Significance
Fibrosis is a leading cause of death in industrialized countries. Until now, there has been no effective therapy to prevent or counteract the fibrotic process. This article describes the effect of the blockade of a late costimulatory molecule to prevent inflammation-driven skin, lung, and vessel fibrosis and to induce regression of established dermal fibrosis in vivo in complementary murine models of systemic sclerosis, a prototypic autoimmune fibrotic disease. This article also reveals an unexpected role of this protein as a biomarker of worsening fibrosis that might help delineate the prognosis of patients in clinical practice more accurately.
Keywords: systemic sclerosis, fibrosis, costimulation, OX40L, translational approach
Abstract
Treatment for fibrosis represents a critical unmet need, because fibrosis is the leading cause of death in industrialized countries, and there is no effective therapy to counteract the fibrotic process. The development of fibrosis relates to the interplay between vessel injury, immune cell activation, and fibroblast stimulation, which can occur in various tissues. Immunotherapies have provided a breakthrough in the treatment of immune diseases. The glycoprotein OX40–OX40 ligand (OX40L) axis offers the advantage of a targeted approach to costimulatory signals with limited impact on the whole immune response. Using systemic sclerosis (SSc) as a prototypic disease, we report compelling evidence that blockade of OX40L is a promising strategy for the treatment of inflammation-driven fibrosis. OX40L is overexpressed in the fibrotic skin and serum of patients with SSc, particularly in patients with diffuse cutaneous forms. Soluble OX40L was identified as a promising serum biomarker to predict the worsening of lung and skin fibrosis, highlighting the role of this pathway in fibrosis. In vivo, OX40L blockade prevents inflammation-driven skin, lung, and vessel fibrosis and induces the regression of established dermal fibrosis in different complementary mouse models. OX40L exerts potent profibrotic effects by promoting the infiltration of inflammatory cells into lesional tissues and therefore the release of proinflammatory mediators, thereafter leading to fibroblast activation.
Although they contribute to up to 45% of deaths in the industrialized countries, until now little progress has been made in deciphering fibrotic diseases (1). Nonetheless, it has been demonstrated that pulmonary, renal, hepatic, and even dermal fibrosis share common pathways that drive the pathologic events. In all these tissues, the development of fibrosis relates to the interplay between immune cell activation and fibroblast stimulation, and perpetuation of the fibrotic process leads to progressive impaired organ function. Systemic sclerosis (SSc) is an autoimmune T-cell disease that is defined by pathological fibrosis of the skin and also of internal organs such as lungs (2). Therefore SSc is considered a prototype entity for studying the pathogenesis of fibrosis in fibrotic diseases and particularly the links between inflammation/autoimmunity and fibrosis. Immunotherapies have provided a breakthrough in several autoimmune diseases, such as rheumatoid arthritis, but are associated with increased risk of infections. An emerging therapeutic approach for T-cell–mediated diseases is targeting the antigen-specific T cells involved in the disease without leading to generalized immunosuppression (3). The glycoprotein OX40–OX40 ligand (OX40L) pair, which is involved in late T-cell costimulatory signaling and is transiently expressed following antigen recognition, fits these criteria (4). Blocking OX40–OX40L was effective in preventing the development of disease in several in vivo animal models of autoimmune and inflammatory diseases (5). This strategy has not yet been evaluated in fibrotic conditions such as SSc. Genetic data have shown that TNFSF4, which encodes for OX40L, is an SSc susceptibility gene, suggesting a potential role of this pathway in SSc, although the functional effects of the gene variants are not known (6–8). Preliminary results have shown that serum soluble OX40 levels were significantly increased in SSc patients as compared with healthy individuals (9). OX40L blockade was shown to be more effective than OX40 blockade in reducing autoimmunity in the murine model of collagen-induced arthritis, suggesting that signaling via OX40L (and not via OX40) promotes inflammation and autoimmunity (10). Therefore we chose to study the role of OX40L in patients with SSc and in vivo in complementary murine models of SSc. The role of OX40L in SSc was highlighted by the overexpression of the protein in the skin and serum of patients with SSc, particularly in patients with diffuse cutaneous SSc, the most severe and fibrotic form of the disease. Soluble OX40L also was identified as a promising biomarker predictive of fibrosis worsening in patients with SSc. In vivo, blocking OX40L prevented inflammation-driven dermal fibrosis, fibrosing alveolitis, and lung vessel remodeling in complementary murine models of SSc, suggesting that the anti-OX40L antibody offers a promising strategy for the treatment of the inflammatory stages of fibrotic diseases.
Results
OX40L Is Overexpressed in the Skin of Patients with SSc.
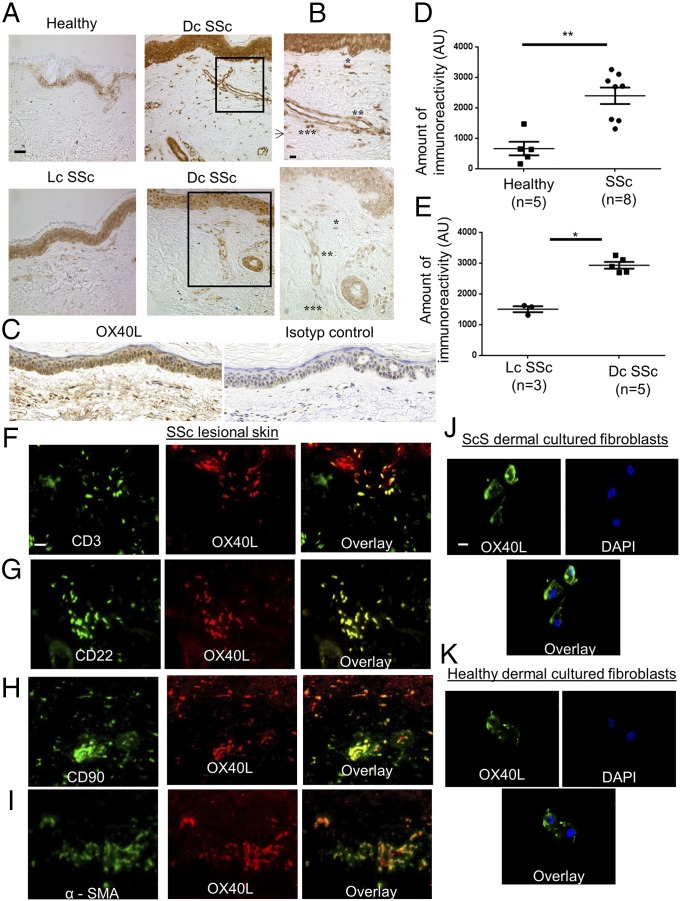
The expression of OX40L protein was 3.6-fold higher in fibrotic skin of patients with SSc, particularly in patients with diffuse cutaneous forms (Fig. 1 A–E), than in the skin of healthy controls (P = 0.003). As expected, OX40L was expressed by CD3+ T cells and CD22+ B cells (Fig. 1 F and G) as well as by endothelial cells (Fig. 1B) (4). OX40L staining also was identified in CD90+ and α-smooth muscle actin (α-SMA)-positive cells in SSc skin (Fig. 1 H and I), suggesting that OX40L is expressed by fibroblasts and myofibroblasts in fibrotic skin. The expression of OX40L by dermal fibroblasts from both patients with SSc and healthy controls was confirmed in vitro (Fig. 1 J and K).
Fig. 1.
OX40L is overexpressed by B cells, T cells, and fibroblasts in fibrotic skin of patients with SSc. (A) Sections of skin biopsies stained for OX40L were obtained from healthy controls and from the lesional skin of patients with SSc. Four representative tissue sections are shown. (Magnification: 200×.) (Scale bar: 50 µm.) Dc SSc: diffuse cutaneous systemic sclerosis. Lc SSC, limited cutaneous systemic sclerosis. (B) Sections of diffuse SSc lesional skin stained for OX40L. (Magnification: 400×.) (Scale bar: 25 µm.) Single asterisks indicate fibroblasts (spindle-shape aspect); double asterisks indicate endothelial cells; triple asterisks indicate inflammatory cells (spherical/ovoid aspect and localized in perivascular area). (C) Skin biopsies from a patient with SSc stained for OX40L and for an isotype control. (Magnification: 200×.) (D and E) Semiquantitative analysis of immunostaining intensity using ImageJ software. Values are represented by dot blots with the mean ± SEM. *P < 0.05; **P < 0.01; two-tailed Mann–Whitney test. AU: arbitrary units. (F–I) Sections of skin biopsies from patients with SSc stained by immunofluorescence for OX40L and CD3 (F), CD22 (G), CD90 (H), and α-SMA (I). (Magnification: 400×.) (Scale bar: 10 µm.) (J and K) Fibroblasts obtained from lesional skin of patients with scleroderma (J) and from healthy controls (K). Nuclei were stained by DAPI. (Magnification: 630×.) (Scale bar: 5 µm.) The staining was performed in three independent series.
Soluble OX40L as a Biomarker of Fibrosis.
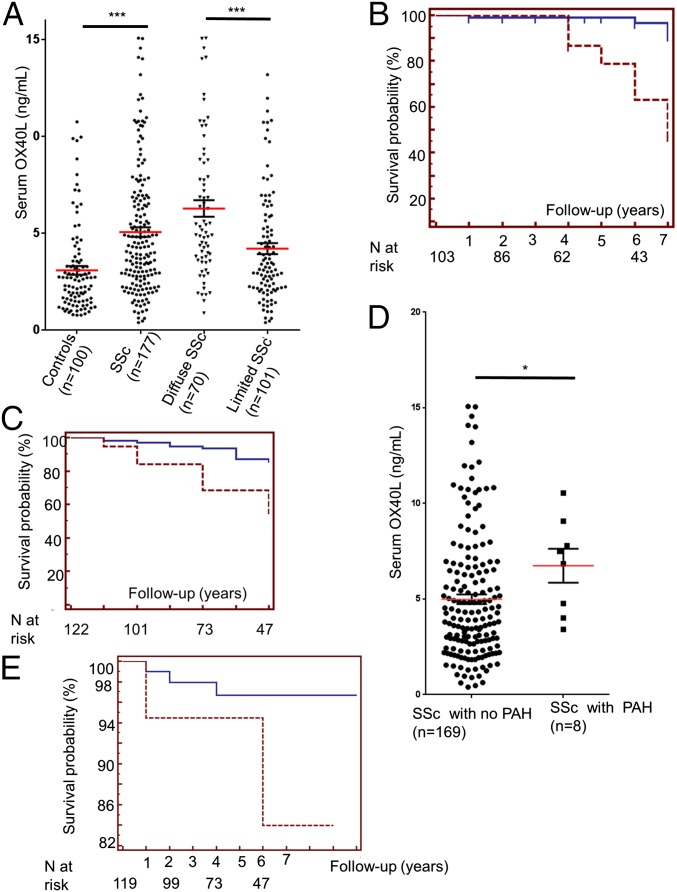
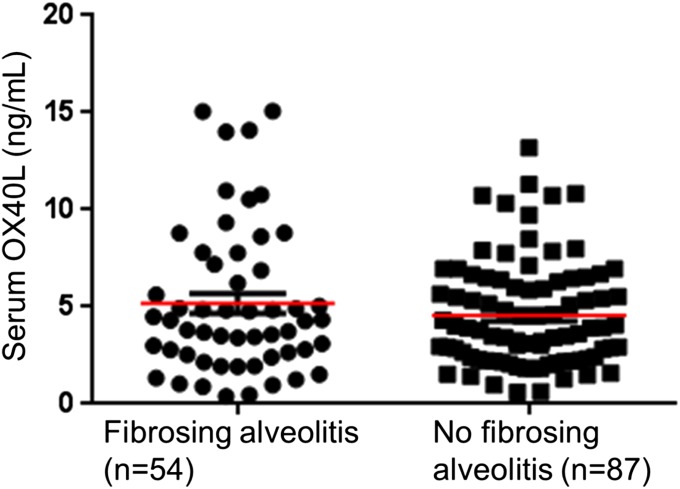
In a cross-sectional analysis soluble OX40 levels were significantly higher in patients with SSc (n = 177), particularly in patients with the diffuse cutaneous subset, than in healthy controls (n = 100) (Fig. 2A). Longitudinal analyses showed that a high OX40L level in serum at baseline was highly predictive of worsening of dermal fibrosis during the follow-up period [hazard ratio (HR): 8.28, 95% confidence interval (CI): 2.11–32.50; P < 0.001] (Fig. 2B). The association between fibrosing alveolitis and baseline OX40L serum levels was nonsignificant P = 0.266 (Fig. S1), but a high OX40L serum level at baseline was highly predictive of pulmonary worsening during the follow-up period (HR: 4.60, 95% CI:1.52–12.18, P < 0.001) (Fig. 2C). Patients with SSc and pulmonary arterial hypertension (PAH) had higher OX40L serum levels at baseline (6.74 ± 3.31 ng/mL versus 4.99 ± 3.29; P < 0.05) (Fig. 2D). A trend was seen for patients with elevated serum OX40L levels at baseline to develop de novo PAH more frequently than patients with a low OX40L concentration (HR: 3.64, 95% CI: 0.32–41.02; P = 0.13) (Fig. 2E), but the small number of patients preclude any firm conclusions.
