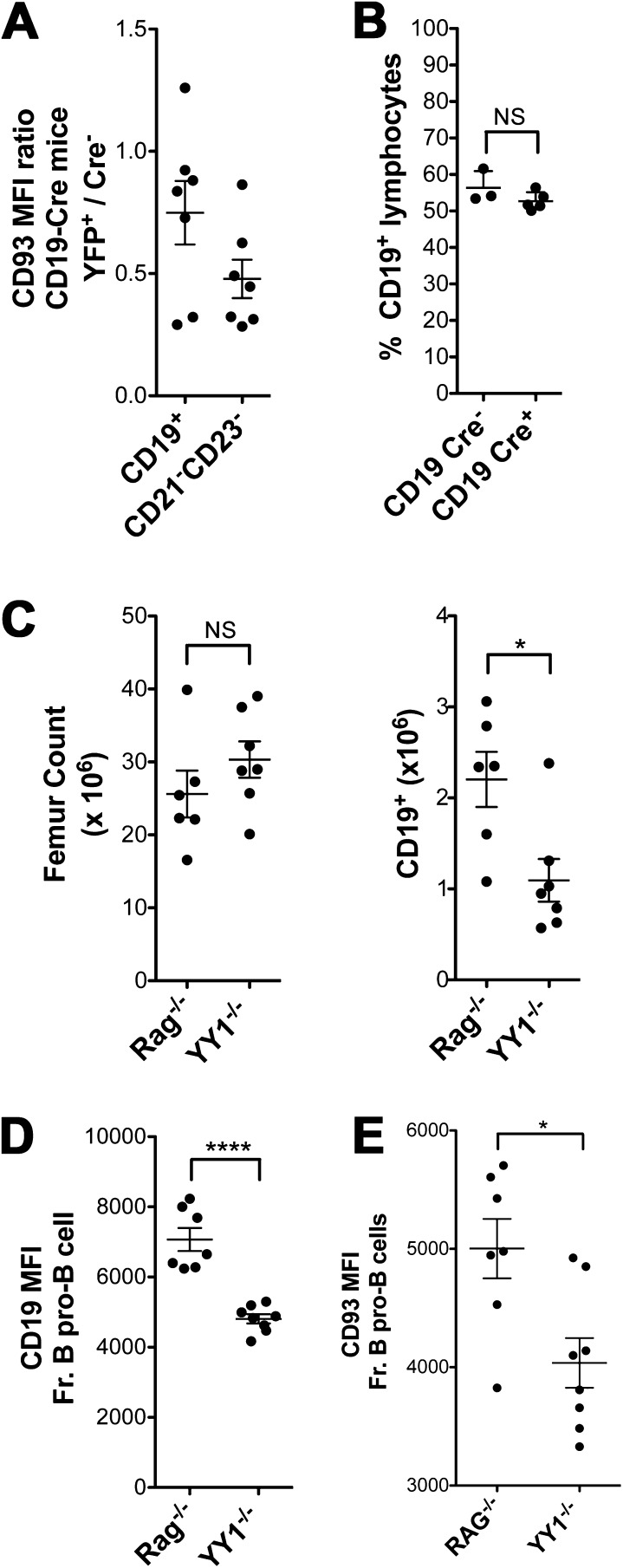
Flow cytometric analysis of YY1f/f × CD19-Cre+ mice versus control Cre− littermates indicated a roughly sixfold drop in the absolute number of splenic B cells (Table 2). Most of the remaining cells were YFP−, indicating that YY1 had not been deleted in them. This number is in accord with the published data for incomplete Cre activation with CD19-Cre+ (23). Importantly, there was a 23-fold drop in the absolute number of YFP+CD19+ B cells compared with CD19+ cells in Cre− littermates, indicating the essential role of YY1 in peripheral B-cell differentiation (Fig. 2A and Table 2). Within the small number of remaining CD19+YFP+ B cells, there were only modest perturbations among more mature splenic B-cell subsets: transitional 2/follicular (T2/FO) or MZ B cells. However, we observed a very significant drop in the percentage of transitional 1 (T1) B cells (Fig. 2A). To analyze T1 B cells, we used various aspects of the gating strategies described by Allman et al. (25, 26) and Loder et al. (27). We first gated on CD21− and CD23− cells and then gated on CD93+ cells, as shown in Fig. S1B (Left). We chose to gate CD93 last because we observed YY1-deficient splenic B cells have reduced surface CD93 expression (Fig. S2A). This strategy prevented inadvertent inclusion of mature B cells into our T1 gates. Additionally, back-gating confirmed that these T1-gated cells were IgMhi. We also used a second gating strategy developed by Su and Rawlings (28) that does not involve the use of CD93. This strategy uses CD21 and CD24 markers, where T1 cells are easily discernible as CD21− and CD24hi (Fig. S1B, Right). In fact, this gating scheme has recently been adopted to characterize human transitional B cells (29). Both gating schemes yielded similar percentages of T1 B cells, thus clearly demonstrating that YY1 deletion results in significantly fewer T1 B cells. Heterozygous mice had only slightly reduced B-cell numbers compared with YY1f/f × CD19-Cre− mice, and this nonsignificant reduction was similar to the slight reduction due to Cre expression alone in YY1+/+ × CD19-Cre+ mice (Fig. S2B). Importantly, most of the CD19+ cells in the heterozygous mice were YFP+ (Fig. 2A), indicating that a half-dosage of YY1 is largely sufficient to reconstitute the peripheral B-cell compartment. In fact, the only significant effect of the decreased dosage of YY1 was the marked decrease in the proportion of T1 B cells, indicating again the sensitivity of this immature peripheral subset to decreases in YY1 levels. Thus, CD19-Cre–mediated YY1 deletion reduces overall splenic B-cell numbers profoundly, with the most pronounced effect on T1 B-cell development.
Table 2.
Absolute number of splenic B cells
| Cre | YY1 | Total CD19+ | YFP+CD19+ |
| CD19-Cre− | f/f | 6.22 ± 0.56† | |
| CD19-Cre+ | f/+ | 5.60 ± 1.77 | 5.11 ± 1.75 |
| CD19-Cre+ | f/f | 1.08 ± 0.14**** | 0.27 ± 0.03**** |
| hCD20-Tam-Cre− | f/f | 8.91 ± 0.64 | |
| hCD20-Tam-Cre+ | f/f | 8.39 ± 0.59 | 5.37 ± 0.45**** |
× 107 ± SEM.
P < 0.0001.
Fig. 2.
(A and B) YY1 deletion regulates all stages of B-cell development. YY1 deletion in peripheral B cells is driven by CD19-Cre. (Left to Right) Scatter dot plots display CD19+ percentage and B-cell subset percentage. Black circles represent Cre− control littermates, open circles with middle dots represent YY1 heterozygotes, and open circles represent homozygous mutants. YY1 deletion is monitored by Rosa-EYFP fluorescence. The percentage of YFP+ heterozygotes or YFP+ homozygotes (i.e., YY1-deleted) is shown as green circles. Data represent seven independent experiments, except for T1 CD21−CD24hi data (A), which are from two independent experiments. PEC, peritioneal cavity. (C) YY1 deletion in BM pro-B cells was induced by mb1-Cre. These mice were compared with Rag1−/− mice in which B-cell differentiation is blocked at the pro-B–to–pre-B transition. The percentage of BM CD19+ pro-B cells (Left); the percentage of BP-1+ pro-B cells, characteristic of later stage pro-B cells (Center); and the percentage of c-kithigh early pro-B cells (Right) are shown. BM data represent five independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Fig. S2.
Effect of YY1 deletion in YY1f/f × CD19-Cre and YY1f/f × mb1-Cre mice. (A) CD93 expression is lower in YY1f/f × CD19-Cre+ total B cells as well as CD21−CD23− B cells. Scatter dot plot displays the ratio of CD93 surface expression in YFP+ (Cre+) cells relative to Cre− cells. (B) Cre enzyme expression does not alter B-cell percentage. Splenocytes from CD19-Cre− or CD19-Cre+ mice were stained for CD19, and the percentage of CD19+ cells in the lymphocyte size gate is displayed. CD19-Cre data are representative of two independent experiments. (C) YY1f/f × mb1-Cre mice have fewer BM B cells versus Rag−/− mice. Femur cells counts (Left) and CD19+ B-cell numbers (Right) are displayed for BM from Rag−/− and YY1−/− mice. CD19 MFI (D) and CD93 MFI (E) scatter dot plots are shown for Rag−/− and YY1−/− BM fraction B pro-B cells (BP-1−). Data are representative of two independent experiments. *P < 0.05; **P < 0.01; ****P < 0.0001.
YY1 Is Important for the B1-Cell Lineage.
B1 B cells, located predominantly in the peritoneal cavity, are an important source of natural antibodies that help protect against infectious agents. Because YY1 deficiency prevented mature splenic B-cell development, we asked whether YY1 had a similar effect on peritoneal B cells (gating scheme is shown in Fig. S1C). Sixty-two percent of peritoneal cavity exudate lymphocyte-sized cells were CD19+ in wild-type littermates compared with 21.4% in YY1f/f × CD19-Cre/ROSA26.EYFP mice, but only 1.3% of B cells from Cre+ mice were YFP+, indicating a severe reduction in peritoneal cavity B cells in the absence of YY1 (Fig. 2B). Heterozygotes displayed a modest decrease in the percentage of B cells, with almost all being YFP+, indicating that a half-dosage of YY1 is largely sufficient for the differentiation of peritoneal B cells. Within the few remaining CD19+YFP+ cells, the B1a cells were the most sensitive to the absence of YY1. Both heterozygotes and YY1f/f × CD19-Cre+ mice had significantly reduced B1a cells, with the B2 B-cell percentage therefore increasing in these mice.
YY1 Pro-B Cells Do Not Fully Differentiate in Vivo.
It has been reported that YY1f/f × mb1-Cre mice have a block in their differentiation at the pro–B-cell stage, resulting in twice the number of pro-B cells, and essentially no pre-B cells or later stages of differentiation (13). Because it is extremely difficult to discriminate accurately between pro-B cells and pre-B cells by FACS due to incomplete separation of the CD43+ pro-B cells from the CD43− pre-B cells, we compared YY1−/− BM cells with Rag−/− BM cells. With this approach, we have observed that there are fewer CD19+ cells in the BM of YY1−/− mice compared with Rag−/− mice (Fig. 2C and Fig. S2C) and all of the CD19+ cells are CD43+CD2−, the phenotype of pro-B cells, in both strains. Among the CD19+ pro-B cells, there is a higher percentage of c-kithigh pro-B cells in YY1−/− mice compared with Rag−/− mice, with c-kit being a marker of early pro–B-cell differentiation (Fig. 2C, Right, and Fig. S1D). In the Hardy fractionation scheme of pro-B cells, fraction C is the most mature subset, and it is marked by the acquisition of BP-1 (30). We observed fewer BP-1+ fraction C pro-B cells in the CD19+ cells of YY1−/− mice compared with Rag−/− mice (Fig. 2C, Center, and Fig. S1D). We also observed that YY1-deficient pro-B cells have lower surface CD19 (Fig. S2D), potentially hampering their ability to signal through pre-BCR complex and progress to the pre–B-cell stage (31). We also observed reduced surface CD93 expression in YY1-deficient pro-B cells consistent with the decrease in CD93 mean fluorescence intensity in transitional cells in YY1f/f × CD19-Cre+ mice (Fig. S2E). Thus, by surface expression of both c-kit and BP-1, pro-B cells in YY1−/− mice appear more immature than pro-B cells in Rag−/− mice. We conclude that even in vivo, YY1 deletion by mb1-Cre results in a decrease in the most mature pro-B cells. Thus, YY1 is necessary for proper differentiation of all B-cell subsets that we have examined in vivo.
