Abstract
Background
Ovarian cancer patients have a high risk of developing venous thrombosis. The membrane lipid bilayer of platelets and platelet-derived microparticles (PMP) provides a platform for assembly of coagulation proteins and generation of blood clots.
Methods
We compared the lipid composition of platelets and PMPs in patients with ovarian cancer to those in healthy subjects. We used shotgun lipidomics to quantify 12 classes and 177 species of lipids.
Results
We found a significant change in 2 classes of lipids in platelets and PMPs isolated from ovarian cancer patients: higher phosphatidylinositol and lower lyso-phosphatidylcholine. The level of 28 species of lipids was also significantly altered in the direction of an increase in the pro-coagulant and a reduction in the anticoagulant lipids. We found that cancer platelets expressed less lipid phosphate phosphatase 1 (LPP1), a key enzyme in phospholipid biosynthesis pathways, than normal platelets. The reduction in LPP1 might contribute to the changes in the lipid profile of cancer platelets.
Conclusion
Our results support a procoagulant lipid profile of platelets in ovarian cancer patients that can play a role in the increased risk of venous thrombosis in these patients.
General significance
As far as we are aware, our study is the first study on platelet lipidomics in ovarian cancer. The importance of our findings for the future studies are: 1) a similar change in lipid profile of platelets and PMP may be responsible for hypercoagulability in other cancers, and 2) plasma level of high-risk lipids for venous thrombosis may be useful biomarkers.
Keywords: Platelet, Lipidomics, Venous thrombosis, Hypercoagulability, Ovarian cancer, Lipid phosphate phosphatase 1
Highlights
-
•
Lipid composition of platelet and PMP is altered in ovarian cancer.
-
•
The change in lipid composition of platelet and PMP is in a procoagulant direction.
-
•
LPP1 enzyme is reduced in cancer platelets.
1. Introduction
Hypercoagulability, an activated state of coagulation that can be detected in a majority of cancer patients [6], [17], [28], can remain as a subclinical condition or results in thrombosis. Venous thromboembolism (VTE) is a challenging complication of malignancy and the second leading cause of death in cancer patients. Several studies have identified a variety of risk factors for VTE [9], however, the exact molecular mechanisms for the cancer-associated hypercoagulable state and venous thrombosis remain unknown.
Coagulation is the final result of a close interaction between coagulation cascade and platelet membrane. Membrane lipids of activated platelets, particularly phosphatidylserine (PS), mediate binding and assembly of coagulation factors, which in turn promote generation of thrombin and fibrin. Other lipids in the platelet membrane interact with PS, and have regulatory roles as either procoagulant or anticoagulant lipids [24]. Phosphatidylinositol (PI), phosphatidic acid (PA), and phosphatidylglycerol (PG) were demonstrated to be procoagulants; while phosphatidylcholine (PC), acyl-carnitines (CAR) [4], and sphingomyelins (SM) [12] are anticoagulants. The effect of the other lipid classes on coagulation is not clear. The role of ceramides (CER) in platelet function is uncertain [16]. Cardiolipins (CL) are almost exclusively present in mitochondria [5]. The net effect of phosphatidylethanolamines (PE) on coagulation is hard to assess because initially PE synergizes with PS to promote coagulation, but later inactivates FVa [19], [20]. Lyso-PE (LPE) have not been reported to be involved in coagulation. There are controversial reports on the role of LPCs in coagulation [25].
Each of these lipid classes includes several lipid species. Lipid species within a lipid class have similar structural features and usually similar functional roles in coagulation [24], [25]. Activated platelets also release small vesicles encircled by a lipid bilayer, known as platelet-derived microparticles (PMP), that are highly procoagulant and can initiate and propagate coagulation [22]. In cancer patients, the crosstalk between platelets and cancer cells results in platelet activation and in a repertoire of molecular changes in platelets and cancer cells [2]. We speculated that cancer-related changes in platelet composition of molecules, and consequently in PMPs, might contribute to a hypercoagulable state and increased risk of thrombosis in cancer patients. We compared the lipid content of platelets and PMPs in patients with ovarian cancer to that in healthy controls. We identified alterations in lipid content of platelets and PMPs in cancer patients at both lipid class and species levels, with a majority of changes in the direction of promoting coagulation. Furthermore, we found that microparticle generation by cancer patients' and healthy subjects' platelets result in a similar pattern of enrichment of the lipids content in PMPs.
2. Materials and methods
All of the studies were approved by the Institutional Review Board of The University of Texas M. D. Anderson Cancer Center and in accordance with an assurance approved by the US Department of Health and Human Services.