Fig. 2.
Soluble OX40L is overexpressed in the serum of patients with SSc and is predictive of worsening of fibrosis. (A) Serum OX40L levels in patients with SSc (n = 177) and in healthy controls (n = 100). (B, C, and E) Kaplan–Meier survival curves for worsening of dermal fibrosis (B), lung fibrosis (C) and de novo development of PAH (E) during the follow-up period according to OX40L levels in serum at baseline. The dashed line represents survival probability in patients with high OX40L levels in serum at baseline (concentration equal to or above the 95th percentile in healthy controls, i.e., 8.4370). The solid line represents survival probability in patients with low OX40L levels in serum at baseline (concentration below the 95th percentile in healthy controls, i.e., 8.4370). The number of patients with SSc at risk is noted below the curves. (D) Serum OX40L levels in patients with SSc with (n = 8) and without (n = 169) PAH. *P < 0.05.
Fig. S1.
Serum OX40L levels are not significantly different between SSc-patients with and without fibrosing alveolitis.
In the replication cohort (n = 241), OX40L was highly associated with lung fibrosis in the cross-sectional analysis (regression coefficient B: 0.003, SE 0.001, P = 0.025). At follow-up, OX40L was predictive for worsening lung fibrosis both in univariate (P = 0.002) and multivariate linear regression models (HR: 1.5, 95% CI: 1.03–2.31; P = 0.037) (Table S1). These results emphasize the role of OX40L in SSc and in fibrosis and suggest that OX40L might be used as a biomarker to delineate the prognosis of patients with SSc more accurately in clinical practice. To assess the role of OX40L in fibrosis further, the effect of OX40L blockade on fibrosis was evaluated in vivo in different complementary murine models of SSc.
Table S1.
Association of extensive disease at follow-up, FVC% decline >5% and FVC <70% at follow-up, circulating Ox40L, and clinical parameters in the Oslo University Hospital SSc cohort (n = 241) in multivariable logistic regression
| FVC <70% at follow-up (range) | P value | Annual FVC decline >5% (range) | P value | Extensive lung disease* (range) | P value | |
| OX40l | 1.3 (1.01–1.80) | 0.047 | 1.4 (1.06–1.79) | 0.017 | 1.5 (1.03–2.31) | 0.037 |
| Anti-centromere Ab | NS | NS | NS | NS | NS | |
| dcSSc | 4.1 (1.22–13.81) | 0.027 | NS | NS | NS | |
| Fibrosis at baseline | 1.2 (1.09–1.22) | <0.001 | NS | NS | 1.3 (1.14–1.45) | <0.001 |
| Age at disease onset | NS | NS | 1.04 (1.01–1.07) | 0.019 | NS | NS |
All values are adjusted for gender. Ab, antibody; dcSSc: diffuse cutaneous systemic sclerosis; FVC, forced vital capacity; NS, not significant.
*Defined by Goh and Wells (44) as >20% extent of interstitial lung disease (ILD) on HRCT and 10–30% extent of ILD and FVC <70%.
OX40L-Deficient Mice Are Protected from Bleomycin-Induced Dermal Fibrosis.
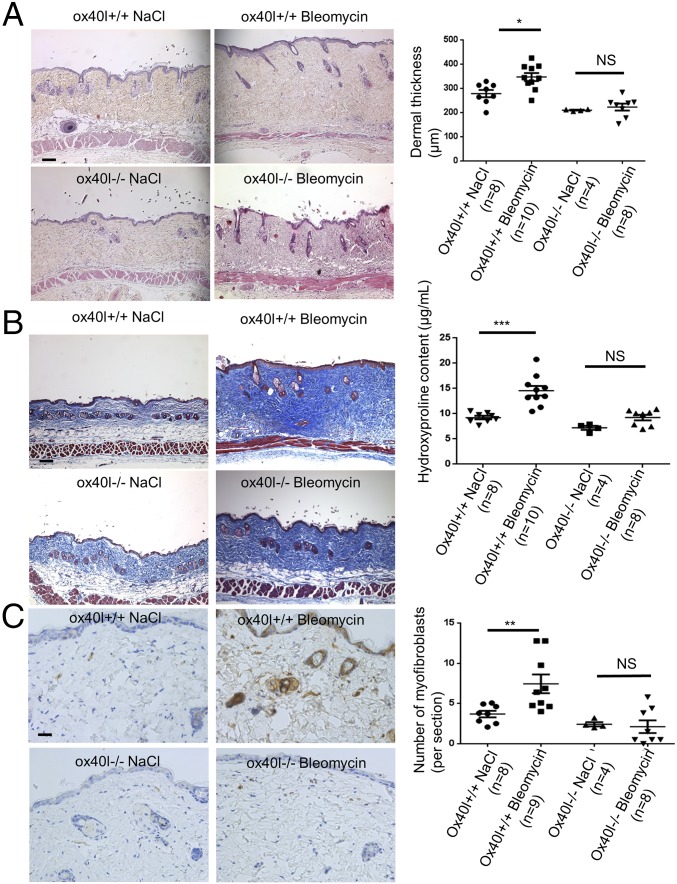
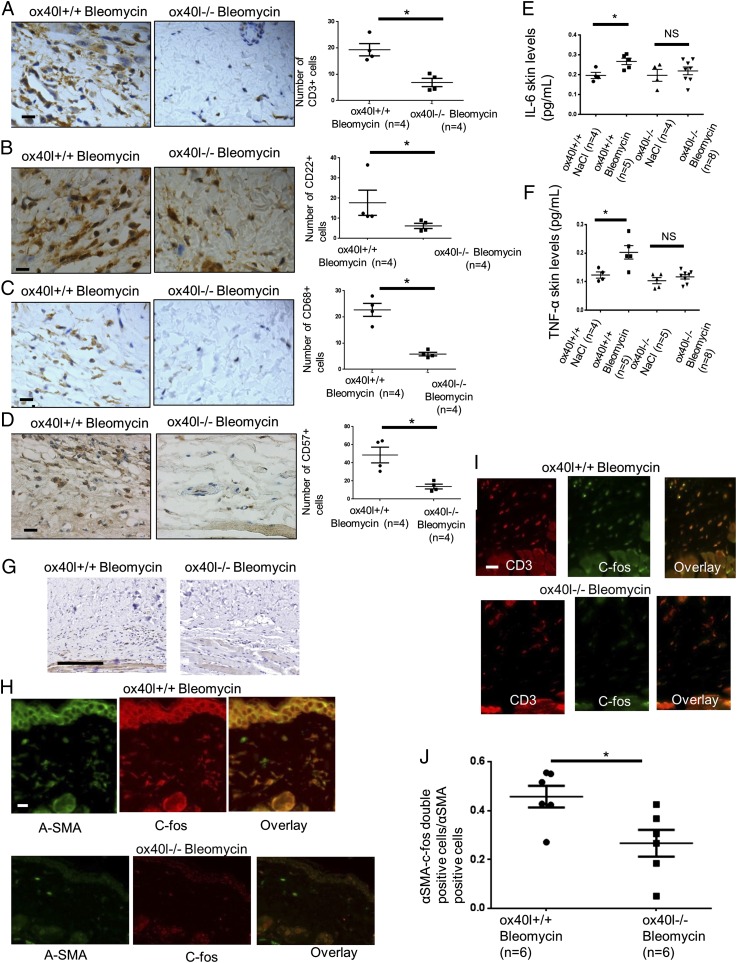
ox40l−/− mice were markedly protected from bleomycin-induced fibrosis with a mean ± SEM decreases of 13 ± 7% in dermal thickness (P = 0.027), 19 ± 8% (P = 0.034) in hydroxyproline content, and 57 ± 32% (P = 0.044) in myofibroblast count in the lesional skin of ox40l−/− mice compared with ox40l+/+ mice after bleomycin injections (Fig. 3).
Fig. 3.
Mice lacking OX40L are protected from dermal fibrosis induced by bleomycin. (A) Representative H&E-stained sections (magnification: 100×) from ox40l+/+ mice injected s.c. with NaCl (n = 8), ox40l+/+ mice injected s.c. with bleomycin (n = 10), ox40l−/− mice injected s.c. with NaCl (n = 4), and ox40l−/− mice injected s.c. with bleomycin (n = 8). (Scale bar: 100 µm.) (B) Representative sections stained by trichrome. (Magnification: 100×.) (Scale bar: 100 µm.) Hydroxyproline content is reduced in lesional skin of ox40l−/− mice. (C) Myofibroblasts were identified by positive staining for α-SMA in slides after counterstaining with hemalun. (Scale bar: 50 µm.) Values are represented by dot blots with the mean ± SEM. The experiment was performed in two independent series. *P < 0.05; **P < 0.01; ***P < 0.001; two-sided Mann–Whitney test. NS: nonsignificant.
OX40L Invalidation Reduces T Cells, B Cells, Natural Killer Cells, and Macrophage Infiltration in Lesional Skin of Mice Challenged with Bleomycin.
CD3+ T cells were reduced by 64 ± 8% (P = 0.029), CD22 B cells were reduced by 71 ± 9% (P = 0.016), natural killer (NK) cells were reduced by 72% ± 26% (P = 0.029), and CD68 macrophages were reduced by 74 ± 3% (P = 0.029) in lesional skin of ox40l−/− mice as compared with ox40l+/+ mice treated with bleomycin (Fig. 4 A–D).
Fig. 4.
Decrease in T cells, B cells, macrophages, NK cells, proinflammatory cytokines, and c-fos expression in lesional skin of OX40L-deficient mice subjected to bleomycin injections. (A–D) The number of CD3+ T cells (A), CD22+ B cells (B), CD68+ macrophages (C), and NK cells (D) by immunohistochemistry in lesional skin of ox40l+/+ and ox40l−/− mice challenged with bleomycin. Ten mice were used for these experiments. Representative sections stained by immunohistochemistry for CD3, CD22, CD68, and CD57 are shown. (Magnification: 400×.) (Scale bars: 10 µm.) (E and F) Skin levels of the cytokines Il-6 (E) and TNF-α (F) in ox40l−/− and ox40l+/+ mice subjected to bleomycin. Sixteen mice were used for these experiments (four mice per group). All cytokine concentrations are normalized on total protein concentration. (G) Representative sections stained by immunohistochemistry for c-fos (magnification: 200×) from ox40l+/+ and ox40l−/− mice receiving s.c. injections of bleomycin. The experiment was performed in two independent series of three mice. (Scale bar: 200 µm.) (H) c-Fos was expressed by CD3+ T cells. The staining was performed in three independent series. (Magnification: 400×.) (Scale bar: 10 µm.) (I) c-Fos was expressed by α-SMA+ cells. The staining was performed in three independent series. (Magnification: 400×.) (Scale bar: 10 µm.) (J) Semiquantitative analysis of intensity of double staining among cells expressing α-SMA using ImageJ software. The staining was performed in six mice from each group (in two independent series). Values in E, F, and J are represented by dot blots with the mean ± SEM; *P < 0.05; two-tailed Mann–Whitney test. NS: nonsignificant.
OX40L Invalidation Reduces Levels of Proinflammatory Cytokines in the Lesional Skin of Mice Challenged with Bleomycin.
