Abstract
Background Antibodies to neuraminidase (NA) contribute to protection during influenza virus infection, but NA inhibition (NI) titers are not routinely analyzed in vaccine trials. One reason is the cumbersome nature of the conventional thiobarbituric acid (TBA) NI assay, which uses chemical methods to quantify free sialic acid following incubation of NA with substrate in the presence of serum. In addition, the assay is complicated by the need to use virus of a hemagglutinin (HA) subtype novel to the host to detect NA‐specific antibodies only.
Objectives Our primary objectives were to miniaturize the colorimetric NI assay to a format suitable for quantitative analysis of large numbers of samples, and validate the specificity and sensitivity of the miniaturized format with ferret and human sera. An additional aim was to use reverse genetics to construct HA‐mismatched viral reagents bearing NA of recent influenza A vaccine strains and H6 HA.
Results Analysis of ferret antisera by the miniaturized assay demonstrated sensitivity and specificity comparable with the conventional assay. Similar increases in the NI titers in sera from vaccinated human volunteers were measured in miniaturized and conventional assays. Inactivated and live‐attenuated vaccines increased NI titers against a given subtype at approximately the same rate.
Conclusions The reagents and miniaturized format of the TBA method described here provide a platform for practical serological monitoring of functional antibodies against NA.
Keywords: Influenza virus, miniaturized assay, neuraminidase, reverse genetics
Introduction
Neuraminidase (NA) is one of the two major surface antigens of influenza viruses. The enzymatic activity of NA contributes to efficient viral replication as it enables spread in a host by cleavage of new virions from infected cells to permit dispersal of aggregated virions. 1 , 2 Neuraminidase may also digest decoy receptors that impede the access of virions to respiratory epithelial cells. 3
Antibodies that inhibit NA activity reduce viral replication in cell culture and decrease the size of plaques formed on cell monolayers. 4 , 5 Animal challenge experiments have shown that the presence of NA‐specific immunity can reduce disease severity upon infection, 6 and retrospective epidemiological studies have supported similar conclusions in the human host. 7 The known influenza A viruses are classified into nine NA subtypes according to serological properties. In the typical serotyping assay, a substrate with complex carbohydrates that contain terminal sialic acid residues, is subjected to digestion by viral NA in the presence or absence of subtype‐specific serum. Detection of liberated sialic acid correlates with NA activity, so that inhibition of this signal by serum is attributed to functional anti‐NA antibodies. In the classical NA assay, sialic acid is cleaved from fetuin, a highly glycosylated protein, and detected by the periodate‐thiobarbituric acid reaction. 8 NA inhibition (NI) can be quantified using the same method to measure NA activity of a defined target virus in the presence of serial dilutions of serum. 9 In conventional protocols for this assay, such as described in the World Health Organization Manual on Animal Influenza Diagnosis and Surveillance, 10 all steps for incubation of virus with serum and substrate and chemical reactions are performed in glass test tubes. Several steps are required after the incubation of virus and serum with fetuin substrate, beginning with addition of periodate to oxidize the free sialic acid product. Subsequent steps include the addition of arsenite to stop the oxidation reaction; addition of thiobarbituric acid (TBA) followed by heating to convert the product to a chromophore (β‐formylpyruvic acid); extraction of the chromophore into an organic phase; and measurement of absorbance. Hence, sample processing is cumbersome and requires significant volumes of multiple hazardous chemicals. It is impractical to assay large numbers of sera by this method. A non‐quantitative microtiter plate form of the assay to expedite NA subtyping was previously reported. 11
The aim of this study was to develop a quantitative, miniaturized format for the classical TBA method which would provide a more practical way to quantify NI antibody titers in large numbers of samples. NI assays can be susceptible to background interference by HA‐specific antibodies that hinder the substrate’s access to the NA catalytic site. 12 , 13 , 14 Therefore, we generated viral reagents by cloning and reverse genetics to contain the relevant NA gene in combination with H6 HA, a novel subtype for humans which has been used in past NI assays. 15 , 16 After establishing optimized conditions for the miniaturized assay, its sensitivity and specificity were tested using ferret and human sera against N1 and N2 antigens of recent seasonal influenza vaccine strains, and compared directly with results obtained using the conventional protocol.
A clinical study in which volunteers were challenged with wild‐type influenza virus showed a correlation between serum NI titer and protection against illness in individuals vaccinated with either an inactivated or live‐attenuated vaccine. 17 To evaluate the use of our miniaturized assay to identify responses following vaccination, we analyzed responses to NA in groups of volunteers immunized with the 2006/2007 formulation of either trivalent inactivated vaccine (TIV) or live‐attenuated influenza vaccine (LAIV).