2.1. Reagents
Chloroform, methanol, and isopropanol were purchased from Burdick and Jackson (Muskegon, MI). Lithium chloride and lithium hydroxide were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO). All of the lipid internal standards were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL) unless otherwise indicated. 1,2-Dimyristoleoyl-sn-glycero-3-phosphocholine (di14:1 PC); 1,2-Dipalmitoleoyl-sn-glycero-3-phosphoethanolamine (di16:1 PE); 1,2-Dipentadecanoyl-sn-glycero-3-phosphoglycerol (sodium salt) (di15:0 PG); 1,2-Dimyristoyl-sn-glycero-3-phospho-l-serine (sodium salt) (di14:0 PS); 1,2-Dimyristoyl-sn-glycero-3-phosphate (sodium salt) (di14:0 PA); 1,1′,2,2′-Tetramyristoyl cardiolipin (T14:0 CL); N-Lauroyl sphingomyelin (N12:0 SM); N-Heptadecanoyl ceramide (N17:0 Cer); 1-Heptadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine (17:0 lysoPC); 1,2,3,4-13C4-Palmitoyl-l-carnitine hydrochloride (13C4-16:0 CN) (Sigma-Aldrich, St. Louis, MO).
2.2. Preparation of platelets and microparticles
Washed platelets were prepared from 3 patients with ovarian cancer and 3 gender and age-matched healthy subjects. Blood samples from cancer patient were obtained prior to any surgical intervention or chemotherapy. Patients with ovarian cancer did not have any detectable metastasis at the time of blood draw, and had normal blood counts and normal routine coagulation parameters. Platelets were prepared from 20 mL of fresh whole blood using a previously described method [21]. PMPs were prepared by activating the washed platelets with a combination of thrombin (0.1 unit/mL) and collagen (50 μg/mL) at 37 °C for 30 min, as described before [3], [18]. Washed platelets and PMPs were pelleted and stored at − 80 °C for lipidomics.
2.3. Shotgun lipidomics
Both platelets and PMPs were resuspended in 300 μL PBS and homogenized for 1 min using a disposable soft tissue homogenizer. An aliquot of 25 μL was pipetted to determine the protein content (BCA protein assay kit, Thermo Scientific, Rockford, IL). The rest of homogenate was transferred into a disposable glass culture test tube. For quantification of all reported lipid species, a mixture of lipid internal standards was added prior to lipid extraction. Lipid extraction was performed by a modified Bligh and Dyer procedure [29]. Each lipid extract was resuspended into a volume of 500 μL of chloroform/methanol (1:1, v/v) per mg of protein and flushed with nitrogen, capped, and stored at − 20 °C for lipid analysis. For direct infusion electrospray ionization (ESI) analysis, lipid extract was further diluted to a final concentration of ~ 500 fmol/μL, and the mass spectrometric analysis was performed on a QqQ mass spectrometer (Thermo TSQ VANTAGE, San Jose, CA) equipped with an automated nanospray device (TriVersa NanoMate, Advion Bioscience Ltd., Ithaca, NY).
2.4. Western blot analysis
Washed platelets were lyzed in a buffer consisting of 1% Triton X-100, 50 mM HEPES, pH 7.4, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 100 mM NaF, 10 mM Na pyrophosphate, 1 mM Na3VO4, 10% glycerol, and freshly added protease and phosphatase inhibitors from Roche Applied Science (Cat. # 05056489001 and 04906837001, respectively). Protein concentrations of the lysates were determined by a BCA Protein Reagent Kit (Pierce Biotech.), and 25 μg of proteins were subjected to gel electrophoresis on 10% SDS-PAGE gels. Antibodies used were against LPP1 (Abcam, ab198280, at 1:250 dilution), and β-ACTIN (Sigma-Aldrich, A5316, 1:5000).
2.5. Statistical analysis
All lipid class or species data were expressed as means ± SE for triplicate measurements. Comparisons between groups were made using the Student's t-test with p < 0.1 being considered statistically significant. For comparable analysis between platelets and PMPs, the measured value for a lipid class or a species was converted to parts per thousand (ppt), or equivalent to nmol per a total of 1000 nmol lipids. Measured values for lipid classes in platelet and PMP samples were subjected to multivariate analysis in the form of unsupervised principal component analysis (PCA), using Partek Genomics Suite 6.6 (Partek, St. Louis, MO). PCA is used to visualize the interrelationship between large numbers of measured (observed) variables and describes the largest variation in data using principal components to plot against each other so that trends and groupings can be detected [10].
3. Results
3.1. Lipid content of platelets/PMPs in ovarian cancer patients and normal subjects
A total of 12 classes and 177 species of lipids were analyzed by shotgun lipidomics. The total amount of lipid content of platelets and PMPs was not different between cancer patients and normal subjects (Fig. S1A). The total amount of lipid content in PMPs was less than in platelets, which is expected given the size difference between platelets and PMPs.
The 2 main lipid classes in both platelets and PMPs were phosphatidylethanolamine (PE), including plasmalogen PE (PE-pl), and phosphatidylcholine (PC) (Fig. 1), similar to the reported lipid composition of cell membrane in eukaryotes [26].