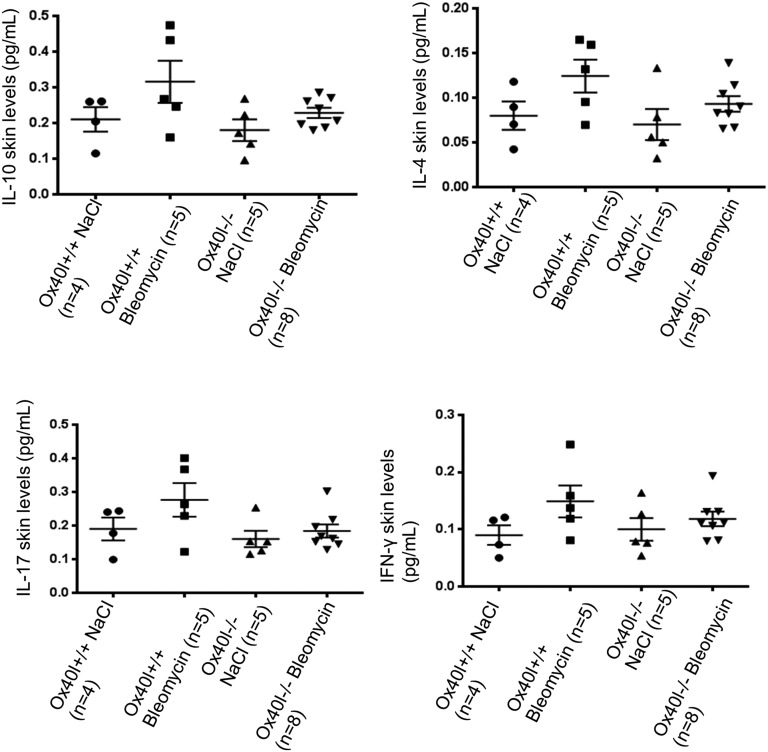
ox40l−/− mice showed reduced levels of IL-6 (56 ± 9%, P = 0.029) and TNF-α (66 ± 2%, P = 0.016) after bleomycin treatment (Fig. 4 E and F). No differences in the levels of IL-4 and IL-10 were observed, but there was a nonsignificant trend for decreased IL-17 and IFN-γ (Fig. S2).
Fig. S2.
Skin levels of the cytokines IL-10, IL-4, IL-17, and IFN-γ did not differ in ox40l−/− mice and ox40l+/+ mice subjected to bleomycin. Twenty-two mice were used. All cytokine concentrations are normalized on total protein concentration.
OX40L Invalidation Induces Gene-Expression Changes in the Lesional Skin of Mice Challenged with Bleomycin.
To identify the downstream pathways affected by OX40L inhibition, gene expression was compared in ox40l+/+ and ox40l−/− mice injected with bleomycin. Five top key cell functions (Table 1) and five canonical pathways (Table 2) were identified by supervised analysis. Inhibition of matrix metalloproteinase (MMP) was a relevant pathway in the context of fibrosis (P = 0.020). The main genes identified in this pathway were MMP-28, thrombospondin-2, and tissue factor pathway inhibitor-2. Gene set enrichment analysis identified the NF-κB, and activator protein 1 (AP-1) pathways as down-regulated in ox40l−/− mice (Table 3).
Table 1.
Ingenuity knowledge base analysis
| Molecular and cellular functions | No. of differentially expressed genes | P value |
| Cellular assembly and organization | 28 | 1.74 × 10−4 |
| Cellular function and maintenance | 20 | 2.59 × 10−4 |
| Molecular transport | 13 | 6.60 × 10−4 |
| Protein trafficking | 12 | 6.60 × 10−4 |
| Posttranslational modification | 13 | 1.47 × 10−3 |
A transcriptomic approach was performed to compare genes differentially expressed between ox40l+/+ (n = 3) and ox40l−/− mice (n = 4) injected with bleomycin.
Table 2.
Interactive pathway analysis
| Top canonical pathways | P value |
| Sulfite oxidation IV | 1.49 × 10−2 |
| Androgen biosynthesis | 1.80 × 10−2 |
| Inhibition of matrix metalloproteases | 2.03 × 10−2 |
| l-DOPA degradation | 2.96 × 10−2 |
| Thiosulfate disproportionation III | 2.96 × 10−2 |
A supervised analysis revealed the up-regulation of five pathways.
Table 3.
Gene set enrichment analysis
| Top gene set enrichment analysis signaling pathways | Enrichment score | P value |
| PDGF receptor → AP-1/MYC | −0.51 | <1 × 10−5 |
| HGFR → AP-1/CREB/MYC | −0.50 | <1 × 10−5 |
| TGFBR → AP-1 | −0.49 | <1 × 10−5 |
| Angiopoietin → AP-1 | −0.47 | <1 × 10−5 |
| Notch → NFκB | −0.45 | <1 × 10−5 |
| IL1R → NFκB | −0.44 | <1 × 10−5 |
A transcriptomic approach was performed to compare genes differentially expressed between ox40l+/+ (n = 3) and ox40l−/− mice (n = 4) injected with bleomycin. Gene Set Enrichment Analysis showed an up-regulation of genes involved in two main pathways: the NFκB and AP1 pathways. CREB: cAMP response element binding protein; HGFR: hepatocyte growth factor receptor; PDGFR, PDGF receptor; TGFBR: TGF-β receptor.
OX40L Regulates Inflammatory and Myofibroblast Lesional Skin Infiltration Through AP-1.
AP-1 regulates the gene expression of MMP1 and TIMP1, which are involved in matrix remodeling. Furthermore, AP-1 is up-regulated in a TGF-β–dependent manner in SSc, and pharmacological inhibition of AP-1 prevents the pathological activation of dermal fibroblasts in vitro and the development of experimental dermal fibrosis in different in vivo models (11) Therefore we focused on the AP-1 pathway by studying the expression of two well-known AP-1 proteins. c-fos and c-jun, in the lesional skin of mice treated with bleomycin. c-Fos was detected on T cells and myofibroblasts, with fewer myofibroblasts expressing c-fos in the lesional skin of ox40l−/− mice than in ox40l+/+ mice (P = 0.0260) (Fig. 4 G–J). A decrease in the expression of c-jun was also observed in ox40l−/− mice treated with bleomycin (Fig. S3).
Fig. S3.
Representative sections stained by immunohistochemistry for c-jun (magnification: 200×) from ox40l+/+ and ox40l−/− mice receiving s.c. injections of bleomycin.
OX40L Blockade Prevents Dermal Fibrosis by Acting on both Hematopoietic and Nonhematopoietic Cells.
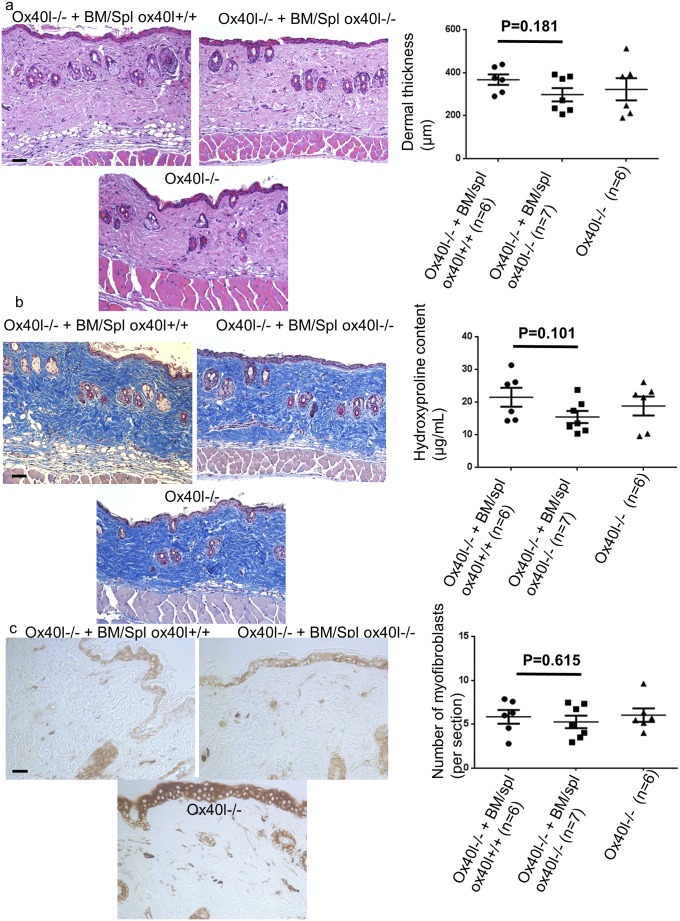
To determine whether OX40L blockade acts predominantly on inflammatory cells or fibroblasts to prevent dermal fibrosis, ox40l−/− mice were lethally irradiated and reconstituted with bone marrow and spleen cells from ox40l+/+ mice or ox40l−/− mice. One mouse died in the group reconstituted with cells from ox40l+/+ mice. A trend was seen for increased thickness and collagen content of lesional skin in irradiated mice reconstituted with bone marrow and spleen cells from ox40L+/+ mice as compared with mice reconstituted with cells from ox40l−/− mice (Fig. S4), but the trend did not reach significance. There was no significant difference between ox40l−/− mice reconstituted with bone marrow from ox40l+/+ or ox40l−/− mice, suggesting that the blockage of OX40L acts on both hematopoietic cells and nonhematopoietic cells to prevent bleomycin-induced dermal fibrosis.
Fig. S4.
Reconstitution of sublethally irradiated ox40l−/− mice with bone marrow and spleen cells from wild-type mice is not sufficient to reverse the protection of dermal fibrosis. (A) Representative H&E-stained sections (magnification: 100×) from sublethally irradiated ox40l−/− mice reconstituted with spleen and bone marrow cells from ox40l+/+ mice (Ox40l−/− + BM/Spl ox40l+/+) (n = 7), sublethally irradiated ox40l−/− mice reconstituted with spleen and bone marrow cells from ox40l−/− mice (Ox40l−/− + BM/Spl ox40l−/−) (n = 7), and nonirradiated ox40l−/− mice (n = 6). All mice received s.c. injections of bleomycin (n = 20). One mouse from the Ox40l−/− + BM/Spl ox40l+/+ group died. (Scale bar: 100 µm.) (B) Representative sections stained by trichrome. (Magnification: 100×.) (Scale bar: 100 µm.) There was a trend for increased hydroxyproline content in lesional skin of Ox40l−/− + BM/Spl ox40l+/+ mice. (C) Myofibroblasts were identified by positive staining for α-SMA in slides. (Scale bar: 50 µm.) Values are represented by dot blots with the mean ± SEM; two-sided Mann–Whitney test.
OX40L mAb Protects Against the Development of Bleomycin-Induced Fibrosis.
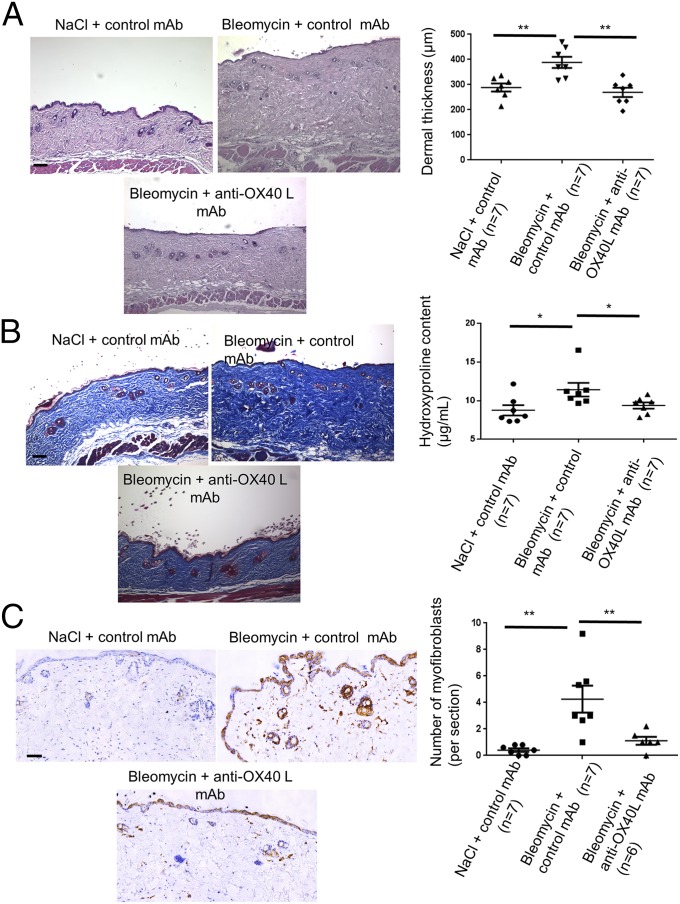
For therapeutic purposes, we assessed the effect of a neutralizing mAb against murine OX40L in this model. Tolerance of the treatment was good. The anti-OX40L mAb significantly reduced dermal thickening by 31 ± 6% (P = 0.002), hydroxyproline content by 18 ± 5% (P = 0.026), and the number of myofibroblasts by 74 ± 67% (P = 0.009) (Fig. 5).
