Significance
Lung cancer is the leading cause of cancer death worldwide. Lung squamous cell carcinoma (SCCa) is classified as central or peripheral, according to the location of the primary tumor. Peripheral SCCa (PSCCa) is becoming as common as central SCCa, but the reasons for this increase are unclear. Smoking and chronic lung inflammation are recognized as risk factors for lung cancer. LXRα,βDko mice accumulate lipid in their lungs and develop M1-macrophage-predominant chronic lung inflammation and eventually lesions resembling PSCCa. This mouse model may provide a tool to study the etiology and progression of lung PSCCa and suggests a need for examination of LXR signaling in human PSCCa.
Keywords: peripheral squamous cell lung carcinoma, Liver X receptor, lipid metabolism, macrophage, inflammation
Abstract
The etiology of peripheral squamous cell lung cancer (PSCCa) remains unknown. Here, we show that this condition spontaneously develops in mice in which the genes for two oxysterol receptors, Liver X Receptor (LXR) α (Nr1h3) and β (Nr1h2), are inactivated. By 1 y of age, most of these mice have to be euthanized because of severe dyspnea. Starting at 3 mo, the lungs of LXRα,βDko mice, but not of LXRα or LXRβ single knockout mice, progressively accumulate foam cells, so that by 1 y, the lungs are covered by a “golden coat.” There is infiltration of inflammatory cells and progressive accumulation of lipid in the alveolar wall, type 2 pneumocytes, and macrophages. By 14 mo, there are three histological lesions: one resembling adenomatous hyperplasia, one squamous metaplasia, and one squamous cell carcinoma characterized by expression of transformation-related protein (p63), sex determining region Y-box 2 (Sox2), cytokeratin 14 (CK14), and cytokeratin 13 (CK13) and absence of thyroid transcription factor 1 (TTF1), and prosurfactant protein C (pro-SPC). RNA sequencing analysis at 12 mo confirmed a massive increase in markers of M1 macrophages and lymphocytes. The data suggest a previously unidentified etiology of PSCCa: cholesterol dysregulation and M1 macrophage-predominant lung inflammation combined with damage to, and aberrant repair of, lung tissue, particularly the peripheral parenchyma. The results raise the possibility that components of the LXR signaling may be useful targets in the treatment of PSCCa.
Lung cancer is the most common cancer and the leading cause of cancer-related death world-wide (1). Nonsmall cell lung carcinomas (NSCLC), the major class of lung cancer, includes squamous cell lung carcinoma (SCCa) and adenocarcinoma (ADCa), which account for approximately 23% and 43% of lung cancer, respectively (2). On the basis of the site of the primary cancer, squamous cell lung cancer is described as central (CSCCa) or peripheral (PSCCa). The incidence of PSCCa is increasing and is reported to be approximately 50% of squamous cell lung cancer (3–6). Although the etiology of squamous cell lung cancer is not known, smoking and chronic lung inflammation have been recognized as predisposing factors (7). Smoking is a major risk factor for chronic obstructive pulmonary disease, interstitial lung diseases, and all types of lung cancer, especially squamous cell lung cancer. Smoking exposure rapidly leads to lipid accumulation in pulmonary macrophages, a condition associated with abnormal lung physiology, including dysregulated pulmonary innate and adaptive immune responses to the environment (8). Abnormal lipid metabolism such as increase in total and esterified cholesterol, increased fatty acid synthesis, and accumulation of lipid-loaded macrophages (9–13) are characteristic of lung cancer.
In mice, inactivation of the kinase activity of IKKα in the NF-κB signaling pathway leads to SCCa (14), which can be prevented by depleting macrophages or reintroducing WT (IKKα active) bone marrow. The data point to dysfunctional macrophages as drivers of SCCa. LXRα and LXRβ are oxysterol-activated transcription factors regulating cholesterol homeostasis by (i) suppressing de novo biosynthesis of cholesterol; (ii) removing cholesterol from cells via their stimulation of four members of the ATP-binding cassette transporter (ABC) family, ABCA1, ABCG5, ABCG8, and ABCG1; and (iii) reducing the level of Niemann-Pick C1-Like 1, a transporter that facilitates uptake of cholesterol into cells. LXRs are widely expressed in the immune system where they have a powerful antiinflammatory function. In the lung, LXRs protect against acute lung injury, chronic obstructive pulmonary disease, asthma, and bacterial infection (15–17).
Through their roles in metabolism of cholesterol and lipids, regulation of the extracellular milieu, and the cell cycle, LXRs have been reported to protect against cancer (18). Although LXRα and LXRβ are expressed in pneumocytes and bronchial epithelium (19), little has been reported on the role of LXRs in lung carcinogenesis. In the present study, we examined the lungs of LXRα; LXRβ double null mutant (LXRα,βDko) mice on the C57BL/6 background that were fed a regular diet. We found chronic lung inflammation and disturbed lung lipid homeostasis characterized by progressive and massive accumulation of lipid in type 1 and type 2 pneumocytes, fibroblasts, and macrophages. By 14 mo of age, mice spontaneously developed lesions resembling peripheral squamous cell carcinoma. We conclude that LXRs normally play a key role in maintaining lung epithelial homeostasis and preventing epithelial hyperplasia, metaplasia, and squamous cell cancer.
Results
Squamous Cell Lung Carcinoma-Like Lesions Develop in LXRα,βDko Mice Fed Regular Diet.
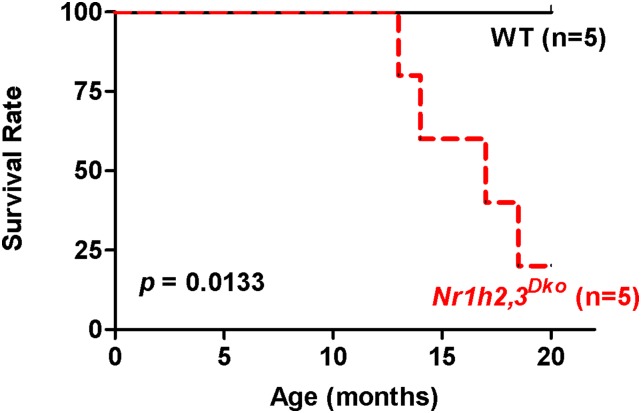
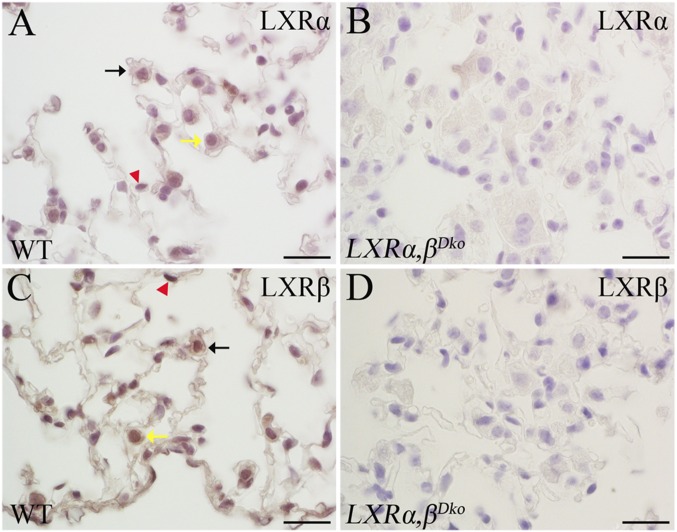
The overall survival of (LXRα,βDko) (n = 5) and wild-type (WT) mice (n = 5) was followed up to 20 mo. After 12 mo of age, LXRα,βDko mice began to die, whereas all WT mice were still alive at 20 mo of age. The overall survival of the LXRα,βDko mice was 16.5 ± 2.9 mo. The difference in survival rates was significant (Fig. S1, P = 0.0133). LXRα,βDko mice were found dead in their cages or had to be euthanized because of severe dyspnea. Both LXRα (Fig. S2A) and LXRβ (Fig. S2C) are expressed in the nuclei of alveolar macrophages, type 2 pneumocytes, and type 1 pneumocytes. In the lungs of LXRα,βDko mice, no LXRα or LXRβ protein was detectable (Fig. S2 B and D).
Fig. S1.
Survival curves of and WT mice. LXRα,βDko (n = 5) and WT mice (n = 5) were used to measure the lifespan. The mean survival of LXRα,βDko mice was 16.5 ± 2.96 mo; all WT mice were still alive at 20 mo of age. The LXRα,βDko mice showed increased mortality after 12 mo of age.
Fig. S2.
Immunohistochemical study of the expression of LXRα and LXRβ in lung. Both LXRα (A) and LXRβ (C) were expressed in the nuclei of alveolar macrophage (black arrow), type 2 pneumocyte (yellow arrow), and type 1 pneumocyte (red arrowhead). In the lung of LXRα,βDko mice, neither LXRα nor LXRβ could be detected (B and D). (Scale bars: 10 μm.)