Materials and methods
Viruses
Influenza A virus A/New Caledonia/20/1999 (H1N1) was supplied by the United States Centers for Disease Control and Prevention (CDC); and A/Wisconsin/67/2005 NYMC X161B (H3N2) was supplied by Dr. Galina Vodeiko (FDA Center for Biologics Evaluation and Research). A virus derived by traditional reassortment between H6N1 virus A/turkey/Massachussettes/3740/1975 (A/tk/Mass/75) and A/Puerto Rico/8/34 (PR8) was contributed by Dr. Doris Bucher (New York Medical College). For reverse genetics‐derived viruses used, the NA gene segments of A/New Caledonia/20/99 and A/Wisconsin/67/2005 and the HA segment of A/tk/Mass/75 were amplified by PCR with universal primers 18 and cloned into the plasmid pHW2000. Sequences of cloned inserts matched the intended viral genes at the amino acid level. The empty plasmid and plasmids encoding PR8 genes were provided by Dr. Robert Webster (St. Jude Children’s Research Hospital). Reassortant viruses bearing NA of a given vaccine strain, H6 HA of A/tk/Mass/75, and the complementary six gene segments of PR8 were rescued by 8‐plasmid reverse genetics, as previously described. 19 The viral constructs bearing NA of A/New Caledonia/20/1999 and A/Wisconsin/67/2005 are referred to as H6N1NewCal/99 and H6N2Wis/05, respectively. HA and NA genes of the rescued viruses were confirmed by sequencing. HA cross‐reactivity between the H6 viral constructs and ferret sera specific to A/New Caledonia/20/1999 or A/Wisconsin/67/2005 was undetectable by standard HA inhibition tests 20 using chicken erythrocytes. All viruses were propagated in 10‐day fertilized chicken eggs, harvested, and concentrated by ultracentrifugation through 25% sucrose.
Serum specimens
Ferret sera supplied by the CDC, Influenza Virus Surveillance and Diagnosis Branch, were collected from naïve (normal) or virus‐infected animals. Human serum specimens were collected from volunteers before and 4 weeks after immunization with (split) TIV or LAIV seasonal vaccines (2006–2007 season) at Brooke Army Medical Center (Fort Sam Houston, TX, USA) under an IRB‐approved protocol and after obtaining informed consent.
Neuraminidase inhibition assays
The miniaturized format of the NI assay was developed by reduction and optimization of the conventional assay described in the 2002 WHO Manual on Animal Influenza Diagnosis and Surveillance 10 and is summarized below. Fetuin was diluted in phosphate‐buffered saline (PBS) to 25 mg/ml, except where noted. Each virus was titrated in PBS (pH 7·4) containing 0·1% bovine serum albumin (PBS–0·1% BSA) to determine the dilution that yields an optical density at wavelength 550 nm (OD550) of 1·0 in the final extracted chromophore layer. Please note that NAs of some other H1N1 and H3N2 strains have shown a marked dependence on divalent cations in diluent (e.g., saline with titrated CaCl2 or Dulbecco’s PBS with Ca2+and Mg2+) for optimal activity. Twofold serial dilutions of serum were made in duplicate across wells of a 96‐well polypropylene PCR plate (Eppendorf, Hamburg, Germany). Six sera were typically titrated across seven dilutions in one plate that included controls. In each well, 5 μl serum diluted in PBS was mixed with 5 μl virus and incubated for 30–45 minutes at room temperature. Volumes were dispensed by a low‐volume multi‐channel pipette. Next, 5 μl fetuin (25 mg/ml) was added per well. Four fetuin control wells for background signal contained the substrate alone with respective buffers in place of serum and virus, and four virus control wells for non‐inhibited signal contained all components except serum (PBS in place of serum). The plates were sealed, mixed, and incubated at 37°C in a PCR thermocycler or cabinet incubator for 15–16 hours or for 2 hours, where noted. Detection of free sialic acid was initiated with addition of 5 μl periodate reagent per well (200 mm NaIO4, 53% H3PO4, stored in the dark) for a 15–20 minutes incubation at room temperature. Each well then received 25 μl arsenite reagent (1M AsNaO2, 700 mm Na2SO4, 0·3% concentrated H2SO4), and the plate was agitated until the yellow color disappeared. Next, 50 μl of TBA reagent (50 mm TBA, 625 mm Na2SO4,) was added per well, wells were closed with cap strips, and the plate was incubated 15 minutes at 99°C on a PCR thermocycler block with heated lid. Plates were chilled on an ice bath for 5–10 minutes. Warrenoff reagent (95% 1‐butanol, 5% concentrated HCl) was dispensed at 75 μl per well, wells were sealed with cap strips or MicroAmp optical adhesive film (Applied Biosystems, Foster City, CA, USA), and each plate was vortexed vigorously until the extraction of pink chromophore to the organic layer was clearly evident. Plates were centrifuged at 250 x g for 5 minutes to separate phases, and 50 μl of the upper phase per well was transferred to Costar 96‐well half area flat bottom plates (Corning Life Sciences, Corning, NY, USA). Sample absorbance was analyzed on a Victor3 V multi‐label reader with a 550 nm filter (PerkinElmer, Waltham, MA, USA). BSA, fetuin, and chemical compounds, including pure N‐acetyl neuraminic acid (NANA), were purchased from Sigma‐Aldrich (St. Louis, MO, USA).