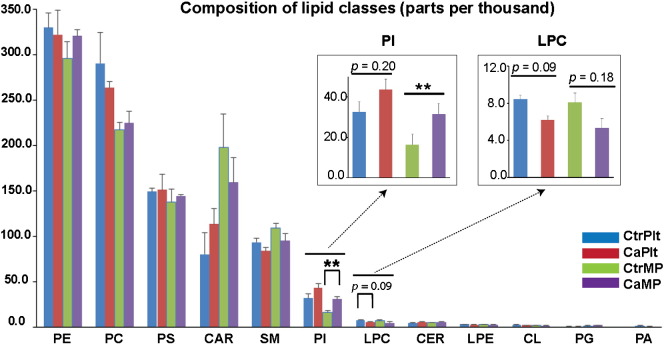
Fig. 1.
Lipid class distribution in platelets and PMPs and comparison between the cancer and control groups. Phosphatidylinositol (PI) class content in PMPs from cancer samples was ~ 2 fold higher than that from the controls with a significance level p = 0.01. Lyso-phosphatidylcholine (LPC) class content in platelets from cancer samples was reduced by ~ 26% at a significance level of 0.09. ** indicates p ≤ 0.01. PE: phosphatidylethanolamine; PC: phosphatidylcholine; PS: phosphatidylserine; CAR: acyl-carnitine; CER: ceramide; LPE: lyso-phosphatidylethanolamine; CL: cardiolipin; PG: phosphatidylglycerol; PA: phosphatidic acid. CtrPlt: control platelets; CaPlt: cancer platelets; CtrPMP: control PMPs; CaPMP: cancer PMPs.
Lipid profiles (classes and species) of platelets and PMPs were compared between cancer and control groups. Phosphatidylinositol (PI) class in PMPs was significantly higher (~ 2-fold) in cancer patients than in controls, and a similar trend was observed in platelets although statistically non-significant (p = 0.20). Lyso-phosphatidylcholine (LPC) class was ~ 26% lower in cancer platelets compared to controls (p = 0.09), and a similar trend was observed in PMPs (p = 0.18) (Fig. 1). In cancer platelets, a trend toward reduction in PC, a precursor of LPC, was observed.
Changes in lipid species in platelets or PMPs of ovarian cancer patients as compared to healthy subjects were compiled in Table 1. To conduct a meaningful comparison of lipid species, we focused on lipid species in 7 lipid classes with a known regulatory role in coagulation (PS, PI, PA, PG, PC, CAR, and SM), and eliminated the other 5 lipid classes (ceramides, cardiolipins, PE, lyso-PE, Lyso-PC) from our analysis. In the procoagulant PI class, 9 of 11 lipid species increased in cancer platelets. Of note, the predominant PI species 18:0–20:4 doubled in PMPs of cancer patients (Fig. S1B and Table 1). The procoagulant change in a lipid species is highlighted in red and the anticoagulant change in green. The procoagulant changes involved ~ 20% of total species (24 out of 115 species in the 7 lipid classes) (Table 1). Considering different abundance of lipid species, the procoagulant changes involved 28% of lipid molecules (179.95 nmol out of 649.27 nmol of the 7 classes of lipids). The anticoagulant changes involved a residual 0.6% of lipids. The total pro-coagulant/anti-coagulant lipid ratio was calculated, and found for cancer platelets to be 0.425 ± 0.401 and for normal platelets 0.395 ± 0.028; the same ratio for cancer and normal PMP was 0.377 ± 0.012, and 0.307 ± 0.049, respectively.
