Abstract
Please cite this paper as: Toback et al. (2012) Maternal outcomes among pregnant women receiving live attenuated influenza accine. Influenza and Other Respiratory Viruses 6(1), 44–51.
Background Although the live attenuated influenza vaccine (LAIV) prescribing information contains warnings/precautions against use during pregnancy, administration of LAIV to pregnant women does occur. Data regarding maternal outcomes after LAIV administration during pregnancy are limited.
Objectives Maternal outcomes after LAIV vaccination during pregnancy were examined.
Methods Data from a health insurance claims database that covers approximately 50 million individuals were analyzed for the six influenza seasons from 2003–2004 through 2008–2009. Emergency department (ED) visits and hospitalizations occurring within 42 days of vaccination were analyzed by primary diagnosis; outcomes were categorized as cardiopulmonary, obstetric, and other. Cohort characteristics were analyzed using descriptive statistics.
Results Of 834 999 pregnancies identified, 138 (0·017%) were among women who received LAIV vaccinations. Of the 138 pregnant women, 13% were ≤19 years, 67% were 20–34 years, and 20% were ≥35 years of age. Eight events occurred within 42 days of vaccination: one ED visit for bronchitis, two hospitalizations for hyperemesis gravidarum and premature labor, and five ED visits/hospitalizations for common medical conditions. All outcomes identified after LAIV exposure occurred at rates similar to rates in unvaccinated pregnant women reported in the medical literature.
Conclusions Administration of LAIV to pregnant women is rare; the rate has remained constant since 2004–2005. In this cohort, there was no evidence of significant maternal adverse outcomes after receipt of LAIV. These data may offer some reassurance to providers and pregnant women in the event of inadvertent LAIV administration, but do not support the routine use of LAIV in pregnant women.
Keywords: Inactivated influenza vaccine, live attenuated influenza vaccine, pregnancy, safety
Introduction
Women who are pregnant during the influenza season are at increased risk for severe complications from influenza infections. 1 , 2 , 3 For this reason, influenza vaccination at any time during pregnancy is recommended by the US Advisory Committee on Immunization Practices (ACIP) and the American College of Obstetricians and Gynecologists (ACOG). 1 , 4 Two types of influenza vaccines are approved in the United States: injectable trivalent inactivated influenza vaccines (TIV) and the intranasal live attenuated influenza vaccine (LAIV). The safety of TIV during pregnancy has been well established. 5 , 6 Accordingly, the US Centers for Disease Control and Prevention (CDC) recommend that TIV are used to vaccinate pregnant women during the influenza season. As with all live vaccines, LAIV is not recommended for use during pregnancy. 1 , 7 LAIV is approved in the United States for use in eligible children and adults 2–49 years of age, and more than 40 million doses of seasonal trivalent LAIV have been distributed for use in the United States from licensure in 2003 through the 2010–2011 influenza season (MedImmune, data on file).
It is not known whether LAIV administration during pregnancy is associated with any maternal or fetal risks. To date, there has been little information available. A 2010 analysis of data from the Vaccine Adverse Events Reporting System (VAERS) reported 27 pregnant women from 2003 through 2009 who received LAIV, 60% of whom did not report any associated adverse event. 8 Of those reporting adverse events, the most common were spontaneous abortion (n = 3), fever (n = 3), vomiting (n = 3), headache (n = 3), and sore throat (n = 2). No congenital anomalies or adverse fetal events were reported. Additionally, a study by Piedra et al. 9 described fetal outcomes among six adolescents who were inadvertently vaccinated with LAIV while pregnant. Among these pregnancies, there were five full‐term healthy infants and one preterm delivery. The lack of available information regarding maternal and fetal risks after LAIV vaccination and concerns regarding inadvertent vaccination of pregnant women has complicated vaccination efforts in some settings. 10
Although LAIV is not recommended to be administered to pregnant women, administration of LAIV to pregnant women does rarely occur. The purpose of this study was to quantify, using healthcare claims data, the frequency of LAIV vaccination among pregnant women and to assess maternal adverse events after vaccination with LAIV.
Methods
Anonymized patient‐specific claims data were obtained from the LifeLink™ Health Plan Claims Database (IMS Health, Norwalk, CT, USA). At the time of data acquisition, the database included medical and pharmacy claims from more than 50 million unique members from over 90 U.S. health plans. The database includes inpatient and outpatient claims, including diagnoses in ICD‐9‐CM format, procedures in CPT‐4 and HCPCS formats, and retail and mail‐order pharmacy claims containing National Drug Codes (NDC). Date and place of service are included with all claims. Information regarding influenza vaccinations for which claims were not submitted was unavailable.
