Abstract
Lay Abstract
Individuals with autism spectrum disorder (ASD) show deficits in acquiring a wide range of skills including social communication skills, a defining feature of ASD, as well as basic motor skills. Development of even the most basic skill involves integrating information from multiple senses. Learning skilled social gestures is particularly reliant on integration of visual and tactile information. To better understand this process, we used a serial response time task to explore how adults with ASD use visual and proprioceptive input to promote motor learning as compared to healthy controls. In line with prior studies of motor adaptation, we find that individuals with ASD show an anomalous pattern of learning. While both groups learned the implicit motor sequence during training there was decreased reliance on visual input during generalization, as compared to healthy controls. The findings have important implications for understanding the brain basis of impaired skill learning and for understanding approaches for improving these skills in individuals with ASD. Effective learning strategies may leverage guided performance and proprioceptive input or facilitate development of visual-motor connections.
Scientific Abstract
In addition to defining impairments in social communication skills, individuals with autism spectrum disorder (ASD) also show impairments in more basic sensory and motor skills. Development of new skills involves integrating information from multiple sensory modalities. This input is then used to form internal models of action that can be accessed when both performing skilled movements, as well as understanding those actions performed by others. Learning skilled gestures is particularly reliant on integration of visual and proprioceptive input. We used a modified serial reaction time task (SRTT) to decompose proprioceptive and visual components and examine whether patterns of implicit motor skill learning differ in ASD participants as compared to healthy controls. While both groups learned the implicit motor sequence during training, healthy controls showed robust generalization whereas ASD participants demonstrated little generalization when visual input was constant. In contrast, no group differences in generalization were observed when proprioceptive input was constant, with both groups showing limited degrees of generalization. The findings suggest, when learning a motor sequence, individuals with ASD tend to rely less on visual feedback than do healthy controls. Visuomotor representations are considered to underlie imitative learning and action understanding and are thereby crucial to social skill and cognitive development. Thus, anomalous patterns of implicit motor learning, with a tendency to discount visual feedback, may be an important contributor in core social communication deficits that characterize ASD.
Keywords: Serial Reaction Time Task, ASD, Motor Learning, Proprioception
Generation of internal models of action, or sensorimotor representations, is critical to social, communicative, and motor skill acquisition. Recently, our understanding of altered motor development in autism spectrum disorder (ASD) and its relationship to anomalous sensorimotor representations and the core deficit of social communication (APA, 2013) has substantially increased.
Motor function difficulties in ASD are evident in infancy (Landa, 2008), persist into adulthood (Jansiewicz et al., 2006; Sutera et al., 2007, Dziuk et al., 2007), and effectively distinguish children with ASD from healthy controls (HC) and children with other developmental disabilities (McNeil & Mostofsky, 2012; Wyatt & Craig, 2012; Ament et al., 2014). Furthermore, children with ASD struggle identifying representations of gestures accurately (Dowell et al., 2009). Sensorimotor representation when learning novel movements may play a causative role with motor function and social communication deficits.
Sensorimotor representations form during basic motor skill development. Procedural learning is implicit skill acquisition through repeated exposure and practice in which an individual’s motor command is associated with sensory feedback (Squire, 1986; Willingham et al., 1989). This association produces a sensorimotor representation, during action observation, enabling interpretation of meaning from another’s actions through self-other mapping (Rizzolatti et al., 2002; Mattar & Gribble, 2005; Klin et al., 2003). Procedural learning underlies social, communicative, and motor skill acquisition. Atypical motor skill development may lead to atypical sensorimotor representations (Gidley Larson & Mostofsky 2008, Klinger & Dawson, 2001, Walenski et al., 2008). Models formed from an atypical system have far reaching consequences in this framework.
Converging evidence suggests visual and proprioceptive sensory processing systems leveraged in manual gesture procedural learning are utilized to varying degrees in ASD. ASD Adults perform worse at object identification than controls when the task requires visual-temporal integration (Nakano et al., 2010) and perform better when similar tasks require somatomotor (haptic) integration (Nakano et al., 2012). Furthermore, studies examining visual and proprioceptive contributions to motor adaptation in controls, ASD, and ADHD children consistently reveal anomalous patterns of motor learning, with increased reliance on proprioceptive input and decreased reliance on visual input in ASD children (Haswell et al., 2009; Izawa et al., 2012; Marko et al., in press). These biases are strongly associated with motor and social deficits in ASD (Haswell et al., 2009; Izawa et al., 2012). Divergent motor learning biases may put complex skills necessary for adaptive social-communicative behavior at a developmental disadvantage.