The mean background absorbance obtained in fetuin control wells (no virus or serum) was subtracted from readings of all other wells. Based on the results of an empirical test to determine the ratio of sample absorbance of 50 μl in a 96‐well half area plate and in a standard spectrophotometer (data not shown), the background‐corrected readings were multiplied by 3·1 to derive OD values as defined in a 1‐cm cuvette spectrophotometer. Assay results were accepted if the mean OD550 values of virus control samples (no serum) was 0·7–1·3 and fetuin control samples were <0·1. NI titers were defined as the inverse of the highest serum dilution at which the mean absorbance was ≤50% of the mean signal of virus controls. Human samples with titers <5 were assigned a value of 2·5 for the purpose of calculating geometric mean titers.
The conventional NI assay was performed in test tubes as described in the 2002 WHO manual, 10 with the following adjustments and differences from the miniaturized assay. Each virus preparation was diluted in PBS–0·1% BSA to a concentration providing NA activity that yielded an end OD549 reading of 0·5 by a cuvette spectrophotometer in the absence of serum (using a fetuin control sample to blank the instrument). Following incubation of 50 μl virus with an equal volume of each serial dilution of serum for 30 minutes at room temperature, 100 μl fetuin solution (12·5 mg/ml) was added per sample and incubated at 37°C for 15–16 hours. Volumes of periodate, arsenite, TBA, and Warrenoff reagents added per sample were 0·1, 1·0, 2·5, and 3·0 ml, respectively. Arsenite reagent contained 770 mm AsNaO2 and 500 mm Na2SO4, with 0·3% H2SO4, while TBA reagent contained 42 mm TBA and 500 mm Na2SO4. Incubation of samples with TBA reagent was performed in a boiling water bath. Absorbance was measured in 1‐cm cuvettes with a SmartSpec 3000 spectrophotometer (Bio‐Rad Laboratories, Hercules, CA, USA) at wavelength 549 nm.
Statistical analysis
The intra‐assay variability of the miniaturized assay was determined by performing 12 replicate titrations of a human serum sample, prepared separately and tested against H6N1NewCal/99 and H6N2Wisc/05 antigens. To generate continuous data sets for analysis of variance, dilution factors were transformed (log2) and 50% endpoint titers were determined using non‐linear regression (GraphPad Prism, GraphPad Software, La Jolla, CA, USA). In accordance with the previous analysis of traditional NI assay data, 21 the standard deviation (SD) of log2‐transformed NI titers from each data set was calculated by sum of squares analysis.
Results
The TBA method was miniaturized by reducing volumes of all constituents sufficiently for a 96‐well plate format. Viral antigen concentrations were standardized for a balance of robust signal quantification and sensitivity to serum inhibition. Because the miniaturized assay was conducted in wells of a PCR plate, the incubation step conventionally performed in a boiling water bath could be performed effectively in a thermocycler block. Sealing plates with cap strips or adhesive film permitted all incubations and the extraction of chromophore to be conducted within a closed container. Rapid colorimetric analysis of samples was performed with a microplate reader.
To assess the linear range for free sialic acid quantification in the miniaturized NA assay format, we analyzed twofold serial dilutions of NANA that were dispensed in duplicate wells (15 μl/well). OD550 signal increased in direct proportion to sialic acid quantity across a 0·7–20 nmol range (0·1–2·7 OD550, Figure 1A). The levels of sialic acid detected after overnight fetuin incubation with serially diluted H6N1NewCal/99 and H6N2Wis/99 were proportional to virus concentration within approximately the same OD550 limits (Figure 1B). A value of 1·0 OD550 was selected as the target for NA antigen dilutions in the miniaturized NI assay.
Figure 1.
