Abstract
BACKGROUND
Functional and structural liver abnormalities may be found in patients with advanced heart failure (HF). The Model of End-Stage Liver Disease Excluding INR (MELD-XI) score allows functional risk stratification of HF patients on and off anti-coagulation awaiting heart transplantation (HTx), but these scores may improve or worsen depending on bridging therapies and during time on the waiting list. Liver biopsy is sometimes performed to assess for severity of fibrosis. Uncertainty remains whether biopsy in addition to MELD-XI improves prediction of adverse outcomes in patients evaluated for HTx.
METHODS
Sixty-eight patients suspected of advanced liver disease underwent liver biopsy as part of their HTx evaluation. A liver risk score (fibrosis-on-biopsy + 1) × MELD-XI was generated for each patient.
RESULTS
Fifty-two patients were listed, of whom 14 had mechanical circulatory support (MCS). Thirty-six patients underwent transplantation and 27 patients survived ≥1 year post-HTx (74%, as compared with 88% average 1-year survival in HTx patients without suspected liver disease; p < 0.01). Survivors had a lower liver risk score at evaluation for HTx (31.0 ± 20.4 vs 65.2 ± 28.6, p < 0.01). A cut-point of 45 for liver risk score was identified by receiver-operating-characteristic (ROC) analysis. In the analysis using Cox proportional hazards models, a liver risk score ≥45 at evaluation for HTx was associated with greater risk of death at 1 year post-HTx compared with a score of <45 in both univariable (HR 3.94, 95% CI 1.77–8.79, p < 0.001) and multivariable (HR 4.35, 95% CI 1.77–8.79, p < 0.001) analyses. Patients who died <1 year post-HTx had an increased frequency of acute graft dysfunction (44.4% vs 3.7%, p = 0.009), longer ventilation times (55.6% vs 11.1%, p = 0.013) and severe bleeding events (44.4% vs 11.1%, p = 0.049). The liver risk score at evaluation for HTx also predicted 1-year mortality after HTx listing (p < 0.001).
CONCLUSIONS
Patients with HF and advanced liver dysfunction are high-risk HTx candidates. Liver biopsy in addition to MELD-XI improves risk stratification of patients with advanced HF and suspected irreversible liver dysfunction.
Keywords: congestive hepatopathy, fibrosis, heart failure, liver disease, risk stratification
Heart transplantation (HTx) remains the only curative therapy for patients with end-stage heart failure (HF).1,2 Organ scarcity continues to have a major adverse impact on morbidity and mortality in patients awaiting HTx, as extended waiting time has been associated with higher risk for adverse outcomes.2,3 Use of mechanical circulatory support (MCS) to bridge patients has been shown to prevent irreversible end-organ dysfunction and death in patients awaiting HTx.4–6 However, complications related to MCS adversely impact on the success of bridge-to-transplantation strategies. Severe end-organ dysfunction before MCS is an established risk factor for poor outcome after MCS and after HTx.2,7
Evidence of significant liver dysfunction is a frequent finding in patients with advanced HF. Etiology of liver dysfunction may be directly related to HF, manifested as congestive hepatopathy and cardiac cirrhosis, or from other liver disease, such as non-alcoholic fatty liver disease (NAFLD), amiodarone toxicity and viral hepatitis. The clinical presentation of patients with liver dysfunction includes jaundice, ascites, elevated bilirubin and transaminases, low albumin, abnormal hepatic imaging, increased transhepatic pressure gradient and abnormal liver biopsy findings.8,9 Inadequate hepatic synthetic function, with abnormal levels of albumin, coagulation factors and acute-phase proteins, has been associated with an increased risk of bleeding and infection. Increased nitric oxide and a decreased response to endogenous vasopressors foster a vasodilatory state,10 which may initially improve HF hemodynamics, but ultimately precipitates hepatorenal syndrome and complicates the peri-operative management of these patients. We and others have shown that liver function abnormalities correlate with poor outcomes in ambulatory HF patients11–13 and in patients after MCS surgery13,14 and HTx.7
Functional liver abnormalities are reliably identified in high-risk patients using the Model of End-stage Liver Dysfunction (MELD) scoring system and its variations in patients on and off oral anti-coagulation (MELD eXcluding International normalized ratio, or MELD-XI). These parameters are dynamic and may improve or worsen under HF therapies and during progression of HF.10,15,16 In patients with evidence of liver fibrosis or cirrhosis on imaging, liver biopsy is performed to determine the extent of fibrosis and other structural changes that may impact clinical management and prognosis. Herein we analyzed the impact of liver fibrosis of any etiology, in combination with MELD scores, on the outcome of patients undergoing HTx at our center.