Consistent with past motor adaptation studies, we hypothesize that individuals with ASD will show anomalous motor sequence learning using a modified version of Nissen and Bullemer’s (1987) serial reaction time task (SRTT). Previous SRTT studies demonstrate ASD participants have impaired implicit motor sequence learning (Mostofsky et al., 2000; Gidley Larson & Mostofsky, 2008), although findings are not consistent throughout the field (Travers et al., 2010; Gordon & Stark, 2007). Discrepancies across studies may reflect differences in how ASD participants perform the task, such as sensory biases, rather than overall ability, for acquiring motor sequences. We used a modified SRTT to examine generalization of learned sequences across hemispheres in visual versus proprioceptive coordinates (Cohen et al., 2005), and hypothesized the ASD group would demonstrate differences in learning patterns reflecting enhanced generalization from proprioceptive input and impaired generalization from visual input.
Methods
Participants
Thirty-five adults participated in this study. Three participants were excluded for left-hand-dominance and an additional three for having incomplete task data. Data were analyzed from the remaining 18 ASD participants (16 male, 38.72±18.362 years SD) and 11 controls (6 male, 36.36±17.42 years SD). Diagnosis was determined by an independent clinician using DSM-IV criteria. Handedness was defined using the Edinburgh Handedness Inventory (Oldfield, 1971). IQ in the ASD cohort was assessed using the WASI (Wechsler, 1999) (See samples characteristics in Table 1). Participants were recruited through approved advertisements on community bulletin boards and a local ASD organization’s site: The Asperger/Autism Network.
Table 1.
| Participant Characteristics: | ||||||
|---|---|---|---|---|---|---|
| Subject | Diagnosis | Age | Gender | FSIQ | VIQ | PIQ |
| A1 | PDD-NOS | 33 | M | 117 | 117 | 114 |
| A2 | AS | 18 | M | 73 | 67 | 83 |
| A3 | AS | 25 | F | 128 | 132 | 118 |
| A4 | AS | 42 | M | 124 | 131 | 111 |
| A5 | AS | 54 | F | 128 | 121 | 129 |
| A6 | PDD-NOS | 64 | M | 117 | 111 | 119 |
| A7 | PDD-NOS | 56 | M | 94 | 75 | 119 |
| A8 | AS | 50 | M | 124 | 121 | 119 |
| A9 | AS | 62 | M | 134 | 131 | 129 |
| A10 | AS | 64 | M | 140* | ||
| A11 | AS | 19 | M | 127 | 121 | 126 |
| A12 | AS | 20 | M | 96 | 103 | 89 |
| A13 | PDD-NOS | 21 | M | 120 | 109 | 128 |
| A14 | Autism | 17 | M | 81 | 82 | 84 |
| A15 | AS | 62 | M | 123 | 118 | 121 |
| A16 | AS | 24 | M | 123 | 113 | 127 |
| A17 | AS | 22 | M | 110 | 119 | 117 |
| A18 | AS | 44 | M | 102 | 83 | 91 |
| C1 | 53 | F | ||||
| C2 | 47 | M | ||||
| C3 | 26 | F | ||||
| C4 | 32 | M | ||||
| C5 | 19 | M | ||||
| C6 | 39 | M | ||||
| C7 | 61 | M | ||||
| C8 | 19 | M | ||||
| C9 | 21 | F | ||||
| C10 | 19 | F | ||||
| C11 | 64 | F | ||||
FSIQ was acquired from clinical report that did not contain subscales
Paradigm
A modified SRTT assessed visual and proprioceptive motor generalization components in procedural learning. Tasks were introduced to participants as reaction time (RT) tests. Certain task sections contained a repeating sequence to be implicitly learned. Throughout the task, a circular stimulus (diameter 20mm) would appear in one of four positions on a Dell Latitude D831 laptop with a 15.3in display, corresponding to one of four buttons on a Cedrus RB-410 response pad. Participants situated 800mm away from the screen were instructed to respond by pressing the appropriate button when stimuli appeared. Following a correct response, a 400ms interval passed before the next stimulus presentation. Stimuli remained on screen until a correct response appeared. The task was coded, performed, and recorded using SuperLab 2 (Cedrus Corporation, 2010). Motor sequence learning was assessed by comparing RTs during the last sequence and random blocks. Only correct responses were included in analyses. Participants were trained using their dominant right hand and tested using their untrained hand, similar to Cohen and colleagues (2005). Generalization of learning from trained to untrained hand was used as a probe of reliance on visual and proprioceptive input (Masterton & Biederman 1983; Willingham, 1998; Cohen et al., 2005).