Role of YY1 in B-Cell Subset Maintenance.
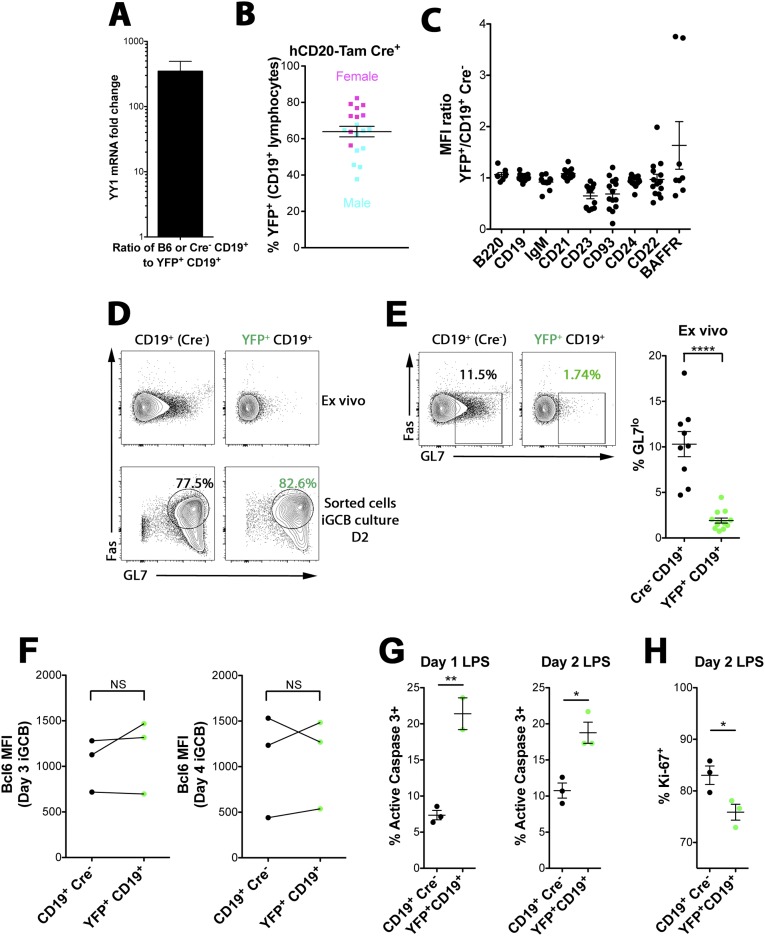
We determined that YY1 is needed for the differentiation of B cells, but to ask if it is also required for their maintenance in vivo, we treated YY1-floxed mice bearing a B-cell–specific tamoxifen-responsive Cre transgene (hCD20.Tam-Cre) and the ROSA-EYFP reporter gene with tamoxifen for 3 consecutive days, and then assayed the spleens 7 d after the last tamoxifen injection (32). We verified that YFP+ cells had efficiently deleted the YY1 gene by assaying for YY1 mRNA expression using quantitative PCR (qPCR) from sorted cells (Fig. S3A). We observed no significant effect of tamoxifen treatment on overall B-cell percentage and B-cell numbers (Table 2), although only about 40–65% of the CD19+ spleen cells were YFP+ (Fig. 3A and Fig. S3B). We attribute this lower percentage to incomplete deletion by hCD20.Tam-Cre at the tamoxifen dosage used. The most striking phenotype was the sharp decrease in T1 B cells when comparing control littermate B cells versus YFP+ cells (Fig. 3A). Because we observed a decrease in surface CD93 expression (Fig. S3C), we used the two independent gating schemes described above to gate T1 B cells. Both gating schemes yielded similar percentages of T1 B cells and clearly demonstrate that acute YY1 deletion results in significantly fewer T1 B cells. The percentage of mature B cells (T2/FO) within the YFP+ cells increased slightly, presumably due to available space created by T1 depletion. We observed no effect of acute YY1 deletion on peritoneal or recirculating fraction F BM B-cell percentages (Fig. 3 B and C), further demonstrating that YY1 is not needed for short-term maintenance of most mature B-cell subsets other than T1 cells, at least within the 1-wk period after Cre activation that we analyzed here.
Fig. S3.
Effect of YY1 depletion on differentiation, survival, and proliferation. (A) Tamoxifen treatment results in efficient YY1 gene deletion. YY1f/f × hCD20-Tam-Cre mice were injected intraperitoneally with tamoxifen in olive oil for three consecutive days, and spleens were harvested 7 d after the last injection. Splenocytes were sorted for either CD19+ (Cre− or B6 control mice) or YFP+CD19+ (Cre+ mice). RNA from these cells was used for qPCR of the YY1 transcript. Displayed is the mRNA fold change of control CD19+ to YFP+CD19+ YY1 transcript abundance. Data are representative of four independent experiments, one of which used B6 B cells as the control. (B) Percentage of splenocytes from tamoxifen (Tam)-treated YY1f/f × hCD20-Tam-Cre mice that were YFP+ in either female mice (pink squares) or male mice (aqua squares) is displayed. (C) MFI ratio from CD19+YFP+ (Cre+) relative to CD19+ (Cre−) B cells is displayed for multiple cell surface proteins. Data are representative of at least two independent experiments for each surface protein. (D–F) iGCB cultured cells express Fas, GL7, and Bcl6. YY1f/f × hCD20-Tam-Cre mice were treated with tamoxifen as described above. Splenocytes were sorted for CD19+ (Cre− mice) or YFP+CD19+ (Cre+ mice) and cultured under iGCB conditions. (D) Fas and GL7 surface expression is shown in FACS plots for unsorted ex vivo splenocytes gated on CD19+ (Cre− mouse) or YFP+CD19+ (Cre+ mouse) (Upper), as well as sorted cells iGCB-cultured for 2 d (Lower). Data are representative of four independent experiments. (E) Ex vivo splenocytes shown in D are displayed with gating on GL7lo cells. Quantification of ex vivo GL7lo cells representing six independent experiments is shown next to FACS plots. Numbers inside FACS plots in D and E represent cell percentages within respective cell populations, with green numbers representing the percentage within YFP+CD19+ cells. (F) Sorted splenocytes were cultured under iGCB conditions and stained for intracellular Bcl6 expression. MFI quantification of intracellular Bcl6 expression from three independent experiments for both day 3 and day 4 iGCB cultures is shown. (G) Similar to YY1f/f × hCD20-Tam-Cre mice, YY1-deficient B cells in the CD19-Cre system undergo more apoptosis and less proliferation relative to littermate control Cre−/CD19+ cells. YY1f/f × CD19-Cre+ or Cre− splenocytes were cultured for 1 or 2 d with 10 μg/mL LPS. Cells were then assayed for apoptosis using active caspase 3 (G) or for proliferation using Ki-67 (H). Cells in this experiment were not sorted because prefixing before permeabilization retained YFP expression. Data are from one experiment, with three mice per genotype. An unpaired two-tailed t test (E, G, and H) or paired two-tailed t test (F) was used to calculate statistical significance. *P < 0.05; **P < 0.01; ****P < 0.0001.
Fig. 3.
YY1 regulates T1 B-cell maintenance. B-cell–specific tamoxifen-responsive hCD20.Tam-Cre mice (YY1f/f × hCD20.Tam-Cre.Rosa26.EYFP) were induced by three daily intraperitoneal injections of tamoxifen, followed by FACS analysis 7 d after the last injection. Scatter dot plot analysis of B cells and/or subsets from the spleen (A), PEC (B), or BM recirculating fraction F (Fr. F) B cells (C). In A and B, black circles represent Cre− control littermates and open circles represent homozygous mutants. The cell percentages within the YFP+ homozygotes are illustrated by green circles. Scatter dot plots displaying the median fluorescence intensity (MFI) of CD19 (D) or IgM (E) expressed as the ratio of YFP+CD19+ to CD19+/Cre− from spleens of tamoxifen-treated YY1f/f × hCD20.Tam-Cre.Rosa26.EYFP mice are shown. Data are shown for total splenic B cells and for B-cell subsets, and the data are from two to eight independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. NS, not significant.
T1 B cells are acutely sensitive to BCR signaling intensities, undergoing negative selection when too self-reactive (28). Using both T1 B-cell gating schemes (Fig. S1B), we observed down-regulated expression of IgM in YY1-deficient T1 B cells (Fig. 3D). Surprisingly, we observed up-regulated co-BCR receptor CD19 expression specifically in YY1-deficient T1 B cells (Fig. 3E). CD19 and IgM levels reverted back to levels observed in control mice after the T1 stage of differentiation (Fig. S3C). Thus, BCR component expression is dysregulated in YY1-deficient T1 B cells, but these aberrant cells are presumably rapidly eliminated because the T1 compartment is very small and the subsequent cells have normal levels of CD19 and IgM.
YY1-Deficient Cells Do Not Persist During in Vitro Culture.
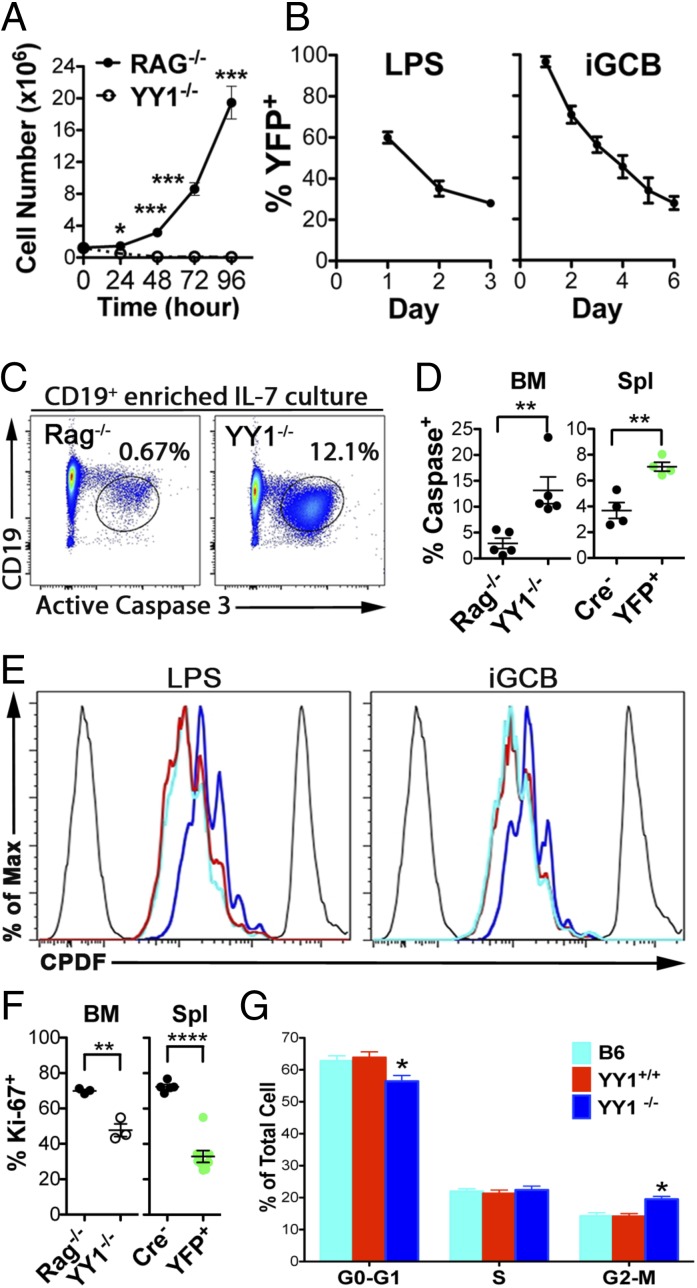
Due to the observed defect in B-cell development using multiple mouse lines, we next asked if YY1-deficient B cells could persist during in vitro culture. Pro-B cells normally proliferate after 2–3 d in culture with IL-7 and continue to expand for a week. In Fig. 4A, the Rag−/− pro-B cells expanded 20-fold in 4 d. However, YY1f/f × mb1-Cre pro-B cells are unable to expand in culture, and the majority were dead within 2 d (Fig. 4A).