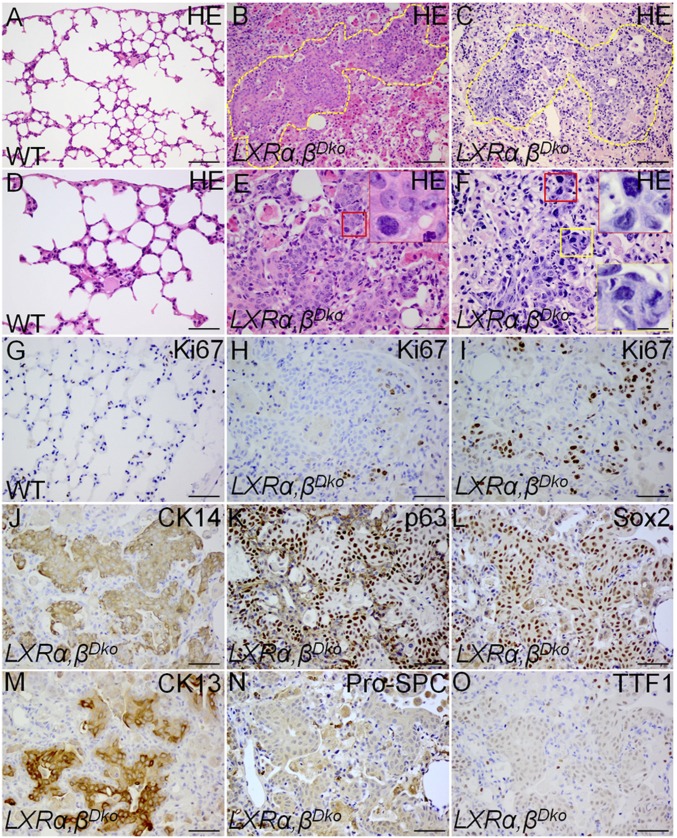
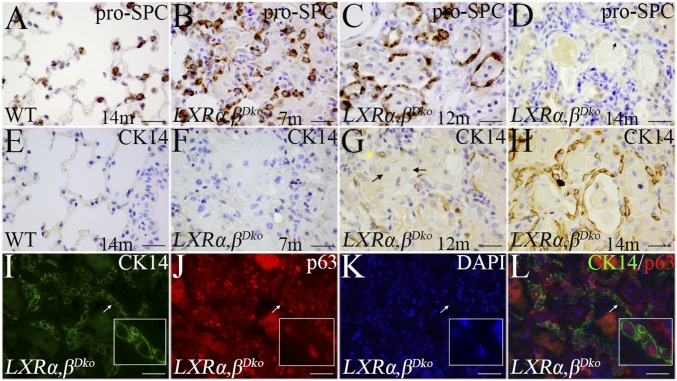
Multiple lesions with a high density of disorganized epithelial cells were found in the lungs of all LXRα,βDko mice at 14 mo of age. The lesions were present in the alveolar space (Fig. 1 B and E) and in the lung parenchyma (Fig. 1 C and F). Significantly, many of the cells within the lesions were Ki67+ and had hyperchromatic, atypical nuclei of median to large size with irregular nuclear contours, prominent nucleoli, and abnormal mitotic figures (Fig. 1 E, F, H, and I), all characteristics of cancer tissue. By contrast, the lung parenchyma of WT littermates had a normal structure with clear alveolar spaces (Fig. 1 A and D) and few Ki67+ nuclei (Fig. 1G). To further analyze the lesions, we used sequential sections for immunohistochemical staining for transformation-related protein 63 (p63), cytokeratin 14 (CK14), cytokeratin 13 (CK13), sex determining region Y-box 2 (Sox2), thyroid transcription factor 1 (TTF1), and prosurfactant protein C (pro-SPC). The lesions were p63, CK14, CK13, and Sox2 positive, but negative for TTF1 and pro-SPC (Fig. 1 J–O), indicating SCCa and not adenocarcinoma.
Fig. 1.
Tumors in the lungs of 14-mo-old LXRα,βDko mice. In 14-mo-old LXRα,βDko mouse lungs (B and C), the parenchyma close to pleura and the alveolar spaces were filled with disorganized cells with hyperchromatic atypical nuclei, prominent nucleoli, and irregular mitotic figures. (E and F) High-magnification insets show abnormal mitoses. A few Ki67+ cells are present in the normal WT lung (A and G), but many more Ki67+ cells are present in the epithelial lesions of LXRα,βDko mice (H and I). Yellow lines circle the lesion field in B and C. E and F are magnified areas of labeled boxes. Immunohistochemical study to differentiate between squamous cell lung cancer and adenocarcinoma in sequential sections with different lung cancer markers. Cells in the lesions of the LXRα,βDko mouse lung were CK14+ (J), p63+ (K), Sox2+ (L), CK13+ (M). They did not express pro-SPC (N) or TTF1 (O), but there were pro-SPC and TTF1-positive cells scattered around the lesion. (Scale bars: A–C, 100 μm; D–O, 50 μm.)
Progressive Lipid Accumulation in Lungs of LXRα,βDko Mice.
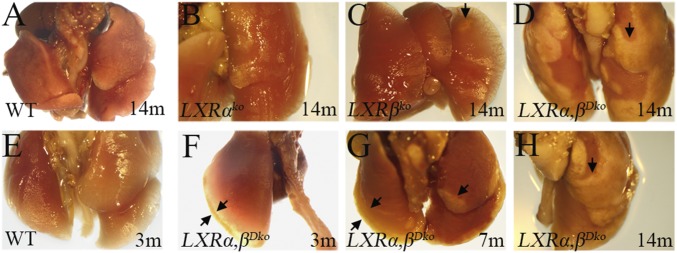
At the level of gross morphology, focal golden spots were observed along the margin of LXRα,βDko lungs at 3 mo of age (Fig. 2F), and by 7 mo of age, these lesions had spread from the periphery toward the center (Fig. 2G, black arrows). At 14 mo, the lungs of WT and LXRαko mice showed normal gross morphology (Fig. 2 A and B), whereas in LXRβko mice, there were scattered golden patches on the surface (Fig. 2C, black arrow). Strikingly, in LXRα,βDko mice, most of the lung was covered by a “golden coat” of lipid (Fig. 2 D and H, black arrows).
Fig. 2.
Gross morphological changes in the lungs of WT, LXRαko, LXRβko, and LXRα,βDko mice fed with regular diet. The lungs of 14-mo-old WT mice (A) and those of LXRαko (B) showed a normal gross appearance. There were golden patches in the periphery of the lungs of LXRβko mice (C), and these patches covered most of the lung of 14-mo-old LXRα,βDko mice (D). From 3 to 14 mo of age, there was a progressive expansion of the golden lesion from the margin of lung toward the center (E–H). Arrows show the margins of the lesions.