Fig. 5.
Bleomycin-induced skin fibrosis is prevented upon OX40L inhibition with a neutralizing mAb. (A) Representative H&E-stained sections (magnification: 100×) from C57/Bl6 mice injected s.c. with NaCl and injected i.p. with IgG (control) (n = 7); C57/Bl6 mice injected s.c. with bleomycin injected i.p. with IgG (control) (n = 7); and C57/Bl6 mice injected s.c. with bleomycin and injected i.p. with anti-OX40L neutralizing mAb (n = 7). (Scale bar: 100 µm.) (B) Representative sections stained by trichrome. (Magnification: 100×.) (Scale bar: 100 µm.) The hydroxyproline assay evaluates collagen content. (C) Myofibroblasts were identified by positive staining for α-SMA in slides after counterstaining with hemalun. (Scale bar: 100 µm.) Values are represented by dot blots with mean ± SEM. The experiment was performed in two independent series. *P < 0.05; **P < 0.01; two-sided Mann–Whitney test.
OX40L mAb Induces the Regression of Established Fibrosis in the Modified Bleomycin Model.
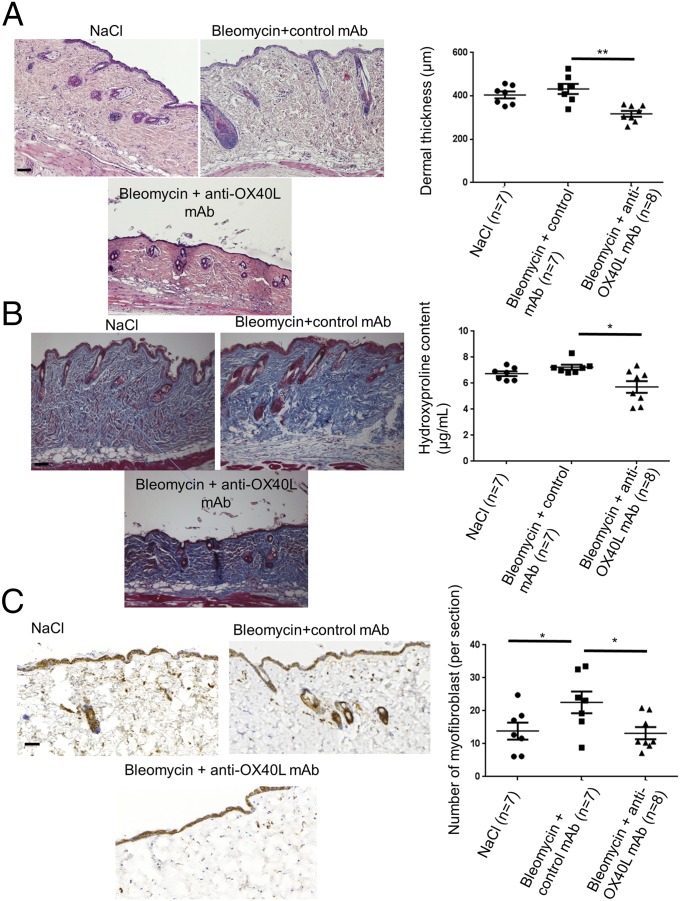
Although the prevention of fibrosis is a major aim in the treatment of SSc, the clinical situation is most often characterized by patients who present with already established fibrosis. We addressed the antifibrotic effects of the anti-OX40L mAb in a model of established fibrosis. Treatment with the anti-OX40L mAb induced a regression of established fibrosis, with a decrease in dermal thickness by 26 ± 3% (P = 0.002), in collagen content by 22 ± 7% (P = 0.020), and in myofibroblasts recruitment by 42 ± 6% (P = 0.027) (Fig. 6).
Fig. 6.
Inhibition of OX40L with a neutralizing mAb induces regression of established fibrosis in the bleomycin mouse model. (A) Representative H&E-stained sections (magnification: 100×) from C57/Bl6 mice injected s.c. with bleomycin for 6 wk and injected i.p. with IgG (control) for the last 3 wk (n = 7); C57/Bl6 mice injected s.c. with bleomycin for 6 wk and injected i.p. in parallel with anti-OX40L neutralizing mAb for the last 3 wk (n = 7); and C57/Bl6 mice injected s.c. with bleomycin for 3 wk followed by s.c. injections of NaCl for the last 3 wk (n = 8). (Scale bar: 100 µm.) (B) Representative sections stained by trichrome. (Magnification: 100×.) (Scale bar: 100 µm.) The hydroxyproline assay evaluates collagen content. (C) Myofibroblasts were identified by positive staining for α-SMA in slides after counterstaining with hemalun. (Scale bar: 100 µm.) Twenty-two mice were used for these experiments. Results are represented by dot blots with mean ± SEM. The experiment was performed in two independent series. *P < 0.05; **P < 0.01; two-sided Mann–Whitney test.
OX40L mAb Does Not Protect Against Noninflammatory Skin Fibrosis in the Tight Skin 1 Mouse Model.
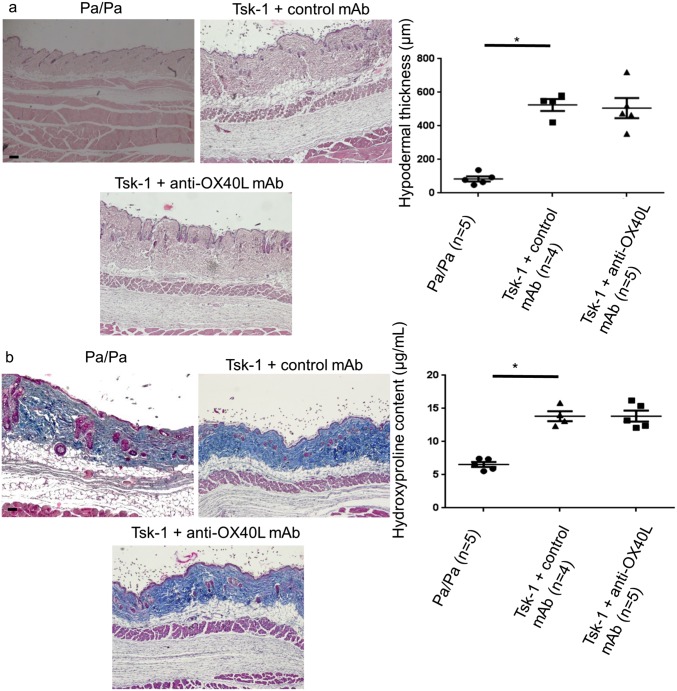
Next, we aimed to determine whether OX40L inhibition was efficient in an inflammation-independent model of skin fibrosis, the tight skin 1 (Tsk-1) mouse model (12, 13). We did not observe any difference in hypodermal thickening and collagen content between Tsk-1 mice treated with the anti-OX40L mAb or with a control mAb (Fig. S5).
Fig. S5.
OX40L inhibition has no effect in the Tsk-1 mouse model. (A) Representative H&E-stained sections (magnification: 40×) from control pa/pa mice (n = 5), Tsk-1 mice treated with control mAb (n = 4), and Tsk-1 mice treated with anti-OX40L mAb (n = 5). (Scale bar: 250 µm.) (B) Hydroxyproline content is not significantly different in Tsk-1 mice treated with anti-OX40L mAb or with control mAb. Representative samples stained by trichrome are shown. (Magnification: 40×.) Values are represented by dot blots with means ± SEM. Control mice were pa/pa mice. The experiment was performed in two independent series. *P < 0.05; two-sided Mann–Whitney test.
OX40L mAb Protects Against Fibrosing Alveolitis in the Fra-2 Transgenic Mouse Model.
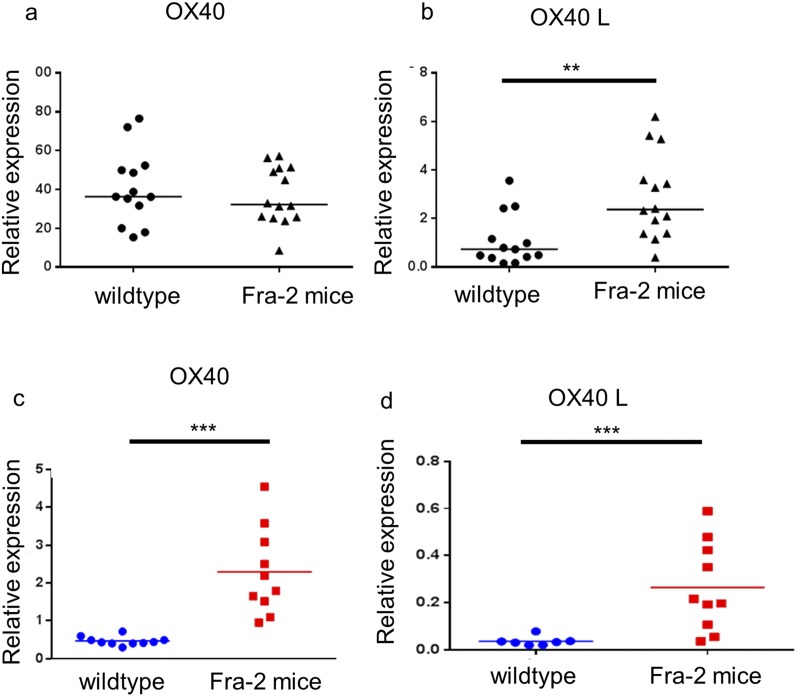
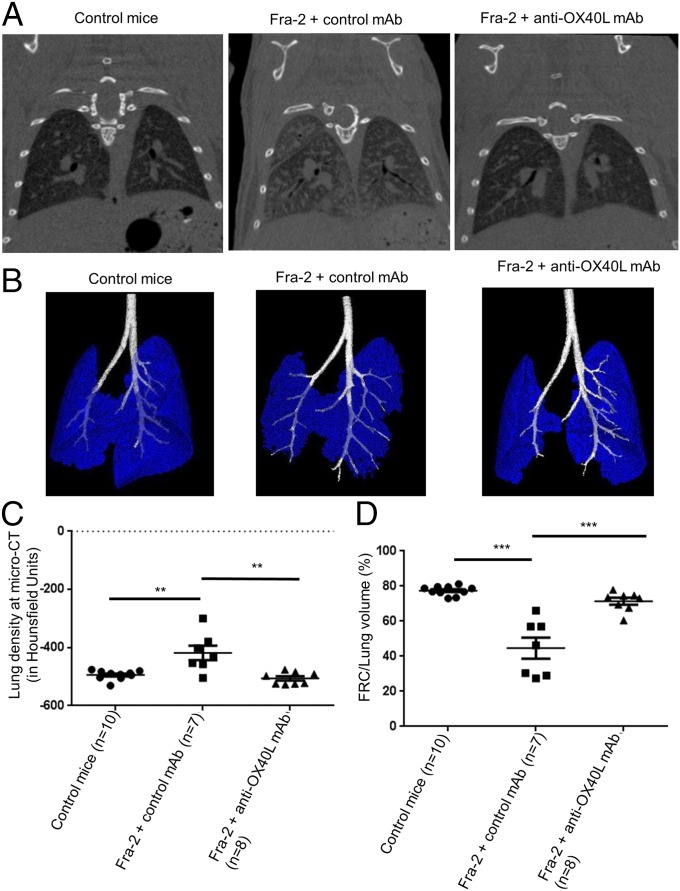
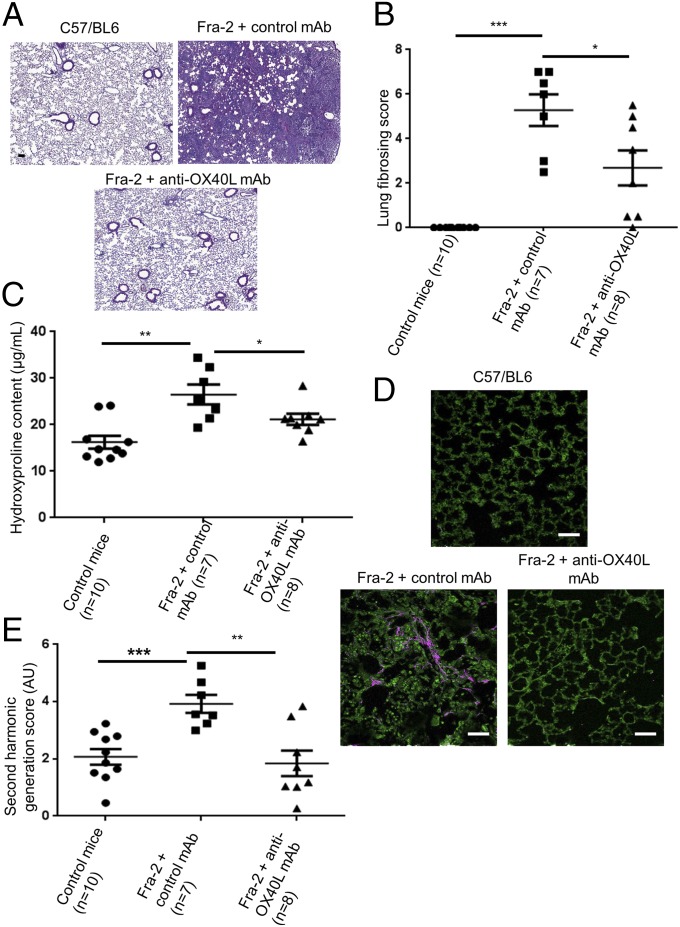
Because interstitial lung involvement and PAH are key prognostic factors in SSc (14), we aimed to assess the effects of the OX40L mAb in the Fra-2 mouse model, which exhibits these severe involvements (15, 16). Fra-2 transgenic mice displayed an increased expression of OX40 and OX40L in the skin and in the lungs as compared with control mice (Fig. S6). Treatment by anti-OX40L mAb was well tolerated. Micro-computed tomography (micro-CT) revealed higher lung density consistent with fibrosing alveolitis in Fra-2 mice treated with control mAb than in C57/BL6 mice (P = 0.007); this lung density was decreased significantly in Fra2 mice treated with the anti-OX40L mAb (P = 0.004). Restrictive lung disease was observed in Fra-2 mice treated with control mAb; these mice had a functional residual capacity equal to 44.7% of lung volume versus 77.4% in control mice (P < 0.001). Fra-2 mice receiving anti-OX40L mAb had a functional residual capacity of 71.4% of lung volume (P < 0.001). There was no significant difference at micro-CT between Fra-2 mice treated with anti-OX40L mAb and wild-type mice (Fig. 7). Fra-2 mice treated with control IgG were characterized by SSc-like features of nonspecific interstitial pneumonia at histology, with large patchy areas of lung parenchyma characterized by both diffuse cellular inflammation and collagen deposition (17). The lung fibrosis histological score was significantly higher in Fra-2 mice treated with control IgG than in Fra-2 mice receiving anti-OX40L mAb (mean score: 4.69 versus 2.69; P = 0.032) (Fig. 8 A and B). The lung fibrosis score was highly correlated with CT data (correlations with density score: ρ: 0.470, P = 0.018) and with forced residual capacity/lung volume (ρ: −0.846, 95% CI: −0.930–0.677; P < 0.001). Consistently, hydroxyproline content was reduced by 23 ± 9% (P = 0.029) (Fig. 8C). Second harmonic generation (SHG) microscopy showed a preferential perivascular distribution of fibrosis, suggesting fibrosing alveolitis (Fig. 8D). Scoring of fibrillar collagen deposits confirmed an increase in collagen scoring in Fra-2 mice receiving control IgG as compared Fra-2 mice treated with the anti-OX40L mAb (P = 0.009) (Fig. 8E).