The experiment was divided into two sessions with a break in between. Each session had two right hand training periods, one short and one long. A test period followed during which participants used the left hand to perform the same task with the original sequence in one session and the mirror of the original sequence in the other. Visual-based generalization using external coordinates was assessed using the original sequence during the test period. Thus, the visual goal remained constant from the training phase but required a different motor pattern when performed by the left rather than the right hand. Proprioceptive-based generalization using internal coordinates was assessed using the mirrored sequence during the test period. This configuration maintained the same motor pattern as the trained hand while changing the visual input (See Figure 1 for experimental setup). Session order was randomized within groups. For each participant, two unique sequences were randomly assigned to either the visual or proprioceptive SRTT sessions to avoid sequence-learning effects across sessions.
Figure 1.
Experimental Design. (A) Design used to isolate motor learning components. Participants implicitly learn a sequence of visual cues (i.e., -1-4-3) and series of finger movements (i.e., -index-pinky-ring) from embedded repeating patterns of stimuli during the training period. The right hand was trained and the learning effects that transferred to the left hand were tested. Visual and proprioceptive configurations of the test period were used to decompose motor learning. Using the left hand while maintaining the visual sequence (i.e., -1-4-3) requires different finger movements (i.e., -pinky-index-middle) and provides a measure of the visual-based component of motor learning. Alternatively, mirroring the visual sequence (i.e., -4-1-2) requires the same finger movements on the new hand (i.e., -index-pinky-ring) and provides a measure of the proprioceptive component of motor learning. (B) Each participant had two configurations of the SRTT. Both had two training periods, short and long, using the right hand and a final test period using the left hand. Within each period random trials (grey blocks) bookended the sequence trials (white blocks). The motor learning score, an assessment of skill transferred to the untrained hand, was calculated using the last 50 random trials and last 50 sequence trials of the test period.
The short training, long training, and test periods of each session had a 50-trial random block before and after the sequence trial blocks. Random blocks contained pseudo-random sequences with no repeats. The 400-trial long training period sequence block contained 25 repetitions of the 12-trial sequence (300 trials). The 280-trial short training period and test period sequence blocks contained 15 repetitions of the same 12-trial sequence (180 trials).
Data Analysis
Learning occurs when RT decreases in sequenced trials and increases, or rebounds, upon the random sequence reintroduction. Rebound disambiguates sequence specific learning from general task adaptation. Learning scores were calculated by subtracting the average correct RT of the last 50 trials of the sequence block from the average correct RT from the 50 trials of the subsequent random block during the test period. Trials that fell below 200ms or beyond 2.7 standard deviations (Cohen et al., 2005; Cohen & Robertson, 2007) of the mean were removed from learning score calculation to eliminate anticipatory errors and attention lapses.
Differences in motor generalization during visual and proprioceptive sessions between groups were assessed using a repeated measures two-way ANOVA on learning scores. Planned simple effect comparisons were used to assess group differences in visual-based and proprioceptive-based skill learning scores.