The study population consisted of females who were 12–49 years of age at the time they had a claim for delivery of a child. For inclusion, individuals were required to have at least 270 days of continuous enrollment in the database before delivery. Data from October 2003 through September 2009 were included in the analysis. Because the database was unable to link claims data for a mother and her offspring, no fetal outcomes could be examined.
Pregnant women were identified by a claim for a delivery procedure code or an ICD‐9‐CM diagnosis code for a delivery. Women could contribute more than one pregnancy and delivery to the analysis as long as the aforementioned criteria were met for each included delivery. For pregnancies with multiple delivery codes during the period of pregnancy, the investigators manually reviewed the claims to ascertain the correct delivery date based on the date of the delivery procedure code; if no delivery procedure code was present, the delivery date was assigned to be the date of the first ICD‐9‐CM diagnosis code for a delivery. LAIV exposure during pregnancy was identified by the presence of CPT code 90660 or an NDC code of 66019010001, 66019020001, 66019030001, 66019040001, 66019050001, or 66019060001. TIV exposure was defined by the presence of an insurance claim with a CPT code of 90655, 90656, 90657, 90658, 90659, or relevant NDC code. The 2009 monovalent H1N1 pandemic influenza vaccines were not in use at the time of the study and were not included in the analysis.
For each pregnancy, a date of conception was calculated by estimating the duration of pregnancy from ICD‐9‐CM codes associated with the delivery. If an ICD‐9‐CM code for gestational age (765·2x) was recorded at the time of delivery, the conception date was assumed to precede delivery by the upper limit of the range of weeks associated with the specific code. We used the upper limit to maximize the detection of LAIV vaccination during pregnancy. If the 765·2x codes were not present, but premature delivery was indicated by codes 644·20 or 644·21, the date of conception was assumed to be 245 days before delivery, the approximate gestational age for children born <37 weeks as described by Martin et al. 11 If neither the 765·2x nor the 644·2x codes were present, but the birth involved multiple infants, then the conception date was assumed to be 256 days before delivery, the midpoint of the range of days used by Cole et al. 12 For all other pregnancies, the conception date was estimated as the date 270 days before delivery. 13 The end of the first and second trimesters was defined as 90 and 180 days after conception, respectively; the end of the third trimester was the delivery date. The gestational day at the time of vaccination was defined as the number of days between LAIV vaccination and the estimated date of conception.
To evaluate the presence of underlying high‐risk medical conditions, available ICD‐9‐CM codes from claims as early as January 1997 to the time of vaccination were reviewed. To qualify as having a high‐risk condition, a subject had to have at least one hospital/emergency department (ED) claim or two outpatient claims on separate dates for the condition on or before the delivery date. The following ICD‐9‐CM codes were used to define high‐risk disease groups: chronic cardiac disease (093, 393–398, 402–404, 410–414, 416, 424–425, 428–429, 440, and 745–746); chronic pulmonary disease (277·0, 491–496, 500–506, 515–517, and 519·9); diabetes mellitus (250 and 648·0); chronic renal disease (581–583, 585, and 587, or CPT codes 800, 801, 90935, 90937, 90940); malignancy (140–199 [except 173] and 200–208); and immunosuppressive disorders (042–044 and 136·3). 3
Live attenuated influenza vaccine was first widely available in the United States in the fall of 2003. To calculate the proportion of deliveries with LAIV vaccination during pregnancy, we divided the number of eligible deliveries with LAIV vaccination during pregnancy by the total number of eligible deliveries that occurred on or after October 1, 2003, through September 2009. The number of pregnant women vaccinated with LAIV in each influenza season was divided by the number of doses distributed each season to estimate the number of vaccinations per million doses distributed for each season.