Table 1.
List of lipid class and species with compositional changes in platelets and/or MPs between cancer patients and healthy controls.
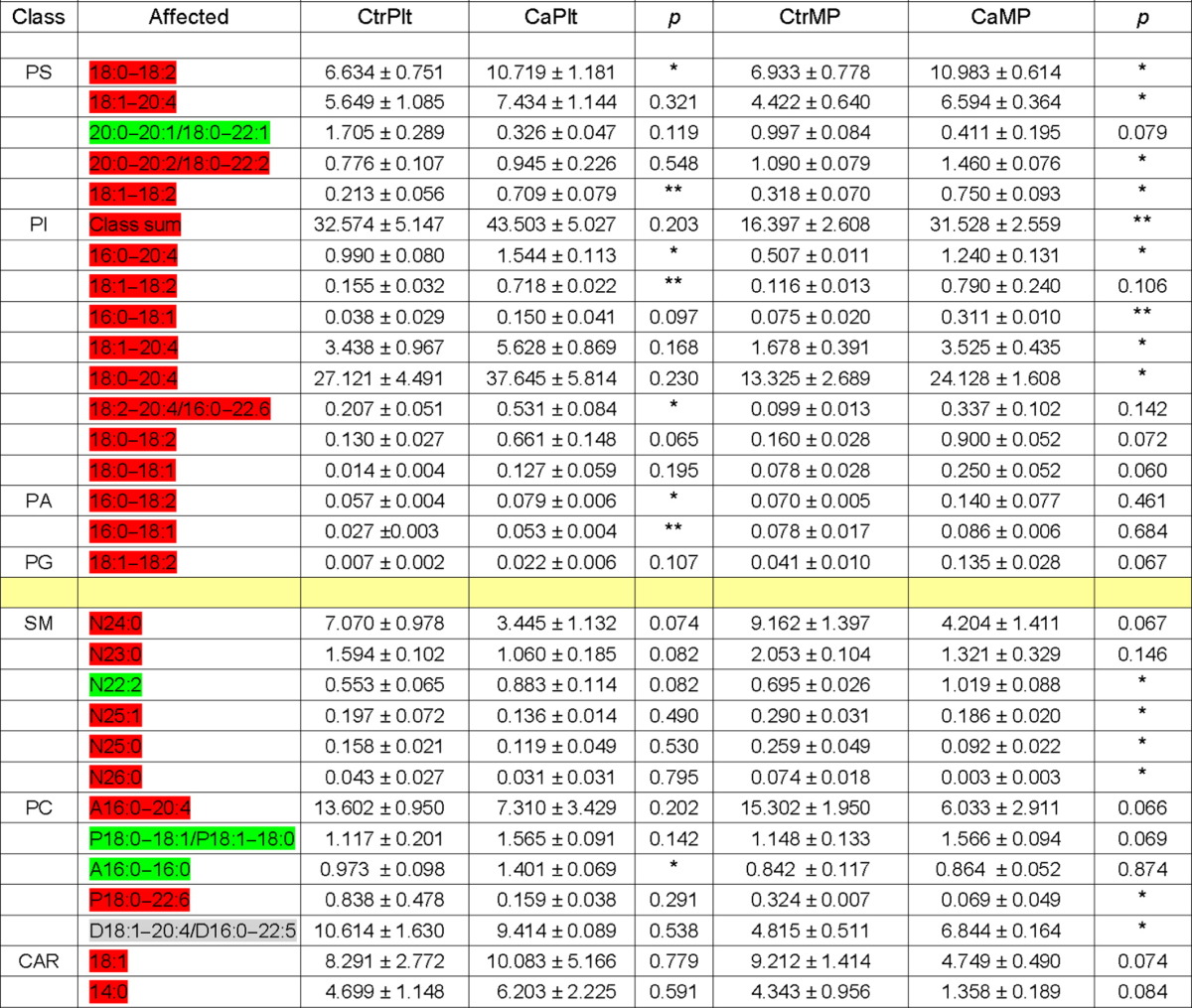
Two out of 5 CAR species, CAR 18:1 and CAR 14:0, were reduced in PMPs of cancer patients by 1.9-fold (p = 0.07) and 3.2-fold (p = 0.08), respectively. The other 3 CAR species showed downward trend that was not statistically significant; e.g., CAR 20:4 was reduced by 1.9 folds (p = 0.14). Interestingly, in a previous study on venous thromboembolism (VTE) within general population, several species of CAR were identified to have lower plasma levels in VTE patients as compared to control subjects. Further mechanistic study demonstrates that CAR acts as anticoagulants and inhibits Factor Xa-induced clotting [4].
3.2. Lipid enrichment/depletion in PMPs of control subjects and cancer patients
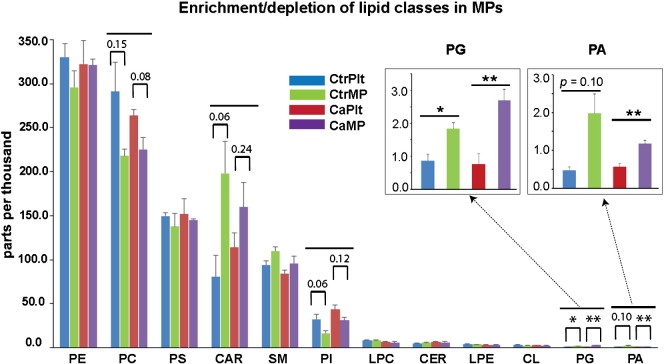
We compared lipid content of PMPs to those of intact platelets in patients with ovarian cancer and in healthy subjects. The enriched or depleted lipid classes or species with p ≤ 0.10 are presented in Table S1. We found similar pattern but different magnitude of enrichment or depletion of lipids in PMPs from cancer patients and control subjects. Three out of 12 lipid classes (CAR, PG, PA) were enriched, and 2 of 12 (PC, PI) were depleted in PMPs (Fig. 2). Additionally, 6 lipid species from PE, PS, and Lyso-PC classes were either enriched or depleted in PMPs. There was no changes in the lipid species of SM, CER, PE, CL classes in PMPs (Table S1). Although PMPs from both cancer patients and controls showed similar and consistent trend but the magnitude of enrichment or depletion of lipid species was different between them. For example, procoagulant PG class was significantly enriched in PMPs from control and cancer groups by 2.2-fold and 3.6-fold, respectively. Anticoagulant CAR class was enriched in PMPs of control subjects by 2.5 folds (p = 0.06), whereas in cancer patients CAR increased by 1.4-fold (p = 0.24). The procoagulant class PI depleted in PMPs of both groups. PMPs in control subjects had a 2-fold decrease (p = 0.06) in PI, and in cancer patients had a 1.4-fold decrease (p = 0.12). Overall, in cancer patients the procoagulant changes in lipid content of PMPs were more pronounced, and the anticoagulant changes were smaller in magnitude as compared to control subjects.