Fig. S6.
OX40 and OX40L are overexpressed in skin and lungs of Fra-2 transgenic mice. (A and B) Expression of OX40 (A) and OX40L (B) was assessed in the skin of 14 Fra-2 mice and 10 wild-type control mice by quantitative PCR (Taqman). (C and D) OX40 (C) and OX40L (D) expression also was assessed in the lungs of 10 wild-type mice and 10 Fra-2 mice. **P < 0.01; ***P < 0.001.
Fig. 7.
Inhibition of OX40L prevents the development of fibrosing alveolitis: CT-scan data. (A) Fibrosing alveolitis was observed in Fra-2 mice receiving control IgG. Representative micro-CT images are shown. (B) Representative images of functional residual capacity (in blue) in different mice; bronchi are in white. (C) Increased lung density at micro-CT in Fra-2 transgenic mice treated with control IgG (n = 7) compared with Fra-2 mice treated with anti-OX40L mAb (n = 8) or C57/BL6 wild-type (control) mice (n = 10). (D) Residual lung volume, expressed as the percentage of functional residual capacity on total lung volume. Values are dot blots with the mean ± SEM; **P < 0.01; ***P < 0.001; two-sided Mann–Whitney test.
Fig. 8.
Inhibition of OX40L prevents the development of fibrosing alveolitis: histological analysis. (A) Representative H&E-stained lung sections from Fra-2 mice treated with control IgG (n = 7), Fra-2 mice treated with anti-OX40L mAb (n = 8), and control C57/BL6 mice (n = 10). (Scale bar: 100 µm.) (B) Lung fibrosing score was significantly higher in Fra-2 mice receiving control mAb than in Fra-2 mice treated with anti-OX40L mAb. (C) The hydroxyproline assay evaluates collagen content. (D) SHG showed fibrillar collagen (in pink) in Fra-2 mice treated with control IgG. (Scale bar: 50 µm.) (E) Second harmonic scores were higher in Fra-2 mice receiving control IgG than in Fra-2 mice treated by anti-OX40L mAb or in control mice. Twenty-five mice were used for these experiments. Values in B, C, and E are represented by dot blots with means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; two-sided Mann–Whitney test. AU: arbitrary units.
OX40L mAb Protects Against PAH in the Fra-2 Transgenic Mouse Model.
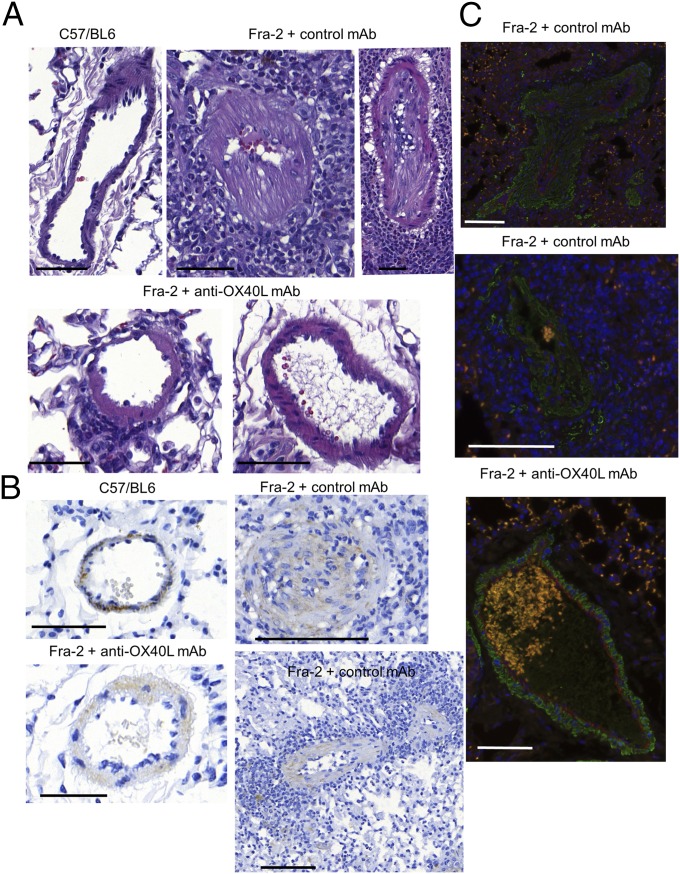
An increase in wall thickness and occlusion of pulmonary arteries, associated with massive perivascular inflammatory infiltrates, resembling SSc-associated PAH, were detected more frequently in Fra-2 mice treated with control antibody than in wild-type mice or Fra-2 mice receiving the anti-OX40L antibody (Fig. 9A). Obliterated vessels were undetectable in wild-type mice and in Fra-2 mice treated with anti-OX40L (0%) but were observed in 42.9% of Fra-2 mice receiving control antibody (P = 0.02). Completely muscularized or occluded vessels were observed in five of seven (71.4%) Fra-2 mice treated with control IgG and none of 10 wild-type and eight Fra-2 mice treated with anti-OX40L mAb (P = 0.003 and P = 0.007, respectively) (Fig. 9B). We did not observe coexpression of CD31 and α-SMA in the vessel walls of Fra-2 mice treated with control mAb, suggesting that PAH in this model was explained by the differentiation of resident fibroblasts into myofibroblasts rather than by endothelial-to-mesenchymal transition. This proliferation was decreased in Fra-2 mice treated with anti-OX40L antibody (Fig. 9C).
Fig. 9.
Inhibition of OX40L prevents PAH in Fra-2 mice. (A) Representative H&E-stained sections from Fra-2 mice treated with control antibody (two representative pictures are shown: one vessel is almost occluded, and one is completely occluded), from C57/BL6 (control) mice, and from Fra-2 mice receiving anti-OX40L antibody (two representative pictures are shown: one vessel with a mild infiltrate, and one vessel without vasculopathy or inflammatory infiltrate). (Scale bars: 100 µm.) (B) Remodeling of the vessel was assessed by staining for α-SMA (immunohistochemistry) and was more pronounced in Fra-2 mice receiving control IgG (vessel occluded or almost completely occluded with a massive inflammatory infiltrate on two representative pictures) than in Fra-2 mice treated with anti-OX40L mAb or C57/BL6 (control) mice. Twenty-five mice were used for this experiment. (Scale bars: 100 µm.) (C) There was no costaining for CD31 (red) or α-SMA (green) in vessels from Fra-2 mice treated with control antibody or anti-OX40L antibody. Vasculopathy in Fra-2 mice receiving control antibody was related to the prominent proliferation of fibroblasts (marked by α-SMA) rather than endothelial-to-mesenchymal transition (no coexpression of CD31 and α-SMA). A massive perivascular infiltrate was noticed (DAPI). (Scale bars: 100 µm.)
Discussion
Our study provides the first evidence, to our knowledge, that OX40L, a costimulatory molecule required for full activation of T cells, is implicated in the development of inflammation-driven skin, lung, and vessel fibrosis. SSc is the ideal disease for studying the events preceding the onset of fibrosis because the immune component and typical fibrotic events coincide in this condition. We identified increased OX40L expression in the lesional skin and serum of patients with SSc, particularly in patients with diffuse cutaneous SSc. Diffuse SSc, defined by the highest extent of skin fibrosis, is associated with high morbidity and mortality (18). In addition to the expected expression of OX40L in perivascular T cells and B cells (4), we observed an expression, not previously reported, of OX40L by activated dermal fibroblasts, suggesting that mesenchymal cells can be directly activated by the OX40/OX40L pathway and thus promote collagen synthesis and fibrosis. Next, we showed that the inhibition of OX40L through complementary gene inactivation and targeted molecular strategies prevented and even induced the regression of bleomycin-induced dermal fibrosis. This widely used model mimics early and inflammatory stages of SSc. Blocking OX40L exerted antifibrotic effects in this model by decreasing the infiltration of T cells into lesional skin, as is consistent with previous reports in other preclinical models of autoimmune disorders (3, 19–22).
Our data support the notion that OX40L regulates the cytokine balance toward a proinflammatory and profibrotic profile, because lower levels of TNF-α and IL-6 were detected in ox40l−/− mice. This finding is consistent with the previous description of enhanced production of TNF-α and IL-6 through OX40/OX40L interaction (23). Reduced IL-6 release after OX40L invalidation also has been described in the murine model of encephalomyelitis (20). Herein, we confirmed that NF-κB is one of the central mediators of OX40L signaling (24). We also identified AP-1 as a downstream signaling pathway of OX40L. This finding has been scarcely reported to date but is consistent with the up-regulation of c-jun and c-fos mRNA observed in activated endothelial cells after binding OX40L (25). Interestingly, AP-1 is implicated both in inflammatory response and fibrosis. AP-1 is up-regulated in a TGF-β–dependent manner in SSc, and pharmacological inhibition of AP-1 prevents the pathological activation of dermal fibroblasts and the development of experimental dermal fibrosis (11). The reduced expression of c-jun and c-fos observed on T cells and fibroblasts from ox40l−/− mice upon bleomycin challenge suggests that OX40L may regulate inflammation and fibroblast activation through AP-1 signaling in inflammation-driven skin fibrosis. Despite its known predominant role in hematopoietic cells and the immune response, our results indicate that OX40L blocking does not act only through hematopoietic cells. Indeed, the results obtained in irradiated mice reconstituted with bone marrow and spleen cells from ox40l+/+ mice and the in vitro data presented herein show that both hematopoietic and nonhematopoietic cells, including fibroblasts, are involved in the effects mediated by OX40L in the context of fibrosis.
