Highlights
-
•
Sexual problems are related to three components: physiology, sociocultural background, and neurological location of injury.
-
•
Studying the neural basis of sexual response and preference is crucial for exposing pathways involved in sexual disorders.
-
•
Literature case reports have shown that sexual disinhibition is associated with basal frontal lobe dysfunction.
Keywords: Brain injury, Hypersexuality, Pedophilia
1. Introduction
Sexual problems post-injury are found to be the result of three components of the injured person: physiological makeup, sociocultural background, and neurological location of the injury [1]. Among many common disorders, hypersexual behavior disorder is one we seldom hear about in humans [2]. Hypersexuality has been defined as the subjective experience of loss of control over sexuality. This disorder has appeared in animals due to the production of experimental brain injury, but has rarely been found in humans. A great deal of our current understanding regarding this disorder has come from studies of non-traumatic brain injury [3]. The appearance of this offers an interesting understanding of the anatomical basis of human sexual behavior [4]. It also provides us with important verification regarding the neurological basis of irregular behavior. This study describes a patient who developed hypersexuality behavior toward children, following a brain injury.
2. Case presentation
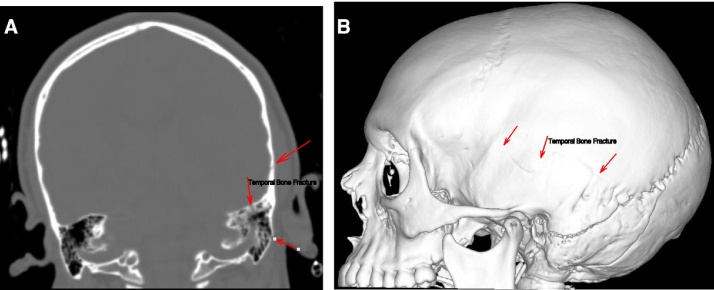
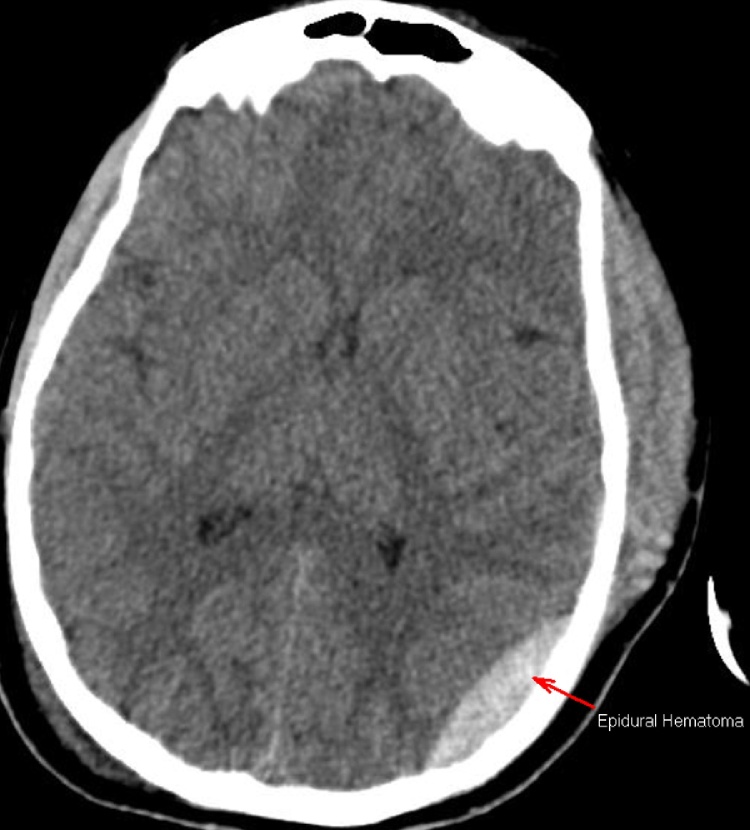
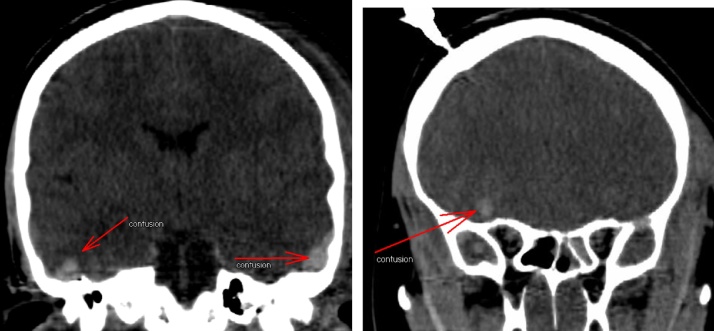
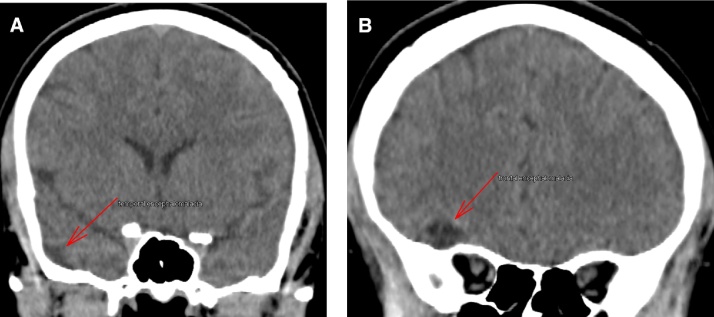
A patient in their early twenties was hospitalized following a traumatic brain injury incurred during a horseback-riding event, which resulted in a left temporal bone fracture (Fig. 1) and led to the development of a left epidural hematoma (Fig. 2). The brain CT scan showed left basal frontal and bilateral temporal contusions (Fig. 3). The patient had a craniotomy and evacuation of the epidural hematoma. Following the surgery the patient was doing very well; the patient’s only complaint was with regard to having difficulty sleeping. A brain CT scan at the visit showed no bleeding, no