Fig. 2.
Enrichment or depletion of lipid classes in PMPs. Comparison was conducted between PMPs and platelets for both control and cancer samples and 5 classes of lipids displayed enrichment or depletion in MPs of either or both origins. The numbers on top of the cluster bars are p values for enrichment or depletion. * indicates p ≤ 0.05 and ** indicates p ≤ 0.01.
3.3. Distinct lipid profiles of platelets and PMPs in cancer patients and controls
We used principal component analysis (PCA) to summarize and visualize the large number of data generated by comparing lipid classes between platelets and PMPs of cancer patients and controls [10]. As a result, data generated from 6 subjects can be categorized into 4 distinct groups of cancer platelets, control platelets, cancer PMPs, and controls PMPs; with clear proximity between same type of samples from different individual, and little overlap between different groups (Fig. 3.). The first principal component (PC1) accounted for 34.4% of the total variance in the data and the second principal component explained ~ 22.1% of the total. PCA results showed that the differences in lipid profiles between different groups are recurrent and not due to random differences.
Fig. 3.
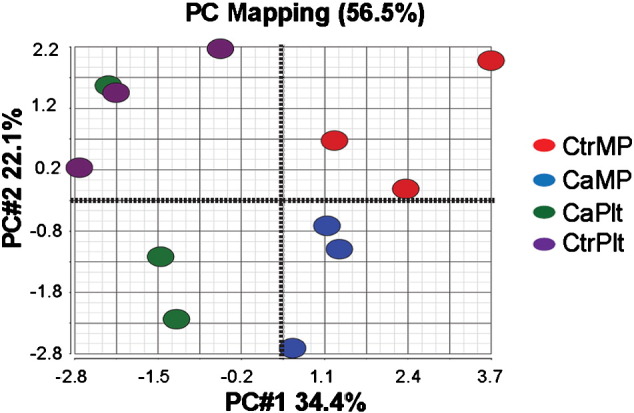
PCA score scatter plot indicated distinct categories for the 4 analyzed samples CaPlt, CaPMP, CtrPlt, and CtrPMP. PC#1: first principal component; PC#2: second principal component.
3.4. Lipid phosphate phosphatase 1 (LPP1) in platelets of control subjects and cancer patients
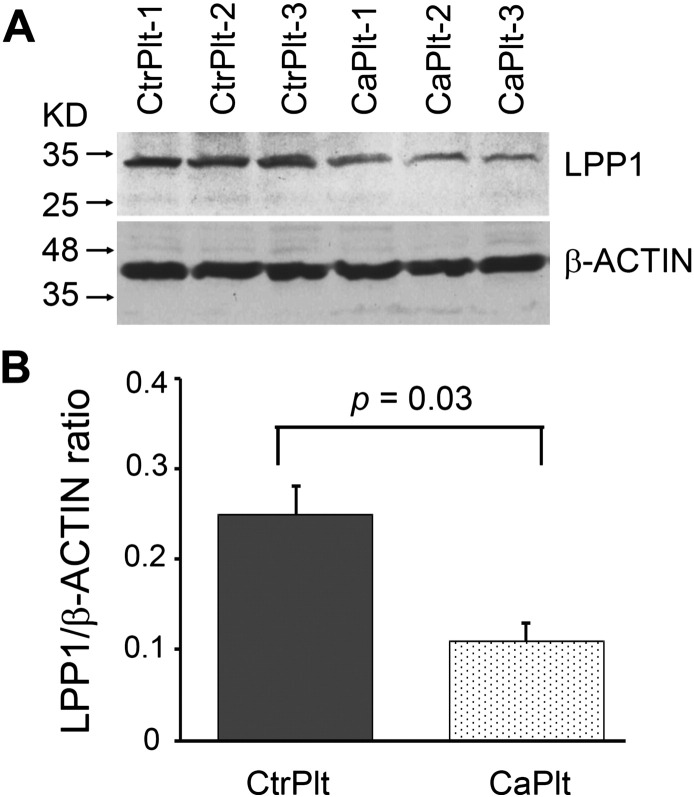
We compared expression of LPP1, a key enzyme in the phospholipid metabolism, in platelets of ovarian cancer patients to that of healthy subjects by Western-blotting. Relative LLP1 protein level was measured as the ratio of intensity of band representing LPP1 to β-ACTIN in platelet lysates of 3 patients with ovarian cancer and 3 control subjects. Platelets from cancer patients showed > 50% reduction in the level of LPP1 as compared to control platelets (0.25 vs 0.11, p = 0.03, n = 3 in each group) (Fig. 4A and B).
Fig. 4.
Platelets of cancer patients expressed less than half of the LPP1 protein of control platelets. (A) Western blot analysis of LPP1 protein expression level in platelets of both control subjects and cancer patients. (B) Quantification of relative protein expression level and comparison between the control and cancer groups. Data presented as mean ± sem.