To assess potential adverse outcomes among the cohort of women vaccinated with LAIV during pregnancy, all health insurance claims for hospitalization or ED visits occurring within 42 days post‐vaccination were reviewed. Because the sample of LAIV‐vaccinated pregnant women was small, a qualitative description of adverse outcomes in comparison with the rates published in the medical literature was provided. For each hospitalization or ED visit, authors reviewed the primary discharge diagnoses and grouped events into three categories: obstetric outcomes (those not related to delivery), cardiopulmonary outcomes, and other medical outcomes. For hospitalizations within 42 days post‐vaccination listing a primary discharge diagnosis of normal delivery, all additional codes associated with that hospitalization were also reviewed. Any diagnosis code for a previously unrecorded medical condition that was not related to routine labor and delivery and not related to a fetal condition was considered to be a potential outcome of interest. To evaluate any potential long‐term side effects of vaccination, primary discharge diagnoses associated with hospitalizations from the time of vaccination up until, but not including, the hospitalization at the time of delivery were analyzed. To focus on serious adverse events, only hospitalizations were considered in this analysis. If a subject had a claim for an ED visit and hospitalization on the same day, a single hospitalization event was counted. The number and percentage of women who experienced either a hospitalization or ED visit was stratified by trimester of vaccination. Women with high‐risk medical conditions were included in the analysis and examined separately.
Because the delivery of a child was an entry criterion and delivery data were only available through September 2009, the 2008–2009 cohort would not include first‐trimester vaccinations of women with deliveries after September 2009. All analyses were performed using SAS 9.1.3 (SAS Institute Inc., Cary, NC, USA) or Microsoft Excel (Microsoft, Redmond, WA, USA). Because data were anonymized, the protocol was granted Institutional Review Board (IRB) review exemption by the RTI International IRB.
Results
The database search identified 834 999 women with a pregnancy resulting in a delivery between October 2003 and September 2009. Of these, 138 women (0·017%) had a claim for LAIV vaccination while pregnant. The number of vaccinations increased throughout the study period with 14, 12, 17, 32, 22, and 41 occurring in the 2003–2004, 2004–2005, 2005–2006, 2006–2007, 2007–2008, and 2008–2009 seasons, respectively. In the first year LAIV was available, there was a disproportionally high number of vaccinations during pregnancy, namely 31·1 per million LAIV doses distributed. Since the 2003–2004 season, the rate of LAIV vaccination during pregnancy per million LAIV doses distributed has remained constant with an average of 7·9 per season (range: 5·7–12·3 per season). The characteristics of those vaccinated with LAIV relative to those not vaccinated with LAIV were comparable (Table 1), with the exception of LAIV‐vaccinated women having a greater likelihood of being ≤19 years of age (13% versus 5%; P < 0·0001) and of having >10 prenatal visits (21% versus 13%; P < 0·01). LAIV‐vaccinated women were also less likely to have a claim for vaccination with TIV (4% versus 12%; P < 0·01). Of those vaccinated with LAIV, 47%, 27%, and 26% were vaccinated in the first, second, and third trimesters, respectively. Among the 65 first‐trimester exposures, 42 occurred during the first 6 weeks of pregnancy. When trimester of vaccination was analyzed by influenza season, no trends were discernible among the small number of vaccinations occurring each season (Fig. 1). Women vaccinated in each trimester were comparable with similar mean age, number of high‐risk medical conditions, and number of prenatal visits.
Table 1.
Characteristics of LAIV‐vaccinated and LAIV‐unvaccinated cohorts
| LAIV vaccinated (n = 138) | LAIV non‐exposed (n = 834 861) | |
|---|---|---|
| n (%) | n (%) | |
| Maternal age at delivery, year | ||
| 14–19 | 18 (13)* | 45 447 (5) |
| 20–34 | 93 (67) | 629 545 (75) |
| 35–43 | 27 (20) | 159 869 (19) |
| Gestational age at birth, week | ||
| ≤36 | 7 (5) | 66 003 (8) |
| >36 | 131 (95) | 768 858 (92) |
| High‐risk medical condition† | ||
| Cardiac disease | 5 (4) | 19 172 (2) |
| Pulmonary disease | 10 (7) | 42 491 (5) |
| Diabetes mellitus | 7 (5) | 25 649 (3) |
| Renal disease | 0 (0) | 1096 (0) |
| Malignancy | 1 (1) | 4752 (1) |
| Immunosuppresive disorder | 0 (0) | 444 (0) |
| Any high‐risk condition | 21 (15) | 86 666 (10) |
| Claim for TIV during pregnancy | 6 (4)† | 96 600 (12) |
| Prenatal visit claims during pregnancy | ||
| 0 | 7 (5) | 48 041 (6) |
| 1–3 | 33 (24) | 225 294 (27) |
| 4–6 | 43 (31) | 291 776 (35) |
| 7–10 | 26 (19) | 164 468 (20) |
| >10 | 29 (21)‡ | 105 282 (13) |
| Trimester at vaccination | ||
| 1st | 65 (47) | NA |
| 2nd | 37 (27) | NA |
| 3rd | 36 (26) | NA |
LAIV, live attenuated influenza vaccine; NA, not applicable; TIV, trivalent inactivated influenza vaccine.