epidural hematoma, and no sign of infection. Three months later, the patient came back with a family member for a follow. At this visit the patient was active and doing well, and reported no neurologic deficits. The patient denied having a headache, dizziness, nausea, vomiting, or visual problems. However, the patient reported having attention deficit, difficulty sleeping, irritability, and behavioral changes. The patient’s family member noticed an increase in the patient’s sexual desire, especially towards children and teenagers. There was no evidence of seizures at this time. However, the brain CT showed a left basal temporal and left basal frontal encephalomalacia (Fig. 4).
Fig. 1.
(A) Coronal temporal bone fracture. (B) Temporal bone fracture 3D.
Fig. 2.
Epidural hematoma.
Fig. 3.
CT scan on the day of the injury show a basal frontal contusion.
Fig. 4.
(A) Basal temporal contusion. (B) Three months after injury show a basal temporal encephalomalacia.
3. Discussion
Studying the neural basis of the human sexual response and sexual preference is crucial for exposing the pathways involved in various sexual disorders, ranging from hyposexual desire disorder to pedophilia. The normal sexual response has been described as a multifaceted process that includes arousal, copulation, and orgasm [4]. Despite the unprecedented progress that medicine has witnessed over the past few decades, the medical community’s knowledge currently falls short of adequately explaining and describing the neural pathways involved in sexual response, primarily the arousal component.
Most of our understandings of sexual behavior have been largely based on lesion studies in primates [1], [5]. Although we share 96% of our genome with other species, studies in animals cannot be generalized to explain human behavior. On the other hand, injuries to certain areas and structures in the human brain, as a result of traumatic brain injury, stroke, or tumor-mediated damage, have resulted in an alteration of normal sexual behavior, response, and preference. Such reports can help tremendously in suggesting which brain areas and structures are mostly involved in determining normal sexual behavior.
