If we want to grow vegetables in a garden, we pick a nice sunny spot and clear a space free from other plants that might shade them. Sunlight provides the energy for photosynthesis: the more light, the bigger they grow. But plants are not so simple; they never are. Growth is an investment. More leaves mean more photosynthesis and greater returns, but all good investors will tell you to keep a little back for a rainy day. In PNAS, Yang et al. (1) show that plants manage this balance between saving and investment depending on the quality of light, not just the quantity. In plants, the phytochrome photoreceptors detect red and far-red (near infrared) light. Yang et al. (1) show that loss of phytochrome results in a general risk-averse strategy to growth. Instead of allocation toward growth, more resources are allocated toward resilience, and at the heart of this change in strategy there is a change in metabolism at a quite fundamental level.
The phytochrome photoreceptors are proteins that bind a tetrapyrrole chromophore, allowing them to absorb light. These phytochromes exist in two photo-interconvertible forms: an inactive, red-absorbing “Pr” form and an active, far-red–absorbing “Pfr” form. Absorption of light by the chromophore causes it to change conformation and this, in turn, causes a change in conformation of the phytochrome protein from the Pr form to the Pfr form or vice versa (2). The active Pfr form is translocated from the cytoplasm to the nucleus, where it interacts with a number of transcription factors to mediate changes in plant physiology (3). One of the earliest demonstrations of phytochrome action was in the germination of lettuce seeds, where pulses of red light were found to trigger germination, while pulses of far-red light inhibited germination (4). Along with a suite of other plant photoreceptors absorbing in the blue and UV regions of the spectrum, phytochrome acts throughout the life of a plant, in processes such as seedling establishment and photoperiodic regulation of flowering time. However, the dual red and far-red absorption peaks of the two forms of phytochrome mean that the phytochromes are uniquely suited to one particular additional role: the detection of neighboring vegetation. This role is vitally important to plants that are adapted to growing in open fields, as most of our crop plants are, because it carries a potentially very significant threat: that of shading. Direct sunlight contains a high proportion of red light, whereas light reflected from neighboring vegetation is depleted in red and relatively rich in far red (Fig. 1 A and B). This far-red–rich light causes the removal of the active Pfr form of phytochrome and, in plants native to an open canopy, the result is what is known as the “shade-avoidance response.” Shade avoidance involves a dramatic promotion of elongation growth so as to prevent overtopping by neighboring plants (5). The Pfr form of phytochrome normally suppresses this elongation and its removal in far-red–rich light releases this suppression. Harry Smith, who sadly died last year, was one of the key pioneers in this field. McLaren and Smith showed some time ago that this elongation growth involves a reallocation of resources away from leaves, the structures dedicated to resource acquisition (5, 6). Less leaf material, of course, means less photosynthesis and less biomass. Shade avoidance is, consequently, a major factor limiting planting density because individual plant yield reduces at higher densities of planting.