*Significantly different than LAIV non‐exposed, P < 0·0001.
†At least one hospital/emergency department claim or two outpatient claims anytime on or before delivery date.
‡Significantly different than LAIV non‐exposed, P < 0·01.
Figure 1.
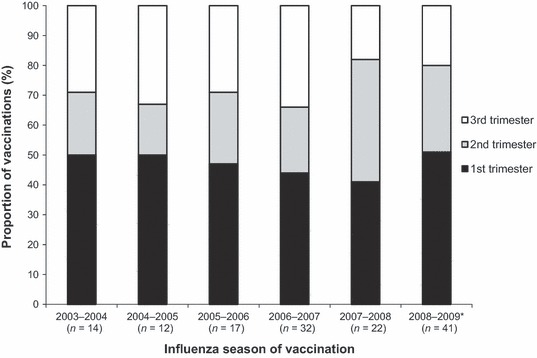
Trimester of vaccination with LAIV by influenza season (season defined as September–February). LAIV, live attenuated influenza vaccine. *Because the data analyzed included deliveries through September 2009, the 2008–2009 cohort may be missing some first‐trimester vaccinations (see Methods for full description).
In addition to pregnancy, 21 women had an underlying high‐risk medical condition, specifically cardiac disease, pulmonary disease, diabetes mellitus, or malignancy. Six patients vaccinated with LAIV also had a claim for TIV during pregnancy; in 5 of the 6 patients, the TIV claim occurred on the same date as the LAIV claim. All LAIV vaccinations occurred between September and February of each influenza season, with 87% occurring in October through December. The specialty of the vaccinating physician was only available in 26 of the 138 cases and therefore was not evaluated.
Eight unique individuals were found to have a claim for a hospitalization or ED visit within 42 days post‐vaccination with LAIV (three hospitalizations, five ED visits; Table 2). Two events were obstetric in nature (hyperemesis gravidarum and threatened premature labor), one event was cardiopulmonary in nature (bronchitis), and five events were because of other medical conditions (pyelonephritis, epigastric symptoms, limb pain, diarrhea, and chest pain). One woman was hospitalized twice for the same diagnosis (threatened premature labor without delivery) on days 9 and 12 post‐vaccination, which was counted as a single hospitalization. The three women with ED visits for limb pain, chest pain, and bronchitis, respectively, had pre‐existing diagnoses of degenerative thoracic scoliosis, diabetes mellitus, and asthma with congestive heart failure, respectively. Overall, 5·8% of women experienced a hospitalization or ED visit within 42 days post‐vaccination with LAIV; 1·4%, 0·7%, and 3·6% experienced an obstetric event, cardiopulmonary event, and other medical event, respectively. The rates of hospitalization and/or ED visits within 42 days post‐vaccination were higher among women vaccinated in the first and second trimesters (6·2% and 8·1%, respectively) than among women vaccinated in the third trimester (2·8%). Similarly, 14·3% of women with high‐risk underlying conditions experienced an event (three events among 21 women) compared with 4·3% of women without high‐risk conditions (five events among 117 women).
Table 2.