Fig. 4.
YY1 regulates B-cell survival and proliferation. (A) Rag1−/− pro-B cells and YY1f/f × mb1-Cre pro-B cells were cultured for 4 d in IL-7 culture media and counted daily. Data represent two independent experiments. (B) CD19+-purified YY1f/f × hCD20.Tam-Cre+ cells containing both YFP+ and YFP− were cultured in either 10 μg/mL LPS (Left) or iGCB (Right) culture conditions. Cells were assayed for the presence of YFP at indicated times, and the YFP+ percentage decrease relative to the starting YFP+ percentage over time is plotted. The LPS data represent three independent experiments. The iGCB data represent five independent experiments. BM cells were cultured for 24 h with IL-7. A FACS plot (C) and scatter dot plot (D, Left) show the percentage of active caspase 3 in Rag−/− versus YY1−/− pro-B cells. (D, Right) Scatter dot plot showing the percentage of active caspase 3+ cells comparing sorted YFP+ (YY1f/f × hCD20.Tam-Cre mice) versus CD19+ (Cre− littermates) spleen (Spl) cells cultured for 24 h with 10 μg/mL LPS. Data represent three independent experiments. (E) Naive splenocytes purified by negative selection from tamoxifen-treated YY1f/f × hCD20.Tam-Cre mice were loaded with CPDF and cultured for 2.5 d in either LPS (Left) or iGCB (Right) culture. Proliferation is detected by dye dilution. The aqua line is B6, the blue line is Cre+ YY1-deficient B cells, and the red line is B cells from Cre− littermates. Freshly labeled cells (Right) and unlabeled cells (Left) are shown. (F) Scatter dot plot of the percentage of Ki-67+CD19+ B cells from total BM cells cultured for 2 d in IL-7 (Left) or the percentage of Ki-67+CD19+ B cells from total splenocytes (tamoxifen-treated YY1f/f × hCD20.Tam-Cre mice) cultured for 2 d with LPS (Right). Green dots indicate the percentage of Ki-67+ from YFP+ B cells. Ki-67 data represent two independent experiments. (G) YFP+ (YY1-deficient) cells are delayed in the G2-M cell cycle stage. Naive splenocytes purified by negative selection as in E were cultured for 4 d in iGCB culture conditions and assessed for cell cycle using Hoechst 33342 staining. Colors are the same as in E. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
To investigate the ability of splenic B cells to persist in response to LPS, we injected YY1f/f × hCD20.Tam-Cre mice with tamoxifen for 3 consecutive days, and then splenic CD19+ B cells were purified 7 d later. Cells from Cre+ mice (containing both YFP+ and YFP−) were cultured with LPS and analyzed by flow cytometry at days 1, 2, and 3 after the start of the culture. It can be seen that the percentage of EYFP+ (YY1-deleted) splenic B cells precipitously drops during LPS-stimulated cultures (Fig. 4B, Left).
CD19+-enriched Cre+ splenocytes were then used in “induced GC B-cell” (iGCB) cultures (33). In this culture system, B cells are cultured on 3T3 cells that have been transfected with CD40 ligand and BAFF in the presence of IL-4. Normal B cells can be very efficiently induced to express Fas and GL7, the canonical markers of GC B cells, within 2 d in this culture system. Using this iGCB culture system, we observed that even the EYFP+ (YY1-deficient) cells became Fas+GL7+ (Fig. S3D), which is in contrast to the in vivo situation in which Fas+GL7+ cells do not develop in YY1f/f × Cγ1-Cre–immunized mice. In addition, YY1-deficient iGCB-cultured cells (YFP+) also became Bcl6+ to the same extent as Cre−/CD19+ cells (Fig. S3F). Despite the ability of YY1-deficient B cells to differentiate into cells with the phenotype of GC B cells in vitro, they decreased in percentage over time relative to the YFP− (YY1-sufficient) cells (Fig. 4B, Right). Therefore, YY1-deficient B cells do not persist in culture in this iGCB system, or in IL-7– or LPS-stimulated culture.
YY1-Deficient B Cells Display Defective Proliferation and Survival.
Defective proliferation and apoptosis are two possible explanations of our in vitro and in vivo data. To test for YY1-regulated apoptosis, we assayed for active caspase 3, which detects cells actively undergoing caspase-dependent apoptosis, under different culture conditions. First, we cultured CD19+-enriched BM B cells from both Rag−/− and YY1−/− mice in IL-7 for 24 h and assessed active caspase 3 levels. YY1-deficient BM pro-B cells were significantly more prone to undergoing apoptosis relative to their Rag-deficient BM pro–B-cell counterparts (Fig. 4 C and D, Left). We also sorted YY1-deleted (CD19+YFP+) or CD19+ (YFP−) Cre− littermate control splenic B cells from tamoxifen-injected YY1f/f × hCD20.Tam-Cre mice, and subsequently cultured the sorted cells for 24 h with LPS. Again, peripheral YY1-deficient B cells displayed enhanced apoptosis relative to control Cre− B cells (Fig. 4D, Right). We observed the same effect of YY1-deficient–mediated apoptosis in peripheral B cells using sorted B cells from YY1f/f × CD19-Cre+ or Cre− mice (Fig. S3G). Our data show that YY1 normally promotes BM and peripheral B-cell survival.
To test for any proliferative defect, we cultured purified naive B cells from tamoxifen-treated YY1f/f × hCD20.Tam-Cre/ROSA26.EYFP mice in which purified cells were labeled with Cell Proliferation Dye eFluor450 (CPDF) (eBioscience). Labeled cells were cultured for 2.5 d in either LPS or iGCB culture conditions, and CPDF dye dilution was assayed as a readout for proliferation. In both cases, we observed slower proliferation in the EYFP+ (YY1-deleted) cells than in the wild-type C57BL/6 (B6) B cells (Fig. 4E). The EYFP− cells (i.e., those cells that did not delete the floxed YY1 gene) from the YY1f/f × hCD20.Tam-Cre/ROSA26.EYFP naive B cells proliferated identical to those from B6 mice. We also assessed proliferation of both BM and peripheral B cells using the proliferation marker Ki-67. YY1-deficient (mb1-Cre) BM B cells were cultured for 48 h in the presence of IL-7 and stained for Ki-67. There was a significant reduction in Ki-67+ cells in YY1-deficient BM B cells relative to Rag−/− BM B cells, suggesting a defect in proliferation (Fig. 4F, Left). We observed a similar phenomenon among LPS-cultured splenocytes (tamoxifen-treated YY1f/f × hCD20.Tam-Cre/ROSA26.EYFP mice) in which YFP+ B cells had less Ki-67+ staining (Fig. 4F, Right). LPS-cultured CD19-Cre splenocytes also displayed the same trend (Fig. S3H). We next assessed the stages of the cell cycle using Hoechst 33342 on purified naive splenocytes cultured for 4 d in the iGCB culture system. We observed a delay of passage through the G2/M phase in EYFP+ (YY1-deleted) cells compared with B6 or EYFP− (YY1-intact) naive splenic B cells (Fig. 4G). Overall, our data illustrate a role for YY1 in promoting normal B-cell proliferation and survival. Therefore, in its absence, YY1-deficient B cells are defective in proliferation and succumb to apoptosis quicker than wild-type cells.
YY1 Binds Predominantly to Promoters.
We performed ChIP-seq for YY1 with chromatin from Rag1−/− pro-B cells and Rag1−/−μ+ pre-B cells on the C57BL/6 background, and cell sorter-purified follicular B cells and GC B cells from B6 mice. Rag−/− background mice were used for the pro-B cells and pre-B cells because, for other work in the laboratory, we are interested in the binding of YY1 to the Ig loci, and Rag−/− B-cell precursors have not undergone any deletion of genomic DNA from Ig loci by V(D)J recombination. Also, importantly for this study, the use of a genetic mutation that blocks differentiation cleanly, as the Rag deficiency does, ensures that the populations of pro-B cells and pre-B cells that we use for ChIP-seq are 100% pure. This finding is particularly important for the pro-B vs. pre-B separation for which CD43, even when coupled with CD2, does not give good enough discrimination.
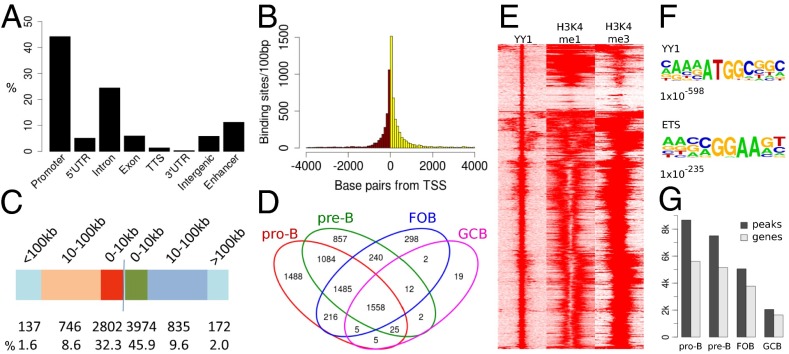
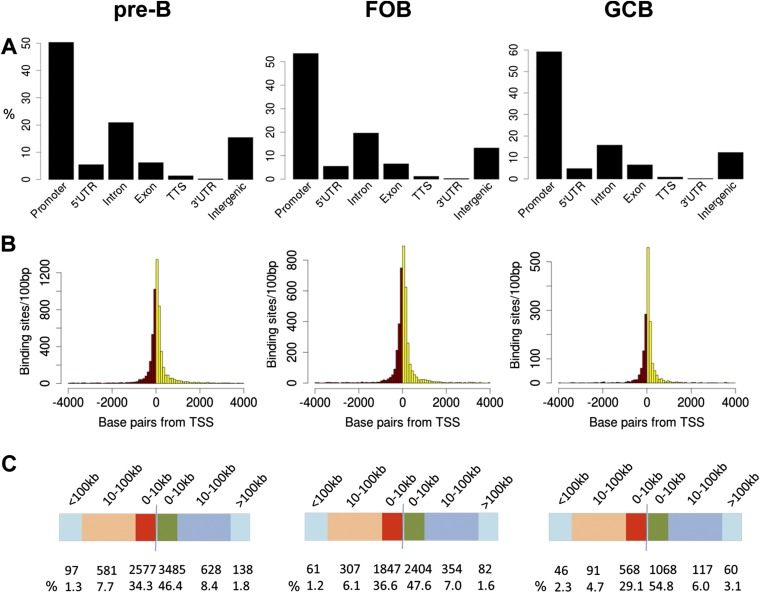
The locations of the YY1 sites relative to the closest genes were determined using the HOMER algorithm (34) (Fig. 5A). HOMER defines the promoter as −1 kb to +100 bp of the transcription start site (TSS). (Note that this result is different from many of our other analyses in which we have defined the promoter-related region as ±2.5 kb of the TSS.) The only exception to the HOMER classifications is that HOMER does not include a category for enhancer, so we have fractionated the “intergenic” category that we get from HOMER into the regions with or without H3K4me1, the epigenetic mark of enhancers. The data for pro-B cells are shown in Fig. 5, and data for other cell types are shown in Fig. S4. In all four cell types, ∼50% of the YY1 peaks were located in promoters (Fig. 5A and Fig. S4). Analyzing just the distance from the TSS of the closest gene to the location of the bound YY1, we observed that 67–84% of the YY1-bound sites are within 10 kb of the TSS in the four cell types (Fig. 5 B and C and Fig. S4). Fig. 5E also demonstrates that the majority of YY1 sites correlate with H3K4me3, the mark of promoters, and only a small subset of YY1 sites are present in regions marked by high H3K4me1 but lower H3K4me3 (enhancers) or regions with neither of these two epigenetic marks. Using HOMER, we searched in a 200-bp window surrounding the bound YY1 sites to see if any other transcription factor binding sites colocalized with YY1, but the only significant factor was the Ets family motif (Fig. 5F).