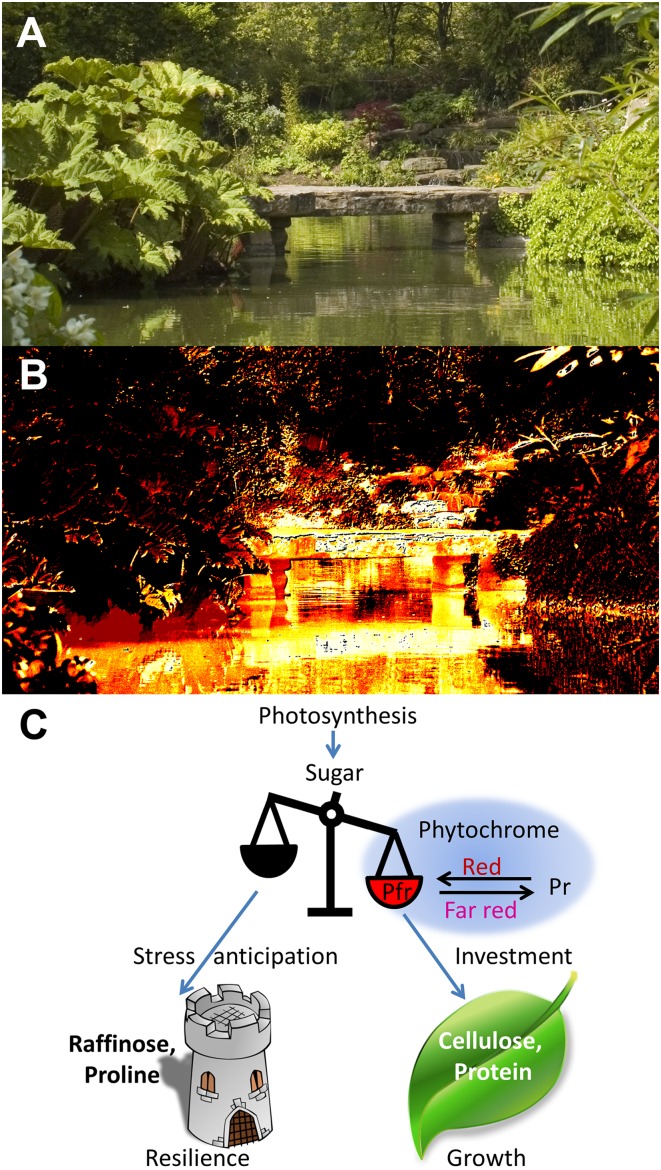
Fig. 1.
Plants perceive the quality of light in their environment to detect potential competing neighboring vegetation. The photoreceptor, phytochrome, photo-converts between an inactive red light-absorbing Pr form and an active far-red light-absorbing Pfr form, and this allows plants to perceive the red to far-red ratio (R:FR) of incident light. Direct sunlight is rich in red light, whereas light reflected from neighboring vegetation is depleted in red and rich in far red. (A) The world as we see it. (B) The world according to phytochrome. A representation of the R:FR for the same scene, produced by digitally subtracting the pixel values from a photograph taken using a camera with an infrared filter from those of a copy taken with a red filter. Darker colors show areas of low R:FR; bright colors are areas of high R:FR. Note the difference between the light reflected from the stone bridge and the light reflected from the vegetation. This distinction is vital to a plant facing potential competition. The original photographs were taken by Glasgow-based photographer James Gillies. (C) The active Pfr form of the photoreceptor, phytochrome, swings the balance between stress physiology and growth in plants. Pfr, formed in red-rich direct sunlight, favors the channeling photosynthetic products toward investment in growth. In contrast, light reflected from neighboring vegetation, rich in far red, removes active Pfr causing photosynthetic products to be channeled toward stress physiology, making the plant more resilient in anticipation of multiple possible stresses.
Yang et al. (1) used phytochrome mutants in the model plant, Arabidopsis, to examine the way in which phytochrome regulates biomass in more detail. Phytochrome mutants, of course, lack both forms of phytochrome but, importantly, they lack the active Pfr form and so they behave as if they were constantly shaded. There are actually multiple different phytochromes in higher plants, the products of distinct genes. Each shows the same red/far-red reversibility between an inactive Pr form and an active Pfr form but they have both distinct and overlapping roles, with each regulating a slightly different set of responses (7). Arabidopsis possesses five phytochromes, named phyA to phyE. Phytochromes A, B, D, and E have all been shown to play roles in shade avoidance, phyA as more of a gauge of total light intensity and phyB, phyD, and phyE as classic red/far-red reversible photoreceptors (7). Yang et al. (1) used mutants lacking all of these phytochromes. The image of the phyABDE mutant in their paper beautifully shows the classic constitutively shade-avoiding phenotype whereby resources have been channeled into elongation growth rather than the production of leaf material.