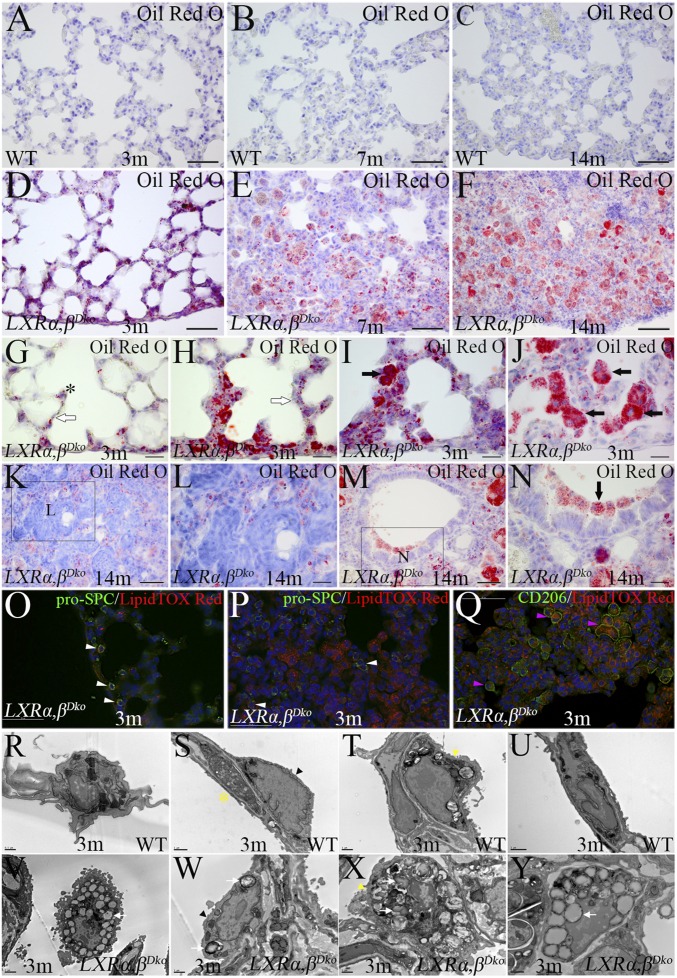
We examined the time course of appearance of lipid laden cells within the lung by two methods; Oil Red O and HCS LipidTOX Deep Red staining and transmission electron microscopy (TEM). In the lungs of WT mice, there was no obvious staining with Oil Red O between 3 and 14 mo of age (Fig. 3 A–C at 20× magnification), although fibroblasts with lipid inclusions could be seen in the alveolar wall at 100× magnification. By contrast, in the lungs of LXRα,βDko mice (Fig. 3D), lipid deposits were clearly evident along the pleura and in the alveolar walls close to the pleura at 3 mo of age and by 7 and 14 mo, foci of densely packed cells including enlarged foamy cells completely obscured normal alveolar structures (Fig. 3 E and F). In the lungs of 3-mo-old LXRα,βDko mice before the appearance of foam cells in the alveolar space, there was lipid accumulation in type 2 pneumocytes and in the alveolar wall (Fig. 3 G and H). As lipid deposition increased, lipid-loaded foamy cells began to accumulate in the alveolar space (Fig. 3 I and J). Thus, as evidenced by microscopic observation, type 2 pneumocytes are the first responders in these mice, with lipid accumulation occurring before the invasion of macrophages. Massive lipid accumulation was seen in cells surrounding the hyperplasic epithelial lesions in 14-mo-old mice (Fig. 3 K and L) and in epithelial cells in the bronchioles (Fig. 3 M and N). To identify the cell types with lipid accumulation, we combined staining with HCS LipidTOX Deep Red and immunofluorescence for the macrophage marker CD206, and the type 2 pneumocyte marker, pro-SPC. In the lungs of 3-mo-old LXRα,βDko mice, CD206 and pro-SPC were coexpressed with HCS LipidTOX Deep Red (Fig. 3 O–Q). TEM was used to observe the ultrastructures of subpleural lungs of 3-mo-old WT and LXRα,βDko mice. In WT lung, the alveolar macrophage and type 1 and type 2 pneumocytes did not show obvious lipid inclusions and some fibroblasts showed small size lipid droplets in cytoplasm (Fig. 3 R–U); in LXRα,βDko mice, the alveolar macrophages and type 1 and type 2 pneumocytes showed obvious lipid accumulation, the type 2 pneumocytes also showed abnormal lamellar bodies, and the lipofibroblasts showed increased size of lipid droplets around the nucleus (Fig. 3 V–Y).
Fig. 3.
Progressive accumulation of lipid in the lung of LXRα,βDko mice with age. There was no positive staining for lipid with Oil Red O in the lungs of WT mice from 3 to 14 mo of age (A–C). In lungs of 3-mo-old LXRα,βDko mice, scattered lipid deposits were found along the pleura and the alveolar walls (D). In 7- and 14-mo-old LXRα,βDko mice, there were dense lesions full of enlarged foamy cells (E and F). At 3 mo of age, there was lipid accumulation in type 2 pneumocytes (black asterisk) and in the alveolar wall (white arrow). In some areas, the alveolar space was clear (G and H), but in others, there was an accumulation of lipid-loaded foamy cells (black arrow) (I and J). In 14-mo-old mice, there was lipid accumulation around the tumor masses (K and L), and foamy cells were present in the bronchioles (M and N). L and N are magnified views of the boxes in K and M. In 3-mo-old LXRα,βDko mice, pro-SPC (type 2 pneumocyte marker) positive cells (white arrowhead) stained positively for HCS LipidTOX Deep Red (neutral lipid) (O and P). CD206 (macrophage marker) cells also stained positively for HCS LipidTOX Deep Red (pink arrowheads) (Q). EM is to show different cells close to pleura in lung of WT mice and mutants at 3 mo of age. (R–U) WT lung: alveolar macrophage (R); type 1 cell, black arrowhead and lipofibroblast with small lipid inclusion, yellow star (S); type 2 cell, yellow arrowhead (T); fibroblast without lipid inclusion (U). (V–Y) LXRα,βDko lung: alveolar macrophage, white arrow (V); type 1 cell with lipid inclusion, white arrow (W); type 2 cell with abnormal lamellar bodies, yellow arrowhead and lipid inclusion shown by white arrow (X); lipofibroblast, white arrow (Y). (Scale bars: A–F, K, M, and O–Q, 50 μm; G–J, L, and N, 20 μm; R, 0.5 μm; V, 2 μm; S–U and W–Y, 1 μm.)
Lipid Dysregulation in the Lung Induces Chronic Lung Inflammation.
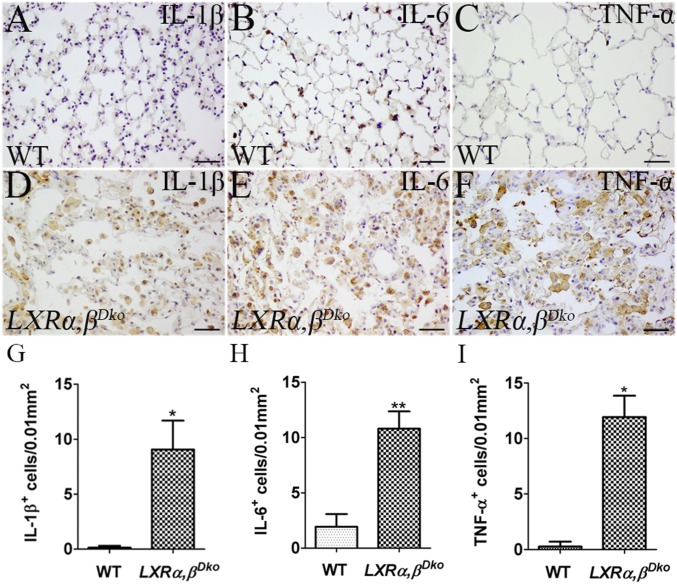
At 12 mo of age, there was no evident inflammation in the lungs of WT mice (Fig. 4A). However, in the lungs of LXRα,βDko mice, there was lymphoid hyperplasia around the vessels and CD3+ inflammatory T cells infiltrating the parenchyma (Fig. 4 B, C, G, and J). Scattered CD206+ macrophages were found in the alveolar space and interstitium of WT mice (Fig. 4E), whereas large clusters of macrophages occupied the alveolar spaces in LXRα,βDko mice (Fig. 4 H and K). Myeloperoxidase (MPO), a neutrophil marker, was highly expressed in lung macrophages of LXRα,βDko mice (Fig. 4 I and L), but not in WT lungs (Fig. 4F). There were many cells expressing the inflammatory cytokines IL-1β and TNF-α in lungs of LXRα,βDko (Fig. 5 D–I) but not in WT mice (Fig. 5 A, C, G, and I). IL-6 was expressed in the cytoplasm of macrophages of both LXRα,βDko and WT mice (Fig. 5 B and H).
Fig. 4.
Increased inflammatory cell infiltration in the lung of LXRα,βDko mice at 12 mo of age. In the lungs of 12-mo-old LXRα,βDko (B and C) but not WT mice (A), there was lymphoid hyperplasia around the vessels and inflammatory cells infiltrating into the parenchyma with foamy cells filling the alveolar space. A few CD3+ T cells (D) were found along the WT vessels, but there was a clear increase in the number of CD3+ T-cells in LXRα,βDko lungs (G). In WT mice, scattered CD206+ macrophages were found in the lung interstitium (E) and on the alveolar wall and few MPO+ cells were detectable (F). In LXRα,βDko mice, clustered CD206+ macrophages with enlarged cell bodies filled the alveolar space (H) and MPO was found in the cytoplasm of macrophages (I). The densities of CD3+ T cells, CD206+ macrophages, and MPO+ cells in lungs of WT and LXRα,βDko mice are depicted in J–L. (Scale bars: 50 μm.) *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. 5.
Increased cytokines in the lung of 12-mo-old LXRα,βDko mice. Macrophages in the alveoli of 12-mo-old LXRα,βDko but not WT mice (A) strongly expressed IL-1β (D). Few scattered IL-6–expressing cells (B) but no TNF-α+ cells (C) were detected in WT mice. However, in LXRα,βDko mice, clusters of IL-6+ cells were found in alveoli (E), and macrophages strongly expressed TNF-α (F). The densities of cytokine (IL-1β, IL-6, and TNF-α) positive cells are depicted in lung of WT and LXRα,βDko mice (G–I). LXRα,βDko (Scale bars: 50 μm.) *P < 0.05, **P < 0.01.
Evidence for Lung Injury and Alveolar Remodeling in Response to Chronic Lung Inflammation.