Fig. 5.
YY1 ChIP-seq analysis. (A) Percentage of significant YY1 binding sites in Rag−/− pro-B cells for each of the genomic features from the Reference Sequence (RefSeq) database as identified by HOMER. YY1 binding sites identified as intergenic were divided into those sites that overlap with significant H3K4me1 sites (called “enhancer” in A) and those sites that do not (called “intergenic” in A). (B) Histogram of distances between YY1 binding sites and nearest TSS in pro-B cells. Bin sizes of 100 bp were used. Red, YY1 sites upstream of the TSS; yellow, YY1 sites downstream of the TSS. (C) Distribution of YY1 binding sites in pro-B cells within 0–10 kb, 10–100 kb, and farther than 100 kb from the nearest TSS. (D) Venn diagram of overlaps between the genes bound by YY1 in the four different cell types. Genes bound by YY1 were defined as genes that had the YY1 binding site ± 2.5 kb from the TSS. (E) Heat map of ChIP-seq data showing distribution of the deposition of YY1, H3K4me1, and H3K4me3 in Rag−/− pro-B cells, assessed in a 6-kb window across YY1-bound sites, gated on genome-wide YY1-bound sites. (F) Sequence logos corresponding to enriched sequence elements identified by de novo motif analysis of YY1 binding sites using HOMER. Motifs were identified by analyzing a 200-bp window surrounding the bound YY1 site and comparing the motif frequency identified in that window with the motif frequency of randomly selected genomic regions. The number beneath the logo motif is the P value. (G) Number of significant YY1 total bound sites (peaks) and number of sites present within 2.5 kb of a gene (genes) from the four cell types.
Fig. S4.
YY1 ChIP-seq analysis. (A) Percentage of significant YY1 binding sites in RAG−/−μ+ pre-B cells, follicular B (FOB) cells, and GC B (GCB) cells from B6 mice for each of the genomic features from RefSeq as identified by HOMER. Because we did not do ChIP-seq for H3K4me1 for all of these cell types, we did not divide the intergenic category into enhancer or nonenhancer as we did in Fig 5A. (B) Histogram of distances between YY1 binding sites and nearest TSS. Bin sizes of 100 bp were used. Red, YY1 sites upstream of the TSS; yellow, YY1 sites downstream of the TSS. (C) Distribution of YY1 binding sites within 0–10 kb, 10–100 kb, and farther than 100 kb bins from the nearest TSS.
Analysis of the significant peaks in the ChIP-seq from all four cell types showed that pro-B cells harbor the most YY1 peaks, followed by pre-B cells and then follicular B cells (Fig. 5G). GC B cells had the lowest number of YY1 peaks. The vast majority (81%) of YY1 sites bound in GC B cells were also bound in the other three cell types, and GC B cells had the fewest significant sites (Fig. 5D). For follicular B cells, 33% of sites were common to all four cell types, or common with pro-B and pre-B cells (34%). However, both pro-B and pre-B cells also had many stage-specific YY1 sites, as well as a large number of sites common to just those two cell types (Fig. 5D).
Genes Bound by YY1 Are Involved in Common Cellular Functions.
We performed analysis of pathways and functions of the genes bound by YY1 using the “GREAT” algorithm (35) (Dataset S1). Despite the different numbers of genes bound by YY1 in the four different cell types (Fig. 5G), the functions and pathways were quite similar in all four. Common functions among YY1-bound genes are those functions related to ribosomes, the site of translation; functions related to mitochondria, such as NADH dehydrogenase activity, respiratory chain, and cytochrome oxidase electron transfer; and functions related to transcription, such as mRNA splicing, transcriptional elongation, and metabolism of RNA (Table 3).
Table 3.
Enrichment analysis of YY1 binding sites in murine pro-B cells
| Biological function | Gene ontology term | P value | FDR | Observed region hits |
| Ribosome function | Translation | 8.14E-19 | 7.53E-17 | 104 |
| Structural constituent of ribosome | 2.51E-16 | 2.04E-13 | 155 | |
| Ribosome biogenesis | 9.70E-12 | 1.66E-09 | 114 | |
| rRNA processing | 8.34E-09 | 9.42E-07 | 81 | |
| Mitochondria | Mitochondrial membrane part | 1.66E-15 | 5.92E-14 | 105 |
| Respiratory chain | 1.99E-10 | 4.86E-09 | 60 | |
| NADH to cytochrome oxidase electron transfer | 9.70E-08 | 3.13E-05 | 41 | |
| Transcription | Gene expression | 2.88E-51 | 4.53E-48 | 341 |
| Metabolism of RNA | 2.22E-42 | 1.74E-39 | 271 | |
| Formation and elongation of mRNA transcript | 3.5E-39 | 1.84E-36 | 192 | |
| mRNA splicing | 2.31E-36 | 7.29E-34 | 119 | |
| Nucleosome assembly | 4.59E-20 | 5.55E-18 | 44 | |
| NF-κB binding | 1.11E-06 | 9.53E-05 | 28 |
FDR, false-discovery rate.
Differential Gene Expression in Pro-B Cells.
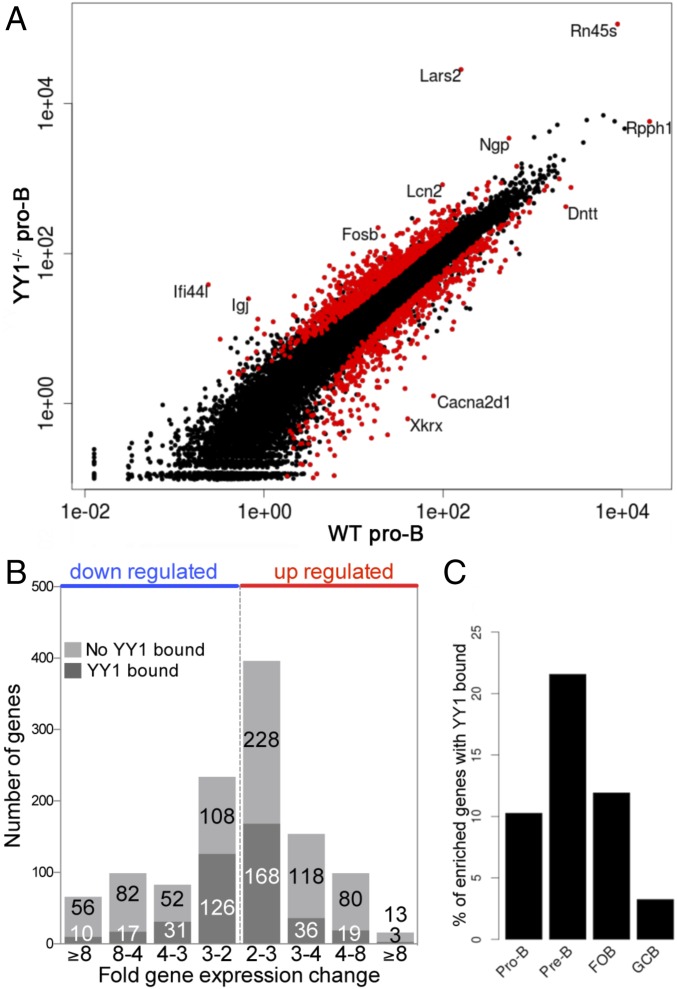
We performed RNA-seq on CD19+ pro-B cells isolated from Rag−/− or from Rag−/− YY1f/f × mb1-Cre mice, and differential gene expression was analyzed using EdgeR (36). As described above, we used mice that were on the Rag−/− background to obtain a pure population of pro-B cells uncontaminated with any pre-B cells. qPCR of several genes validated the accuracy of our RNA-seq data (Fig. S5). The RNA-seq results are displayed in Fig. 6A. A total of 1,147 genes were greater than twofold differentially expressed (Fig. 6B). Genes that were higher in the YY1−/− pro-B RNA compared with the wild-type pro-B RNA include Lars2, leucyl-tRNA synthetase that is located in mitochondria; Rn45s, 45S preribosomal RNA; Ifi44l, an IFN-induced protein encoding a minor histocompatibility antigen; Igj, J chain, or joining chain, which links monomer units of IgM or IgA into multimers in mature B cells; and Fosb, a transcription factor that interacts with Jun proteins forming AP-1 complexes. Genes that were higher in the wild-type (Rag−/−) pro–B-cell RNA include Rpph1, ribonuclease P RNA component; Dntt, terminal deoxynucleotidyl transferase; and Cacna2d1, a subunit of a voltage-dependent calcium channel.
Fig. S5.
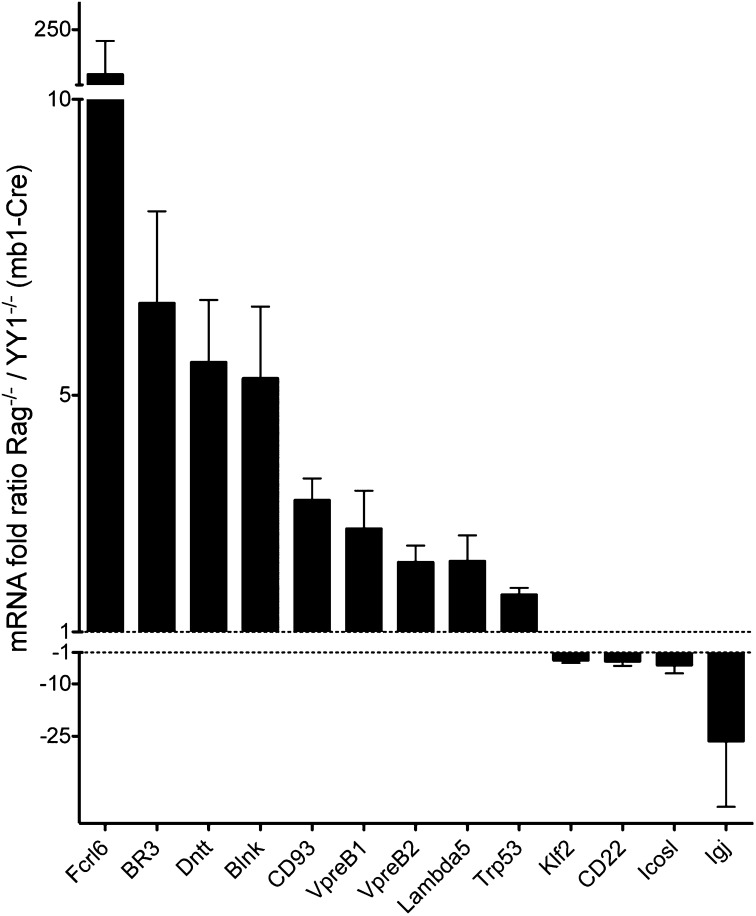
qPCR validation of RNA-seq data. RNA from CD19+-enriched pro-B cells from either Rag−/− or YY1−/− (mb1-Cre) mice was extracted, and qPCR was performed for the indicated genes. The bar graph shows genes elevated in Rag−/− above the dotted lines, whereas genes elevated in YY1−/− are shown below the dotted lines. Data for each gene are from two to three independent samples.
Fig. 6.
Differential gene expression in pro-B cells. (A) Scatter plot of gene expression differences observed between wild-type and YY1−/− pro-B cells, both on the Rag1−/− background. The normalized expression value of each gene in the two pro–B-cell types was plotted as reads per gene per million mapped sequence reads (RPM). Genes with a nominal false-discovery rate (FDR) ≤ 0.1 for differential expression (glmQLFTest) (SI Materials and Methods) are highlighted in red. Outliers are labeled with gene symbols. (B) Histogram of fold gene expression change for YY1-bound (black) and unbound (gray) genes. (C) Percentage of B-cell stage–specific enriched genes that have YY1 bound within 2.5 kb of the TSS.