Methods
Patient cohort and data collection
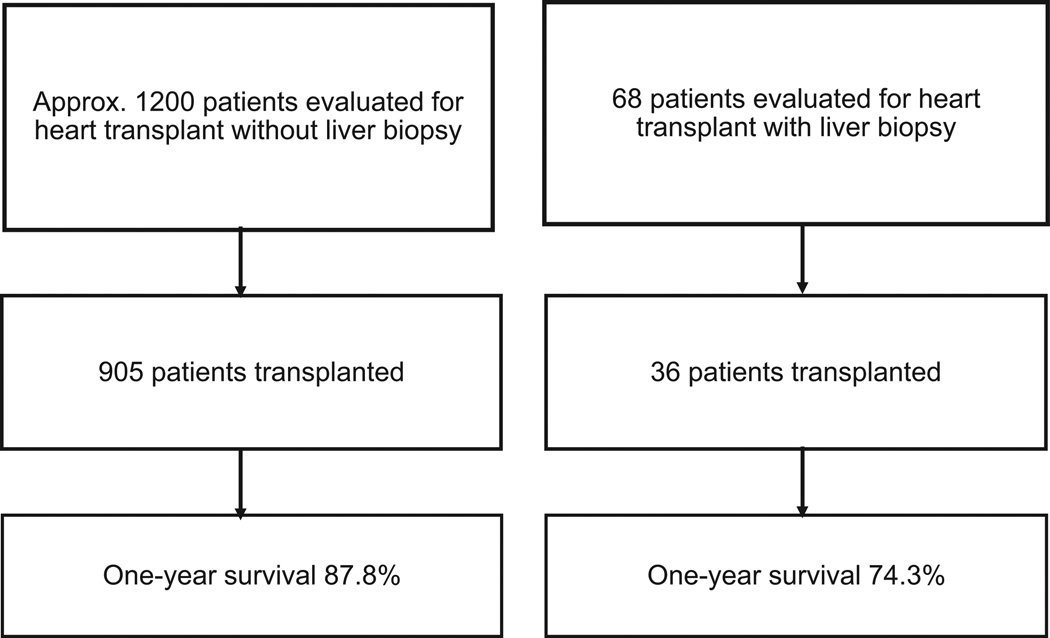
During the study period, from January 1, 2000 to May 1, 2013, approximately 1,200 patients underwent HTx evaluation at the Columbia University Medical Center. Sixty-eight of these patients underwent liver biopsy for suspected advanced liver dysfunction (Figure 1). Need for liver biopsy was based on history of liver disease, including viral hepatitis, NAFLD, alcoholism and congestive hepatopathy or cardiac cirrhosis. The clinical characteristics of this population included recurrent ascites, low albumin, sustained elevation in bilirubin or transaminases despite optimized medical therapy, and/or evidence of fibrosis or cirrhosis on liver sonogram. Other data collected included age, race, gender, etiology of HF, medications at time of transplant evaluation, echocardiogram parameters, invasive cardiac and liver hemodynamic measurements, laboratory analysis, liver sonogram, listing decision, need for MCS, post-HTx complications and survival. Patients were accepted or rejected for HTx based on consensus of the heart transplant selection committee of Columbia University.
Figure 1.
Study population and outcomes in patients with and without liver biopsies.
The study protocol was approved by the institutional review board of Columbia University and complied with the Health Insurance Portability and Accountability Act regulations and the ethical guidelines outlined in the 1975 Helsinki Declaration.
Liver biopsy procedure
All patients underwent transjugular liver biopsy with a 2–3-cm liver biopsy specimen obtained using a standard biopsy needle.17 Specimens were fixed in 10% neutral-buffered formalin, prepared in paraffin and stained with hematoxylin and eosin, Masson’s trichrome and reticulin.