Yang et al. (1) confirm that these phyABDE mutants have greatly reduced biomass, but their key finding is that this reduced biomass is not just a result of their rate of photosynthesis being lower. A lower rate of photosynthesis would be expected, given their reduced leaf material, and phytochrome mutants did, indeed, show lower chlorophyll levels and reduced CO2 assimilation through photosynthesis. However, the mutants actually showed a higher daytime accumulation of the products of photosynthesis: organic sugars and starch. This result demonstrates a key change in investment strategy in the phytochrome mutants, with resources seemingly held in reserve during the day rather than invested in growth. This finding was true in both shoot and root tissues, meaning that this is not simply an issue of altered shoot to root transport. In agreement with this finding, growth rate of phyABDE mutants is slower than that of wild-type plants, specifically during the day.
Cell walls and proteins form a significant proportion of plant biomass, and investment in these building blocks is a major part of growth. Yang et al. (1) found that expression of several genes involved in cell wall synthesis and reorganization is reduced in phytochrome mutants. Expression of these genes normally peaks at dawn and, although this is still true in the phyABDE mutants, the peak level was reduced in the mutants for each of the genes examined. Similarly, total protein levels were much lower in the phytochrome mutants. Together, these findings suggest that phytochrome affects the rate of allocation of carbon into these key building blocks of growth. The authors point out that one of the key transcription factors that interacts with phytochrome Pfr in the nucleus, phytochrome-interacting factor 4, has been shown to directly bind to the promoter of one of the genes involved in cell wall reorganization, providing a possible mechanism by which part of this difference could be regulated.
A gas chromatography-mass spectrometry analysis revealed more about the fates of the carbon acquired in photosynthesis. Tricarboxylic acid cycle intermediates, amino acids, and sugar derivatives all accumulated to higher levels in the phytochrome mutants. Accumulation of such metabolic pathway intermediates would be consistent with reduced investment in cellulose and proteins. Surprisingly, though, raffinose and proline were two of the most dramatically up-regulated metabolites in the phytochrome mutants. Consistent with this accumulation, RAFFINOSE SYNTHASE 6 gene expression was elevated in phyABDE mutants, whereas expression of the gene encoding the proline catabolic enzyme, PROLINE DEHYDROGENASE, was suppressed.
Accumulation of both raffinose and proline is associated with stress response in plants (8). Similarly, stress commonly causes a reduction in growth and developmental processes in plants (9). It is believed that such a reduction in growth allows the plant to save and redirect resources to allow adaptation to the stress. Thus, the phenotype of the phytochrome mutants suggests that loss of phytochrome signaling has caused a switch at the metabolic level, from a strong investment in growth to a more cautious preparedness for difficult times. Plants have an extensive network of stress responses but many of these responses are common to several stressors and, in fact, there appears to be a core generic stress response as well as a set of more specific responses to each particular stress (10). Indeed, many stresses do actually present a common challenge as part of their impact, requiring a similar adaptation. Notably, salt, drought, and freezing stress all result in a difficulty for the plant in acquiring water and, ultimately, in osmotic stress within the plant. Proline and raffinose both accumulate rapidly in plants in response to a range of stresses and appear to be part of this core stress response (11, 12). Proline and raffinose are thought to enhance plant resilience to stress, at least in part by acting as osmoprotectants: small, relatively inert molecules that balance the osmotic difference between the inside and the outside of the cell so that water is not lost through osmosis. However, both proline and raffinose have also been shown to have more general antioxidant effects and to act as a source of carbon storage, providing resources for the resumption of growth once stress is removed (11, 12).
Given this metabolic profile, Yang et al. (1) examined the resilience of phytochrome mutants to stress. To look at stress more globally, one treatment used was application of the stress hormone abscisic acid (ABA). ABA is induced by many stresses in plants as part of the common core stress response pathway and triggers a wide range of adaptations, including a pronounced reduction in growth rate (9). Yang et al. (1) confirmed that application of the stress hormone, ABA, or growth at high salt concentrations, both caused a reduction in fresh weight in wild-type plants, but they found that these stresses had little effect in phyABDE mutants, indicating that these mutants are already adapted to these stresses. Similarly, phytochrome mutants were shown to have constitutively high levels of expression of a number of genes associated with a wide range of different abiotic stresses. This finding is also consistent with a previous observation that phytochrome mutants have been shown to possess improved resistance to freezing through constitutive induction of genes involved in the cold-response pathway (13), further confirming the wide-ranging effects of this phytochrome response.