We divided the differentially expressed genes into those genes that were normally up-regulated by YY1 (i.e., lower in the YY1−/− pro-B cells) and those genes that were normally down-regulated by YY1 (i.e., higher in the YY1−/− pro-B cells). The genes were further divided into those genes that had YY1 bound ±2.5 kb from the TSS, because over half of the YY1 sites were located within close proximity to the promoters. If YY1 is bound near the promoter of a differentially expressed gene, it is likely that YY1 directly regulates that gene. However, if YY1 is not bound near the differentially expressed gene, it either means that it is indirectly regulated, presumably by a transcription factor that is directly regulated by YY1 or that YY1 is bound to an enhancer or other regulatory element outside of our narrow ±2.5-kb window surrounding the TSS. Fig. 6B shows that of the genes that have YY1 bound in their promoters, and therefore are apparently direct targets of YY1, approximately equal proportions are up-regulated and down-regulated by YY1. A larger proportion of differentially expressed genes did not have YY1 bound within the proximal promoter region. We therefore performed gene ontology analyses on the four groups of genes (up- or down-regulated in the YY1−/− pro-B cells, direct or indirect regulation) separately using topGO and signaling pathway impact analysis (SPIA) (37, 38). Both programs, and others that we used, including GSEA (data not shown), identified similar categories and pathways regulated directly or indirectly by YY1, the highlights of which are summarized in Table 3. The full topGO and SPIA results are shown in Datasets S2 and S3. All gene ontology and pathway analyses identified mitochondrial functions among the genes that bind YY1 and that are normally up-regulated by YY1. These pathways include the TCA cycle, respiratory electron transport, and ATP synthesis. Genes that are directly down-regulated by YY1 in wild-type cells involve functions including regulation of transcription, mRNA splicing, NF-κB signaling pathways, Jak-STAT signaling pathways, AP-1 transcription factor network, and chromatin remodeling. Cell proliferation and c-Myc targets are also directly regulated by YY1. Gene pathways that are indirectly down-regulated by YY1 include G protein-coupled receptor pathways, cell adhesion and integrin pathways, and some cytokine signaling pathways.
Some genes of relevance for B-cell differentiation that are normally moderately up-regulated by YY1 include components of the pre–B-cell receptor, such as lambda5, Vpreb1, and Vpreb2 (Table 4). BAFF-receptor (BAFF-R), terminal deoxynucleotidyl transferase (Dntt/TdT) and CD5 are also up-regulated by YY1. Several genes that are normally not expressed until B cells differentiate into naive peripheral cells, such as ICOS ligand, CIITA, BTLA, and CD22, begin to be up-regulated prematurely in the absence of YY1. J chain (Igj) is normally only up-regulated significantly in GC B cells, but it is prematurely up-regulated 36.6-fold in YY1-deficient pro-B cells. However, only the Vpreb1 and Vpreb2 genes had YY1 bound at their promoters; the rest of the genes must be indirectly up-regulated. Other genes relevant for B cells that are normally negatively regulated by YY1, and that have YY1 bound in their promoters, include some NF-κB genes and Arid3A (also known as BRIGHT), as well as others listed in Table 3. The moderate down-regulation of the pre-BCR components in YY1-deficient pro-B cells may partially explain why a rearranged IgH as a transgene does not efficiently rescue pre–B-cell differentiation (13); however, alternatively, the lack of rescue could be due to the fact that other cellular functions regulated by YY1 preclude differentiation to the late pro–B-cell stage, where the pre-BCR gene products are necessary, as suggested in Fig. 4.
Table 4.
Differentially expressed genes relevant for B-cell differentiation
| Genes | Fold change in YY1−/− pro-B cells |
| Normally up-regulated by YY1 | Decrease |
| Direct (YY1 bound) | |
| Vpreb1 | 2.8 |
| Vpreb2 | 2.5 |
| Indirect | |
| Fcrl6 | 62 |
| Dntt (TdT) | 5.5 |
| CD93 (AA4.1) | 2.7 |
| TNFRSF13C (BAFF-R) | 2.6 |
| Igll1 (Lambda 5) | 2.6 |
| Id3 | 2.5 |
| Blnk | 2.1 |
| Normally down-regulated by YY1 | Increase |
| Direct (YY1 bound) | |
| Egr1 | 12 |
| Klf2 | 7.9 |
| Nfkbiz | 3 |
| Cebpb | 2.9 |
| Notch 2 | 2.9 |
| Brd4 | 2.7 |
| Trp53 (p53) | 2.4 |
| Drosha | 2.2 |
| Arid3a (BRIGHT) | 2.2 |
| RelA | 2.2 |
| Indirect | |
| Igj (J chain) | 36.6 |
| CD22 | 7.1 |
| Arid3b | 3.7 |
| Icosl | 3.5 |
| CIITA | 3.1 |
| BTLA | 2.8 |
| Id2 | 2.7 |
YY1 Binding to B-Cell Stage-Enriched Genes.
We identified stage-specific signature genes, or B-cell stage-enriched genes, using the following criteria. We called a gene a stage-enriched gene of pro-B, pre-B, follicular, or GC B cells if its expression was threefold higher in that cell type than in any other three cell types, and had at least one read per million in each of the samples. We then determined if YY1 was bound to these stage-specific–enriched genes, but only 3.2–21.6% of the genes had YY1 bound (Fig. 6C). We conclude that although YY1 regulates some B-lineage stage-specific genes, its main functions appear to be in the regulation of more general cellular functions.
YY1 Does Not Colocalize with H3K27me3 or with H3K9me3, but Does Partially Colocalize with CHD4.
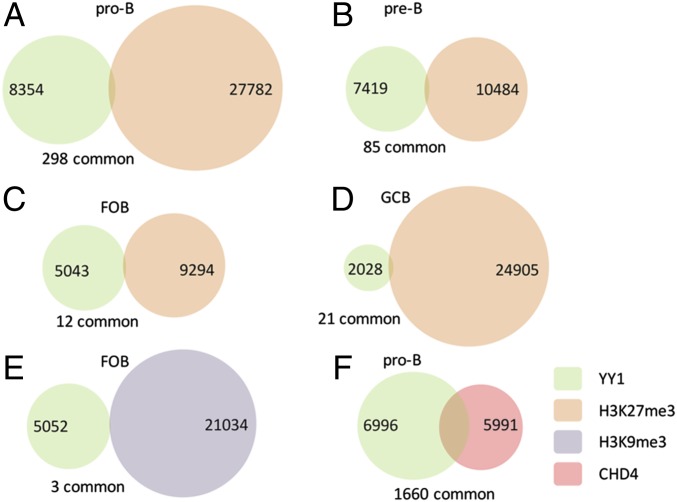
YY1 can repress genes as well as activate them. YY1 has been shown to be the mammalian homolog of the Drosophila Pho, the protein that binds to the repressive Polycomb response DNA elements (PREs) in Drosophila (10). YY1 has been shown to be able to rescue gene silencing in Drosophila lacking Pho, and thus it was proposed that YY1, which clearly functions as a Polycomb group protein, may be a DNA binding protein that recruits PRC2 to mammalian genomes, because there are no apparent PRE sequences in the mammalian genome (8, 9). Also, YY1 is present at high levels in myoblasts, and YY1 plays an essential role in the recruitment of the Polycomb repressive complex 2 (PRC2) in these cells that will result in methylation of H3K27 by Ezh2, the catalytic component of PRC2, which results in repression of several H3K27me3-marked muscle-specific genes (39). YY1 has also been demonstrated to bind directly to Suz12, another PRC2 component, in human cancer cell lines (40). Together, these data and other literature clearly implicate YY1 in recruitment of PRC2, and thus in the deposition of H3K27me3, at some locations in the mammalian genome. However, more recent genome-wide studies in ES cells and in myoblasts do not show significant overlap of YY1 binding with Ezh2/PRC2 sites (41–43). We performed ChIP-seq for H3K27me3 on all four stages of B-cell development studied here, and asked if YY1 sites overlapped with H3K27me3. It can be seen that there is almost no overlap of YY1 binding with the presence of H3K27me3 (Fig. 7 A–D). Thus, in B-lineage cells, as with ES cells and muscle precursors, PRC2 is largely recruited to the genome by other mechanisms (41), and the gene repression mediated by YY1 must be, in general, carried out through different mechanisms. We also compared the binding pattern of our YY1 ChIP-seq on follicular B cells with a previously published H3K9me3 ChIP-seq done on splenic B cells (44), and there was no overlap at all (Fig. 7E). Thus, there is little overlap of YY1 with either of these repressive histone marks, and no evidence for genome-wide recruitment of the PRC2 complex by YY1, although such recruitment has been observed on individual genes.
Fig. 7.
Overlap of YY1 binding sites with histone modifications and NuRD complex. Overlap of YY1 binding sites with H3K27me3 regions in Rag−/− pro-B (A), Rag−/−μ+ pre-B (B), follicular B cells (C), and GC B cells (D) is shown. (E) Overlap of YY1 binding with H3K9me3 in splenic B cells. H3K9me3 ChIP-seq data are from GSM902932 (44), performed on mouse splenic B cells. (F) Overlap of YY1 binding sites with the NuRD complex component CHD4 in pro-B cells. CDH4 data are from GSM1296550 (48). Numbers in circles indicate nonoverlapping gene number. The overlapping gene number is indicated below.
YY1 has been demonstrated to repress individual genes through the recruitment of nucleosome remodeling and histone deacetylase (NuRD) complexes and HDACs (45, 46). CHD4 is the Mi-2β component of the NuRD complex (47). We therefore compared the binding pattern of YY1 from our YY1 ChIP-seq on pro-B cells with the published ChIP-seq data for CHD4 performed on pro-B cells (48), and observed a 28% overlap of the binding sites (Fig. 7F). Thus, it is possible that YY1 recruits the NuRD complex genome-wide to repress some of its target genes.
Discussion
Through the use of various Cre transgenes that are activated at different times during B-cell differentiation, we show here that YY1 is needed for all stages of B-cell differentiation, and not specifically for the differentiation of naive splenic B cells into GC B cells as was suggested previously. The differentiation of naive B cells into GC B cells, PCs, and class-switched cells was completely prevented by deletion of YY1 with Cγ1-Cre, which is expressed soon after cells become activated by antigen (21). Our data build on the predictions of the study by Green et al. (19) and provide evidence for the crucial functional role of YY1 in B-cell–mediated humoral immunity.
CD19-Cre deletion of YY1 illustrated that normal development of all peripheral B-cell subsets requires YY1, with splenic T1 B cells and peritoneal B1a being most sensitive to YY1 dosage. However, use of a B-cell–specific tamoxifen-responsive Cre demonstrated that YY1 is not essential for the relatively short-term maintenance of mature B cells during the 1-wk time period we analyzed, with the exception of T1 B cells, although the fitness of YY1-deficient mature B cells in culture was diminished relative to YY1-“sufficient” cells. In contrast to the persistence of YY1− mature B cells, T1 splenocytes were completely dependent on YY1 for development and maintenance, with even a half-dose of YY1 causing a significant decrease. Acutely YY1-deficient T1 B cells had reduced surface IgM yet increased surface CD19 levels. CD19 forms part of the co–B-cell receptor complex along with CD21 and CD81, although CD19 is the only coreceptor with intracellular signaling capabilities (49). During receptor editing, CD19 surface levels decrease along with surface IgM (50). B cells at the T1 stage develop from the receptor-editing competent immature B cells, and they are programmed to respond to antigen by apoptosis rather than receptor editing (28). CD19 deficiency in splenic B cells results in significantly defective BCR signaling initiation (49). It might be that increased levels of CD19 occur in cells where BCR receptor is uncoupled from coreceptors to diminish downstream signal intensity to avoid negative selection. Decreased surface IgM may be a result of encounter with antigen. In any case, the disconnect between CD19 and IgM surface levels that we observed in YY1-deficient T1 cells offers a possible explanation for why T1 B cells are most sensitive to YY1 levels. YY1 depletion at this stage may result in dysregulated BCR signals, possibly by uncoupling BCR and CD19.