Histopathologic scoring
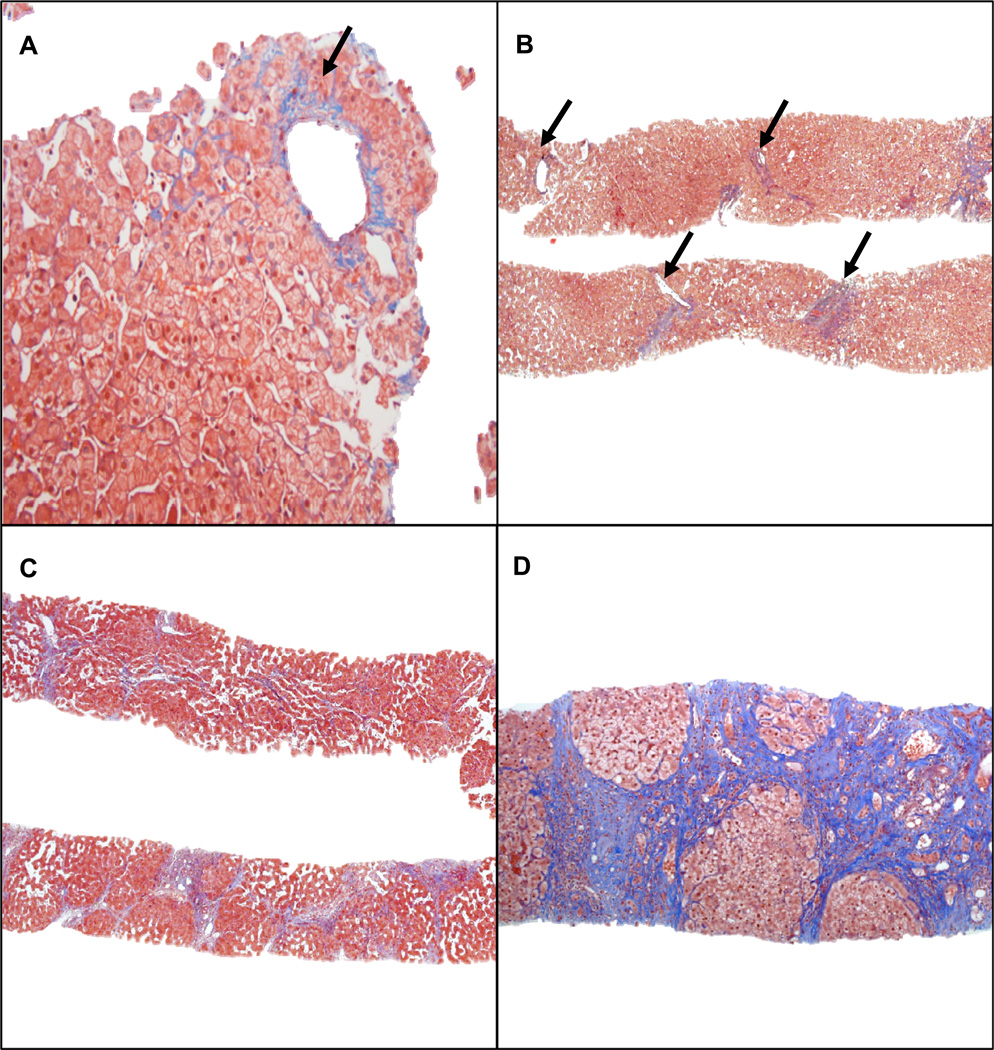
All liver biopsy specimens were assessed by 2 independent liver pathologists. Basic clinical information was available to accurately interpret the biopsies, such as history of viral hepatitis. All slides were assessed for fibrosis of any etiology and quantified per standard pathology algorithms.18–22 The degree of centrilobular fibrosis seen in the context of congestive hepatopathy or cardiac cirrhosis was scored on a scale of 0–4 (Figure 2). Patients with underlying chronic viral hepatitis, NAFLD or amiodarone toxicity were scored for fibrosis in reference to these specific disease pathologies. In some patients there was evidence of 2 forms of fibrosis (e.g., periportal fibrosis related to viral hepatitis and centrilobular fibrosis related to cardiac congestion) with the overall score also adjudicated as 0–4 by 2 independent pathologists.
Figure 2.