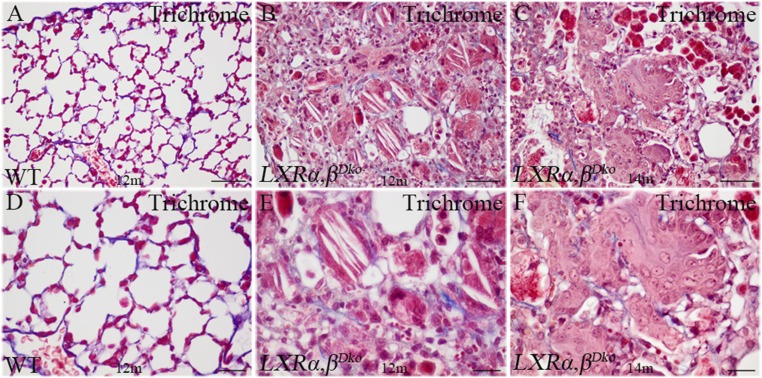
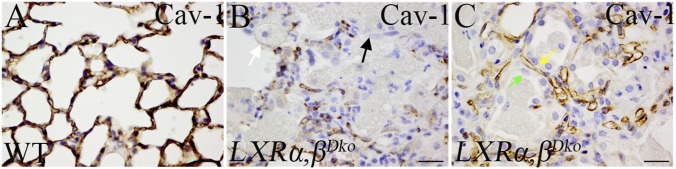
With Masson's trichrome stain, there was no indication of fibrosis in either WT (Fig. S3 A and D) or LXRα,βDko mice (Fig. S3 B, C, E, and F). However, histological examination showed evidence for extensive remodeling of the alveolar regions of the lung. For example, type I pneumocytes, which express high levels of caveolin-1 (Cav-1), normally cover approximately 95% of the alveolar surface and are tightly apposed to capillary vessels that are also Cav-1 positive (Fig. 6A). In 12-mo-old LXRα,βDko mice, Cav-1 staining was seen in the endothelial cells but the type 1 pneumocyte staining was discontinuous in areas where there was infiltration of macrophages (Fig. 6B). In neighboring areas, the luminal sides of the alveoli were lined by simple cuboidal epithelial cells that did not express Cav-1 (Fig. 6C). Immunohistochemistry for pro-SPC indicated some of the cuboidal cells populating the remodeled alveoli are type 2 pneumocytes. At 7 mo of age, there was evidence for hyperplasia of these cells (Fig. 7B), and by 12 mo, pro-SPC+ cells were observed lining alveoli-like structures (Fig. 7C). Remarkably, by 14 mo, the epithelial cells covering the alveolar surface did not express pro-SPC (Fig. 7D) but expressed CK14 (Fig. 7H). Earlier, in 12-mo-old LXRα,βDko, only scattered CK14+ cells were present on the luminal surface of those alveoli that were full of foamy cells (Fig. 7 E and G). Immunofluorescence staining showed that the CK14+ cells in lung parenchyma were also positive for p63, a marker for basal cells in the lung (Fig. 7 I–L). The time course of appearance of these cells was followed in more detail below.
Fig. S3.
No significant fibrosis in the lungs of LXRα,βDko mice. Masson's trichrome stain was used to study the lung fibrosis. The WT lung at 12 mo of age did not show fibrosis (A and D). No obvious fibrosis was found in the lesion of LXRα,βDko mice at 12 or 14 mo of age (B and E and C and F). The alveolar space was filled with foamy cells and cholesterol clefts. The tumor mass and foamy cells filled the alveolar space (C and F). D–F are magnified views for A–C, respectively. (Scale bars: A–C, 50 μm; D–F, 20 μm.)
Fig. 6.
Injury and abnormal repair in the lungs of LXRα,βDko mice. In lungs of WT mice, there was a continuous band of caveolin-1 staining along the alveolar wall (A), but this staining of caveolin-1 was discontinuous in LXRα,βDko mice (B). Regular lung structure was lost (black arrow), and alveoli were filled with foamy cells (white arrow). The luminal side of the alveoli around the injured lung parenchyma was lined by simple cuboidal epithelial cells, which did not express caveolin-1 (C, green arrow). Caveolin-1 expression was maintained in the capillaries (C, yellow arrow). (Scale bars: 20 μm.)
Fig. 7.
Loss of pro-SPC and gain of CK14 in cuboidal epithelia of alveoli in LXRα,βDko mice. The lung of WT mice showed well-distributed type 2 pneumocytes expressing pro-SPC (A). In 7-mo-old LXRα,βDko mice, clusters of pro-SPC cells were seen (B). In 12-mo-old LXRα,βDko mice, pro-SPC+ cells covered alveolar walls (C), but by 14 mo, these cells did not express pro-SPC (D), but expressed CK14 and p63 (white arrows) (G–L). CK14+ cells were not detected in the alveolar region of WT mice (E) nor in the lesions of 7-mo-old LXRα,βDko mice (F). (Scale bars: 20 μm.)
Appearance of Ectopic CK14+p63+ Cells in the Lungs of LXRα,βDko Mice.
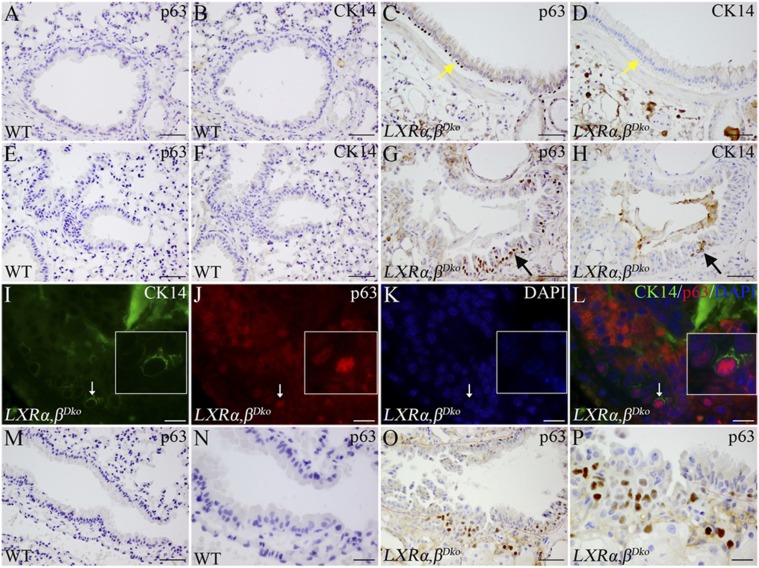
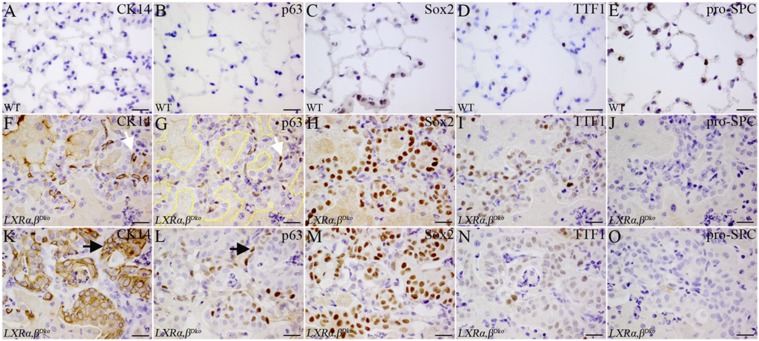
In WT adult mice, no CK14+ or p63+ cells were detectable in the epithelium of the bronchioles or lung parenchyma (Fig. S4 A, B, E, and F). However, in 12-mo-old LXRα,βDko mice, p63+ cells were located basally in some bronchioles (Fig. S4C). Significantly, these cells were not CK14+ (Fig. S4D). p63+ cells were also seen in terminal bronchioles in the neighborhood of the areas of focal hyperplasia and PSCCa-like lesions. Analysis of sequential sections showed that only some of the p63+ cells expressed CK14 (Fig. S4 G and H), whereas all CK14+ cells were p63+ (Fig. S4 I–L). In some areas near terminal bronchioles, clusters of p63+ cells appeared to be infiltrating into the lung parenchyma (Fig. S4 O and P). In some areas at 14 mo of age, there were “glandular-like structures” composed of epithelial cells positive for p63, CK14, Sox2, and TTF1 but negative for pro-SPC (Fig. S5 F–J). Within the lesions designated histologically as squamous metaplasia with dysplasia, all layers of squamous epithelia expressed CK14 and Sox2. At 14 mo, approximately one-third of these epithelial cells expressed p63, but not TTF1 or pro-SPC (Fig. S5 K–O).
Fig. S4.
The changes of p63+ CK14+ cells in bronchiole of LXRα,βDko mice at 12 mo of age. In WT adult mice, expression of CK14 and p63 cannot be detected in the epithelia of different-level bronchioles or in lung parenchyma (A and E and B and F). In some bronchioles of LXRα,βDko mice at 12 mo of age, p63+ cells were located at the basal layer (C); however, sequential sections of the bronchiole showed that p63+ cells were CK14− (D, yellow arrows). In the terminal bronchioles adjacent to the lesions, the basal cells and focal hyperplasic epithelial cells expressed p63 (G); however, sequential sections showed that only some p63+ basal cells expressed CK14 (black arrows) (H). Some p63+ basal cells coexpressed CK14; the white boxes show the areas pointed out by the white arrows (I–L). The terminal bronchiole of WT mice was lined by simple cuboidal epithelia, and p63 was not detected (M and N). The epithelial cells of the bronchiole were hyperplastic at one side, and clusters of p63+ cells (O and P). N and P are magnified views for M and O, respectively. (Scale bars: A–H, M, and O, 50 μm; I–L, N, and P, 20 μm.)