It has been previously reported that deletion of YY1 with mb1-Cre blocked differentiation of pro-B cells into pre-B cells (13). In that study, it was not clear why the cells were blocked at the pro–B-cell stage of differentiation. Crossing mice with an Igh transgene into the YY1f/f × mb1-Cre mice only partially rescued pre–B-cell differentiation. Our differentially expressed gene analysis showed that some genes critical for pro-B to pre-B differentiation, such as components of the pre–B-cell receptor (VpreB1, VpreB2, lambda5, and Blnk) are moderately decreased in the absence of YY1, and ChIP-seq shows that VpreB1 and VpreB2 genes have YY1 bound, and thus are most likely directly regulated by YY1. Down-regulation of the pre-BCR components, as well as lower CD19 coreceptor (Fig. S2D), could explain why a rearranged IgH transgene was not sufficient to promote effective differentiation to the pre–B-cell stage. However, an alternative explanation is that YY1 pro-B cells do not mature to the stage in which pre-BCR signaling normally occurs. The original study of YY1f/f × mb1-Cre mice indicated that pro–B-cell differentiation, per se, was intact (13). However, it is very difficult to assess pro–B-cell numbers accurately by precisely discriminating the small CD43+ pro–B-cell compartment from the large CD43− pre–B-cell compartment, particularly in mice on the B6 background. We therefore compared YY1f/f × mb1-Cre pro-B cells with Rag−/− pro-B cells, because the latter progress normally through pro–B-cell differentiation but cannot progress into pre-B cells. In this way, we were able to determine that YY1 deficiency results in incomplete differentiation to the more mature pro–B-cell stages defined by BP-1+ and c-kitlo. Interestingly, this premature block in the differentiation of the pro-B cells is similar to the premature block in Pax5-deficient pro-B cells, and both YY1- and Pax5-deficient cells display a lack of contraction of the Igh locus and inability for any but the most proximal Vh genes to undergo V(D)J rearrangement (51). Thus, we favor the explanation that the premature block in the midst of the pro–B-cell stage of differentiation may be a significant reason for the observed inability of an IgH transgene to rescue development efficiently and for the lack of Igh locus compaction in YY1-deficient pro-B cells. Recently, it has been demonstrated that early thymic T cells do not survive due to up-regulated p53 expression (52), and we observed increased p53 mRNA levels in YY1-deficient pro-B cells. We also found that YY1 bound to p53 in pro-B cells, and thus YY1 may directly regulate p53, which may offer an explanation for why YY1-deficient cells do not persist in culture and cannot fully differentiate in vivo.
We analyzed the genes that were differentially expressed between wild-type and YY1-deficient pro-B cells and the pathways that were affected (Table 3 and Datasets S1–S3). In addition to lymphoid-specific pathways, such as cytokine signaling in immune system cells, IFN signaling, inflammatory response, and innate immune response, there were many pathways of a more general nature. Notable among the general pathways were genes involved in transcription, translation, and ribosomal functions; cell adhesion; proliferation; and especially mitochondrial bioenergetics functions. These latter pathways suggest major cellular defects in YY1-deficient cells that may overshadow any specific immune system defects. Interestingly, we observed that YY1f/f Rosa26-EYFP × hCD20-Tam-Cre YFP+ splenocytes (YY1-deficient) had significantly less of the low-level GL7 staining observed in unimmunized mice compared with littermate control B cells (Fig. S3E). GL7 was originally characterized as an activation marker (53). These data suggest that YY1-deficient B cells may be less primed to participate in an immune response, possibly due to defective immune signaling.
YY1 has been shown to regulate individual genes that are specific for differentiation in several different cell types, and down-regulation or deletion of YY1, or overexpression of YY1, has been shown to dramatically affect the ability of precursor cells to undergo differentiation in several in vitro systems (54). For example, YY1 regulates the in vitro differentiation of chondrocytes from mesenchymal stem cells, in part, by regulating the expression of a cartilage-specific gene, chondromodulin-I (55). YY1 also regulates the differentiation of muscle cells from myoblasts, in part, by repressing muscle-specific genes, such as skeletal α-actin (56), and YY1 negatively regulates Runx2 transcription, which is required for osteoblast differentiation (57). Similarly, YY1 was shown to regulate adipocyte differentiation, in that overexpression of YY1 in 3T3-L1 cells decreased differentiation and YY1 knockdown increased differentiation (58). More global gene expression analyses in cells from mice that conditionally deleted YY1 specifically in muscle cells, or in intestinal epithelial stem cells, indicated that the major pathways down-regulated in both types of knockout mice were mitochondrial bioenergetics pathways (59, 60). The intestinal epithelial stem cells also revealed up-regulation of cell cycle genes, and ChIP-seq in these cells showed enrichment for RNA processing and mitochondrial functions. These results in muscle and epithelial stem cells are similar to the pathways and gene groups that we found to be most affected in mouse B-cell progenitors lacking YY1, and, together, these data indicate a common set of cellular functions and pathways in a variety of cell types that are profoundly regulated by YY1. These common sets of biological processes, including those biological processes related to proliferation and survival, provide a general set of mechanisms for why YY1 is required for all steps of B-cell differentiation.
Materials and Methods
The Scripps Research Institute Institutional Animal Care and Use Committee approved all mouse protocols in this project. Details on animals; reagents; FACS analysis protocols and subset classifications; cell culture conditions, including apoptosis and proliferation studies; ChIP-seq and RNA-seq, including the bioinformatics analysis pipeline; and qPCR can be found in SI Materials and Methods. See Tables S1 and S2 for antibodies and primers used.
Table S1.
Antibody list
| Antibody | Conjugation | Company | Catalog no. |
| CD19 | BV421 | Biolegend | 115549 |
| CD19 | PE-Cy7 | Biolegend | 115519 |
| CD19 | FITC | Biolegend | 115505 |
| GL7 | Alexa Fluor 647 | Biolegend | 144605 |
| IgM | FITC | Biolegend | 406506 |
| IgD | FITC | Biolegend | 405704 |
| IgG1 | BV510 | Biolegend | 400171 |
| AA4.1 (CD93) | APC | Biolegend | 136510 |
| AA4.1 (CD93) | PE-Cy7 | Biolegend | 136506 |
| CD23 | PE-Cy7 | Biolegend | 101614 |
| CD21 | Pacific Blue | Biolegend | 123414 |
| B220 | PE-Cy7 | Biolegend | 103222 |
| CD5 | APC | eBioscience | 17-0051-82 |
| C-kit | PE-Cy7 | Biolegend | 105814 |
| BP-1 | PE | eBioscience | 12-5891-83 |
| CD138 | APC | Biolegend | 142506 |
| CD19 | PerCP Cy5.5 | eBioscience | 45-0193-82 |
| CD19 | BV510 | Biolegend | 115545 |
| CD23 | PE | Biolegend | 101608 |
| IgM Fab fragment | Alexa Fluor 647 | Jackson ImmunoResearch | 115-607-020 |
| CD24 | eFluor450 | eBioscience | 48-0242-82 |
| CD21 | PE | Biolegend | 123410 |
| Fas | PE | eBioscience | 12-0951-81 |
| CD2 | PE | Biolegend | 100108 |
| CD43 | BV510 | BD Bioscience | 563206 |
| Active caspase 3 | PE | BD Bioscience | 561011 |
| Bcl6 | PE | BD Bioscience | 561522 |
| Ki-67 | Alexa Fluor 647 | Biolegend | 652407 |
| Live/dead | Near-IR | Life Technologies | L34976 |
| CD22 | APC | eBioscience | 17-0221-82 |
| BAFF-R | APC | eBioscience | 51-5943-82 |
| CD43 | Biotin | BD Bioscience | 553269 |
| AA4.1 (CD93) | Biotin | eBioscience | 13-5892-82 |
| Ter119 | Biotin | Biolegend | 116204 |
| CD5 | Biotin | Biolegend | 100604 |
APC, allophycocyanin; PE, phycoerythrin; PerCP, peridinin chlorophyll protein.
Table S2.
Primer list
| Gene | Forward primer 5′ to 3′ | Reverse primer 5′ to 3′ |
| Fcrl6 | CTACCAGTCTCTCTCTCCCCAG | CTGTCAGCTCAGGATTTGGGA |
| BR3 (BAFF-R/Tnfrsf13c) | AGTGAGTCTGGTGAGCTGGA | GGGTTTCTGAGGAGGGTACA |
| Dntt | GCCACACACGAGAGGAAGAT | ACACCCTCAAGGCTTTCTGT |
| Blnk | AGTCAAAGGCCCTCCAAGTG | TTCATAGGAATCGTCGCCCG |
| CD93 | GTTCCAGAGCGAGCAGAGAG | AGAATGATGTGGCTTCCCCC |
| VpreB1 | CTCTCCCCAAAGCAGGGAGG | GTGGCTCCAAGGGAAGAAGA |
| VpreB2 | CCCACCTCACAGGTTGTGG | CGTTGCTCAGGGTACAGGAG |
| Lambda5 | TGCTGCTGTTGGGTCTAGTG | CCCGTGGGATGATCTGGAAC |
| Trp53 | GTGCTCACCCTGGCTAAAGT | TCCGACTGTGACTCCTCCAT |
| Klf2 | CACCAAGAGCTCGCACCTAA | GGCACTGAAAGGGTCTGTGA |
| CD22 | TGGCCAAGCGTGTGAGACTT | GCCTGTTTCCTCCTGTGTTCG |
| Icosl | CGTCCCCACCGAAGCTATAC | GGGGGTCCACTCTGAAGTTG |
| Igj | AGGATCATCCCTTCCACCGA | CCACTTCCACAGGATCGCAT |
| YY1 | TTGGAGAGAACTCACCTCCTG | AGCCTTTATGAGGGCAAGCTA |
SI Materials and Methods
Animals.
All mouse strains were maintained on the C57BL/6 background. Mice with loxP-flanked YY1 gene (YY1f) were kindly provided by Yang Shi, Department of Cell Biology, Children’s Hospital Boston, Harvard Medical School, Boston (61). These mice were crossed with the following Cre transgenic mice: mb1-Cre, CD19-Cre, hCD20.Tam-Cre, and Cγ1-Cre (21–23, 32). Some of these mice were further crossed to the Rosa26.EYFP reporter mice (24). To deplete YY1 acutely, hCD20.Tam-Cre × YY1f/f mice were injected intraperitoneally with 1 mg of tamoxifen (Sigma) dissolved in olive oil daily for 3 d. Seven days after the last injection, the spleens were harvested. For BM subset purification, we used B6.Rag1−/− mice and B6.Rag1−/−μ+ mice (62). All mice were bred and maintained under specific pathogen-free conditions at the animal facility of The Scripps Research Institute. The Scripps Research Institute Institutional Animal Care and Use Committee approved all mouse protocols in this project. Analyzed animals ranged from 6 to 14 wk old, including both males and females.
FACS Analysis.