Stages of fibrosis in congestive hepatopathy. (A) Isolated central vein shows perivenular fibrosis (arrow) (Stage 1) (trichrome stain; magnification ×20). (B) Fibrosis affects the majority of the central veins (arrows) (Stage 2) (trichrome stain; magnification ×4). (C) Extensive bridging fibrosis and multifocal nodularity without cirrhosis (Stage 3) (trichrome stain; magnification ×4). (D) Cirrhosis with diffuse fibrosis surrounding regenerative nodules (Stage 4) (trichrome stain; magnification ×10).
MELD scores
The MELD score is calculated as: 3.78 × Ln(total bilirubin) + 11.2 × Ln(INR) + 9.57 × Ln(creatinine) + 6.43.15 MELD-XI is calculated as 5.11 × Ln(total bilirubin) + 11.76 × Ln(creatinine) + 9.44, where total bilirubin and creatinine are equal to 1 if the raw laboratory values are <1. Previous studies have demonstrated a high correlation between MELD and MELD-XI scoring.15 The MELD-XI score was generated at the time of HTx evaluation and just before HTx surgery. The modified MELD (modMELD) is calculated as a standard MELD score, except that albumin is substituted for INR.7
Liver risk score
The liver risk score was generated using (fibrosis-on-biopsy score + 1) × (MELD-XI) and expressed as a unitless value. The fibrosis variable was represented as value + 1 to avoid possible multiplication by 0. The liver risk score was calculated at the time of evaluation and pre-operatively using the degree of fibrosis on the liver biopsy in combination with the respective MELD-XI score (at evaluation or before HTx).
Statistical analysis
Data are presented as mean ± SD for continuous variables and as frequency and percent for categorical variables. Variables were compared between the groups with Student’s unpaired 2-tailed t-test for continuous variables and with chi-square or Fisher’s exact test for categorical variables. Uni- and multivariable logistic regression models were used to calculate odds ratios (ORs) and corresponding 95% confidence intervals (CIs) to identify risk factors for 1-year survival after transplant. p < 0.05 was considered statistically significant. In addition, the receiver-operating-characteristic (ROC) analysis was used to identify the optimal cut-off of liver risk score to differentiate 1-year survivors from those who died within 1-year post-transplant. The Kaplan–Meier method and log-rank test were used to assess and compare survival among groups. Cox proportional hazards regression models were used for the analysis of survival after listing and transplant. Clinical correlates with fibrosis score or MELD-XI were examined with Spearman’s correlation coefficient. All data were analyzed using SPSS version 20 (SPSS IBM). Intra- and interobserver reproducibility of fibrosis on biopsy readings was analyzed in 10 randomly selected patients, with slides repeatedly shown to a single reader (J.H.L.) and then adjudicated findings compared with scores from a second reader (J.M.).
Results
Study cohort
Sixty-eight patients (56.5 ± 11.8 years of age, 79.4% male, 58.8% Caucasian) underwent liver biopsy as part of their HTx evaluation (Table 1A and 1B and Figure 1). Etiology of HF was 29.4% ischemic, 22.1% dilated and 48.5% other (which included congenital, restrictive and infiltrative cardiomyopathies). These patients presented with abnormal hepatic function and had persistently abnormal liver function tests or abnormal abdominal sonogram findings, such as cirrhosis or nodularity or serologic evidence of past or present viral hepatitis. Comparison with a random sample of 200 patients who were evaluated, listed and transplanted during the same time period revealed differences in parameters associated with liver function, such as lower albumin (p = 0.04), a greater incidence of viral hepatitis (p < 0.001) and more abnormal liver imaging (fibrosis or cirrhosis on sonogram, p < 0.001), but no significant difference in MELD-XI or total bilirubin (p = 0.33; Table 2). Of the 68 patients, those rejected for HTx listing had higher MELD-XI (20.2 ± 12.5 vs 17.2 ± 7.7, p = 0.37) and more fibrosis on liver biopsy (>2–4, 68.8% vs 21.2%, p < 0.003; Table 1B).