Results
Groups were matched on age (t(27)=.775, p=.445, d= 0.298) but not gender (X2=4.398, p=.036, ϕ=.389). Age and performance were not significantly correlated (G: r=−.071, p=.713; M: r=.060, p=.758) across groups or within groups. Error rates were low in both groups (ASD: M=6.21%, SD=4.90%; HC: M=7.95%, SD=7.88%) with no significant between-group differences (t(96)=−1.610, p=.111, d=.329). Removed RTs as anticipatory errors or attention lapses only encompassed 1.07% of all data and did not significantly differ across groups (t(172)=0.277, p=.781, d=.0422). Baseline RT during the initial random block of each condition did not differ between groups (t(27)=0.208, p=0.262, d=0.46). Both groups demonstrated sequence learning: across training periods the average sequenced trial performance was significantly faster than average random trial performance (ASD: t(17)=−2.692, p=.015; HC: t(10)=−3.369, p=.007).
For both test conditions, overall RT for all blocks did not differ between groups (Visual: F(1,27)=1.929, p=0.176, ηp2=0.067; Proprioceptive: F(1,27)=1.069, p=0.310, ηp2=0.038). A repeated measures two-way ANOVA, with motor component (visual, proprioceptive) as the within-subject factor and diagnostic group (controls, ASD) as the between-subject factor, revealed a significant interaction effect between motor component and group membership on learning score (F(1,27)=11.06, p=0.003, ηp2=0.291). The significant motor component effect on learning scores (F(1,27)=5.626, p=0.025, ηp2=0.172) is explained by the significant simple effect of motor component (t(10)=3.52, p=0.006, d=1.06) in controls and not ASD participants (t(17)=−.787, p=0.442, d=−0.186). There was no main effect of diagnosis on learning score (F(1,27)=1.197, p=0.284, ηp2=0.042).
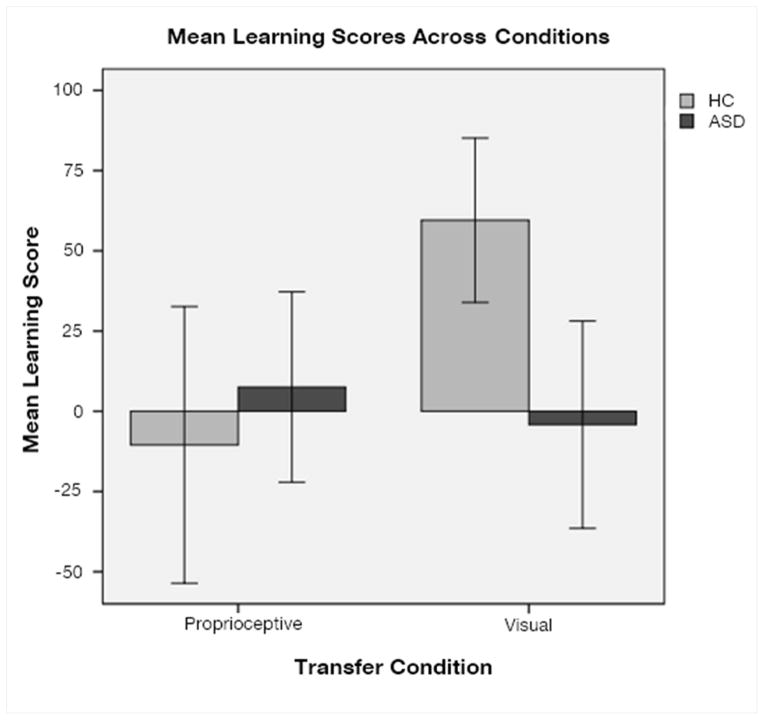
Follow-up analyses of skill component learning scores revealed controls showed greater improvement from sequence learning than ASD participants (t(27)=2.765, p=0.010, d=1.23) during the visual component. Single-sample t-tests revealed only controls showed a visual learning score significantly different from zero (HC: t(10)=4.650, p=0.001, d=2.941; ASD: t(17)=−0.259, p=0.799, d=−0.126). The two groups do not significantly differ during the proprioceptive component (t(27)=−0.711, p=0.483, d=0.27), and neither group had a movement learning score significantly different from zero (HC: t(10)=−0.486, p=0.637, d=−0.31; ASD: t(17)=0.508, p=0.618, d=0.246) (Figure 2).
Figure 2.