BM, spleen, and peritoneal cavity cells were analyzed by FACS analysis on an LSR II instrument (BD Biosciences), and data were analyzed with FlowJo. Cell surface markers used to define different subpopulations are shown in Fig. S1. From the spleen, T1 B cells are CD19+AA4+CD21−CD23− or CD19+CD21−CD24hi, T2/FO B cells are CD19+CD23+CD21+, and MZ B cells are CD19+CD21hiCD23lo. From the peritoneal cavity exudate cells, we identified B1a cells as CD19hiB220loCD5+, B1b cells as CD19hiB220loCD5−, and B2 cells as CD19+B220hiCD5−. In the BM, pro-B cells were identified as CD19+AA4+CD43+IgM− and further subdivided using BP-1 and c-kit. Recirculating BM B cells [fraction F (63)] were characterized as CD19+IgM+CD93lo. GC B cells and PCs were obtained from spleens of mice 8 d after intraperitoneal immunization with 100 μg of OVA and 10 μg of LPS in alum. GC B cells were identified as CD19+Fas+GL7+, and PCs were identified as CD19+CD138+. IgG1 isotype-switched B cells from the same spleens were identified as CD19+IgG1+. Antibody information is listed in Table S1.
B-Cell Subset Purification for ChIP-Seq and RNA-Seq.
For BM subset purification for RNA-seq or ChIP-seq, we used B6.Rag1−/− mice to obtain pro-B cells. B6.Rag1−/−μ+ mice bearing a rearranged IgM transgene were used to obtain pre-B cells (62). Obtaining a pure population of pro-B cells free of any pre-B cells from wild-type mice is very difficult, especially in mice of the B6 background, so use of Rag1−/− mice ensures pure populations. Pro-B cells and pre-B cells were purified using CD19-conjugated model-based analysis of ChIP-seq (MACS) magnetic beads (Miltenyi). YY1−/− pro-B cells were obtained in a similar manner from YY1f/f × mb1-Cre mice also on a Rag1−/− background. Follicular B cells and GC B cells were sorted from B6 mice on a FACS Aria II instrument using the cell surface phenotypes described above. The GC B cells were obtained from B6 mice that had been immunized 8 d earlier as described above.
Cell Culture, Proliferation, and Apoptosis Analysis.
Pro-B cells were cultured in Opti-MEM medium, 10% (vol/vol) FCS, and 5 × 10−5 M 2-mercaptoethanol with IL-7 [1% (vol/vol) of supernatant of a J558L cell line stably expressing IL-7]. Cultured BM cells were counted each day to monitor cellular proliferation and viability. Splenocyte cultures were performed using hCD20.Tam-Cre × YY1f/f mice that were injected intraperitoneally with 1 mg of tamoxifen daily for 3 d, and 7 d later, the spleens were harvested. Splenic B cells were purified by positive cell enrichment, negative cell depletion, or cell sorting as indicated. Positive cell enrichment was performed by using CD19-conjugated Miltenyi MACS magnetic beads (CD19+-purified). Negative cell depletion was performed by incubating splenocytes with biotin-conjugated anti-CD43, anti-CD5, anti-CD93 (AA4.1), and anti-Ter119 antibodies, followed by depletion using magnetic beads conjugated with antibiotin (Miltenyi).
Splenocytes were cultured either in LPS or induced GC conditions. LPS cultures contained 10 μg/mL LPS (L2880; Sigma) in RPMI-1640 medium supplemented with 10% (vol/vol) FBS, 1% penicillin/streptomycin, and 5 × 10−5 M 2-mercaptoethanol. The iGCB cultures were performed as previously described (33). Briefly, splenic B cells were cultured in the presence of 10 ng/mL recombinant mIL-4 (Biolegend) on irradiated 3T3 cells that had been stably transfected with CD40L and BAFF (40LB cells), kindly provided by Daisuke Kitamura, Division of Molecular Biology, Research Institute for Biological Sciences, Tokyo University of Science, Noda, Chiba, Japan (33). To assess cell cycling, cells were stained with Hoechst 33342 (Cell Signaling Technologies) on day 4 of culture. To assess proliferation, cells were labeled with Cell Proliferation Dye eFluor450 (CPDF) (eBioscience) before culture and analyzed on day 2.5. To be able to track YFP+ cells using intracellular staining, CD19+-enriched B cells were sorted on a FACS Aria II instrument because YFP expression is lost when fixation and permeabilization are combined in the first step. However, a few intracellular staining experiments were performed without sorting by preserving YFP expression with a 2% paraformaldehyde prefix for 10 min at 37 °C (64), followed by use of the BD Cytofix/Cytoperm Kit (BD Biosciences). The percentage of cells that were YFP+ during culture was assessed, as well as active caspase 3 and Ki-67, using a BD LSR II instrument (BD Biosciences).
ChIP-Seq.
ChIP-seq was performed as previously described (65). Next-generation sequencing was performed on the Illumina HiSeq system at the Scripps Next-Generation Sequencing Core. Anti-YY1 (SC-1703) was purchased from Santa Cruz Biotechnology (SC-1703), H3K27me3 from EMD Millipore, H3K4me3 from Active Motif, and H3K4me1 from Abcam. All ChIP-seq data have been deposited in the Gene Expression Omnibus database.
Preliminary quality control over raw sequence data was performed with FastQC 0.11 (66). Duplicate reads were removed before mapping, and TruSeq adapter sequences were removed with the HOMER trim tool (34). Experimental and input control fastq tags were aligned to the mouse reference genome (mm9) using Bowtie 1.1.2 (alignment parameters: -a -v 2 -m 3 --best –strata) (67). Tags were extended 300 bp upstream or downstream from the alignment start site, depending on the strand orientation of the mapping, and normalized to 10 M reads according to the protocol described by Bardet et al. (68). Confident YY1 peaks were called using MACS (v1.4.2) (69), with a false-discovery rate (FDR) ≤ 1% and the default P value (1E-5). Confident histone modification peaks were called using HOMER. Downstream analysis and manipulation of the data, including annotation of peaks, motif finding, quantification of data at peaks/genomic regions, and overlap analysis, were performed with HOMER 4.7 and R/Bioconductor (70). In particular, peaks were annotated with genomic features obtained from RefSeq release 66 through homer.salk.edu/homer/data/genomes/mm9.v5.7.zip. Overlap between YY1 peaks and H3K27me3 regions was computed using the HOMER mergePeaks tool (parameter used: -d for literal overlaps). Finally, we used GREAT (background: whole genome, default settings) for functional interpretation of confident peaks in each cell type (35).
RNA-Seq.
Total RNA was prepared from Rag1−/− pro-B cells, Rag1−/− YY1f/f × mb1-Cre pro-B cells, Rag1−/−μ+ pre-B cells, C57BL/6 follicular B cells, and C57BL/6 GC B cells. RNA was extracted using TRIzol (Life Technologies), and genomic DNA was eliminated using the genomic DNA wipeout buffer in the QuantiTect Reverse Transcription Kit (Qiagen). A final purification of the RNA was performed with the RNeasy Kit (Qiagen). Ribosomal RNA was eliminated using the Ribo-Zero Magnetic Gold Kit (Illumina). RNA samples were submitted to the Scripps Next-Generation Sequencing Core, where they were processed with the NEBNext Ultra Directional RNA Library Prep Kit for Illumina and sequenced on the Illumina HiSeq system. Three independent RNA-seq samples were used for Rag1−/− pro-B and Rag1−/− YY1f/f × mb1-Cre pro-B cells, and two samples were used for the other cell types. RNA-seq data have been deposited in the Gene Expression Omnibus database.
After quality control as described above for ChIP-seq, raw data were aligned to the mouse reference genome (mm9) using TopHat 2.1.0 and Bowtie 2 2.2.6 (67, 71). Count-based differential expression analysis was conducted according to a published protocol (72). Briefly, reads were counted with HTSeq (parameters: -s no -m intersection-nonempty) (73). A quasi-likelihood negative binomial generalized log-linear model (74) was fitted to count data using EdgeR (36) with a simple design (i.e., two-group comparison, wild-type vs. YY1−/−). Differentially expressed genes were called using a nominal FDR threshold of 0.1. For functional analysis, Gene Ontology enrichment was performed with topGO (38) and pathway enrichment was performed with SPIA (37) in an R/Bioconductor environment.
Signature genes for each cell type were identified as follows:
(i) Filter weakly expressed genes. Using a table of counts per million for each gene in each replicate experiment and cell type, we removed genes without at least one read per million in each sample.
(ii) Select genes with at least a threefold change in a specific cell type, but lower fold changes in the remaining conditions. Select genes with an average fold change in a specific cell type at least threefold higher than the average fold change in any of the remaining conditions.
RNA-seq differentially expressed genes were validated with qPCR validation using pro–B-cell RNA. qPCR was performed using SYBR green master mix (Biotool) with primers listed in Table S2.
Statistical Analysis.
Statistical analysis was performed using an unpaired, two-tailed t test unless otherwise noted using Prism v5.0 statistical software (GraphPad) (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
Supplementary Material
Acknowledgments
We thank Amy Baumgart, Timothy Wong, and Felicia Han for expert technical help. This research was supported by NIH Grants R03AI115127 and R01 AI082918 (to A.J.F.). A.I.S. was supported in part by NIH Grant UL1TR001114.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE73534).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1606297113/-/DCSupplemental.