Fig. S5.
p63+CK14+ cells in glandular structures and squamous cell metaplasia of lung parenchyma in LXRα,βDko mice at 14 mo of age. In the lung parenchyma of WT mice, no CK14+ cells (A) or p63+ cells were detectable (B). Sox2 was expressed in the cuboidal epithelia of terminal bronchioles, but not by the cells in parenchyma (C). TTF1+ cells (D) and pro-SPC–positive cells (E) were scattered and well distributed in WT lung. In the lesions of LXRα,βDko mice, there were “glandular structures” lined with hyperplastic cuboidal epithelia. The yellow dashes outline these “glandular structures”. CK14-positive fusiform shaped cells, similar to basal cells, surrounded the cuboidal epithelia (F). Sequential sections showed that CK14+ cells also expressed nuclear p63 (F and G, white arrow). The cuboidal epithelia slightly expressed TTF1 (I), strongly expressed nuclear Sox2 (H), but were negative for pro-SPC (J). Adjacent to glandular structures, there were regions of CK14+ squamous metaplasia with dysplasia (K). Approximately one-third of the epithelia at basal side expressed p63 (L). All layers of the epithelia were strongly Sox2 (M). The squamous metaplasia was negative for TTF1 (N) and pro-SPC (O). (Scale bars: 20 μm.)
RNA Sequencing of WT and LXRα,βDko Mice at 3 and 12 Mo of Age (Figs. S6–S8).
Fig. S6.
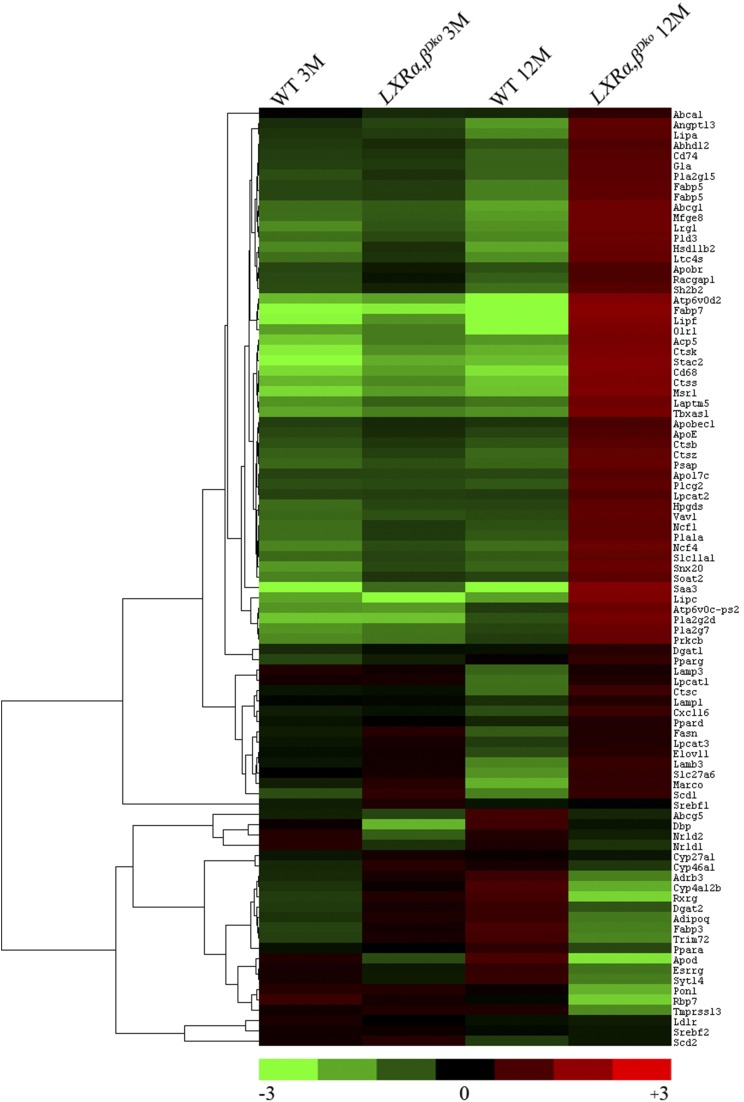
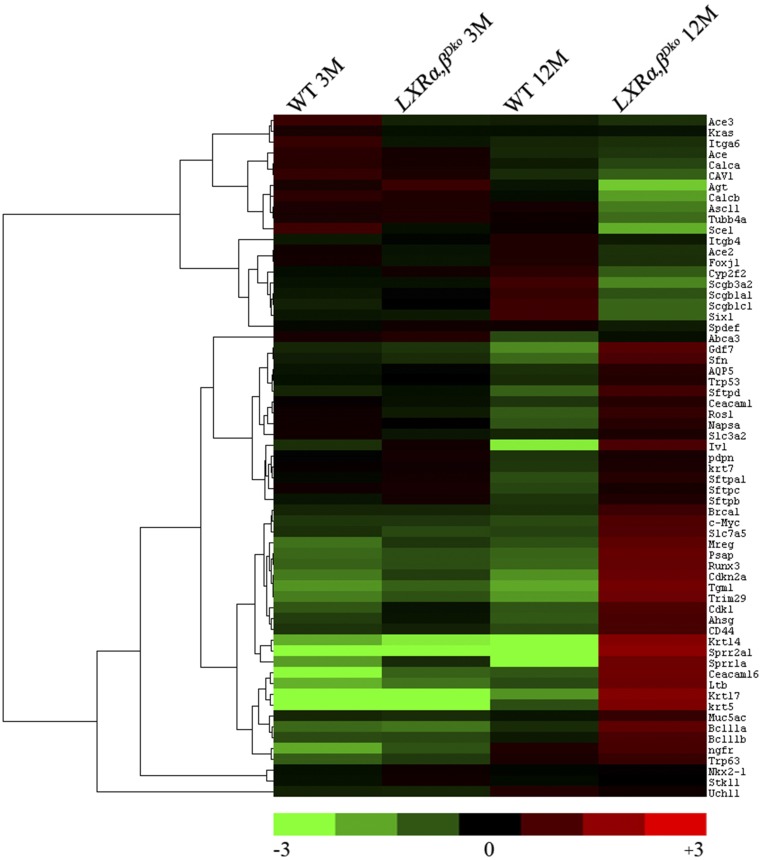
Heat map of selected genes related to lung lipid metabolism from RNA sequencing in WT and LXRα,βDko mice at 3 mo and 12 mo of age. The genes for lung lipid metabolism were selected (fold change ≤ −4-fold or ≥ 4-fold and P < 0.05) at 12 mo old. To determine fold change for each gene, the mean was calculated from transcripts of the four mouse groups. Each point represents one gene plotted as log2 fold change from the mean. Results were visualized by using Treeview.
Fig. S8.
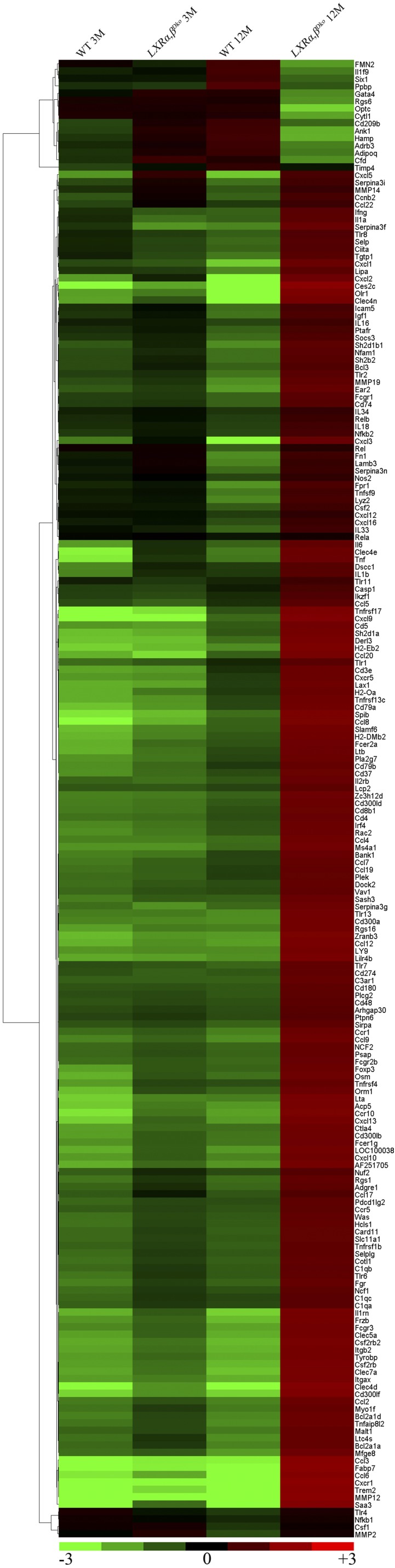
Heat map of selected genes related to lung injury and repair from RNA sequencing in WT and LXRα,βDko mice at 3 mo and 12 mo of age. The genes related to lung injury and repair were selected (fold change ≤ −4-fold or ≥ 4-fold and P < 0.05) at 12 mo old. To determine fold change for each gene, the mean was calculated from transcripts of the four mouse groups. Each point represents one gene plotted as log2 fold change from the mean. Results were visualized by using Treeview.