Primary diagnoses for emergency department visit or hospitalization within 42 days after LAIV vaccination
| Event | Events* Among LAIV‐Vaccinated Women, n | Proportion of LAIV‐vaccinated women with ED visit or hospitalization, %, (95% CI) | ||
|---|---|---|---|---|
| ED visit | Hospitalization | ED visit or hospitalization | ||
| Any complication | 5 | 3 | 8 | 5·8 (2·5–11·1) |
| Obstetrical complications | ||||
| Any | 0 | 2 | 2 | 1·4 (0·2–5·1) |
| 643·13 – Hyperemesis gravidarum with metabolic disturbance, antepartum | 0 | 1 | 1 | 0·7 (0·02–4·0) |
| 644·03 – Threatened premature labor, without delivery | 0 | 1 | 1 | 0·7 (0·02–4·0) |
| Cardiopulmonary conditions | ||||
| Any | 1 | 0 | 1 | 0·7 (0·02–4·0) |
| 466·0 – Acute bronchitis | 1 | 0 | 1 | 0·7 (0·02–4·0) |
| Other conditions | ||||
| Any | 4 | 1 | 5 | 3·6 (1·2–8·3) |
| 590·80 – Pyelonephritis, unspecified | 0 | 1 | 1 | 0·7 (0·02–4·0) |
| 789·06 – Epigastric symptoms involving abdomen and pelvis | 1 | 0 | 1 | 0·7 (0·02–4·0) |
| 729·5 – Pain in limb | 1 | 0 | 1 | 0·7 (0·02–4·0) |
| 787·91 – Diarrhea | 1 | 0 | 1 | 0·7 (0·02–4·0) |
| 786·59 – Chest pain; other | 1 | 0 | 1 | 0·7 (0·02–4·0) |
ED, emergency department; LAIV, live attenuated influenza vaccine.
*Excludes visits for delivery. Each woman could contribute only one ED visit and one hospitalization per ICD‐9 code.
Nineteen women were hospitalized for a delivery within 42 days post‐vaccination. Among these women, four maternal medical conditions that had not been previously diagnosed during pregnancy and that were unrelated to routine delivery were noted (polyhydramnios, anemia of pregnancy, premature rupture of membranes, and transient hypertension). Five additional hospitalizations that occurred more than 42 days post‐vaccination were noted before delivery (Table 3). Diagnoses included three obstetric conditions (cervical carcinoma in situ, other placental condition, and breech presentation) and two infections (pyelonephritis and influenza). The subject who was hospitalized for pyelonephritis had a history of recurrent urinary tract infections, before, during, and after pregnancy and was the same subject who was hospitalized for pyelonephritis within 42 days post‐vaccination. The subject hospitalized for influenza was vaccinated with LAIV in December 2008 and diagnosed with influenza in August 2009.
Table 3.
Listing of primary diagnoses associated with all hospitalizations occurring any time between LAIV vaccination and delivery*
| Diagnosis | Day of occurrence† | Length of stay, days |
|---|---|---|
| 0–42 days post‐vaccination | ||
| 590·80 – Pyelonephritis, unspecified | 6 | 3 |
| 643·13 – Hyperemesis gravidarum with metabolic disturbance, antepartum | 7 | 3 |
| 644·03 – Threatened premature labor, without delivery | 9,12‡ | 1 |
| ≥43 days post‐vaccination | ||
| 233·1 – Carcinoma in situ of cervix uteri | 51 | 1 |
| 590·1 – Acute pyelonephritis | 62§ | 5 |
| 656·73 – Other placental conditions affecting management of mother, antepartum | 126 | 4 |
| 652·23 – Breech presentation without version, antepartum | 184 | 1 |
| 487·1 – Influenza with other respiratory manifestations | 240 | 1 |
LAIV, live attenuated influenza vaccine.
*Excludes hospitalizations for delivery.
†Day of occurrence relative to LAIV vaccination (1 = day of vaccination).
‡Both hospitalizations for code 644·03 occurred in the same subject on separate days.
§Same subject hospitalized for diagnosis code 590·80 on day 6 post‐vaccination.
Discussion
This analysis of health insurance claims from a large U.S. population during the 2003–2004 through 2008–2009 influenza seasons demonstrates that LAIV vaccination during pregnancy is rare. The number of LAIV vaccinations during pregnancy increased throughout the study period, commensurate with the total number of LAIV doses distributed each season. When LAIV use in pregnancy does occur, it is more likely to occur in adolescents, which is not surprising given the extensive use of the vaccine in children and adolescents in the U.S. Additionally, LAIV use in pregnancy is most likely to occur very early in the pregnancy, when a pregnancy may be unrecognized. For second‐ and third‐trimester vaccinations, it is likely that the pregnancy was known and LAIV was administered owing to a lack of knowledge regarding the recommended use of the vaccine. Interestingly, LAIV vaccination in the second and third trimesters has not decreased in later years as LAIV use has become more widespread, which suggests that some groups of healthcare providers would benefit from increased education regarding the recommended use of LAIV.