4. Discussion
In this study, we have investigated the effect of ovarian cancer on lipid profile of platelets, and the effect of platelet microvesiculation on the lipid profile of PMPs in cancer patients and control subjects. We identified recurrent and persistent changes in lipid composition of cancer platelets and PMPs. Platelets of cancer patients have a higher amount of PI class of lipids, lower Lyso-PC, and a similar amount of phosphatidic acid (PA). In phospholipid metabolism phosphatidic acid (PA), although comprises only a minute amount of the total amount of cellular lipid content, plays an important role as the initial substrate in phospholipids biosynthesis. Lipid phosphate phosphatase 1 (LPP1) removes a phosphate group from PA to generate diacylglycerol that can be used for synthesis of phosphatidylcholine (PC). The alternative route for PA is to be converted to CDP-diacylglycerol to be used for generation of PI. LPP1 is decreased in the majority of ovarian cancers [23]. We speculated that a reduction in LPP1 in ovarian cancer platelets divert limited available PA in platelets away from PC synthesis and toward PI generation. In fact, our data demonstrated that LPP1 is reduced by ~ 56% in cancer platelets. This significant reduction in LPP1 might explain the increase in PI in cancer platelets. Lyso-PC is the product of hydrolysis of PC and a reduction in Lyso-PC in cancer platelets, as a part of a trend of PC reduction, was also detected in our study.
In addition to the two major classes of lipid, we identified changes in 28 lipid species (Table 1). Considering the importance of platelets and PMPs lipid bilayer in assembly of coagulation factors and increased risk of venous thrombosis in ovarian cancer patients, we investigated the changes in lipid species within lipid classes with a known regulatory role in coagulation (PS, PI, PA, PG, PC, CAR, and SM). The other lipid classes were either strictly mitochondrial and were physically distant from coagulation factors or did not have a well-established effect on coagulation. We identified that the overwhelming majority of changes in lipid species in cancer platelets were toward an increase in procoagulant lipids and a reduction in anticoagulant lipids. Although the trend toward more procoagulant and less anticoagulant lipids in cancer platelets is important, but perhaps the change in one key lipid moiety rather than a small amount of change in the total sum of several procoagulant or anticoagulant lipids can have more clinical impact.
Activation of platelets by various platelet agonists or calcium ionophore results in generation of microparticles from platelet surface. These PMPs are encircled by lipid bilayer and are highly procoagulant. We compared the lipid content of PMPs with the original platelets and identified enrichment and depletion of several procoagulant and anticoagulant lipid classes and species. Although the procoagulant changes in lipid profile of PMPs from ovarian cancer patients were more pronounced than those in normal PMPs, we did not detect an overwhelming dominant change in procoagulant or anticoagulant lipids. We speculate that the increase in the procoagulant activity of PMPs might be due the change in distribution of lipid classes rather than the quantity of lipids, i.e. distribution of procoagulant lipids to the outer leaflet of lipid bilayer. Only few studies have investigated the lipid content of platelets and PMPs in healthy human subjects [15], [16]. As far as we are aware, our study is the first one comparing lipid profile of platelets in cancer patients and normal subjects. Our results demonstrated that the procoagulant lipids were increased and anticoagulant lipids decreased in platelets and/or PMPs of cancer patients. This might contribute to a hypercoagulable state in cancer patients. We recently reported that platelet of patients with ovarian cancer are not hyperreactive and have similar aggregation response to platelet agonists as platelets of healthy subjects [7]. This further support the possibility that contribution of cancer platelets to a hypercoagulable state might be related to an aggregation-independent effect of platelets in hemostasis via increasing coagulation.
Platelets and PMPs play important role in tumor progression [8], [27]. Alteration in the lipid content of platelets, and as a result in the lipid content of PMPs, may contribute to the progression of ovarian cancer via an interaction between platelets and cancer cells. Both platelets and PMPs can fuse to cell membrane and be internalized by cancer cells [1], [13], [14]. In addition to their cytoplasmic content, PMPs can also transfer their lipid contents to cancer cells. Elevated concentration of PI in PMPs can provide a source for synthesis of phosphoinositides and PI3K signaling molecules in cancer cells, and decrease the relative concentration of Lyso-PC and PC in these cells. Both Lyso-PC and PC have been demonstrated to play antitumor roles in lung cancer and pancreatic cancer murine models, respectively [11].
Crosstalk between platelets and cancer cells can change their compositions. It has been shown that tumor cells alter platelet mRNA profile [2]. Reduction in LPP1 in ovarian cancer cells may result in reduction in LLP1 and altering phospholipid composition in platelets. Fusion of PMP and internalization of PMP lipid content into ovarian cancer cells, in turn, might change the lipid profile of cancer cells.
Our study is the first study to correlate the presence of cancer with specific changes in the lipid composition of platelets. We identified potential links between alteration in lipids in platelets and PMPs and an increased risk of thrombosis or a progrowth effect of platelets on cancer. These links need to be validated in larger studies and can provide preliminary information for development of new biomarkers, such as a low concentration of CAR in plasma PMPs, as predictive of development of venous thrombosis in cancer patients.