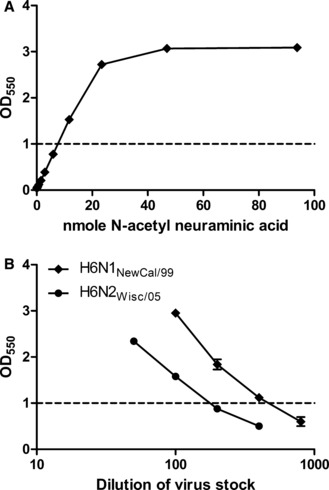
Colorimetric detection of sialic acid in a miniaturized assay format. (A) Serial dilutions of purified N‐acetyl neuraminic acid were added to duplicate wells of a 96‐well PCR microplate, converted to chromophore by the miniaturized periodate‐thiobarbituric acid reactions, extracted, and measured in terms of absorbance at 550 nm. (B) Allantoic fluid stocks of reverse genetics‐derived viruses H6N1NewCal/99 and H6N2Wis/05 were serially diluted and incubated overnight with fetuin substrate in duplicate microplate wells. Liberated sialic acid was assayed in the same manner as N‐acetyl neuraminic acid was assayed in (A). For subsequent NI antibody analysis, each virus was diluted to the concentration which yielded a reading of 1·0. Error bars represent the standard deviation (SD) of replicate wells. At some data points, the SD is not large enough for bars to be visible.
To demonstrate specificity of the assay, sera harvested from ferrets previously infected with A/New Caledonia/20/1999 or A/Wisconsin/67/2005 vaccine strain viruses were tested for NI activity against H6N1NewCal/99 and H6N2Wis/05 viral antigens. Each ferret antiserum inhibited the homologous NA, but did not inhibit NA of the other subtype (Figure 2). The 50% inhibition titers determined with the miniaturized assay equaled or exceeded the corresponding titers obtained using the conventional assay (Table 1).
Figure 2.

Analysis of ferret antiserum NI activity against homologous and heterosubtypic NA antigens in miniaturized colorimetric assay. Serial dilutions of normal ferret serum (NFS), ferret anti‐A/New Caledonia/20/1999, and ferret anti‐A/Wisconsin/67/2005 were incubated for 30 minutes with (A) H6N1NewCal/99 or (B) H6N2Wis/05 antigens in duplicate wells of a 96‐well microplate. Fetuin substrate was added, and samples were incubated 15 hours at 37°C. Sialic acid cleavage from the substrate was quantified using the miniaturized colorimetric assay.
Table 1.
Comparison of NI results using conventional and miniaturized assays
| NA source | Serum | NI titers by two assays | |
|---|---|---|---|
| Conventional | Miniaturized | ||
| H6N1NC/99 | Normal ferret | <5 | <5 |
| Ferret α‐H1N1* | 320 | 1280 | |
| Ferret α‐H3N2** | <5 | <5 | |
| H6N2Wis/05 | Normal ferret | <5 | <5 |
| Ferret α‐H1N1* | <5 | <5 | |
| Ferret α‐H3N2** | 40 | 80 | |
| Human | Pre | Post*** | Pre | Post | |
|---|---|---|---|---|---|
| H6N2Wis/05 | Volunteer 1 | <5 | 5 | 5 | 20 |
| Volunteer 4 | <5 | 5 | 10 | 20 | |
| Volunteer 6 | <5 | 5 | 10 | 20 | |
| Volunteer 7 | 5 | 5 | 20 | 20 |
NI, neuraminidase inhibition; NA, neuraminidase.
*Ferret anti‐A/New Caledonia/20/1999.
**Ferret anti‐A/Wisconsin/67/2005.
***Sera were collected 4 weeks after influenza vaccination.
Neuraminidase activity in the miniaturized assay was also measurable using a 2‐hour incubation of virus with fetuin substrate, and this signal was enhanced moderately by doubling the fetuin concentration (data not shown). However, an increased amount of virus was necessary in the 2‐hour fetuin incubation to provide adequate dynamic range for reliable quantification of serum NI activity (e.g., a signal of at least 0·5 OD550). A consequence of increased virus concentration is reduction in sensitivity to serum‐mediated inhibition. For example, assaying ferret anti‐A/New Caledonia/20/1999 in the 2‐hour format, with H6N1NewCal/99 antigen at a sixfold greater concentration than in the overnight format, resulted in a reduction of NI titer from 1280 to 320. Similarly, assaying ferret anti‐A/Wisconsin/67/2005 in the 2‐hour format, with H6N1Wis/05 at a threefold greater concentration than in the overnight format, resulted in a reduction of NI titer from 80 to 20.