Hospitalizations or ED visits were uncommon within 42 days of LAIV vaccination. All three hospitalizations that occurred were because of common causes of antepartum hospitalization: hyperemesis gravidarum, threatened premature labor, and pyelonephritis (all at a rate of 0·7%). Hyperemesis gravidarum has been reported to occur in 0·3% to 1·5% of all live births, with most references reporting an incidence of 0·5%. 14 Threatened premature labor is the most common non‐delivery cause of hospitalization among pregnant women, 15 with an incidence rate of first‐time hospitalization of 5·7% occurring in women from 24 weeks gestational age through delivery. 16 Antepartum hospitalization for pyelonephritis has been reported to occur in 1–2% of all pregnancies. 17 , 18 The five ED visits within 42 days post‐vaccination were because of common medical conditions, and three of the women had underlying illnesses that may have played contributing roles. The ED visit for bronchitis is notable given that LAIV is a live attenuated virus vaccine. It is not possible to determine whether there was a causal relationship between LAIV and the episode of bronchitis. Acute respiratory infections are common complications of pregnancy, and bronchitis has been found to occur in 1% of all pregnancies. 19 While LAIV can cause minor upper respiratory symptoms such as rhinorrhea or sore throat, clinical studies of LAIV in healthy adults, 20 , 21 children and adolescents with asthma, and older adults with chronic obstructive pulmonary disease have not shown an increase in medically attended respiratory events after vaccination. 22 , 23 For the women hospitalized for delivery within 42 days of vaccination, the four diagnoses that were potentially new (polyhydramnios, anemia of pregnancy, premature rupture of membranes and transient hypertension) were all common obstetric complications that can be noted for the first time during a hospitalization for delivery. 6 , 24
For the five hospitalizations occurring later than 42 days post‐vaccination, but before delivery, three were because of common obstetric conditions, 6 , 25 one was because of a reoccurrence of pyelonephritis, and one was because of influenza. For the influenza hospitalization, the dates of vaccination and hospitalization suggest that the woman was immunised with 2008–2009 trivalent seasonal LAIV in December 2008 and diagnosed in August 2009 with pandemic H1N1 influenza. The 2008–2009 seasonal formulation of LAIV would not be expected to provide protection against pandemic H1N1 illness. 26
This analysis has several limitations. Despite the very large sample size, the actual number of women in the study who were exposed to LAIV during pregnancy was small. The available sample size was sufficient, with 95% probability, to detect at least one event for outcomes occurring at a frequency of 2·2% or greater. Additionally, the precision of rate estimates for hospitalization and ED visits because of specific adverse events among these vaccinated women is limited. Consequently, rates of events occurring after vaccination with TIV were not generated for comparison. Furthermore, claims data may not include all diagnoses experienced by a patient, and the codes may lack sufficient specificity for some conditions. Also, because the date of conception was estimated in this study, some misclassification of vaccine exposure by pregnancy status likely occurred. Our study also assumes that there is accurate coding of medical conditions and procedures via specific coding systems. Coding errors could result in classifying another medication or vaccine as LAIV or classifying LAIV as another vaccine. Generally, the rare nature of such errors has minimal impact on quantifying the rate of common events. However, for rare events, such as LAIV vaccination among pregnant women, such errors can erroneously over‐ or underestimate the rate of the event of interest. Coding errors may explain the five subjects who had claims for TIV and LAIV on the same day. Additionally, as noted previously, the 2008–2009 cohort would not include first‐trimester vaccinations of women with deliveries after September 2009; however, these vaccinations would have been expected to occur in January through June, months in which LAIV vaccination was rare in the other influenza seasons evaluated. Consequently, the number of 2008–2009 vaccinations that were not captured is expected to be low.