References
- 1.Shi Y, Lee JS, Galvin KM. Everything you have ever wanted to know about Yin Yang 1..... Biochim Biophys Acta. 1997;1332(2):F49–F66. doi: 10.1016/s0304-419x(96)00044-3. [DOI] [PubMed] [Google Scholar]
- 2.Montalvo EA, Shi Y, Shenk TE, Levine AJ. Negative regulation of the BZLF1 promoter of Epstein-Barr virus. J Virol. 1991;65(7):3647–3655. doi: 10.1128/jvi.65.7.3647-3655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seto E, Shi Y, Shenk T. YY1 is an initiator sequence-binding protein that directs and activates transcription in vitro. Nature. 1991;354(6350):241–245. doi: 10.1038/354241a0. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y, Seto E, Chang LS, Shenk T. Transcriptional repression by YY1, a human GLI-Krüppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991;67(2):377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- 5.Park K, Atchison ML. Isolation of a candidate repressor/activator, NF-E1 (YY-1, delta), that binds to the immunoglobulin kappa 3′ enhancer and the immunoglobulin heavy-chain mu E1 site. Proc Natl Acad Sci USA. 1991;88(21):9804–9808. doi: 10.1073/pnas.88.21.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon S, Akopyan G, Garban H, Bonavida B. Transcription factor YY1: Structure, function, and therapeutic implications in cancer biology. Oncogene. 2006;25(8):1125–1142. doi: 10.1038/sj.onc.1209080. [DOI] [PubMed] [Google Scholar]
- 7.Thomas MJ, Seto E. Unlocking the mechanisms of transcription factor YY1: Are chromatin modifying enzymes the key? Gene. 1999;236(2):197–208. doi: 10.1016/s0378-1119(99)00261-9. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson FH, Park K, Atchison ML. Polycomb recruitment to DNA in vivo by the YY1 REPO domain. Proc Natl Acad Sci USA. 2006;103(51):19296–19301. doi: 10.1073/pnas.0603564103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atchison L, Ghias A, Wilkinson F, Bonini N, Atchison ML. Transcription factor YY1 functions as a PcG protein in vivo. EMBO J. 2003;22(6):1347–1358. doi: 10.1093/emboj/cdg124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown JL, Mucci D, Whiteley M, Dirksen ML, Kassis JA. The Drosophila Polycomb group gene pleiohomeotic encodes a DNA binding protein with homology to the transcription factor YY1. Mol Cell. 1998;1(7):1057–1064. doi: 10.1016/s1097-2765(00)80106-9. [DOI] [PubMed] [Google Scholar]
- 11.Satijn DP, Hamer KM, den Blaauwen J, Otte AP. The polycomb group protein EED interacts with YY1, and both proteins induce neural tissue in Xenopus embryos. Mol Cell Biol. 2001;21(4):1360–1369. doi: 10.1128/MCB.21.4.1360-1369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donohoe ME, et al. Targeted disruption of mouse Yin Yang 1 transcription factor results in peri-implantation lethality. Mol Cell Biol. 1999;19(10):7237–7244. doi: 10.1128/mcb.19.10.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, et al. Yin Yang 1 is a critical regulator of B-cell development. Genes Dev. 2007;21(10):1179–1189. doi: 10.1101/gad.1529307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sayegh CE, Jhunjhunwala S, Riblet R, Murre C. Visualization of looping involving the immunoglobulin heavy-chain locus in developing B cells. Genes Dev. 2005;19(3):322–327. doi: 10.1101/gad.1254305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roldán E, et al. Locus ‘decontraction’ and centromeric recruitment contribute to allelic exclusion of the immunoglobulin heavy-chain gene. Nat Immunol. 2005;6(1):31–41. doi: 10.1038/ni1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma-Gaur J, et al. Noncoding transcription within the Igh distal V(H) region at PAIR elements affects the 3D structure of the Igh locus in pro-B cells. Proc Natl Acad Sci USA. 2012;109(42):17004–17009. doi: 10.1073/pnas.1208398109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerasimova T, et al. A structural hierarchy mediated by multiple nuclear factors establishes IgH locus conformation. Genes Dev. 2015;29(16):1683–1695. doi: 10.1101/gad.263871.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan X, et al. YY1 controls Igκ repertoire and B-cell development, and localizes with condensin on the Igκ locus. EMBO J. 2013;32(8):1168–1182. doi: 10.1038/emboj.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green MR, et al. Signatures of murine B-cell development implicate Yy1 as a regulator of the germinal center-specific program. Proc Natl Acad Sci USA. 2011;108(7):2873–2878. doi: 10.1073/pnas.1019537108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaprazna K, Atchison ML. YY1 controls immunoglobulin class switch recombination and nuclear activation-induced deaminase levels. Mol Cell Biol. 2012;32(8):1542–1554. doi: 10.1128/MCB.05989-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casola S, et al. Tracking germinal center B cells expressing germ-line immunoglobulin gamma1 transcripts by conditional gene targeting. Proc Natl Acad Sci USA. 2006;103(19):7396–7401. doi: 10.1073/pnas.0602353103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25(6):1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobeika E, et al. Testing gene function early in the B cell lineage in mb1-Cre mice. Proc Natl Acad Sci USA. 2006;103(37):13789–13794. doi: 10.1073/pnas.0605944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allman D, et al. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167(12):6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 26.Allman D, Srivastava B, Lindsley RC. Alternative routes to maturity: Branch points and pathways for generating follicular and marginal zone B cells. Immunol Rev. 2004;197:147–160. doi: 10.1111/j.0105-2896.2004.0108.x. [DOI] [PubMed] [Google Scholar]
- 27.Loder F, et al. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190(1):75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su TT, Rawlings DJ. Transitional B lymphocyte subsets operate as distinct checkpoints in murine splenic B cell development. J Immunol. 2002;168(5):2101–2110. doi: 10.4049/jimmunol.168.5.2101. [DOI] [PubMed] [Google Scholar]
- 29.Benitez A, et al. Differences in mouse and human nonmemory B cell pools. J Immunol. 2014;192(10):4610–4619. doi: 10.4049/jimmunol.1300692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otero DC, Rickert RC. CD19 function in early and late B cell development. II. CD19 facilitates the pro-B/pre-B transition. J Immunol. 2003;171(11):5921–5930. doi: 10.4049/jimmunol.171.11.5921. [DOI] [PubMed] [Google Scholar]
- 32.Khalil AM, Cambier JC, Shlomchik MJ. B cell receptor signal transduction in the GC is short-circuited by high phosphatase activity. Science. 2012;336(6085):1178–1181. doi: 10.1126/science.1213368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nojima T, et al. In-vitro derived germinal centre B cells differentially generate memory B or plasma cells in vivo. Nat Commun. 2011;2:465. doi: 10.1038/ncomms1475. [DOI] [PubMed] [Google Scholar]
- 34.Heinz S, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38(4):576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLean CY, et al. GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol. 2010;28(5):495–501. doi: 10.1038/nbt.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson MD, McCarthy DJ, Smyth GK. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarca AL, et al. A novel signaling pathway impact analysis. Bioinformatics. 2009;25(1):75–82. doi: 10.1093/bioinformatics/btn577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alexa A, Rahnenführer J, Lengauer T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics. 2006;22(13):1600–1607. doi: 10.1093/bioinformatics/btl140. [DOI] [PubMed] [Google Scholar]
- 39.Caretti G, Di Padova M, Micales B, Lyons GE, Sartorelli V. The Polycomb Ezh2 methyltransferase regulates muscle gene expression and skeletal muscle differentiation. Genes Dev. 2004;18(21):2627–2638. doi: 10.1101/gad.1241904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ko CY, Hsu HC, Shen MR, Chang WC, Wang JM. Epigenetic silencing of CCAAT/enhancer-binding protein delta activity by YY1/polycomb group/DNA methyltransferase complex. J Biol Chem. 2008;283(45):30919–30932. doi: 10.1074/jbc.M804029200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mendenhall EM, et al. GC-rich sequence elements recruit PRC2 in mammalian ES cells. PLoS Genet. 2010;6(12):e1001244. doi: 10.1371/journal.pgen.1001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vella P, Barozzi I, Cuomo A, Bonaldi T, Pasini D. Yin Yang 1 extends the Myc-related transcription factors network in embryonic stem cells. Nucleic Acids Res. 2012;40(8):3403–3418. doi: 10.1093/nar/gkr1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu L, et al. Genome-wide survey by ChIP-seq reveals YY1 regulation of lincRNAs in skeletal myogenesis. EMBO J. 2013;32(19):2575–2588. doi: 10.1038/emboj.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santoni de Sio FR, et al. KAP1 regulates gene networks controlling mouse B-lymphoid cell differentiation and function. Blood. 2012;119(20):4675–4685. doi: 10.1182/blood-2011-12-401117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun JM, Chen HY, Davie JR. Differential distribution of unmodified and phosphorylated histone deacetylase 2 in chromatin. J Biol Chem. 2007;282(45):33227–33236. doi: 10.1074/jbc.M703549200. [DOI] [PubMed] [Google Scholar]
- 46.Sankar N, et al. p300 provides a corepressor function by cooperating with YY1 and HDAC3 to repress c-Myc. Oncogene. 2008;27(43):5717–5728. doi: 10.1038/onc.2008.181. [DOI] [PubMed] [Google Scholar]
- 47.Tong JK, Hassig CA, Schnitzler GR, Kingston RE, Schreiber SL. Chromatin deacetylation by an ATP-dependent nucleosome remodelling complex. Nature. 1998;395(6705):917–921. doi: 10.1038/27699. [DOI] [PubMed] [Google Scholar]
- 48.Schwickert TA, et al. Stage-specific control of early B cell development by the transcription factor Ikaros. Nat Immunol. 2014;15(3):283–293. doi: 10.1038/ni.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Depoil D, et al. CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nat Immunol. 2008;9(1):63–72. doi: 10.1038/ni1547. [DOI] [PubMed] [Google Scholar]
- 50.Lamoureux JL, et al. Reduced receptor editing in lupus-prone MRL/lpr mice. J Exp Med. 2007;204(12):2853–2864. doi: 10.1084/jem.20071268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuxa M, et al. Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene. Genes Dev. 2004;18(4):411–422. doi: 10.1101/gad.291504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen L, Foreman DP, Sant’Angelo DB, Krangel MS. Yin Yang 1 promotes thymocyte survival by downregulating p53. J Immunol. 2016;196(6):2572–2582. doi: 10.4049/jimmunol.1501916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laszlo G, Hathcock KS, Dickler HB, Hodes RJ. Characterization of a novel cell-surface molecule expressed on subpopulations of activated T and B cells. J Immunol. 1993;150(12):5252–5262. [PubMed] [Google Scholar]
- 54.Kurisaki K, et al. Nuclear factor YY1 inhibits transforming growth factor beta- and bone morphogenetic protein-induced cell differentiation. Mol Cell Biol. 2003;23(13):4494–4510. doi: 10.1128/MCB.23.13.4494-4510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aoyama T, et al. Histone modifiers, YY1 and p300, regulate the expression of cartilage-specific gene, chondromodulin-I, in mesenchymal stem cells. J Biol Chem. 2010;285(39):29842–29850. doi: 10.1074/jbc.M110.116319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacLellan WR, Lee TC, Schwartz RJ, Schneider MD. Transforming growth factor-beta response elements of the skeletal alpha-actin gene. Combinatorial action of serum response factor, YY1, and the SV40 enhancer-binding protein, TEF-1. J Biol Chem. 1994;269(24):16754–16760. [PubMed] [Google Scholar]
- 57.Jeong HM, Choi YH, Lee SH, Lee KY. YY1 represses the transcriptional activity of Runx2 in C2C12 cells. Mol Cell Endocrinol. 2014;383(1-2):103–110. doi: 10.1016/j.mce.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 58.Han Y, et al. Yin Yang 1 is a multi-functional regulator of adipocyte differentiation in 3T3-L1 cells. Mol Cell Endocrinol. 2015;413:217–227. doi: 10.1016/j.mce.2015.06.035. [DOI] [PubMed] [Google Scholar]
- 59.Blättler SM, et al. Defective mitochondrial morphology and bioenergetic function in mice lacking the transcription factor Yin Yang 1 in skeletal muscle. Mol Cell Biol. 2012;32(16):3333–3346. doi: 10.1128/MCB.00337-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perekatt AO, et al. YY1 is indispensable for Lgr5+ intestinal stem cell renewal. Proc Natl Acad Sci USA. 2014;111(21):7695–7700. doi: 10.1073/pnas.1400128111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Affar B, et al. Essential dosage-dependent functions of the transcription factor yin yang 1 in late embryonic development and cell cycle progression. Mol Cell Biol. 2006;26(9):3565–3581. doi: 10.1128/MCB.26.9.3565-3581.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nussenzweig MC, et al. Allelic exclusion in transgenic mice that express the membrane form of immunoglobulin mu. Science. 1987;236(4803):816–819. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- 63.Li Y-S, Hayakawa K, Hardy RR. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993;178(3):951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grupillo M, et al. An improved intracellular staining protocol for efficient detection of nuclear proteins in YFP-expressing cells. Biotechniques. 2011;51(6):417–420. doi: 10.2144/000113780. [DOI] [PubMed] [Google Scholar]
- 65.Degner SC, et al. CCCTC-binding factor (CTCF) and cohesin influence the genomic architecture of the Igh locus and antisense transcription in pro-B cells. Proc Natl Acad Sci USA. 2011;108(23):9566–9571. doi: 10.1073/pnas.1019391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andrews S. FastQC: A quality control tool for high throughput sequence data. 2015 Available at www.bioinformatics.babraham.ac.uk/projects/fastqc. Accessed May 5, 2015.
- 67.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bardet AF, He Q, Zeitlinger J, Stark A. A computational pipeline for comparative ChIP-seq analyses. Nat Protoc. 2011;7(1):45–61. doi: 10.1038/nprot.2011.420. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9(9):R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gentleman RC, et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004;5(10):R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim D, et al. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anders S, et al. Count-based differential expression analysis of RNA sequencing data using R and Bioconductor. Nat Protoc. 2013;8(9):1765–1786. doi: 10.1038/nprot.2013.099. [DOI] [PubMed] [Google Scholar]
- 73.Anders S, Pyl PT, Huber W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31(2):166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lund SP, Nettleton D, McCarthy DJ, Smyth GK. Detecting differential expression in RNA-sequence data using quasi-likelihood with shrunken dispersion estimates. Stat Appl Genet Mol Biol. 2012;11(5):11. doi: 10.1515/1544-6115.1826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.