To compare the sensitivity of conventional and miniaturized NI assay methods, both were used to analyze ferret and human sera. NI titers determined for ferret anti‐H1N1 and anti‐H3N2 sera against homologous antigens were four‐ and twofold lower, respectively, when measured by the conventional assay (Table 1). Four pairs of adult human serum collected before and after vaccination with a seasonal LAIV (containing the A/Wisconsin/67/2005 strain), were analyzed for NI titers against H6N2Wis/05 by the two methods. Higher anti‐N2 NI titers were obtained with the miniaturized assay, and shifts in titer following vaccination using either of the two assays were comparable (Table 1). For some of the pre‐vaccination serum samples the conventional assay yielded no detectable titer at the lowest tested dilution of serum (<5), whereas the miniaturized assay was sensitive enough to determine a titer of 5 or 10. The increased sensitivity of the miniaturized assay did not diminish its capacity to detect a rise in NI titer following vaccination; in one case it measured a greater increase than the conventional assay.
The effect of HA subtype on NI titer determinations with the miniaturized assay was assessed in the post‐vaccination sera of nine human volunteers. Most of these sera caused more inhibition of NA when assayed against HA‐matched H1N1 virus than against HA‐mismatched H6N1 virus (Table 2), which is evidence that the H6N1 antigen permits more accurate determination of anti‐NA antibody activity. The impact of HA specificity when assaying N2‐specific titers was less pronounced, but still evident, as three of the nine sera mediated greater inhibition of HA‐matched H3N2 than HA‐mismatched H6N2 neuraminidase activity. Results of these tests are consistent with a moderate exaggeration of NI titers in assays against viruses with matched HA subtypes, because of HA antibody‐mediated interference, as previously reported. 12 , 13 , 14
Table 2.
Neuraminidase inhibition assay comparison using antigens with native viral HA versus HA novel to host
| Human volunteer | NI titers to N1NewCal/99 | NI titers to N2Wis/05 | ||
|---|---|---|---|---|
| H1N1* | H6N1** | H3N2*** | H6N2† | |
| #3 | 160 | 80 | 40 | 20 |
| #4 | 20 | 10 | 20 | 20 |
| #5 | 5 | <5 | 40 | 20 |
| #6 | 40 | 20 | 10 | 10 |
| #7 | 20 | 10 | 20 | 20 |
| #13 | 160 | 40 | 80 | 40 |
| #18 | 80 | 80 | 20 | 20 |
| #25 | ≥320 | 160 | 20 | 20 |
| #26 | 40 | 5 | 80 | 80 |
NI, neuraminidase inhibition; HA, hemagglutinin.
*Tested against wild‐type A/New Caledonia/20/1999.
**Tested against reverse genetics‐derived virus with H6 HA and N1NewCal/99 genes.
***Tested against A/Wisconsin/67/2005 × 161B.
†Tested against reverse genetics‐derived virus with H6 HA and N2Wis/05 genes.
We analyzed the intra‐assay variability of NI readings in the miniaturized assay by performing 12 replicate titrations of one human serum sample, each of these in duplicate. The replicates were prepared separately and tested against both H6N1NewCal/99 and H6N2Wisc/05 antigens. To generate appropriately distributed data sets for analysis of variance, the dilution factors were transformed (log2) and 50% endpoint titers were determined using non‐linear regression. The mean NI titer against H6N1NewCal/99 was 4·16 log2, with SD 0·182 log2. Against H6N2Wisc/05 the mean titer was 5·55 log2, with SD 0·175 log2. In both cases, two SDs equal approximately 0·36 log2, or 1·3. Thus, >1·3‐fold difference in NI titers between two sera tested in the same assay is considered significant. We intend to confirm this analysis of intra‐assay variability using multiple samples during inter‐laboratory validation studies.
Finally, using the miniaturized NI assay with HA‐mismatched viruses, NI titers against N1NewCal/99 and N2Wis/05 homologous to the vaccine antigens were determined in sera from human volunteers, before and after immunization with TIV or LAIV vaccines (Table 3). Prior to vaccination, NI titers against N1 ranged from <5 to 160, whereas titers against N2 ranged from <5 to 80. Increases in NI antibody titers against N1 were detected in five of 16 LAIV recipients and six of 16 TIV recipients. Titers against N2 were increased in six of 16 LAIV recipients and seven of 16 TIV recipients. In the two groups combined, there were six individuals with rises in titer against both N1 and N2, five with a rise in titer against only N1, and seven with a rise in titer against only N2. There were 14 individuals (44%) in this study with no rise in titer against either NA. Despite the considerable range in NI titers detected among individuals, most of the responders to either antigen had only twofold increases in NI titer, with a few instances of fourfold increases (three of 64 total comparisons). There were also three instances in which an NI titer in post‐vaccination serum was twofold lower than the titer in pre‐vaccination serum.