Comparison of RNA transcripts in lungs of LXRα,βDko and WT mice at 3 mo of age revealed few differences. However, in lungs of 12-mo-old LXRα,βDko mice, there was clear evidence for M1 macrophage-predominant immune invasion and a down-regulation of markers of Club cells. The most up-regulated genes were (i): the cell surface markers CD180, 209, 274, 300, 37, 3, 4, 48, 5, 74, 79, and 8; (ii) the cytokines CXCl1, 2, 3, 5, 10, 12, 13, 16, and 19; (iii) cytokine receptors CXCR1 and 5; (iv) interleukins 1a, 1b, 6, 16, 18, 33, 34; (v) IL2RB; (vi) metalloproteases MMP 2, 8, 12, 14, and 19; (vii) NF-κB2; (viii) the tyrosine receptor binding protein Tyrobp and its partner Trem2; and (ix) the acute phase secreted serum amyloid A protein Saa3 (Figs. S6–S8). The most down-regulated genes in the RNAseq were markers of club cells (Cyp2f2, Scgb1a1, Scgb3a2) (Fig. S8). Some genes were chosen to validate the RNA sequencing, which is summarized in Fig. S9. Despite the massive accumulation of lipids in the double mutant mice, there was no up-regulation of the ABC transporters (Figs. S6 and S9B); scavenger receptors CD36, LDLR, SREBP1, and SCAP; or the cholesterol metabolizing genes CYP27A and CYP46A1 (Figs. S6 and S9 B and F).
Fig. S9.
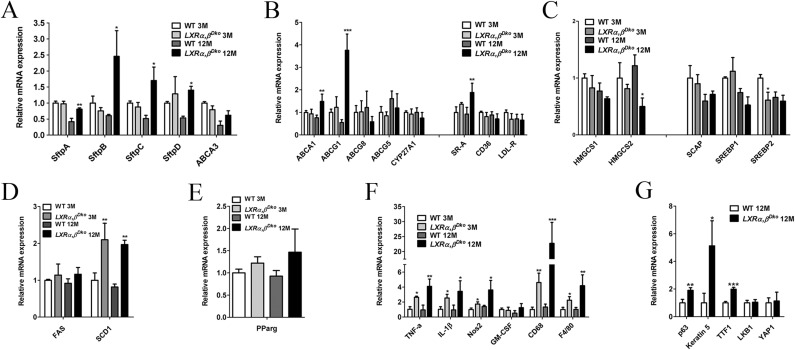
qPCR validation of gene expressions associated with lung lipid metabolism, inflammation, and lung repair in WT and LXRα,βDko mice at 3 and 12 mo of age. (A) The genes of surfactant proteins were increased at 12 mo of age. (B) The gene expression for cholesterol transport in lung of LXRα,βDko mice. The expressions of ABCA1 and ABCG1 increased at 12 mo of age, but no difference at 3 mo of age. (C) The gene expression for cholesterol synthesis in lung of LXRα,βDko mice. The expression of HMGCS2 at 12 mo of age was decreased; SREBP2 was decreased at 3 mo of age. (D) The gene expression for fatty acid synthesis in lung of LXRα,βDko mice. SCD1 was increased at 12 mo of age. (E) Gene expression of PPAR gamma did not show any changes either at 3 mo of age or at 12 mo of age. (F) The gene expression of chronic inflammation in lung of LXRα,βDko mice. (G) The gene expression of p63, keratin 5, and TTF1 was increased at 12 mo of age, the gene expression of LKB1 and YAP1 did not show any changes. Two-way ANOVA followed by Bonferroni post hoc test (A–F) or t test (G) was used. *P < 0.05, **P < 0.01, ***P < 0.001, LXRα,βDko vs. WT at 3 mo of age or LXRα,βDko vs. WT at 12 mo of age.
Fig. S7.
Heat map of selected genes related to lung inflammation from RNA sequencing in WT and LXRα,βDko mice at 3 mo and 12 mo of age. The genes for lung inflammation were selected (fold change ≤ -4-fold or ≥ 4-fold and P < 0.05) at 12 mo old. To determine fold change for each gene, the mean was calculated from transcripts of the four mouse groups. Each point represents one gene plotted as log2 fold change from the mean. Results were visualized by using Treeview.
Discussion
We have shown here a previously unidentified mechanism for development of squamous cell lung cancer-like lesions in mice, namely the inactivation of both LXRα and LXRβ. We have presented evidence that LXRα and LXRβ are essential for lipid homeostasis in the lung. Without these two receptors, there is a massive accumulation of foam cells in the lung accompanied by chronic lung inflammation and eventual development throughout the alveolar region of extensive epithelial dysplasia, squamous metaplasia, and squamous cell cancer-like lesions that result in death when mice are approximately 14 mo old. The findings that, on a regular diet, there is a massive accumulation of lipids in pulmonary type 1 and type 2 pneumocytes, fibroblasts, and macrophages of LXRα,βDko mice identifies a critical defect in the ability of these mice to excrete cholesterol from the lung. Lipid deposits along the alveolar wall indicate that there may be problems in lipid disposition in type 1 pneumocytes. Abca1 and Abcg1, which are LXR-target genes, facilitate cholesterol efflux from cells and are important for the maintenance of normal lung lipid composition, structure, and function. It has been reported that Abca1 or Abcg1 knockout mice develop enlarged foamy macrophages and lipid-filled type 2 pneumocytes with abnormal lung lipid profiles. However, no lung cancer was found in these mice (20–22). In our studies, lipid deposition was much more severe than that reported in the Abc knockout mice and lesions occurred at an earlier age. Despite the massive lipid accumulation, there was no induction of RNA for either Abca1 or Abcg1 in the lungs of LXRα,βDko mice, clearly showing that the essential role for LXR in cholesterol homeostasis of lung is not Abca1 or Abcg1 dependent.
Lipid metabolism is an important modulator of innate and adaptive immune response (23). Modified LDL and LPS activate Toll-like receptors of macrophages, which suppress LXR activity on its target genes, Abca1 and Abcg1, causing decreased macrophage cholesterol efflux (24) and directly triggering proinflammatory signaling to produce cytokines and chemokines. Deficiency of ABCA1 and ABCG1 leads to accumulation of free and esterified cholesterol in macrophages. Cholesterol-laden macrophages release inflammatory cytokines and matrix metalloproteinases and induce lung inflammation (25).
Several mouse models show that macrophages play a vital role in the development of SCCa (14, 26). The susceptibility to and incidence of spontaneous lung tumors, and the chemical induction of lung tumors, vary largely between mouse-inbred strains. The C57BL/6 background is known to confer resistance to spontaneous and chemically induced lung tumors (27), and it has been argued that C57BL/6 mice may not be the appropriate background for studying the contribution of inflammation to lung cancer development (28). Despite this resistance of C57BL/6 mice, the LXRα,βDko mice on the C57BL/6 background developed lung cancer-like lesions after 12 mo of age. It is possible that the defect in lipid metabolism was the factor that overcame the resistance of this mouse strain to the development of lung cancer.
In this report, we have followed the morphological, histological, and genetic changes in LXRα,βDko mice and their WT littermates from 3 to 14 mo of age. Mice were always fed a normal diet and were kept under specific pathogen-free conditions. By 3 mo of age, the LXRα,βDko mice began to accumulate lipids in distal parts of the lung close to the pleural surface. Over the next 12 mo, there followed a progressive and massive lipid deposition in type 2 pneumocytes, in the alveolar wall, and in macrophages. This lipid accumulation of lung was accompanied by loss of Cav-1+ type 1 pneumocytes, the apparent proliferation and dysplasia of type 2 pneumocytes, and the eventual appearance of pro-SPC negative cuboidal epithelial cells lining the alveoli. As the mice aged, the number of small lesions increased, and by 14 mo, 100% of LXRα,βDko mice had developed extensive squamous cell carcinoma-like lesions involving p63, CK14, CK13, Sox2-positive, and TTF1 and pro-SPC-negative epithelial cells throughout the lung parenchyma. An important question, which will require future studies, including genetic lineage tracing, is the origin of these cells, which have some characteristics of basal cells normally present in the pseudostratified epithelium of the trachea and large proximal airways in the mouse lung. One possibility is that they derive from type 2 pneumocytes, which have been shown to proliferate and regenerate type 1 cells in alveolar injury models (29–31). Another possibility is that they arise from the same cells that generate “pods” of p63+ CK5+ cells after H1N1 influenza virus infection (32–34). At present, the precise origin of these cells is a matter of debate, but includes club cells, basal-like cells, and/or lineage-negative progenitors in the distal airways (32–35). However, following influenza infection, these cells do not undergo extensive dysplasia or malignant transformation.