Blocking OX40L with a specific mAb did not display antifibrotic properties in the Tsk-1 mouse model, which is characterized by endogenous activation of fibroblasts independent of inflammation; this result supports the proposition that the inflammatory environment is required for OX40L to exert profibrotic effects.
Our results highlight the substantial effects of blocking OX40L to prevent the severe organ damage characterizing SSc. Treatment with OX40L mAb markedly prevented fibrosing alveolitis in the Fra-2 transgenic mouse model by reducing inflammatory infiltrates, which are prominent features in this model. Consistently, OX40L transgenic mice spontaneous develop interstitial pneumonia accompanied by the significant production of anti-DNA antibody (26). This result is of particular importance because fibrosing alveolitis is the leading cause of death in SSc, and no efficient therapy is yet available to treat this devastating condition (14, 27). Furthermore, Fra-2 transgenic mice receiving anti-OX40L antibody had reduced perivascular inflammatory infiltrates and were protected against the vessel remodeling leading to PAH, the most extreme vascular phenotype of SSc (15, 16). The role of OX40L in PAH is also supported by the observations in OX40L transgenic mice, which spontaneously develop severe PAH associated with massive lymphocytic perivascular infiltration (28). Thus, OX40L inhibition may have the potential to address several aspects of SSc-PAH pathology that are not addressed by current therapies, which act mostly by relieving vasoconstriction. Fighting inflammation is a very appealing complementary strategy because both endothelial dysfunction and inflammation are intertwined with the initiation and progression of PAH.
In idiopathic PAH, proliferative vasculopathy results both from endothelial-to-mesenchymal transition and from the differentiation of resident fibroblasts into myofibroblasts. Our results suggest that the second mechanism is prominent in Fra-2 transgenic mice and that this proliferation was decreased in mice treated with anti-OX40L antibody.
Taken together, our results may have direct implications, because a human mAb, anti-OX40L, is available, and preliminary phase 2 data report that it was well tolerated in patients with mild allergic asthma (29). Fibrosis is the final common pathway hallmarking SSc and many other diseases. Therefore, blocking OX40L appears to be a promising strategy for SSc and for numerous fibrotic disorders. In addition, the anti-OX40L antibody offers the putative advantage of selectively targeting pathogenic T cells without causing generalized immunosuppression, possibly decreasing the risk of infections usually observed with other immunosuppressive drugs (3, 4, 10, 22, 30).
In addition to the lack of an effective therapy, another unmet clinical need in SSc and fibrotic disorders is the absence of reliable biomarkers predicting disease progression. Soluble OX40L appears to be a promising biomarker, because increased serum levels were detected in SSc patients with severe skin or lung disease at baseline, and, of particular interest, high concentrations also were predictive of worsening skin and lung fibrosis during the follow-up period. The data regarding lung fibrosis that were independently replicated in a well-structured cohort support the robustness of OX40L as a prognostic marker for this severe complication, which is the leading cause of death in SSc.
Materials and Methods
Patients and Skin Biopsies.
OX40L was quantified by ELISA in the serum of 177 patients with SSc and 100 healthy age- and sex-matched volunteers using the Human Soluble OX40L (sOX40L) ELISA kit (Cusabio). The replication study cohort was derived from the prospective, observational Oslo University Hospital SSc cohort study dedicated to lung fibrosis assessment (31).
Paraffin-embedded sections of lesional skin biopsies were obtained from eight patients with SSc (three patients with limited SSc and five patients with diffuse cutaneous forms) and from five healthy age- and sex-matched healthy volunteers.
Fibroblast Cultures.
Fibroblast cultures were obtained from biopsies of involved skin of patients with SSc and skin of healthy volunteers. Mouse fibroblasts cultures were obtained from samples taken from the ears of mice. Fibroblasts were prepared by outgrowth cultures. Fibroblasts from passages 2–3 were used for the experiments.
Bleomycin-Induced Dermal Fibrosis in OX40L-Deficient Mice.
Skin fibrosis was induced in 6-wk-old male mice by local injections of bleomycin for 3 wk (32). Injections (s.c.) of NaCl were used as controls. The four groups consisted of 30 mice in total. Validated parameters (11, 33) were used for evaluation. Mice were killed by cervical dislocation after 3 wk of treatment, and the injected skin was processed further for analysis.
Irradiation and Graft.
In all, 20 5- to 7-wk-old ox40l−/− mice (10 females and 10 males) were included. Recipient mice were lethally irradiated and reconstituted with spleen and bone marrow cells from ox40l+/+ mice (n = 7) or from ox40l+/+ mice (n = 7), as previously described (34). Seven ox40l−/− mice were not irradiated and were used as controls. Beginning on the day after irradiation, mice were injected s.c. with bleomycin for 3 wk, as previously described.
Prevention and Treatment of Bleomycin-Induced Fibrosis with anti-OX40L mAb.
In the preventive protocol, one group of seven mice received i.p. injections of 300 µg anti-OX40L mAb (RM134L, rat IgG2b) in 100 µL PBS three times/wk over a 3-wk period starting on the day before the first injection of bleomycin. Two control groups of seven mice were treated with an equivalent amount of control rat IgG (Sigma) and were injected with bleomycin and NaCl, respectively. The three groups consisted of 21 mice in total. In the therapeutic protocol we assessed the effects of OX40L inhibition in established dermal fibrosis. Two groups of mice were challenged with bleomycin for 6 wk and were treated in parallel with i.p. injection of 300 µg anti-OX40L mAb or control IgG three times/wk, beginning after the third week of bleomycin. The third group received bleomycin injections for 3 wk followed by NaCl injections for the next 3 wk to control for the spontaneous regression of fibrosis. Mice were killed by cervical dislocation after 6 wk of treatment.
Evaluation of Dermal Thickness.
Dermal thickness was analyzed at 100× magnification by measuring the distance between the epidermal–dermal junction and the dermal–s.c. fat junction at four sites on H&E-stained skin sections. Measurements were made by two independent blinded examiners (M.F. and M.E.), as previously described (33, 35).
Collagen Measurements.
The collagen content in lesional skin samples was explored by hydroxyproline assay, as previously described (33, 35). For direct visualization of collagen fibers, trichrome staining was performed using the Masson’s Trichrome Staining Kit (Sigma-Aldrich).
Immunohistochemistry for α-SMA, OX40L, c-Fos, c-Jun, CD3, CD22, CD57, and CD68.
Myofibroblasts were identified by staining for α-SMA as previously described (33). T cells, B cells, NK cells, macrophages, and c-fos+ and c-jun+ cells were identified by staining for CD3, CD22, CD57, CD68, c-fos, and c-jun, respectively (all antibodies were from Abcam) (33, 35).
The expression of OX40L in lesional skin from patients with SSc and in skin from controls was detected by staining with polyclonal rabbit anti-human OX40L antibody or isotype control (Sigma-Aldrich). The intensity of OX40L immunostaining was quantified with ImageJ software.
Immunofluorescence for OX40L, CD3, CD22, CD31, α-SMA, c-Fos, and CD90.
For costaining experiments, immunofluorescent staining was performed in lesional skin from patients with SSc. Fibroblasts were identified by staining for CD90 (Abcam). The intensity of α-SMA+ cells expressing c-fos was quantified with ImageJ software.
Inflammatory Cytokine Measurement in Lesional Skin Samples of Bleomycin-Treated Mice.
Cytokine levels were measured in the skin of 16 ox40l−/− and ox40l+/+ mice subjected to bleomycin or NaCl injections (four mice per group), as previously described (33, 35). Protein concentration was determined with the amidoblack method (36). Skin lysates were assayed for TNF-α, IL-6, IFN-γ, IL-4, IL-10, and IL-17.
Effects of OX40L Inhibition in the Tsk-1 Mouse Model.
Starting at 5 wk of age, five Tsk-1 mice were treated with 300 µg anti-OX40L mAb and four Tsk-1 mice were treated with control IgG. A third group consisted of five control mice homozygous for the pallid spontaneous mutation (pa/pa mice). Mice were killed by cervical dislocation at the age of 10 wk to analyze the hypodermal thickness and the hydroxyproline content in lesional skin.
Transcriptomic Approach.
Skin samples from three ox40l+/+ and four ox40l−/− mice treated with bleomycin were defrosted, extracted, and hybridized at the same time. Affymetrix microarray technology was used to analyze gene expression levels.
Extraction protocol.
Total RNA were extracted from lesional skin samples of three ox40l+/+ and four ox40l−/− mice treated with bleomycin using the RNeasy Mini Kit with DNase I digestion according to the manufacturer's instructions.
Transcriptomes preparation and data analysis.
Samples were prepared according to standard practices of the GENOM’IC facility of the Institut Cochin. All microarray data and information have been submitted to the National Center for Biotechnology Information Gene Expression Omnibus site (accession no. GSE73705). Data were robust multiarray normalized with Bioconductor. We first controlled and analyzed data in an unsupervised way by principal components analysis and used ANOVA to extract differentially expressed genes with the Partek Genomics Suite. Enrichment analysis was carried out using Interactive Pathway Analysis and Pathway Studio.
Prevention of Fibrosing Alveolitis and PAH in the Fra-2 Model.
Two groups of Fra-2 transgenic mice were treated by anti-OX40L mAb (n = 8 mice) or control rat IgG (n = 7 mice) beginning on week 13 of development. Two control groups of five C57/BL6 mice were treated with anti-OX40L mAb or control rat IgG, respectively. Mice were killed by cervical dislocation at age 17 wk.
Assessment of Fibrosing Alveolitis by Micro-CT.
Fibrosing alveolitis was evaluated using micro-CT 2 d before mice were killed. Means of lung density of both groups were determined by evaluating all CT scans acquired from the apices to the bases of the lungs. Furthermore, the volume of functional lung parenchyma corresponding to functional residual capacity (FRC) was drawn manually, excluding fibrotic area and vessels. Percentages of FRC on total lung volumes were calculated. The CT expert (J.S.) was blinded to the background of mice, to the treatment, and to the results of the histological assessment.
Histopathologic Assessment of Fibrosing Alveolitis.
Lung sections were stained with H&E. The severity of fibrosing alveolitis was semiquantitatively assessed on a scale of 0–8 according to the method described by Ashcroft et al. (37) by two examiners (O.A. and M.E.) blinded to the genotype and the treatment. Hydroxyproline content in lung biopsies was assessed as previously described.
Nonlinear Microscopy and SHG Processing.
A multiphoton inverted-stand Leica SP5 microscope (Leica Microsystems GmbH) was used for tissue imaging. Two fixed thresholds were chosen to distinguish biological material from the background signal [two-photon excited harmonic (TPEF) images] and specific collagen fibers (SHG images). The SHG score was established by comparing the area occupied by the collagen relative to the sample surface. Image processing and analysis (thresholding and SHG scoring) were performed using ImageJ homemade routines (imagej.nih.gov/ij) as previously described (38). Results were normalized to control C57/BL6 mice.
Vessel Remodeling.
In each mouse, a total of 15 vessels and their accompanying alveolar ducts or alveoli were examined by two observers (O.A. and M.E.) masked to the genotype and treatment. Each vessel was categorized as nonmuscular (no evidence of vessel wall muscularization), partially or completely muscular (smooth muscle cells identifiable in part or in the entire vessel circumference), or occluded. Muscularization was defined as the presence of typical smooth muscle cells stained with α-SMA. All images were taken with a Lamina multilabel slide scanner.
Statistics.
All data analyses were performed using GraphPad. Data are presented as mean (SEM) for continuous variables and as numbers (percentages) for categorical variables. Data were analyzed statistically using Fischer tests for differences in frequency and the Mann–Whitney test for comparisons between two continuous variables. A P value of less than 0.05 was considered statistically significant. Further details regarding materials and methods are provided in SI Materials and Methods.
Study Approval.
All patients and controls signed a consent form approved by the local institutional review boards [CPP (Comité de Protection des Personnes) Paris Ile de France 3]. The local ethical committee [CEEA (comité d’éthique en experimentation animale) 34 of Paris Descartes University] approved all animal experiments [agreements no. CEEA34.JA.023.12 and no. 15-031 (Apafis 2015080901097845 and 2015062619109294)].
SI Materials and Methods
Serum Levels of OX40L.