Table 1.
| A Baseline Characteristics of HTx Candidates Under- going Liver Biopsy | |||
|---|---|---|---|
| Listed (n = 52) |
Not listed (n = 16) |
p-value | |
| Age (years) | 57.3 ± 11.2 | 53.9 ± 13.6 | 0.32 |
| Gender (n, % male) | 43 (82.7%) | 11 (68.8%) | 0.29 |
| Race (n, %) | 0.70 | ||
| White | 30 (57.7%) | 10 (62.5%) | |
| African American | 11 (21.2%) | 2 (12.5%) | |
| Hispanic | 5 (9.6%) | 3 (18.8%) | |
| Other | 6 (11.5%) | 1 (6.2%) | |
| Etiology of heart failure (n, %) |
1.0 | ||
| Dilated cardiomyopathy |
12 (23.1%) | 3 (18.8%) | |
| Ischemic | 15 (28.9%) | 5 (31.3%) | |
| Other | 25 (48.1%) | 8 (50.0%) | |
| Medication at evaluation (n, %) |
|||
| Amiodarone | 11 (21.2%) | 2 (12.5%) | 0.72 |
| Anti-platelets | 19 (36.5%) | 2 (12.5%) | 0.12 |
| β-Blockers | 34 (65.4%) | 12 (75.0%) | 0.55 |
| Coumadin | 17 (32.7%) | 9 (56.3%) | 0.09 |
| Diuretics | 48 (92.3%) | 11 (68.8%) | 0.03 |
| Inotropes | 34 (65.4%) | 9 (56.3%) | 0.51 |
| Laboratory data | |||
| Alkaline phosphatase |
113.6 ± 69.1 | 107.9 ± 40.0 | 0.76 |
| ALT (U/liter) | 29.2 ± 23.1 | 37.2 ± 44.6 | 0.50 |
| AST (U/liter) | 30.6 ± 23.5 | 37.2 ± 48.9 | 0.46 |
| Albumin (mg/dl) | 3.6 ± 0.7 | 3.5 ± 0.7 | 0.44 |
| Na (mEq/liter) | 134.8 ± 3.9 | 133.2 ± 4.8 | 0.18 |
| Creatinine (mg/dl) | 1.59 ± 1.14 | 2.24 ± 1.72 | 0.17 |
| Total bilirubin (mg/dl) |
1.97 ± 2.03 | 4.46 ± 9.83 | 0.35 |
| modMELD | 8.72 ± 2.55 | 10.64 ± 3.43 | 0.02 |
| MELD-XI | 17.16 ± 7.72 | 20.21 ± 12.48 | 0.37 |
| HepBsAg/HepBcAb/ HCVAb |
21 (41.2%) | 5 (31.3%) | 0.57 |
| B Fibrosis, Liver and Central Cardiac Hemodynamics and Cardiac Imaging of HTx Candidates Undergoing Liver Biopsy | |||
|---|---|---|---|
| Listed (n = 52) |
Not listed (n = 16) |
p-value | |
| Fibrosis on liver biopsy | |||
| (0–4) | |||
| 0–2 | 43 (78.8%) | 9 (31.3%) | 0.03 |
| > 2–4 | 9 (21.2%) | 7 (68.8%) | |
| Liver hemodynamics | |||
| Liver wedge pressure (mm Hg) |
21.7 ± 7.0 | 22.2 ± 7.1 | 0.82 |
| Liver free (mm Hg) | 15.5 ± 7.0 | 14.7 ± 6.7 | 0.74 |
| Trans-liver gradient (mm Hg) |
9.3 ± 5.0 | 7.2 ± 3.1 | 0.11 |
| Echocardiogram | |||
| LA (mm) | 51.9 ± 10.9 | 46.1 ± 13.1 | 0.13 |
| LVEDD (mm) | 64.3 ± 11.4 | 62.4 ± 15.2 | 0.61 |
| LVEF (%) | 21.9 ± 10.9 | 18.9 ± 6.9 | 0.32 |
| Severe RV dysfunction (n, %) |
20 (39.22%) | 3 (18.8%) | 0.13 |
| Severe TR (n, %) | 10 (19.2%) | 4 (26.7%) | 0.50 |
| Cardiac hemodynamics | |||
| RA (mm Hg) | 12.8 ± 6.7 | 18.1 ± 6.4 | 0.01 |
| Mean PAP (mm Hg) | 31.9 ± 10.9 | 30.0 ± 7.6 | 0.53 |
| PCWP (mm Hg) | 21.8 ± 8.0 | 22.8 ± 6.5 | 0.67 |
| PVR (Wood units) | 3.23 ± 2.09 | 2.33 ± 1.11 | 0.044 |
| TPG (mPAP–PCWP) (mm Hg) |
9.38 ± 4.95 | 7.20 ± 3.05 | 0.11 |
| RA/PCWP | 0.58 ± 0.28 | 0.85 ± 0.34 | 0.004 |
| PA SatO2 (%) | 56.0 ± 10.7 | 49.9 ± 11.5 | 0.07 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; HCV, hepatitis C virus; HepBcAb, hepatitis B core antibody; HepBsAg, hepatitis B surface antigen; HTx, heart transplantation; MELD-XI, Model of End-stage Liver Dysfunction eXcluding International normalized ratio; modMELD, modified Model of End-stage Liver Dysfunction.