Although hyposexuality is commonly manifested in patients following injury to the brain, studies have shown that a number of variable lesions may bring about such change that it is often not feasible to attribute hyposexuality to just one lesion [6]. Hypersexual behavior and alteration of sexual orientation and preference, in contrast to hyposexuality, are not as common of an occurrence following brain injury [7], [8]. For this reason, hypersexual behavior and alteration of sexual preference may possibly be attributed to specific injury-induced lesions in the brain, and may potentially help in demonstrating which neural pathways are involved in formulating a normal sexual response.
Literature case reports have shown that sexual disinhibition and public exhibitionism of genitals are associated with basal frontal lobe dysfunction [8]. Additionally, hypersexuality has been associated with temporal lobe dysfunction manifested in the post-ictal period or following temporal lobe lobectomy, most probably due to disinhibition of structures in the septum and/or hypothalamus [9]. Pedophilia has also been reported in association with hypothalamic dysfunction as a result of infiltrating gliomas of the midbrain-hypothalamic region, suprasellar meningiomas, and epilepsy [4], [10], [11].
In terms of altered sexual preference following brain injury, Lilly et al. [12] reports that bilateral temporal lobe dysfunction associated with human Kluver-Bucy syndrome is most closely related to this change [13], [14]. In addition, altered sexual preference has been most commonly attributed to the preoptic nucleus of the hypothalamus [15]. This has been demonstrated through in utero hormonal manipulation of the preoptic nucleus, which subsequently reverses the gender preference of male and female rats [15], [16].
Overall, hypersexual behavior and altered sexual preference, although uncommon following brain injury, have been attributed to damage of the basal frontal areas and temporal lobes [8], [12], [13], [14]. These areas have a significant number of connections to elements of the limbic system, namely thalamus and hypothalamus, and appear to play a critical role in determining the sexual response of a human being.
In our patient’s case, damage to the basal frontal area as well as the basal temporal area, following an epidural hematoma, unmasked a previously inhibited sexual preference towards young children and heightened the patient’s sexual desire. Damage to the basal frontal area has been previously described in the literature as a probable cause of hypersexuality and elevated sexual desire [8]. Similarly, damage to the temporal lobe, as seen in Kluver-Bucy syndrome [12], [13] and temporal lobe lobectomy [14], has been associated with an altered sexual preference [10].
It is most probable that the basal frontal lobe and the basal temporal lobe mediate important aspects of the normal human sexual response through their extensive regulatory and modulatory connections with the hypothalamus, and other limbic structures [17]. It is believed that efferent pathways connecting the frontal lobe to the pre-optic region of the hypothalamus contribute to sexual drive and arousal [15].
Temporal lobe dysfunction, on the other hand, as observed in temporal lobe epilepsy [9] and temporal lobe lobectomy [14], has been implicated in altered sexual preference and a heightened arousal response in patients [8], [10]. This phenomenon of altered sexual preference and heightened arousal response has been reported in temporal lobe epilepsy in the post-ictal period due to disinhibition of the pre-optic nucleus of the hypothalamus, or due to continued firing of electrical impulses in the limbic system following the seizure [4].
4. Conclusion
Although altered sexual behavior, in terms of hypersexuality or a change in sexual preference, is not very common sequelae of brain injury, studying the change in sexual behavior can change the way our medical community approaches the assessment and diagnosis of sexual disorders and may eventually lead to breakthroughs in the treatment of such disorders. Additionally, since altered sexual behavior is not always seen with damage to basal frontal and basal temporal areas, this suggests that specific pathways exist between the frontal lobe and temporal lobe with the pre-optic nuclei of the hypothalamus and other limbic structures. Studies and imaging techniques that can potentially expose white matter tracts between the aforementioned areas can present stronger evidence of their involvement in mediating the normal human sexual response.
Conflict of interest
The authors of this work have no conflicts of interest to declare.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this report and accompanying images.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author’s contribution
All authors contributed to data analysis and writing those sections of the manuscript that pertained to their particular area of specialty expertise in this case of polytrauma management.
Guarantor
Daniel Gaudin.
Contributor Information
Ahmed M. Alnemari, Email: Ahmed.Alnemari@rockets.utoledo.edu.
Tarek R. Mansour, Email: Tarek.Mansour2@rockets.utoledo.edu.