Table 3.
Subtype‐specific NI titers in human volunteers pre‐ and post‐immunization with seasonal trivalent influenza vaccines*
| Vaccine group | Human volunteer | NI to N1NewCal/99 | NI to N2Wis/05 | ||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| LAIV | #1 | 5 | 10 | 10 | 10 |
| #3 | 20 | 20 | 40 | 40 | |
| #4 | 10 | 10 | 10 | 10 | |
| #5 | <5 | <5 | 40 | 20 | |
| #6 | 20 | 40 | 5 | 10 | |
| #7 | 5 | 20 | 10 | 20 | |
| #8 | <5 | 5 | 5 | 20 | |
| #9 | 5 | 5 | <5 | <5 | |
| #10 | 10 | 10 | <5 | 5 | |
| #13 | 40 | 40 | 80 | 80 | |
| #14 | <5 | 5 | 5 | 5 | |
| #17 | 10 | 10 | 10 | 5 | |
| #18 | 80 | 80 | 10 | 20 | |
| #19 | 10 | 10 | <5 | 5 | |
| #21 | 20 | 20 | 20 | 20 | |
| #35 | 80 | 80 | 80 | 40 | |
| GMT | 10·9 | 14·1 | 10·9 | 13·0 | |
| Response rate | 31% | 38% | |||
| TIV | #11 | 10 | 20 | 10 | 10 |
| #12 | 5 | 10 | 20 | 40 | |
| #15 | 5 | 10 | 20 | 20 | |
| #20 | 5 | 5 | 10 | 10 | |
| #22 | 20 | 20 | 10 | 10 | |
| #23 | 10 | 20 | 10 | 10 | |
| #24 | 10 | 10 | 10 | 10 | |
| #25 | 160 | 160 | 10 | 10 | |
| #26 | <5 | <5 | 40 | 40 | |
| #28 | 20 | 20 | 10 | 20 | |
| #29 | 10 | 10 | 10 | 20 | |
| #30 | 10 | 10 | 10 | 40 | |
| #31 | 5 | 5 | 10 | 10 | |
| #32 | 5 | 5 | 20 | 40 | |
| #33 | 10 | 20 | 10 | 20 | |
| #34 | 20 | 40 | 40 | 80 | |
| GMT | 10·0 | 13·0 | 13·5 | 19·2 | |
| Response rate | 38% | 44% | |||
LAIV, live‐attenuated influenza vaccine; TIV, trivalent inactivated vaccine; NI, neuraminidase inhibition; GMT, geometric mean titer.
*Sera assayed against H6N1NewCal/99 and H6N2Wis/05 antigens.
Discussion
Influenza vaccine evaluation could be enhanced by monitoring functional antibody responses to NA, in tandem with HA‐specific antibody analysis. One hindrance to the widespread adoption of NA antibody monitoring is the lack of suitable high‐throughput serological assays. In this study, we have successfully reduced the scale of the traditional NI assay in which sialic acid molecules cleaved from fetuin substrate are measured using periodate‐TBA chemical reactions. There have been no fundamental changes in the nature or theory underlying the mini‐assay procedure. We have shown that the miniaturized assay retains the NA subtype specificity of the conventional format, and consistent, robust signals are obtained using proportionally less viral antigen. Consequently, the miniaturized assay is generally more sensitive to antibody‐mediated inhibition. In the event that speed of assay completion is a top priority it is possible to perform the miniaturized assay with a 2‐hour virus‐fetuin incubation, rather than overnight. When using the shorter incubation time, a greater concentration of viral antigen is required for robust signal readings, and this reduces the assay’s sensitivity.
Practical advantages for our miniaturized NI assay format compared with the conventional format include a marked increase in sample throughput, a 10‐fold reduction in the volume of serum required for NI titration, and greater safety in handling samples in 96‐well plates rather than racks of open tubes. The small reaction volume is conducive to handling chemical reagents under the protection of a standard fume hood, and incubations outside of the fume hood can be contained by disposable plate sealers or cap strips. Furthermore, the volume of chemical waste generated per sample analyzed is reduced by approximately 20‐fold. The need for a large capacity boiling water bath is eliminated, and the equipment necessary for the miniaturized assay format, including a PCR thermocycler (or simple heat block) for heating and a photometric plate reader, are generally available.