The lessons from the work of Yang et al. (1) are that phytochrome signaling can act as an important barometer of upcoming environmental stress and that it regulates the well-established switch in plants between stress physiology and growth. The prospect of competition with neighboring vegetation does not just entail a struggle for light but a struggle for all resources—not least, water—and so triggering of a generic stress adaptation will serve the plant well in such an environment. The far-red–rich light reflected from neighboring vegetation removes active phytochrome Pfr and causes a significant slow-down in growth. Under these conditions of impending stress, instead of investment in biomass, greater resources are allocated into pathways enhancing resilience to various abiotic stresses (Fig. 1C). The reduction in leaf biomass, in particular, may lead to reduced potential for future resource acquisition, but that would seem a very fair price to pay for survival. Conversely, signals from active phytochrome Pfr, which persist in the red-rich radiance of direct sunlight, promote vigorous growth and strong investment in new leaf material, and are an indicator of good times ahead.
Footnotes
The author declares no conflict of interest.
See companion article on page 7667.
References
- 1.Yang D, Seaton DD, Krahmer J, Halliday KJ. Photoreceptor effects on plant biomass, resource allocation, and metabolic state. Proc Natl Acad Sci USA. 2016;113:7667–7672. doi: 10.1073/pnas.1601309113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rockwell NC, Su YS, Lagarias JC. Phytochrome structure and signaling mechanisms. Annu Rev Plant Biol. 2006;57:837–858. doi: 10.1146/annurev.arplant.56.032604.144208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu X, Paik I, Zhu L, Huq E. Illuminating progress in phytochrome-mediated light signaling pathways. Trends Plant Sci. 2015;20(10):641–650. doi: 10.1016/j.tplants.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Flint LH, McAlister ED. Wavelengths of radiation in the visible spectrum inhibiting the germination of light sensitive lettuce seed. Smithson Misc Collect. 1935;94(5):9–11. [Google Scholar]
- 5.Smith H, Whitelam GC. The shade avoidance syndrome: Multiple responses mediated by multiple phytochromes. Plant Cell Environ. 1997;20(6):840–844. [Google Scholar]
- 6.McLaren JS, Smith H. The function of phytochrome in the natural environment. VI. Phytochrome control of the growth and development of Rumex obtusifolius under simulated canopy light environments. Plant, Cell and Environment. 1978;1(1):61–67. [Google Scholar]
- 7.Franklin KA, Quail PH. Phytochrome functions in Arabidopsis development. J Exp Bot. 2010;61(1):11–24. doi: 10.1093/jxb/erp304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krasensky J, Jonak C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot. 2012;63(4):1593–1608. doi: 10.1093/jxb/err460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skirycz A, Inzé D. More from less: Plant growth under limited water. Curr Opin Biotechnol. 2010;21(2):197–203. doi: 10.1016/j.copbio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Ma S, Bohnert HJ. Integration of Arabidopsis thaliana stress-related transcript profiles, promoter structures, and cell-specific expression. Genome Biol. 2007;8(4):R49. doi: 10.1186/gb-2007-8-4-r49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayat S, et al. Role of proline under changing environments: A review. Plant Signal Behav. 2012;7(11):1456–1466. doi: 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ElSayed AI, Rafudeen MS, Golldack D. Physiological aspects of raffinose family oligosaccharides in plants: Protection against abiotic stress. Plant Biol (Stuttg) 2014;16(1):1–8. doi: 10.1111/plb.12053. [DOI] [PubMed] [Google Scholar]
- 13.Franklin KA, Whitelam GC. Light-quality regulation of freezing tolerance in Arabidopsis thaliana. Nat Genet. 2007;39(11):1410–1413. doi: 10.1038/ng.2007.3. [DOI] [PubMed] [Google Scholar]