Mark Buehler, Email: mark.buehler@utoledo.edu.
Daniel Gaudin, Email: daniel.gaudin@utoledo.edu, teri.diehl@utoledo.edu.
References
- 1.Sabhesan S., Natarajan M. Sexual behavior after head injury in Indian men and women. Arch. Sex. Behav. 1989;18(4):349–356. doi: 10.1007/BF01541953. [DOI] [PubMed] [Google Scholar]
- 2.Mutarelli E.G., Omuro A.M., Adoni T. Hypersexuality following bilateral thalamic infarction: case report. Arq. Neuropsiquiat. 2006;64(1):146–148. doi: 10.1590/s0004-282x2006000100032. [DOI] [PubMed] [Google Scholar]
- 3.Limbert J. Head injury and sexuality: a literature review. Can. J. Hum. Sex. 1992;1:187–193. [Google Scholar]
- 4.Miller B.L., Cummings J.L., McIntyre H., Ebers G., Grode M. Hypersexuality or altered sexual preference following brain injury. J. Neurol. Neurosurg. Psychiatry. 1986;49(8):867–873. doi: 10.1136/jnnp.49.8.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark J.T., Smith E.R., Davidson J.M. Enhancement of sexual motivation in male rats by yohimbine. Science. 1984;225(4664):847–849. doi: 10.1126/science.6474156. [DOI] [PubMed] [Google Scholar]
- 6.Boller F., Frank E. Sexual dysfunction in neurological disorders: diagnosis, management, and rehabilitation. J. Neurol. Neurosurg. Psychiatry. 1982;45(7):663. [Google Scholar]
- 7.Poeck K., Pilleri G. Release of hypersexual behaviour due to lesion in the limbic system. Acta Neurol. Scand. 1965;41(3):233–244. doi: 10.1111/j.1600-0404.1965.tb04295.x. [DOI] [PubMed] [Google Scholar]
- 8.Blumer D., Benson D.F. Grune and Stratton; New York: 1975. Personality Changes with Frontal and Temporal Lobe Lesions; pp. 151–170. [Google Scholar]
- 9.Blumer D. Hypersexual episodes in temporal lobe epilepsy. Am. J. Psychiatry. 1970;126(8):1099–1106. doi: 10.1176/ajp.126.8.1099. [DOI] [PubMed] [Google Scholar]
- 10.Regestein Q.R., Reich P. Pedophilia occurring after onset of cognitive impairment. J. Nerv. Ment. Dis. 1978;166(11):794–798. doi: 10.1097/00005053-197811000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Berlin F.S., Coyle G.S. Sexual deviation syndromes. Johns Hopkins Med. J. 1981;149(3):119–125. [PubMed] [Google Scholar]
- 12.Lilly R., Cummings J.L., Benson D.F., Frankel M. The human Klüver‐Bucy syndrome. Neurology. 1983;33(9):1141–1145. doi: 10.1212/wnl.33.9.1141. [DOI] [PubMed] [Google Scholar]
- 13.Shraberg D., Weisberg L. The Kluver-Bucy syndrome in man. J. Nerv. Ment. Dis. 1978;166(2):130–134. doi: 10.1097/00005053-197802000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Terzian H., Dalle Ore G. Syndrome of Klüver and Bucy, reproduced in man by bilateral removal of the temporal lobes. Neurology. 2016:1955. doi: 10.1212/wnl.5.6.373. [DOI] [PubMed] [Google Scholar]
- 15.Pardridge W.M., Gorski R.A., Lippe B.M., Green R. Androgens and sexual behavior. Ann. Intern. Med. 1982;96(4):488–501. doi: 10.7326/0003-4819-96-4-488. [DOI] [PubMed] [Google Scholar]
- 16.Gorski R.A., Gordon J.H., Shryne J.E., Southam A.M. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148(2):333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 17.Nauta W.J. Neural associations of the frontal cortex. Acta Neurobiol. Exp. (Wars) 1972;32(2):125–140. [PubMed] [Google Scholar]