Authors' contribution
Q.H. designed and performed experiments and analyzed and interpreted data; M.W. M.S.C., and C.W. performed experiments and analyzed data; A.M.N., P.T., and F.M.A. interpreted data; X.H. and A.K.S. designed experiments and interpreted data; and V.A-K. designed experiments, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure
The authors declare no competing financial interests.
Transparency document
Transparency document
Acknowledgements
This work was supported in part by R01CA177909 (to V.A-K. and A.K.S.), Ovarian Cancer Research Fund (Grant number 258813 to V.A-K. and A.K.S).
Footnotes
The Transparency document associated with this article can be found, in online version.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bbacli.2016.06.003.
Appendix A. Supplementary data
Supplementary material
References
- 1.Al-Nedawi K., Meehan B., Micallef J., Lhotak V., May L., Guha A., Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 2.Best M.G., Sol N., Kooi I., Tannous J., Westerman B.A., Rustenburg F., Schellen P., Verschueren H., Post E., Koster J., Ylstra B., Ameziane N., Dorsman J., Smit E.F., Verheul H.M., Noske D.P., Reijneveld J.C., Nilsson R.J., Tannous B.A., Wesseling P., Wurdinger T. RNA-Seq of tumor-educated platelets enables blood-based pan-cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell. 2015;28:666–676. doi: 10.1016/j.ccell.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasgupta S.K., Abdel-Monem H., Niravath P., Le A., Bellera R.V., Langlois K., Nagata S., Rumbaut R.E., Thiagarajan P. Lactadherin and clearance of platelet-derived microvesicles. Blood. 2009;113:1332–1339. doi: 10.1182/blood-2008-07-167148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deguchi H., Banerjee Y., Trauger S., Siuzdak G., Kalisiak E., Fernandez J.A., Hoang L., Tran M., Yegneswaran S., Elias D.J., Griffin J.H. Acylcarnitines are anticoagulants that inhibit factor Xa and are reduced in venous thrombosis, based on metabolomics data. Blood. 2015;126:1595–1600. doi: 10.1182/blood-2015-03-636761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deguchi H., Fernandez J.A., Hackeng T.M., Banka C.L., Griffin J.H. Cardiolipin is a normal component of human plasma lipoproteins. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1743–1748. doi: 10.1073/pnas.97.4.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards R.L., Rickles F.R., Cronlund M. Abnormalities of blood coagulation in patients with cancer. Mononuclear cell tissue factor generation. J. Lab. Clin. Med. 1981;98:917–928. [PubMed] [Google Scholar]
- 7.Feng S., Kroll M.H., Nick A.M., Sood A.K., Afshar-Kharghan V. Platelets are not hyperreactive in patients with ovarian cancer. Platelets. 2016:1–3. doi: 10.3109/09537104.2016.1174204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco A.T., Corken A., Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126:582–588. doi: 10.1182/blood-2014-08-531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hisada Y., Geddings J.E., Ay C., Mackman N. Venous thrombosis and cancer: from mouse models to clinical trials. J. Thromb. Haemost. 2015;13:1372–1382. doi: 10.1111/jth.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu C., Wang Y., Fan Y., Li H., Wang C., Zhang J., Zhang S., Han X., Wen C. Lipidomics revealed idiopathic pulmonary fibrosis-induced hepatic lipid disorders corrected with treatment of baicalin in a murine model. AAPS J. 2015;17:711–722. doi: 10.1208/s12248-014-9714-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jantscheff P., Schlesinger M., Fritzsche J., Taylor L.A., Graeser R., Kirfel G., Furst D.O., Massing U., Bendas G. Lysophosphatidylcholine pretreatment reduces VLA-4 and P-Selectin-mediated b16.f10 melanoma cell adhesion in vitro and inhibits metastasis-like lung invasion in vivo. Mol. Cancer Ther. 2011;10:186–197. doi: 10.1158/1535-7163.MCT-10-0474. [DOI] [PubMed] [Google Scholar]
- 12.Kasahara K., Kaneda M., Miki T., Iida K., Sekino-Suzuki N., Kawashima I., Suzuki H., Shimonaka M., Arai M., Ohno-Iwashita Y., Kojima S., Abe M., Kobayashi T., Okazaki T., Souri M., Ichinose A., Yamamoto N. Clot retraction is mediated by factor XIII-dependent fibrin-alphaIIbbeta3-myosin axis in platelet sphingomyelin-rich membrane rafts. Blood. 2013;122:3340–3348. doi: 10.1182/blood-2013-04-491290. [DOI] [PubMed] [Google Scholar]
- 13.Kirschbaum M., Karimian G., Adelmeijer J., Giepmans B.N., Porte R.J., Lisman T. Horizontal RNA transfer mediates platelet-induced hepatocyte proliferation. Blood. 2015;126:798–806. doi: 10.1182/blood-2014-09-600312. [DOI] [PubMed] [Google Scholar]