Neuraminidase inhibition assay results can be distorted by HA‐specific antibodies interfering with substrate cleavage by NA. 12 We therefore cloned HA of an H6 virus for co‐transfection with the contemporary N1 or N2 NA genes in construction of reassortant viruses to serve as antigens in the NI assay. Given the general absence of human immunity to H6 influenza viruses, human serological studies performed with these constructs are unlikely to be affected by HA‐specific antibodies. These, or other reverse genetics‐derived viruses designed with alternate HA genes, offer the same utility for quantification of NI antibody responses in laboratory animals following vaccination or challenge.
We utilized the miniaturized assay and reverse genetics‐derived antigen reagents to assay NI titers against N1NewCal/99 and N2Wis/05 in pre‐ and post‐vaccination sera of TIV and LAIV recipients in a small clinical trial. The NA antigens were homologous to the H1N1 and H3N2 strains represented in the vaccines. Most individuals had positive NI titers against both NA subtypes prior to vaccination, but titers varied substantially. Whether examining N1NewCal/99‐ or N2Wis/05‐specific NI titers, both vaccine formulations elicited rises in titer in 30–45% of the recipients, and similar response rates were associated with TIV and LAIV. Among the five individuals without initially detectable titers against N1NewCal/99 or N2Wis/05, three developed low titers after vaccination. Instances of twofold rises in titer against either NA subtype far outnumbered fourfold rises, and none were greater than fourfold, despite the assay’s capacity to detect much wider differences between individuals. Thus, both vaccines showed the capacity to induce NI antibodies, but it does not appear that either vaccine elicited particularly robust NI responses in this small trial. The percentages of volunteers with anti‐H1 and anti‐H3 antibody responses to vaccination – using the conventionally defined parameter of a fourfold rise in HAI titer – were comparable to NI response rates. Probably reflecting pre‐existing titers, both vaccines induced fourfold HAI titer increases in fewer than one‐third of recipients, with respect to both H1 and H3 (Hassantoufighi and Eichelberger, unpublished data). The lower magnitude of NI titers in most human volunteers, even after TIV vaccination, may result from a low quantity of conformationally intact NA protein in the vaccine formulation. This parameter is not standardized or routinely monitored for stability. In the case of LAIV, enzymatically active NA should be expressed by virus replicating in vivo, but the amount of expression is not known.
There were isolated instances of twofold lower NI antibody titers in post‐vaccination serum compared with pre‐vaccination serum. However, calculating 50% endpoint titers for these sera by non‐linear regression showed that the difference in titer was only statistically significant in one of these three pairs. Assigning NI titers as the reciprocal of the highest dilution observed to inhibit NA activity by at least 50% has the advantage of simplicity, but a drawback is that two samples which both reach 50% inhibition very near one of the dilutions may be assigned twofold different titers. Conversely, a pair of samples with a small difference in NI activity may be assigned the same titer. The alternative approach of calculating 50% endpoint titers by non‐linear curve fitting precludes these errors. However, high accuracy with this approach requires assaying across a wide range of dilutions, which restricts the output of the assay. The size and variability of a data set and the aims of a study may be important considerations in the choice of method to assign NI titers.
Development of improved methodologies for evaluation of the immune responses elicited by modern influenza vaccines is clearly a desirable objective. NI antibody level has been shown to correlate with protection against seasonal influenza in clinical trials, 17 , 22 which points to the potential value of monitoring NI titers as part of seasonal and pre‐pandemic influenza vaccine evaluation. It may be worth particular consideration in the context of pre‐pandemic vaccines because the antigenic structure of the HA contained within a stockpiled pre‐pandemic vaccine may differ from the strain that becomes transmissible in the human population, reducing the capacity of HA‐specific antibodies alone to mediate protection.
Because it measures functional inhibition, the conventional TBA assay for antibodies that inhibit NA has clear relevance to immunity in vivo. 22 , 23 , 24 However, the inefficiency of the standard method has been a serious hindrance to its widespread use. By miniaturizing the method to a microplate format – and in the process enhancing its sensitivity to serum NI activity – we have developed a more practical platform for large‐scale serological monitoring. In addition, this provides an effective means for comparing new methods to quantify NA‐specific antibody responses with the traditional assessment of NA inhibition.
Acknowledgements
The authors gratefully acknowledge Dr. Robert Webster for reverse genetics plasmids, Drs. Alexander Klimov and Xiyan Xu for supplying ferret sera, Dr. Doris Bucher for supplying A/tk/Mass/75 (H6N1), and Dr. Galina Vodeiko for supplying A/Wisconsin/67/2005 NYMC X161B. We acknowledge Drs. Judy Beeler and Vladimir Lugovtsev for critical review of the manuscript.