In conclusion, we have shown that inactivation of both Liver X receptors induced progressive lung lipid accumulation, chronic macrophage-predominant lung inflammation, and lesions resembling squamous cell lung cancer. Lung lipid dysregulation and lipid-induced chronic lung inflammation in LXRα,βDko mice could both have contributed to the generation and apparent transformation of p63+CK14+ cells in response to extensive alveolar damage (36). There are several mouse models of lung squamous cell lung cancer, but none for PSCCa as described here in the LXRα,βDko mouse. Defective Liver X receptor signaling has not been studied in human PSCCa, and the LXRα,βDko mouse may help us to understand the etiology, progression, and underlying mechanisms of human peripheral SCCa.
Materials and Methods
Animals and Tissue Preparation.
Male C57BL/6 WT, LXRαko, LXRβko, and LXRα,βDko mice were used for experiments. The generation of LXRαko, LXRβko, and LXRα,βDko mice on the C57BL/6 genetic background has been described (37). Mice were housed in a room of standard temperature (22 ± 1 °C) with a regular 12-h light, 12-h dark cycle and given free access to water and standard rodent chow. The animal care and use program complied with the standards and recommendations set forth in the National Research Council’s 1996 Guide for the Care and Use of Laboratory Animals and in accordance with the University of Houston guidelines on the use of laboratory animals.
All mice were anesthetized deeply with CO2. Blood was withdrawn from inferior vena cava or right ventricle. The right hilum of the lung was ligated; the right lung was removed and frozen in liquid nitrogen. The trachea was intubated, and left lung was inflated with 4% (wt/vol) paraformaldehyde in 0.1 M PBS (pH 7.4). The mice were transcardially perfused with 0.1 M PBS followed by 4% (wt/vol) paraformaldehyde. The trachea was ligated, and the heart and lung were postfixed in the same fixative at 4 °C overnight. Half of the left lung was used for paraffin sections and the other half for frozen sections. Paraffin sections (5 μm) were for hematoxylin and eosin stains, Masson's Trichrome Stain, immunohistochemistry, and immunofluorescence studies. Frozen sections (5 μm) were prepared for Oil Red O staining and HCS LipidTOX Deep Red neutral lipid staining.
Immunohistochemistry and Immunofluorescence.
Paraffin sections were deparaffinized in xylene, rehydrated through graded alcohol, and processed for antigen retrieval in 10 mM citrate buffer (pH 6.0) for 10 min at 97 °C with pretreatment module. Sections were incubated in 0.3% H2O2 in 50% (vol/vol) methanol for 20 min at room temperature to quench endogenous peroxidase. To block nonspecific binding, sections were incubated in 3% BSA for 30 min, and then biotin-blocking system (Dako) was used to block endogenous biotin. The sections were incubated with antibodies specific for p63 (1:1000; Abcam, ab124762), Sox2 (1:1000; Santa Cruz Biotechnology, Sc17320), CK13 (1:500; Thermofisher Pierce, MA1-5764), CK14 (1:1500; Abcam, ab7800), CK8 (1:500; Abcam, ab59400), TTF1 (1:1000; Abcam, ab133638), pro-SPC (1:3000; Chemicon, AB3768), CD3 (1:1000; Santa Cruz Biotechnology, Sc-1127), CD206 (1:1000; Abcam, ab64693), TNFα (1:500; Abcam, ab1793), IL1β (1:1000; Abcam, ab9722), IL6 (1:500; Santa Cruz Biotechnology, E209), MPO (1:1000; Abcam, ab45977), Caveolin 1 (1:1000; BD Transduction Laboratories, 610060), Ki67 (1:1000; Abcam, ab15580), and anti-LXR α and β (1:1000; made in laboratory of J.-Å.G.). Primary antibodies were replaced with BSA as negative controls. Sections were incubated with biotinylated secondary antibody (1:200) for 1 h at room temperature, and then the Vectastain Avidin-Biotin Complex kit (Vector Laboratories) was used according to the manufacturer’s instruction, followed by 3,3-diaminobenzidine staining (Thermo Scientific) and counterstaining with Mayer’s hematoxylin (Sigma-Aldrich).
For immunofluorescence, quenching of endogenous peroxidase and blocking of endogenous biotin were omitted. Sections were incubated with anti-p63, anti-CK14, anti-CD206, and anti-pro SPC. Primary antibodies were detected with fluorescent secondary antibodies. Sections were counterstained with Vectashield mounting medium (Vector Laboratories).
Staining for Neutral Lipid.
Frozen sections were stained with Oil Red O stain Kit (American MasterTech., KTORO) according to the manufacturer's instruction. For fluorescence staining of neutral lipid, the frozen sections were stained with HCS LipidTOX Deep Red neutral lipid stain (1:1000, Invitrogen).
RNA Sequencing and Gene Expression Analysis.
Total RNA of inferior lobe of right lung was isolated by using the Qiagen RNeasy lipid tissue mini kit following manufacturer’s protocol. After paired-end sequencing of samples using the Illumina HiSEq 2000 platform, TopHat v2.0.9 was used to align the sequence reads to the mm10 reference genome. The FastQC package v0.11.2 was used to assess the read quality. Partek Genomics Suite v6.6 was used to assign reads to genes by using RefSeq transcript annotations, generating reads per kilobase of exon model per million mapped reads (38). For quantitative PCR (qPCR), the RNA was reverse-transcribed by using random hexamer primers and a revertAid first-strand cDNA synthesis kit (Invitrogen). qPCR was performed by using iTaq Universal SYBR Green Supermix (Bio-Rad). The sequences of qPCR primers are listed in Table S1.
Table S1.
qPCR primer list
| Genes | Forward (5′-3′) | Reverse (5′-3′) |
| ABCA1 | AAAACCGCAGACATCCTTCAG | CATACCGAAACTCGTTCACCC |
| ABCG1 | GTGGATGAGGTTGAGACAGACC | CCTCGGGTACAGAGTAGGAAAG |
| ABCG5 | AGGGCCTCACATCAACAGAG | GCTGACGCTGTAGGACACAT |
| ABCG8 | ATACCCTGGAGGTCTCATAGCA | ACGTCGAGTAGTGAGGCTCTC |
| SRA | AAGTATCAGCAGAAGTCCAGTCT | TCCTTCAGTCTGAGGTCGTTG |
| CD36 | TGGTCAAGCCAGCTAGAAA | CCCAGTCTCATTTAGCCAC |
| LDLR | CCAACCTGAAGAATGTGGTG | CAGGTCCTCACTGATGATGG |
| Cyp27A1 | GCCTCACCTATGGGATCTTCA | TCAAAGCCTGACGCAGATG |
| HMGCS1 | CGATCAAGCTGAGTTGGAAA | ACAGTCGGGCAAAGAGAGTT |
| HMGCS2 | GGAGGACATCAACTCCCTGT | TTGTCAATGATGGTCTCGGT |
| SCAP | TGACCACAAACAAGGAGAGC | CAGGAACACCAAACAGCAAG |
| SREBP1 | GAAGCTGTCGGGGTAGCGTC | CTCTCAGGAGAGTTGGCACCTG |
| SREBP2 | CCGCTCTCGAATCCTCTTAT | CAGCACCTGACTCCAGTGAC |
| FAS | AGGGGTCGACCTGGTCCTCA | GCCATGCCCAGAGGGTGGTT |
| SCD1 | GCGATACACTCTGGTGCTCA | CCCAGGGAAACCAGGATATT |
| SftpA | CCCTAGAGTCCCATCTGAGC | TCTTCCATGGCCACAATTTA |
| SftpB | TGAGCGCTACACAGTTCTCC | CTATCAGAGGCTCCAGAGCA |
| SftpC | CCCTAGTCTTGAGGCTTTCG | GGAACCAGTATCATGCCCTT |
| SftpD | CTCGCAGAGATCAGTACCCA | CAAACCTGGATCACCCTTCT |
| ABCA3 | GCATTGCCCTCATTGGAGAGCCTG | TCCGGCCATCCTCAGTGGTGGG |
| PParg | AGGCCGAGAAGGAGAAGCTGTTG | TGGCCACCTCTTTGCTCTGCTC |
| P63 | TACTGCCCCGACCCTTACAT | GCTGAGGAACTCGCTTGTCTG |
| KRT5 | TCTGCCATCACCCCATCTGT | CCTCCGCCAGAACTGTAGGA |
| TTF1 | GGTGCTGGGACTGGGATGT | TCAAGATGTCAGACACTGAGAACG |
| TNF-a | CAGGCGGTGCCTATGTCTC | CGATCACCCCGAAGTTCAGTAG |
| IL-1β | GAAATGCCACCTTTTGACAGTG | TGGATGCTCTCATCAGGACAG |
| Nos2 | GTTCTCAGCCCAACAATACAAGA | GTGGACGGGTCGATGTCAC |
| CD68 | ATCCCCACCTGTCTCTCTCA | ACCGCCATGTAGTCCAGGTA |
| F4/80 | GGATGTACAGATGGGGGATG | CATAAGCTGGGCAAGTGGTA |
| LKB1 | TTGGGCCTTTTCTCCGAGG | CAGGTCCCCCATCAGGTACT |
| YAP1 | ACCCTCGTTTTGCCATGAAC | TGTGCTGGGATTGATATTCCGTA |
Data Analysis.