This study is also limited by its lack of claims data regarding birth outcomes. The database we used was unable to link maternal claims data to the claims data describing their offspring. However, existing data from clinical study case reports and spontaneous reports to VAERS have not demonstrated any increased risk of adverse fetal outcomes with LAIV administration during pregnancy. In an analysis of 49 live births whose mothers were vaccinated with LAIV while pregnant, 36 infants were described as ‘healthy’, 11 had no additional details reported, one infant was delivered prematurely (32–33 weeks gestational age), and one infant was born with clinodactyly, 27 a relatively common hand anomaly characterized by curvature of the fifth finger toward the adjacent fourth finger. Also, no vaccine‐related fetal malformations or other evidence of teratogenesis were noted in animal developmental toxicity studies. 28 Despite the lack of data regarding outcomes after fetal exposure to LAIV, it is reassuring to note that there is no known fetal injury that has been linked to maternal exposure to wild‐type influenza, unlike wild‐type viruses for which other live vaccines exist (e.g., varicella and rubella). 29 , 30
Finally, we limited evaluated pregnancies to those resulting in the delivery of a child and did not assess spontaneous abortions. Spontaneous abortions may not result in a medical encounter, making the accuracy of the calculations based on claims data unreliable. However, in an analysis of 67 documented LAIV vaccinations during pregnancy from clinical trials and spontaneous VAERS reports, only eight (11·9%) cases resulted in spontaneous abortions, 27 a proportion that is similar to or below reported national rates of spontaneous abortions and very similar to that reported for TIV in VAERS reports from 1990 to 2009. 8 , 31 , 32
Despite the aforementioned limitations, this study examined a very large database, which provided a broad reflection of real‐world clinical practice within the U.S. The study’s unique contribution is the quantification of the frequency of LAIV vaccination during pregnancy and of medically attended adverse event frequencies, which cannot be estimated from spontaneous reporting systems such as VAERS. In this analysis, vaccination with LAIV during pregnancy was uncommon and did not reveal rates of maternal adverse events that differed from those known to occur in the general population of pregnant women. These data may offer some reassurance to providers and pregnant women in the event of inadvertent LAIV administration during pregnancy; however, these data do not support the routine use of LAIV in pregnant women.
Conflict of Interest
Drs Toback, Sifakis, and Ambrose are employees of MedImmune, LLC, and as such receive a salary from the company. Dr Tennis and Mr Calingaert are employees of RTI Health Solutions and provide customized research services as a condition of employment. Dr Beigi received no compensation for his work on this manuscript.
Acknowledgements
This research was funded by MedImmune, LLC, Gaithersburg, MD. As part of a consulting agreement with RTI Health Solutions, MedImmune provided funding to support protocol development, data collection, analysis, and manuscript development activities associated with this manuscript. Editorial assistance in formatting the manuscript for submission was provided by Sue Myers, MSc, and Gerard P. Johnson, PhD, of Complete Healthcare Communications, Inc. (Chadds Ford, PA) and was funded by MedImmune, LLC.
References
- 1. Fiore AE, Uyeki TM, Broder K et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1–62. [PubMed] [Google Scholar]
- 2. Lindsay L, Jackson LA, Savitz DA et al. Community influenza activity and risk of acute influenza‐like illness episodes among healthy unvaccinated pregnant and postpartum women. Am J Epidemiol 2006; 163:838–848. [DOI] [PubMed] [Google Scholar]
- 3. Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol 1998; 148:1094–1102. [DOI] [PubMed] [Google Scholar]
- 4. American College of Obstetricians and Gynecologists (ACOG) . Immunization updates for pregnant women in influenza season 2010 Available at: http://www.acog.org/acog_sections/dist_notice.cfm?recno=27&bulletin=2592 (Accessed 6 April 2011).
- 5. Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis 2008; 8:44–52. [DOI] [PubMed] [Google Scholar]
- 6. Munoz FM, Greisinger AJ, Wehmanen OA et al. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol 2005; 192:1098–1106. [DOI] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention . Guidelines for vaccinating pregnant women. 2010. Available at: http://www.cdc.gov/vaccines/pubs/preg‐guide.htm (Accessed 6 April 2011).