All patients fulfilled the 2013 European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) classification criteria for SSc (39). Among the patients with SSc, 147 (83.1%) were female and 30 were men. The mean age (± SD) of patients with SSc was 56.9 ± 13.8 y. The mean disease duration was 9.8 ± 8.2 y; 70/171 (41%) patients had the diffuse cutaneous subset, and 101 (59%) had the limited form according to Leroy’s criteria (40). Pulmonary hypertension was defined by precapillary pulmonary hypertension on right heart catheterization (resting mean pulmonary artery pressure ≥25 mmHg together with a pulmonary capillary wedge pressure of ≤15 mmHg) (41). Fibrosing alveolitis was searched by high-resolution CT (HRCT). Among these patients, 124 were followed up for a mean 5.6 ± 2.9 y. A high level of serum OX40L at baseline was considered to be a serum OX40L level higher or equal to that in the 95% percentile of healthy patients, i.e., 8.4370 ng/mL. Progression of dermal fibrosis was defined by worsening of Rodnan skin score by ≥10% and was observed in 14/103 (13.6%) patients with SSc during the follow-up period (42). Worsening of fibrosing alveolitis was defined by new-onset lung fibrosis on HRCT and/or deterioration of lung volume (≥10% of forced vital capacity) during the follow-up period. Progression of fibrosing alveolitis was observed in 28/122 (22.9%) patients with SSc.
The replication study cohort was derived from the ongoing, prospective, observational Oslo University Hospital SSc cohort study and included all cases that met the 2013 EULAR/ACR classification criteria for SSc and for which longitudinal clinical data were available (31, 43). Paired pulmonary function tests (PFTs) and HRCT lung images were obtained at baseline and at the last available follow-up visit, and the extent of fibrosis measured precisely, as previously described (31).
The SSc study cohort included 241 patients. Mean age at disease onset was 48 y (SD 15.4), the mean follow-up period was 3.9 y (SD 2.9), and mean disease duration was 5.9 y (SD 6.1). The baseline PFT and HRCT data were obtained a mean of 1.7 y before the serum used for the cross-sectional OX40L analyses was obtained; the corresponding follow-up data for the predictive model were from a mean of 2.8 y after serum sampling.
mAb Against OX40L.
One hybridoma producing a neutralizing mAb against mouse OX40L (RM134L, rat IgG2b) was used as previously described (7–10). This hybridoma was grown in RPMI 1640 medium with FCS; then the supernatant was purified by protein G chromatography and was quantified using spectrophotometry. The purity of the obtained antibody was assessed by PAGE.
Animal Care.
The animals were kept under standard laboratory conditions. Diet and water were provided ad libitum. Animal welfare was assessed every 2 d, and animals were weighed weekly.
Bleomycin-Induced Dermal Fibrosis in OX40L-Deficient Mice.
OX40L-deficient mice were purchased from Jackson Laboratory. Mice on a C57BL/6 background expressing OX40L (ox40l+/+) were purchased from Janvier. Skin fibrosis was induced in 6-wk-old male mice by local injections of bleomycin for 3 wk; 100 μL of bleomycin dissolved in 0.9% NaCl at a concentration of 0.5 mg/mL was administered every other day by s.c. injection in defined 1-cm2 areas of the upper back (32). Injections of 0.9% NaCl (100 μL s.c.) were used as controls. Four different groups, consisting of two groups of ox40l−/− mice and two groups of ox40l+/+ mice were analyzed. One group of ox40l−/− mice and one group of ox40l+/+ mice were challenged with bleomycin; the other two groups were injected with NaCl. The four groups consisted of 30 mice in total.
Prevention and Treatment of Bleomycin-Induced Fibrosis with Anti-OX40L mAb.
Skin fibrosis was induced in 6-wk-old, pathogen-free, male C57BL/6 mice (Janvier) by injection of bleomycin for 3 wk, as previously described. Injections (s.c.) of 100 μL 0.9% NaCl, the solvent for bleomycin, was used as controls. The weight of the mice was 20–25 g.
Collagen Measurements.
The collagen content in lesional skin samples was explored by hydroxyproline assay, as previously described (33, 35). Briefly, each sample was hydrolyzed and titrated to a pH of 7. This solution was combined with chloramine T and p-dimethylaminobenzaldehyde in perchloric acid and was read at 557 nm with a spectrophotometer (Molecular Devices). Two samples from each mouse were analyzed in this experiment.
Immunohistochemistry for α-SMA, OX40L, c-Fos, c-Fun, CD3, CD22, CD57, and CD68.
Myofibroblasts were identified by staining for α-SMA (33). Cells positive for α-SMA in mouse skin sections were detected by incubation with monoclonal anti–α-SMA antibody (clone 1A4; Sigma-Aldrich) at a dilution of 1:1,000 for 3 h at room temperature. Polyclonal rabbit anti-mouse labeled with HRP (Dako) was used as secondary antibody for 1 h at room temperature. The number of myofibroblasts was determined at 200× magnification in four different sections from each mouse by two blinded examiners (M.F. and M.E.). To quantify the numbers of infiltrating T cells, B cells, NK cells, and macrophages and the expression of c-fos and c-jun, lesional skin sections from ox40l−/− and ox40l+/+ mice were incubated with polyclonal rabbit anti-mouse antibodies for CD3 (dilution 1:50), CD22 (dilution 1:100), or CD57 (1/200) or with polyclonal mouse anti-mouse antibodies for CD68 (dilution 1:100) or c-fos (dilution 1:100), or with monoclonal rabbit anti-mouse antibodies for c-jun (dilution 1:50) (all antibodies from Abcam) overnight at 4 °C, after antigen retrieval, as previously described (33, 35). Polyclonal goat anti-rabbit or rabbit anti-mouse (Dako) was used as the secondary antibody (dilution 1:200). T cells, B cells, and macrophages were counted in eight different sections of lesional skin of each mouse at 400× magnification. Counting was performed in a blinded manner by two examiners (M.F. and M.E.).
The expression of OX40L in lesional skin from patients with SSc and in skin from controls was detected by overnight staining at 4 °C with polyclonal rabbit anti-human OX40L antibody or isotype control at a dilution of 1:100 (Sigma-Aldrich). Polyclonal goat anti-rabbit antibody (Dako) was used as secondary antibody (dilution 1:200). The intensity of OX40L immunostaining was quantified with ImageJ software, as described at rsbweb.nih.gov/ij/docs/examples/stained-sections/index.html.
Immunofluorescence for OX40L, CD3, CD22, CD31, α-SMA, c-Fos, and CD90.
For costaining experiments, immunofluorescent staining was performed in lesional skin from patients with SSc. The protocol and antibodies were similar to the immunohistochemistry protocol. Fibroblasts were identified by staining for polyclonal rabbit anti-human antibodies for CD90 (dilution 1:100) (Abcam). Alexa Fluor 594 goat anti-mouse IgG and Alexa Fluor 488 goat anti-rabbit antibodies (Life Technologies) were used as secondary antibodies for 1 h at room temperature at a dilution of 1:200. Slides were mounted on a coverslip with a drop of mounting medium and were stored in the dark at +4 °C until analysis. OX40L expression also was assessed on SSc fibroblasts and healthy fibroblasts cultured in vitro. Nuclei were stained using DAPI.
For costaining between c-fos and CD3 and α-SMA, as well as CD31 (dilution 1:100) (Abcam) and α-SMA. Alexa Fluor 594 goat anti-rabbit IgG and Alexa Fluor 488 goat anti-rabbit antibodies (Life Technologies) were used as secondary antibodies. The intensity of α-SMA+ cells expressing c-fos was quantified with ImageJ software.
Inflammatory Cytokine Measurement in Lesional Skin Samples from Bleomycin-Treated Mice.
Cytokine levels were measured in the skin of 16 ox40l−/− and ox40l+/+ mice subjected to bleomycin or NaCl injections (four mice per group), as previously described (33, 35). Briefly, mouse skin tissue lysate was prepared by homogenization in modified RIPA buffer with a Precellys 24 tissue homogenizer/grinder (Ozyme). Skin lysates were assayed for the following cytokines by multiplex bead array technology (BD Biosciences): TNF-α, IL-6, IFN-γ, IL-4, IL-10, and IL-17.
Effects of OX40L Inhibition in the Tsk-1 Mouse Model.
Five Tsk-1 mice (purchased from Jackson Laboratory) were treated with 300 µg anti-OX40L mAb, and four Tsk-1 mice were treated with control IgG three times/wk i.p., starting at age 5 wk (before the development of fibrosis). Another group consisted of five pa/pa (control) mice. The hypodermal thickness was determined by measuring the thickness of the s.c. connective tissue beneath the panniculus carnosus at four different sites on the upper back in each mouse at 40× magnification. The measurement was performed by two independent blinded investigators (M.P. and M.E.).
Microscopy.
Images were captured with a Nikon Eclipse 80i microscope equipped with a DSP 3CCD camera (Sony) or with a Lamina multilabel slide scanner (PerkinElmer) or on a Zeiss Axio Observer Z1 microscope with dry 40× and dry 63× objectives and a CoolSNAP HQ2 CCD camera.
Prevention of Fibrosing Alveolitis and PAH in the Fra-2 Model.
Fra-2 transgenic mice were obtained from a collaboration established with Sanofi Genzyme. Two groups of eight and seven Fra-2 transgenic mice, respectively, were treated by anti-OX40L mAb or control rat IgG, as previously described, starting when mice were 13 wk old, before the appearance of fibrosing alveolitis and vasculopathy. Two control groups of five C57/BL6 mice were treated by i.p. injections of anti-OX40L mAb and control rat IgG, respectively. Because results in the two control groups (i.e., C57/BL6 mice injected with anti-OX40L or with control IgG) were similar, these groups were analyzed together. Mice were killed by cervical dislocation at age 17 wk.
Expression of OX40 and OX40L by Fra-2 Transgenic Mice.
Expression of OX40 and OX40L by Fra-2 transgenic mice was assessed in the skin and in the lungs of the mice by PCR (Taqman).
Assessment of Fibrosing Alveolitis by Micro-CT.
For assessment of fibrosing alveolitis, the animals were placed in the supine position on the CT table. CT images were obtained with a PerkinElmer Quantum FX system (Caliper Life Sciences).
Mice were sedated with 3–4% isoflurane anesthesia (0.5–1.5 L/min) for induction via a nose cone. Anesthesia was maintained with 2.5–3% isoflurane (400–800 mL/min) during the acquisition. During image acquisition thoracic breathing movements were recorded, detecting the upward and downward movement of the thorax. Images were acquired throughout the spontaneous respiratory cycle. Only images acquired during expiration were analyzed. Images were acquired with the following parameters: 90 kV X-ray source voltage, 160 µA current. Total scanning time was ∼4.5 min per mouse. Tomograms were reconstructed using Rigaku software. The analysis began with the isolation of lung tissue by a manually drawn volume of interest. Analysis of lung density and drawing was performed with CTAn Bruker software. Lung density was measured in Hounsfield units (HU) after calibration. A phantom calibration was made on the acquisition Rigaku software: a water-filled 1.5-mL tube inside a 2-mL tube was scanned. Based on full-stack histograms of a manually delimited volume-of-interest containing only water or air, the mean grayscale index of water was set at 0 HU, and the grayscale index of air was set at −1,000 HU. This value was reported in the CTAn Bruker software.
Histopathologic Assessment of Fibrosing Alveolitis.
The severity of fibrosing alveolitis was semiquantitatively assessed according to the method described by Ashcroft et al. (37) by two examiners blinded to the genotype and the treatment (O.A. and M.E.). Lung fibrosis was graded on a scale of 0–8 by examining randomly chosen fields of the left upper lobe. The grading criteria were as follows: grade 0: normal lung; grade 1: minimal fibrous thickening of alveolar walls; grade 3: moderate thickening of walls without obvious damage; grade 5: increased fibrosis with definite damage and formation of fibrous bands; grade 7: severe distortion of structure and large fibrous areas; and grade 8: total fibrous obliteration. Grades 2, 4, and 6 were used as intermediate stages between these criteria. All images were taken with a Lamina multilabel slide scanner.
Nonlinear Microscopy and SHG Processing.
A multiphoton inverted-stand Leica SP5 microscope (Leica Microsystems GmbH) was used for tissue imaging. A Ti:Sapphire Chameleon Ultra laser (Coherent) with a center wavelength at 810 nm was used as the laser source for SHG and TPEF signals. The laser beam was circularly polarized to ensure isotropic excitation of the sample regardless of the orientation of fibrillar collagen. A Leica Microsystems HCX IRAPO 25×/0.95 W objective was used to excite and collect SHG and TPEF signals. Signals were detected in epi-collection through 405/15-nm and 525/50-nm bandpass filters, respectively, by non-descanned photomultiplier tube detectors (Leica Microsystems) with a constant voltage supply, at constant laser excitation power, allowing direct comparison of SHG intensity values. Two fixed thresholds were chosen to distinguish biological material from the background signal (TPEF images) and specific collagen fibers (SHG images). The SHG score was established by comparing the area occupied by the collagen relative to the sample surface. Image processing and analysis (thresholding and SHG scoring) were performed using ImageJ homemade routines (imagej.nih.gov/ij) as previously described (38). Results were normalized to control C57/BL6 mice.