Data are expressed as mean ± SD. Statistical comparisons were made by using Student’s t test or two-way ANOVA followed by Bonferroni post hoc test. Survival percent was analyzed by using the Kaplan–Meier method and was compared by using the log rank test. P < 0.05 was considered to indicate statistical significance.
Acknowledgments
We thank our colleagues Dr. Kaberi Das and Christopher A. Brooks for assistance with mice handling, Dr. Bilqees Bhatti for Masson’s Trichrome staining, and Margaret Gondo (College of Optometry, University of Houston) for processing samples of TEM. This study was supported by Emerging Technology Fund of Texas grants under Agreement 18 No. 300-9-1958, Cancer Prevention and Research Institute of Texas Grant RP110444-P1, the Robert A. Welch Fund Grant E-0044, the Swedish Science Council, the Center for Innovative Medicine, and National Eye Institute Grants P30EY007551-29 and EY018239 (to A.R.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607590113/-/DCSupplemental.
References
- 1.Torre LA, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, et al. SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Bethesda, MD, seer.cancer.gov/archive/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site, April 2015.
- 3.Funai K, et al. Clinicopathologic characteristics of peripheral squamous cell carcinoma of the lung. Am J Surg Pathol. 2003;27(7):978–984. doi: 10.1097/00000478-200307000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Koenigkam Santos M, et al. Morphological computed tomography features of surgically resectable pulmonary squamous cell carcinomas: Impact on prognosis and comparison with adenocarcinomas. Eur J Radiol. 2014;83(7):1275–1281. doi: 10.1016/j.ejrad.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Brooks DR, et al. Influence of type of cigarette on peripheral versus central lung cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(3):576–581. doi: 10.1158/1055-9965.EPI-04-0468. [DOI] [PubMed] [Google Scholar]
- 6.Ock SY, et al. Characteristics of peripheral versus central lung cancer since 2000. Kosin Med J. 2014;29(1):47–52. [Google Scholar]
- 7.Takiguchi Y, Sekine I, Iwasawa S, Kurimoto R, Tatsumi K. Chronic obstructive pulmonary disease as a risk factor for lung cancer. World J Clin Oncol. 2014;5(4):660–666. doi: 10.5306/wjco.v5.i4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morissette MC, Shen P, Thayaparan D, Stämpfli MR. Disruption of pulmonary lipid homeostasis drives cigarette smoke-induced lung inflammation in mice. Eur Respir J. 2015;46(5):1451–1460. doi: 10.1183/09031936.00216914. [DOI] [PubMed] [Google Scholar]
- 9.Dessi S, et al. Altered pattern of lipid metabolism in patients with lung cancer. Oncology. 1992;49(6):436–441. doi: 10.1159/000227088. [DOI] [PubMed] [Google Scholar]
- 10.Orita H, et al. Selective inhibition of fatty acid synthase for lung cancer treatment. Clin Cancer Res. 2007;13(23):7139–7145. doi: 10.1158/1078-0432.CCR-07-1186. [DOI] [PubMed] [Google Scholar]
- 11.Sulkowska M, Sulkowski S, Chyczewski L, Nikliński J. Surfactant system in lung cancer. Endogenous lipid pneumonia. Neoplasma. 1997;44(3):167–171. [PubMed] [Google Scholar]
- 12.Sulkowska M, Sulkowski S. Endogenous lipid pneumonia- and alveolar proteinosis-type changes in the vicinity of non-small cell lung cancer: Histiopathologic, immunohistochemical, and ultrastructural evaluation. Ultrastruct Pathol. 1998;22(2):109–119. doi: 10.3109/01913129809032265. [DOI] [PubMed] [Google Scholar]
- 13.Tamura A, Hebisawa A, Fukushima K, Yotsumoto H, Mori M. Lipoid pneumonia in lung cancer: Radiographic and pathological features. Jpn J Clin Oncol. 1998;28(8):492–496. doi: 10.1093/jjco/28.8.492. [DOI] [PubMed] [Google Scholar]
- 14.Xiao Z, et al. The pivotal role of IKKα in the development of spontaneous lung squamous cell carcinomas. Cancer Cell. 2013;23(4):527–540. doi: 10.1016/j.ccr.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delvecchio CJ, et al. Liver X receptor stimulates cholesterol efflux and inhibits expression of proinflammatory mediators in human airway smooth muscle cells. Mol Endocrinol. 2007;21(6):1324–1334. doi: 10.1210/me.2007-0017. [DOI] [PubMed] [Google Scholar]
- 16.Botez G, et al. Age-dependent therapeutic effects of liver X receptor-α activation in murine polymicrobial sepsis. Innate Immun. 2015;21(6):609–618. doi: 10.1177/1753425915569367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korf H, et al. Liver X receptors contribute to the protective immune response against Mycobacterium tuberculosis in mice. J Clin Invest. 2009;119(6):1626–1637. doi: 10.1172/JCI35288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo Sasso G, et al. Liver X receptors inhibit proliferation of human colorectal cancer cells and growth of intestinal tumors in mice. Gastroenterology. 2013;144(7):1497–1507, 1507.e1–1507.e13. doi: 10.1053/j.gastro.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Higham A, et al. The role of the liver X receptor in chronic obstructive pulmonary disease. Respir Res. 2013;14(1):106. doi: 10.1186/1465-9921-14-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNeish J, et al. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc Natl Acad Sci USA. 2000;97(8):4245–4250. doi: 10.1073/pnas.97.8.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bates SR, Tao JQ, Collins HL, Francone OL, Rothblat GH. Pulmonary abnormalities due to ABCA1 deficiency in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289(6):L980–L989. doi: 10.1152/ajplung.00234.2005. [DOI] [PubMed] [Google Scholar]
- 22.Baldán A, et al. Deletion of the transmembrane transporter ABCG1 results in progressive pulmonary lipidosis. J Biol Chem. 2006;281(39):29401–29410. doi: 10.1074/jbc.M606597200. [DOI] [PubMed] [Google Scholar]
- 23.Spann NJ, Glass CK. Sterols and oxysterols in immune cell function. Nat Immunol. 2013;14(9):893–900. doi: 10.1038/ni.2681. [DOI] [PubMed] [Google Scholar]
- 24.Castrillo A, et al. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell. 2003;12(4):805–816. doi: 10.1016/s1097-2765(03)00384-8. [DOI] [PubMed] [Google Scholar]
- 25.Yvan-Charvet L, et al. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117(12):3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nalbandian A, Yan BS, Pichugin A, Bronson RT, Kramnik I. Lung carcinogenesis induced by chronic tuberculosis infection: The experimental model and genetic control. Oncogene. 2009;28(17):1928–1938. doi: 10.1038/onc.2009.32. [DOI] [PubMed] [Google Scholar]
- 27.Meuwissen R, Berns A. Mouse models for human lung cancer. Genes Dev. 2005;19(6):643–664. doi: 10.1101/gad.1284505. [DOI] [PubMed] [Google Scholar]
- 28.Dougan M, et al. A dual role for the immune response in a mouse model of inflammation-associated lung cancer. J Clin Invest. 2011;121(6):2436–2446. doi: 10.1172/JCI44796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barkauskas CE, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123(7):3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adamson IY, Bowden DH. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest. 1974;30(1):35–42. [PubMed] [Google Scholar]
- 31.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507(7491):190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar PA, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147(3):525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaughan AE, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517(7536):621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng D, Yin L, Chen J. Evidence for Scgb1a1(+) cells in the generation of p63(+) cells in the damaged lung parenchyma. Am J Respir Cell Mol Biol. 2014;50(3):595–604. doi: 10.1165/rcmb.2013-0327OC. [DOI] [PubMed] [Google Scholar]
- 35.Zuo W, et al. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517(7536):616–620. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bovenga F, Sabbà C, Moschetta A. Uncoupling nuclear receptor LXR and cholesterol metabolism in cancer. Cell Metab. 2015;21(4):517–526. doi: 10.1016/j.cmet.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Dai YB, et al. Liver X receptors regulate cerebrospinal fluid production. Mol Psychiatry. 2016;21(6):844–856. doi: 10.1038/mp.2015.133. [DOI] [PubMed] [Google Scholar]
- 38.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5(7):621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]