Learning Score Results. Visual and proprioceptive motor learning was tested for each participant. Results showed that the healthy control group demonstrated significantly better generalization of visual motor learning than the ASD group. While only the healthy controls’ visual learning scores significantly differed from 0, neither group’s demonstrated generalization of proprioceptive motor learning.
Discussion
Results from this study partially confirm our hypotheses. Both groups demonstrated sequence learning during training and were adequately engaged throughout the task. Controls utilized extrinsic-visual aspects from training to generalize the learned sequence to the untrained hand; however, ASD participants did not utilize this component to the same advantage. In contrast, neither group showed an ability to utilize the proprioceptive component for motor sequence generalization to the untrained hand.
Our findings are, in part, consistent with those from motor adaptation studies (Haswell et al., 2009; Izawa et al., 2012; Marko et al., 2015), suggesting ASD participants under-utilize visual input during motor learning. However ASD participants are not at an advantage for proprioceptive input in this study. This may be due to limitations in sample size, as indicated by the variability within each population and inability to match for gender. Alternatively, our task may fail to capture proprioceptive capability. Our control findings are consistent with Kirsh & Hoffman (2010), who found visual, not proprioceptive, motor learning transfers to the left hand. Training the non-dominant left hand and transferring to the right hand improved transfer of proprioceptive motor learning but not the visual component. Thus, a right to left transfer design may have biased the findings in favor of visual component transfer of learning. Lastly, insufficient training may have also reduced experimental sensitivity to proprioceptive motor learning. Results from previous studies suggest visual and proprioceptive motor skill learning are simultaneously acquired (see Hikosaka 2002 for review), yet at different rates and using different neural substrates (Bapi et al., 2000). The visual component develops faster and is utilized early in learning while the proprioceptive component develops more slowly. Proposed future research include addressing these limitations and including a more detailed behavioral characterization in order to evaluate how these differences may relate to specific behavioral phenotypes.
It should be noted that ASD-associated difficulties with motor sequence learning has also been explored using oculomotor learning tasks. Slower predictive saccade latency for an anticipated stimuli (Goldberg et al., 2002) and slower systemic error correction during adaptation (Mosconi et al., 2013; Johnson et al., 2012) were found and could help explain our impaired visual-motor transfer findings in ASD. However, given that the oculomotor system lacks proprioceptive feedback, relative biases in sensory feedback during motor learning are best explored using limb motor tasks, such as used in this study and prior studies of limb motor adaption (Haswell et al., 2009; Izawa et al., 2012; Marko et al., 2015).
In summary, consistent with our hypothesis, our findings reveal controls generalized learning in visual external coordinate system to a greater degree than ASD participants. Visuomotor representations are considered to underlie imitative learning and action understanding and are thereby crucial to both social skill and social cognitive development. It follows that anomalous patterns of procedural learning may be an important contributor in the development of core social communication deficits that characterize ASD.
Acknowledgments
Work on the project was supported by grants from the National Institutes of Health and National Institute of Mental Health (F32 MH804932, 1KL2RR025757-01, 1R01MH100186, R01 NS048527-07, UL1 RR025005), Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH 8KL2TR000168-05), and Autism Speaks 2506. LO is further supported by grants from the Harvard Clinical and Translational Science Center (8UL1TR000170-05), the Epilepsy Research Foundation, the Simons Foundation and the Nancy Lurie Marks Family Foundation. APL is further supported by grants from the National Institutes of Health (R01HD069776, R01NS073601, R21 MH099196, R21 NS082870, R21 NS085491, R21 HD07616, UL1 RR025758), Michael J. Fox Foundation and Sidney R. Baer Foundation.