References
- 1. Seto JT, Chang FS. Functional significance of sialidase during influenza virus multiplication: an electron microscope study. J Virol 1969; 4:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dowdle WR, Downie JC, Laver WG. Inhibition of virus release by antibodies to surface antigens of influenza viruses. J Virol 1974; 13:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J Virol 2004; 78:12665–12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jahiel RI, Kilbourne ED. Reduction in plaque size and reduction in plaque number as differing indices of influenza virus‐antibody reactions. J Bacteriol 1966; 92:1521–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kilbourne ED, Laver WG, Schulman JL, Webster RG. Antiviral activity of antiserum specific for an influenza virus neuraminidase. J Virol 1968; 2:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schulman JL, Khakpour M, Kilbourne ED. Protective effects of specific immunity to viral neuraminidase on influenza virus infection of mice. J Virol 1968; 2:778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Monto AS, Kendal AP. Effect of neuraminidase antibody on Hong Kong influenza. Lancet 1973; 1:623–625. [DOI] [PubMed] [Google Scholar]
- 8. Warren L. The thiobarbituric acid assay of sialic acids. J Biol Chem 1959; 234:1971–1975. [PubMed] [Google Scholar]
- 9. Webster RG, Laver WG. Preparation and properties of antibody directed specifically against the neuraminidase of influenza virus. J Immunol 1967; 99:49–55. [PubMed] [Google Scholar]
- 10. Webster RG, Krauss S, ed. WHO Manual on Animal Influenza Diagnosis and Surveillance, World Health Organization Global Influenza Program, 2002. Available at: http://www.who.int/vaccine_research/diseases/influenza/WHO_manual_on_animal‐diagnosis_and_surveillance_2002_5.pdf
- 11. Van Deusen RA, Hinshaw VS, Senne DA, Pellacani D. Micro neuraminidase‐inhibition assay for classification of influenza A virus neuraminidases. Avian Dis 1983; 27:745–750. [PubMed] [Google Scholar]
- 12. Schulman JL, Kilbourne ED. Independent variation in nature of hemagglutinin and neuraminidase antigens of influenza virus: distinctiveness of hemagglutinin antigen of Hong Kong‐68 virus. Proc Natl Acad Sci USA 1969; 63:326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anker WJ, Bakker AK, Masurel N. Cross‐protection in mice after immunization with H2N2, H3N2, and Heq2Neq2 influenza virus strains. Infect Immun 1978; 21:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gerentes L, Kessler N, Aymard M. Difficulties in standardizing the neuraminidase content of influenza vaccines. Dev Biol Stand 1999; 98:189–196. (discussion 197). [PubMed] [Google Scholar]
- 15. Khan MW, Gallagher M, Bucher D, Cerini CP, Kilbourne ED. Detection of influenza virus neuraminidase‐specific antibodies by an enzyme‐linked immunosorbent assay. J Clin Microbiol 1982; 16:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kilbourne ED, Cerini CP, Khan MW, Mitchell JW Jr, Ogra PL. Immunologic response to the influenza virus neuraminidase is influenced by prior experience with the associated viral hemagglutinin. I. Studies in human vaccinees. J Immunol 1987; 138:3010–3013. [PubMed] [Google Scholar]
- 17. Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild‐type virus. J Clin Microbiol 1986; 24:157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. Universal primer set for the full‐length amplification of all influenza A viruses. Arch Virol 2001; 146:2275–2289. [DOI] [PubMed] [Google Scholar]
- 19. Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci USA 2000; 97:6108–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Straight TM, Ottolini MG, Prince GA, Eichelberger MC. Antibody contributes to heterosubtypic protection against influenza A‐induced tachypnea in cotton rats. J Virol 2008; 5:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hennessy AV, Davenport FM. Reproducibility of a neuraminidase inhibition test employed to measure anti‐influenza neuraminidase antibody. Appl Microbiol 1972; 23:827–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ogra PL, Chow T, Beutner KR et al. Clinical and immunologic evaluation of neuraminidase‐specific influenza A virus vaccine in humans. J Infect Dis 1977; 135:499–506. [DOI] [PubMed] [Google Scholar]
- 23. Couch RB, Kasel JA, Gerin JL, Schulman JL, Kilbourne ED. Induction of partial immunity to influenza by a neuraminidase‐specific vaccine. J Infect Dis 1974; 129:411–420. [DOI] [PubMed] [Google Scholar]
- 24. Beutner KR, Chow T, Rubi E, Strussenberg J, Clement J, Ogra PL. Evaluation of a neuraminidase‐specific influenza A virus vaccine in children: antibody responses and effects on two successive outbreaks of natural infection. J Infect Dis 1979; 140:844–850. [DOI] [PubMed] [Google Scholar]