- 8. Moro PL, Broder K, Zheteyeva Y et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990–2009. Am J Obstet Gynecol 2011; 204:146.e1–146.e7. [DOI] [PubMed] [Google Scholar]
- 9. Piedra PA, Gaglani MJ, Riggs M et al. Live attenuated influenza vaccine, trivalent, is safe in healthy children 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age in a community‐based, nonrandomized, open‐label trial. Pediatrics 2005; 116:e397–e407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tran CH, McElrath J, Hughes P et al. Implementing a community‐supported school‐based influenza immunization program. Biosecur Bioterror 2010; 8:331–341. [DOI] [PubMed] [Google Scholar]
- 11. Martin JA, Hamilton BE, Sutton PD et al. Births: Final Data for 2006 National Vital Statistics Reports; vol 57 no 7. Hyattsville, MD, National Center for Health Statistics, 2009. [Google Scholar]
- 12. Cole JA, Modell JG, Haight BR, Cosmatos IS, Stoler JM, Walker AM. Bupropion in pregnancy and the prevalence of congenital malformations. Pharmacoepidemiol Drug Saf 2007; 16:474–484. [DOI] [PubMed] [Google Scholar]
- 13. Andrade SE, Raebel MA, Morse AN et al. Use of prescription medications with a potential for fetal harm among pregnant women. Pharmacoepidemiol Drug Saf 2006; 15:546–554. [DOI] [PubMed] [Google Scholar]
- 14. Verberg MF, Gillott DJ, Al‐Fardan N, Grudzinskas JG. Hyperemesis gravidarum, a literature review. Hum Reprod Update 2005; 11:527–539. [DOI] [PubMed] [Google Scholar]
- 15. Bennett TA, Kotelchuck M, Cox CE, Tucker MJ, Nadeau DA. Pregnancy‐associated hospitalizations in the United States in 1991 and 1992: a comprehensive view of maternal morbidity. Am J Obstet Gynecol 1998; 178:346–354. [DOI] [PubMed] [Google Scholar]
- 16. McPheeters ML, Miller WC, Hartmann KE et al. The epidemiology of threatened preterm labor: a prospective cohort study. Am J Obstet Gynecol 2005; 192:1325–1329; discussion 1329–1330. [DOI] [PubMed] [Google Scholar]
- 17. Jolley JA, Wing DA. Pyelonephritis in pregnancy: an update on treatment options for optimal outcomes. Drugs 2010; 70:1643–1655. [DOI] [PubMed] [Google Scholar]
- 18. Millar LK, Cox SM. Urinary tract infections complicating pregnancy. Infect Dis Clin North Am 1997; 11:13–26. [DOI] [PubMed] [Google Scholar]
- 19. Banhidy F, Acs N, Puho EH, Czeizel AE. Maternal acute respiratory infectious diseases during pregnancy and birth outcomes. Eur J Epidemiol 2008; 23:29–35. [DOI] [PubMed] [Google Scholar]
- 20. Nichol KL, Mendelman PM, Mallon KP et al. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA 1999; 282:137–144. [DOI] [PubMed] [Google Scholar]
- 21. Treanor JJ, Kotloff K, Betts RF et al. Evaluation of trivalent, live, cold‐adapted (CAIV‐T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild‐type influenza A (H1N1), A (H3N2), and B viruses. Vaccine 1999; 18:899–906. [DOI] [PubMed] [Google Scholar]
- 22. Fleming DM, Crovari P, Wahn U et al. Comparison of the efficacy and safety of live attenuated cold‐adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J 2006; 25:860–869. [DOI] [PubMed] [Google Scholar]
- 23. Gorse GJ, O’Connor TZ, Young SL et al. Impact of a winter respiratory virus season on patients with COPD and association with influenza vaccination. Chest 2006; 130:1109–1116. [DOI] [PubMed] [Google Scholar]
- 24. Cardwell MS. Polyhydramnios: a review. Obstet Gynecol Surv 1987; 42:612–617. [DOI] [PubMed] [Google Scholar]
- 25. Giuntoli R, Yeh IT, Bhuett N, Chu W, Van Leewen K, Van der Lans P. Conservative management of cervical intraepithelial neoplasia during pregnancy. Gynecol Oncol 1991; 42:68–73. [DOI] [PubMed] [Google Scholar]
- 26. Hancock K, Veguilla V, Lu X et al. Cross‐reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009; 361:1945–1952. [DOI] [PubMed] [Google Scholar]
- 27. Toback SL, Falloon J, Ambrose CS. Case reports on maternal and fetal outcomes after exposure to LAIV during pregnancy. National Immunization Conference; 2010 April 19–22; Atlanta, GA.
- 28. FluMist® (Influenza Virus Vaccine Live, Intranasal, Full prescribing information) . Full Prescribing Information. Gaithersburg, MD: MedImmune, 2010. [Google Scholar]
- 29. Watson JC, Hadler SC, Dykewicz CA, Reef S, Phillips L. Measles, mumps, and rubella – vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1998; 47:1–57. [PubMed] [Google Scholar]
- 30. Marin M, Guris D, Chaves SS, Schmid S, Seward JF. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56:1–40. [PubMed] [Google Scholar]
- 31. Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. BMJ 1997; 315:32–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilcox AJ, Weinberg CR, O’Connor JF et al. Incidence of early loss of pregnancy. N Engl J Med 1988; 319:189–194. [DOI] [PubMed] [Google Scholar]