References
- Ament K, Mejia A, Buhlman R, Erklin S, Caffo B, Mostofsky S, Wodka E. Evidence for specificity of motor impairments in catching and balance in children with autism. Journal of autism and developmental disorders. 2014:1–10. doi: 10.1007/s10803-014-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: 2013. [Google Scholar]
- Bapi RS, Doya K, Harner AM. Evidence for effector independent and dependent representations and their differential time course of acquisition during motor sequence learning. Experimental Brain Research. 2000;132(2):149–162. doi: 10.1007/s002219900332. [DOI] [PubMed] [Google Scholar]
- Cedrus Corporation. SuperLab (version 2) [Computer software] Phoenix, AZ: Cedrus Corporation; 2010. [Google Scholar]
- Cohen DA, Pascual-Leone A, Press DZ, Robertson EM. Off-line learning of motor skill memory: A double dissociation of goal and movement. Proceedings of the National Academy of Sciences. 2005;102(50):18237–18241. doi: 10.1073/pnas.0506072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DA, Robertson EM. Motor sequence consolidation: constrained by critical time windows or competing components. Experimental brain research. 2007;177(4):440–446. doi: 10.1007/s00221-006-0701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell LR, Mahone EM, Mostofsky SH. Associations of postural knowledge and basic motor skill with dyspraxia in autism: implication for abnormalities in distributed connectivity and motor learning. Neuropsychology. 2009;23(5):563. doi: 10.1037/a0015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziuk MA, Larson JC, Apostu A, Mahone EM, Denckla MB, Mostofsky SH. Dyspraxia in autism: association with motor, social, and communicative deficits. Developmental Medicine & Child Neurology. 2007;49(10):734–739. doi: 10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- Gidley Larson JC, Mostofsky SH. Evidence that the pattern of visuomotor sequence learning is altered in children with autism. Autism Research. 2008;1(6):341–353. doi: 10.1002/aur.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MC, Lasker AG, Zee DS, Garth E, Tien A, Landa RJ. Deficits in the initiation of eye movements in the absence of a visual target in adolescents with high functioning autism. Neuropsychologia. 2002;40(12):2039–2049. doi: 10.1016/s0028-3932(02)00059-3. [DOI] [PubMed] [Google Scholar]
- Gordon B, Stark S. Procedural learning of a visual sequence in individuals with autism. Focus on autism and other developmental disabilities. 2007;22(1):14–22. [Google Scholar]
- Haswell CC, Izawa J, Dowell LR, Mostofsky SH, Shadmehr R. Representation of internal models of action in the autistic brain. Nature neuroscience. 2009;12(8):970–972. doi: 10.1038/nn.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Current opinion in neurobiology. 2002;12(2):217–222. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- Izawa J, Pekny SE, Marko MK, Haswell CC, Shadmehr R, Mostofsky SH. Motor Learning Relies on Integrated Sensory Inputs in ADHD, but Over-Selectively on Proprioception in Autism Spectrum Conditions. Autism research. 2012;5(2):124–136. doi: 10.1002/aur.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa R, Mostofsky SH. Motor signs distinguish children with high functioning autism and Asperger’s syndrome from controls. Journal of autism and developmental disorders. 2006;36(5):613–621. doi: 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Johnson BP, Rinehart NJ, Papadopoulos N, Tonge B, Millist L, White O, Fielding J. A closer look at visually guided saccades in autism and Asperger’s disorder. Frontiers in integrative neuroscience. 2012:6. doi: 10.3389/fnint.2012.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch W, Hoffmann J. Asymmetrical intermanual transfer of learning in a sensorimotor task. Experimental brain research. 2010;202(4):927–934. doi: 10.1007/s00221-010-2184-8. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F. The enactive mind, or from actions to cognition: lessons from autism. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2003;358(1430):345–360. doi: 10.1098/rstb.2002.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger LG, Dawson G. Prototype formation in autism. Development and Psychopathology. 2001;13(01):111–124. doi: 10.1017/s0954579401001080. [DOI] [PubMed] [Google Scholar]
- Landa RJ. Diagnosis of autism spectrum disorders in the first 3 years of life. Nature Clinical Practice Neurology. 2008;4(3):138–147. doi: 10.1038/ncpneuro0731. [DOI] [PubMed] [Google Scholar]