- 14.Liang H., Yan X., Pan Y., Wang Y., Wang N., Li L., Liu Y., Chen X., Zhang C.Y., Gu H., Zen K. MicroRNA-223 delivered by platelet-derived microvesicles promotes lung cancer cell invasion via targeting tumor suppressor EPB41L3. Mol. Cancer. 2015;14:58. doi: 10.1186/s12943-015-0327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Losito I., Conte E., Cataldi T.R., Cioffi N., Megli F.M., Palmisano F. The phospholipidomic signatures of human blood microparticles, platelets and platelet-derived microparticles: a comparative HILIC-ESI-MS investigation. Lipids. 2015;50:71–84. doi: 10.1007/s11745-014-3975-7. [DOI] [PubMed] [Google Scholar]
- 16.O'Donnell V.B., Murphy R.C., Watson S.P. Platelet lipidomics: modern day perspective on lipid discovery and characterization in platelets. Circ. Res. 2014;114:1185–1203. doi: 10.1161/CIRCRESAHA.114.301597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schafer A.I., Adelman B. Plasmin inhibition of platelet function and of arachidonic acid metabolism. J. Clin. Invest. 1985;75:456–461. doi: 10.1172/JCI111720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinauridze E.I., Kireev D.A., Popenko N.Y., Pichugin A.V., Panteleev M.A., Krymskaya O.V., Ataullakhanov F.I. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb. Haemost. 2007;97:425–434. [PubMed] [Google Scholar]
- 19.Smirnov M.D., Esmon C.T. Phosphatidylethanolamine incorporation into vesicles selectively enhances factor Va inactivation by activated protein C. J. Biol. Chem. 1994;269:816–819. [PubMed] [Google Scholar]
- 20.Smirnov M.D., Ford D.A., Esmon C.T., Esmon N.L. The effect of membrane composition on the hemostatic balance. Biochemistry. 1999;38:3591–3598. doi: 10.1021/bi982538b. [DOI] [PubMed] [Google Scholar]
- 21.Stone R.L., Nick A.M., McNeish I.A., Balkwill F., Han H.D., Bottsford-Miller J., Rupairmoole R., Armaiz-Pena G.N., Pecot C.V., Coward J., Deavers M.T., Vasquez H.G., Urbauer D., Landen C.N., Hu W., Gershenson H., Matsuo K., Shahzad M.M., King E.R., Tekedereli I., Ozpolat B., Ahn E.H., Bond V.K., Wang R., Drew A.F., Gushiken F., Lamkin D., Collins K., DeGeest K., Lutgendorf S.K., Chiu W., Lopez-Berestein G., Afshar-Kharghan V., Sood A.K. Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med. 2012;366:610–618. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suades R., Padro T., Vilahur G., Badimon L. Circulating and platelet-derived microparticles in human blood enhance thrombosis on atherosclerotic plaques. Thromb. Haemost. 2012;108:1208–1219. doi: 10.1160/TH12-07-0486. [DOI] [PubMed] [Google Scholar]
- 23.Tanyi J.L., Hasegawa Y., Lapushin R., Morris A.J., Wolf J.K., Berchuck A., Lu K., Smith D.I., Kalli K., Hartmann L.C., McCune K., Fishman D., Broaddus R., Cheng K.W., Atkinson E.N., Yamal J.M., Bast R.C., Felix E.A., Newman R.A., Mills G.B. Role of decreased levels of lipid phosphate phosphatase-1 in accumulation of lysophosphatidic acid in ovarian cancer. Clin. Cancer Res. 2003;9:3534–3545. [PubMed] [Google Scholar]
- 24.Tavoosi N., Davis-Harrison R.L., Pogorelov T.V., Ohkubo Y.Z., Arcario M.J., Clay M.C., Rienstra C.M., Tajkhorshid E., Morrissey J.H. Molecular determinants of phospholipid synergy in blood clotting. J. Biol. Chem. 2011;286:23247–23253. doi: 10.1074/jbc.M111.251769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuda T., Yoshimura H., Hamasaki N. Effect of phosphatidylcholine, phosphatidylethanolamine and lysophosphatidylcholine on the activated factor X-prothrombin system. Blood Coagul. Fibrinolysis. 2006;17:465–469. doi: 10.1097/01.mbc.0000240919.72930.ee. [DOI] [PubMed] [Google Scholar]
- 26.van M.G., Voelker D.R., Feigenson G.W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varon D., Shai E. Platelets and their microparticles as key players in pathophysiological responses. J. Thromb. Haemost. 2015;13(Suppl. 1):S40–S46. doi: 10.1111/jth.12976. [DOI] [PubMed] [Google Scholar]
- 28.von Tempelhoff G.F., Dietrich M., Niemann F., Schneider D., Hommel G., Heilmann L. Blood coagulation and thrombosis in patients with ovarian malignancy. Thromb. Haemost. 1997;77:456–461. [PubMed] [Google Scholar]
- 29.Wang M., Han X. Multidimensional mass spectrometry-based shotgun lipidomics. Methods Mol. Biol. 2014;1198:203–220. doi: 10.1007/978-1-4939-1258-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document
Supplementary material