Statistics.
To analyze whether serum OX40L was predictive of dermal, pulmonary, or vascular worsening, a Cox proportional hazard model was performed and summarized as HR and 95% CI. The correlation between the lung fibrosis score and the data obtained at micro-CT was assessed by calculating the Spearman’s coefficient of rank correlation (ρ) and 95% CI.
Acknowledgments
We thank the following individuals for excellent technical assistance: Avigail Bismuth, Lucille Desallais, Florence Morin, Maryline Favier, and Corinne Lesaffre (Histology Facility of the Cochin Institute); B. Durel (Cochin Imaging Facility); M. Andrieu [Immunology Flow Cytometry Facility (CyBio Platform) of the Cochin Institute]; F. Letourneur and F. Dumont (Genomics Platform of the Cochin Institute); and Prof. Catherine Chaussain (Dental School of the Paris Descartes University, EA 2496). This work was supported by INSERM, ATIP/AVENIR programme, Société Française de Rhumatologie, Fondation pour la Recherche Médicale, the Arthritis Foundation, the Institut Servier, and Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research 26293087.
Footnotes
Conflict of interest statement: A.S. and R.R. work for Sanofi. J.A. has/had consultancy relationship and/or has received research funding in relationship with the treatment of systemic sclerosis from Actelion, Bayer, BMS, Roche-Chugai, Pfizer, and Sanofi. Y.A. has/had consultancy relationship and/or has received research funding in relationship with the treatment of systemic sclerosis from Actelion, Bayer, Biogen Idec, Bristol-Myers Squibb, Genentech/Roche, Inventiva, Medac, Pfizer, Sanofi/Genzyme, Servier, and Union chimique belge.
This article is a PNAS Direct Submission.
Data deposition: All microarray data and information have been submitted to the National Center for Biotechnology Information Gene Expression Omnibus (accession no. GSE73705).
See Commentary on page 7291.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1523512113/-/DCSupplemental.
References
- 1.Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: Nearing the starting line. Sci Transl Med. 2013;5(167):167sr1. doi: 10.1126/scitranslmed.3004700. [DOI] [PubMed] [Google Scholar]
- 2.Varga J, Pasche B. Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol. 2009;5(4):200–206. doi: 10.1038/nrrheum.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004;4(6):420–431. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 4.Ishii N, Takahashi T, Soroosh P, Sugamura K. OX40-OX40 ligand interaction in T-cell-mediated immunity and immunopathology. Adv Immunol. 2010;105:63–98. doi: 10.1016/S0065-2776(10)05003-0. [DOI] [PubMed] [Google Scholar]
- 5.Webb GJ, Hirschfield GM, Lane PJL. OX40, OX40L and autoimmunity: A comprehensive review. Clin Rev Allergy Immunol. 2016;50(3):312–332. doi: 10.1007/s12016-015-8498-3. [DOI] [PubMed] [Google Scholar]
- 6.Gourh P, et al. Association of TNFSF4 (OX40L) polymorphisms with susceptibility to systemic sclerosis. Ann Rheum Dis. 2010;69(3):550–555. doi: 10.1136/ard.2009.116434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bossini-Castillo L, et al. A replication study confirms the association of TNFSF4 (OX40L) polymorphisms with systemic sclerosis in a large European cohort. Ann Rheum Dis. 2011;70(4):638–641. doi: 10.1136/ard.2010.141838. [DOI] [PubMed] [Google Scholar]
- 8.Coustet B, et al. Independent replication and meta analysis of association studies establish TNFSF4 as a susceptibility gene preferentially associated with the subset of anticentromere-positive patients with systemic sclerosis. J Rheumatol. 2012;39(5):997–1003. doi: 10.3899/jrheum.111270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komura K, et al. Increased serum soluble OX40 in patients with systemic sclerosis. J Rheumatol. 2008;35(12):2359–2362. [PubMed] [Google Scholar]
- 10.Gwyer Findlay E, et al. OX40L blockade is therapeutic in arthritis, despite promoting osteoclastogenesis. Proc Natl Acad Sci USA. 2014;111(6):2289–2294. doi: 10.1073/pnas.1321071111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avouac J, et al. Inhibition of activator protein 1 signaling abrogates transforming growth factor β-mediated activation of fibroblasts and prevents experimental fibrosis. Arthritis Rheum. 2012;64(5):1642–1652. doi: 10.1002/art.33501. [DOI] [PubMed] [Google Scholar]
- 12.Beyer C, Schett G, Distler O, Distler JHW. Animal models of systemic sclerosis: Prospects and limitations. Arthritis Rheum. 2010;62(10):2831–2844. doi: 10.1002/art.27647. [DOI] [PubMed] [Google Scholar]
- 13.Green MC, Sweet HO, Bunker LE. Tight-skin, a new mutation of the mouse causing excessive growth of connective tissue and skeleton. Am J Pathol. 1976;82(3):493–512. [PMC free article] [PubMed] [Google Scholar]
- 14.Tyndall AJ, et al. Causes and risk factors for death in systemic sclerosis: A study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69(10):1809–1815. doi: 10.1136/ard.2009.114264. [DOI] [PubMed] [Google Scholar]
- 15.Maurer B, et al. Fra-2 transgenic mice as a novel model of pulmonary hypertension associated with systemic sclerosis. Ann Rheum Dis. 2012;71(8):1382–1387. doi: 10.1136/annrheumdis-2011-200940. [DOI] [PubMed] [Google Scholar]
- 16.Maurer B, et al. Transcription factor fos-related antigen-2 induces progressive peripheral vasculopathy in mice closely resembling human systemic sclerosis. Circulation. 2009;120(23):2367–2376. doi: 10.1161/CIRCULATIONAHA.109.855114. [DOI] [PubMed] [Google Scholar]
- 17.du Bois RM. Mechanisms of scleroderma-induced lung disease. Proc Am Thorac Soc. 2007;4(5):434–438. doi: 10.1513/pats.200608-152MS. [DOI] [PubMed] [Google Scholar]
- 18.Frech TM, et al. Treatment of early diffuse systemic sclerosis skin disease. Clin Exp Rheumatol. 2013;31(2) Suppl 76:166–171. [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins LM, et al. Regulation of T cell activation in vitro and in vivo by targeting the OX40-OX40 ligand interaction: Amelioration of ongoing inflammatory bowel disease with an OX40-IgG fusion protein, but not with an OX40 ligand-IgG fusion protein. J Immunol. 1999;162(1):486–493. [PubMed] [Google Scholar]
- 20.Ndhlovu LC, Ishii N, Murata K, Sato T, Sugamura K. Critical involvement of OX40 ligand signals in the T cell priming events during experimental autoimmune encephalomyelitis. J Immunol. 2001;167(5):2991–2999. doi: 10.4049/jimmunol.167.5.2991. [DOI] [PubMed] [Google Scholar]
- 21.Carboni S, et al. CD134 plays a crucial role in the pathogenesis of EAE and is upregulated in the CNS of patients with multiple sclerosis. J Neuroimmunol. 2003;145(1-2):1–11. doi: 10.1016/j.jneuroim.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Croft M, So T, Duan W, Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev. 2009;229(1):173–191. doi: 10.1111/j.1600-065X.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohshima Y, et al. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159(8):3838–3848. [PubMed] [Google Scholar]
- 24.Song J, So T, Croft M. Activation of NF-kappaB1 by OX40 contributes to antigen-driven T cell expansion and survival. J Immunol. 2008;180(11):7240–7248. doi: 10.4049/jimmunol.180.11.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumura Y, Hori T, Kawamata S, Imura A, Uchiyama T. Intracellular signaling of gp34, the OX40 ligand: Induction of c-jun and c-fos mRNA expression through gp34 upon binding of its receptor, OX40. J Immunol. 1999;163(6):3007–3011. [PubMed] [Google Scholar]
- 26.Murata K, et al. Constitutive OX40/OX40 ligand interaction induces autoimmune-like diseases. J Immunol. 2002;169(8):4628–4636. doi: 10.4049/jimmunol.169.8.4628. [DOI] [PubMed] [Google Scholar]
- 27.Bagnato G, Harari S. Cellular interactions in the pathogenesis of interstitial lung diseases. Eur Respir Rev. 2015;24(135):102–114. doi: 10.1183/09059180.00003214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabieyousefi M, et al. Indispensable roles of OX40L-derived signal and epistatic genetic effect in immune-mediated pathogenesis of spontaneous pulmonary hypertension. BMC Immunol. 2011;12:67. doi: 10.1186/1471-2172-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gauvreau GM, et al. OX40L blockade and allergen-induced airway responses in subjects with mild asthma. Clin Exp Allergy. 2014;44(1):29–37. doi: 10.1111/cea.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Humphreys IR, et al. A critical role for OX40 in T cell-mediated immunopathology during lung viral infection. J Exp Med. 2003;198(8):1237–1242. doi: 10.1084/jem.20030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann-Vold A-M, et al. Predictive value of serial high-resolution computed tomography analyses and concurrent lung function tests in systemic sclerosis. Arthritis Rheumatol. 2015;67(8):2205–2212. doi: 10.1002/art.39166. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto T. Animal model of systemic sclerosis. J Dermatol. 2010;37(1):26–41. doi: 10.1111/j.1346-8138.2009.00764.x. [DOI] [PubMed] [Google Scholar]
- 33.Avouac J, et al. Critical role of the adhesion receptor DNAX accessory molecule-1 (DNAM-1) in the development of inflammation-driven dermal fibrosis in a mouse model of systemic sclerosis. Ann Rheum Dis. 2013;72(6):1089–1098. doi: 10.1136/annrheumdis-2012-201759. [DOI] [PubMed] [Google Scholar]
- 34.Kavian N, et al. Sunitinib inhibits the phosphorylation of platelet-derived growth factor receptor β in the skin of mice with scleroderma-like features and prevents the development of the disease. Arthritis Rheum. 2012;64(6):1990–2000. doi: 10.1002/art.34354. [DOI] [PubMed] [Google Scholar]
- 35.Avouac J, et al. Inactivation of the transcription factor STAT-4 prevents inflammation-driven fibrosis in animal models of systemic sclerosis. Arthritis Rheum. 2011;63(3):800–809. doi: 10.1002/art.30171. [DOI] [PubMed] [Google Scholar]
- 36.Kamel H, et al. Comparison of gold levels and distribution in guinea pig serum. Arthritis Rheum. 1978;21(4):441–446. doi: 10.1002/art.1780210407. [DOI] [PubMed] [Google Scholar]
- 37.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41(4):467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gailhouste L, et al. Fibrillar collagen scoring by second harmonic microscopy: A new tool in the assessment of liver fibrosis. J Hepatol. 2010;52(3):398–406. doi: 10.1016/j.jhep.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 39. Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee (1980) Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 23(5):581–590. [DOI] [PubMed]
- 40.LeRoy EC, et al. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J Rheumatol. 1988;15(2):202–205. [PubMed] [Google Scholar]
- 41.Humbert M, McLaughlin VV. The 4th world symposium on pulmonary hypertension. J Am Coll Cardiol. 2009;54(1 Suppl):S1–117. doi: 10.1016/j.jacc.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 42.Clements PJ, et al. Skin thickness score in systemic sclerosis: An assessment of interobserver variability in 3 independent studies. J Rheumatol. 1993;20(11):1892–1896. [PubMed] [Google Scholar]
- 43.Hoffmann-Vold A-M, Gunnarsson R, Garen T, Midtvedt Ø, Molberg Ø. Performance of the 2013 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Systemic Sclerosis (SSc) in large, well-defined cohorts of SSc and mixed connective tissue disease. J Rheumatol. 2015;42(1):60–63. doi: 10.3899/jrheum.140047. [DOI] [PubMed] [Google Scholar]
- 44.Goh NSL, et al. Interstitial lung disease in systemic sclerosis: A simple staging system. Am J Respir Crit Care Med. 2008;177(11):1248–1254. doi: 10.1164/rccm.200706-877OC. [DOI] [PubMed] [Google Scholar]