- Landa RJ. Diagnosis of autism spectrum disorders in the first 3 years of life. Nature Clinical Practice Neurology. 2008;4(3):138–147. doi: 10.1038/ncpneuro0731. [DOI] [PubMed] [Google Scholar]
- MacNeil LK, Mostofsky SH. Specificity of dyspraxia in children with autism. Neuropsychology. 2012;26(2):165. doi: 10.1037/a0026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marko MK, Crocetti D, Hulst T, Donchin O, Shadmehr R, Mostofsky SH. Behavioural and neural basis of anomalous motor learning in children with autism. Brain. 2015:awu394. doi: 10.1093/brain/awu394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterton BA, Biederman GB. Proprioceptive versus visual control in autistic children. Journal of Autism and Developmental Disorders. 1983;13(2):141–152. doi: 10.1007/BF01531815. [DOI] [PubMed] [Google Scholar]
- Mattar AA, Gribble PL. Motor learning by observing. Neuron. 2005;46(1):153–160. doi: 10.1016/j.neuron.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Luna B, Kay-Stacey M, Nowinski CV, Rubin LH, Scudder C, Sweeney JA. Saccade adaptation abnormalities implicate dysfunction of cerebellar-dependent learning mechanisms in Autism Spectrum Disorders (ASD) PloS one. 2013;8(5):e63709. doi: 10.1371/journal.pone.0063709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Goldberg MC, Landa RJ, Denckla MB. Evidence for a deficit in procedural learning in children and adolescents with autism: implications for cerebellar contribution. Journal of the International Neuropsychological Society. 2000;6(07):752–759. doi: 10.1017/s1355617700677020. [DOI] [PubMed] [Google Scholar]
- Nakano T, Ota H, Kato N, Kitazawa S. Deficit in visual temporal integration in autism spectrum disorders. Proceedings of the Royal Society B: Biological Sciences. 2010;277(1684):1027–1030. doi: 10.1098/rspb.2009.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Kato N, Kitazawa S. Superior haptic-to-visual shape matching in autism spectrum disorders. Neuropsychologia. 2012;50(5):696–703. doi: 10.1016/j.neuropsychologia.2011.12.024. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cognitive psychology. 1987;19(1):1–32. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Motor and cognitive functions of the ventral premotor cortex. Current opinion in neurobiology. 2002;12(2):149–154. doi: 10.1016/s0959-4388(02)00308-2. [DOI] [PubMed] [Google Scholar]
- Squire LR. Mechanisms of memory. Science. 1986;232(4758):1612–1619. doi: 10.1126/science.3086978. [DOI] [PubMed] [Google Scholar]
- Sutera S, Pandey J, Esser EL, Rosenthal MA, Wilson LB, Barton M, Fein D. Predictors of optimal outcome in toddlers diagnosed with autism spectrum disorders. Journal of autism and developmental disorders. 2007;37(1):98–107. doi: 10.1007/s10803-006-0340-6. [DOI] [PubMed] [Google Scholar]
- Teitelbaum P, Teitelbaum O, Nye J, Fryman J, Maurer RG. Movement analysis in infancy may be useful for early diagnosis of autism. Proceedings of the National Academy of Sciences. 1998;95(23):13982–13987. doi: 10.1073/pnas.95.23.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Klinger MR, Mussey JL, Klinger LG. Motor-linked implicit learning in persons with autism spectrum disorders. Autism Research. 2010;3(2):68–77. doi: 10.1002/aur.123. [DOI] [PubMed] [Google Scholar]
- Walenski M, Mostofsky SH, Gidley-Larson JC, Ullman MT. Brief report: enhanced picture naming in autism. Journal of autism and developmental disorders. 2008;38(7):1395–1399. doi: 10.1007/s10803-007-0513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. Psychological Corporation; 1999. [Google Scholar]
- Willingham DB. A neuropsychological theory of motor skill learning. Psychological review. 1998;105(3):558. doi: 10.1037/0033-295x.105.3.558. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Goedert-Eschmann K. The relation between implicit and explicit learning: Evidence for parallel development. Psychological Science. 1999;10(6):531–534. [Google Scholar]
- Willingham DB, Nissen MJ, Bullemer P. On the development of procedural knowledge. Journal of experimental psychology: learning, memory, and cognition. 1989;15(6):1047. doi: 10.1037//0278-7393.15.6.1047. [DOI] [PubMed] [Google Scholar]
- Whyatt CP, Craig CM. Motor skills in children aged 7–10 years, diagnosed with autism spectrum disorder. Journal of autism and developmental disorders. 2012;42(9):1799–1809. doi: 10.1007/s10803-011-1421-8. [DOI] [PubMed] [Google Scholar]