HTx, heart transplantation; LA, left atrium; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; mPAP, mean pulmonary artery pressure; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RA, right atrium; RV, right ventricle; SatO2, oxygen saturation; TPG, transpulmonary gradient; TR, tricuspid regurgitation.
Table 2.
Comparison of HTx Candidates Who Did or Did Not Have Liver Biopsy
| Biopsy (n = 68) |
No biopsy (n = 200) |
p-value | |
|---|---|---|---|
| Total bilirubin (mg/dl) | 2.53 ± 5.0 | 1.7 ± 2.1 | 0.19 |
| Creatinine (mg/dl) | 1.74 ± 1.3 | 1.49 ± 0.8 | 0.14 |
| Albumin (mg/dl) | 3.6 ± 0.7 | 3.78 ± 0.6 | 0.04 |
| MELD-XI | 12.7 ± 3.3 | 13.2 ± 5.2 | 0.33 |
| Fibrosis or cirrhosis on abdominal sonogram (%) |
76.5 | 21.5 | < 0.001 |
| Serologic evidence of hepatitis B or C (%) |
32.4 | 13.5 | < 0.001 |
MELD-XI, Model of End-stage Liver Dysfunction eXcluding International normalized ratio.
Clinical correlates with fibrosis score or MELD-XI
Upon dividing the 68 patients based on degree of liver fibrosis into 0–2 (low fibrosis score) and >2–4 (higher fibrosis score) groups, we found no significant differences in albumin level, MELD-XI score, abnormal liver sonogram findings (fibrosis or cirrhosis), left ventricular end-diastolic dimension (as an index of restrictive cardiomyopathy) or right atrial pressure (see Table S1 in Supplementary Material available online at www.jhltonline.org). MELD-XI at evaluation correlated positively with right atrial (RA) pressure (r = 0.32, p = 0.01) and negatively with albumin (r = −0.24, p = 0.04), but with no other parameter. In 72% of patients liver hemodynamics were also obtained. In those patients, there were no significant correlations except for RA and free hepatic wedge pressure (r = 0.25, p = 0.04).
Outcomes and 1-year survival after HTx
Fifty-two patients were listed for HTx, whereas 16 were considered too ill to undergo HTx. Of the 52 patients listed, 14 underwent MCS (8 left ventricular assist device [LVAD], 5 biventricular assist device [BiVAD] and 1 total artificial heart [TAH]). HTx was performed in 36 patients (Table 3A and 3B) with 1-year post-HTx survival of 75%, which is lower than the expected 1-year survival of about 88–90% at our institution (Figure 1).23 The 1-year post-HTx survivors had a lower left ventricular (LV) ejection fraction (19.1 ± 8.5% vs 31.1 ± 15.8%; p = 0.06) and lower transpulmonary gradient (8.8 ± 4.4 mm Hg vs 12.8 ± 7.2 mm Hg, p = 0.06) at evaluation. One-year post-transplant survivors had a lower MELD-XI score (14.53 ± 4.1 vs 21.63 ± 6.4, p < 0.01) and lower liver risk score (31.0 ± 20.37 vs 65.2 ± 28.58, p < 0.01) at evaluation, as well as a lower pre-operative MELD-XI score (10.46 ± 2.49 vs 14.01 ± 2.87, p < 0.01) and liver risk score (26.8 ± 13.7 vs 54.6 ± 25.1, p = 0.01). There were no significant differences in liver fibrosis (p = 0.15), abnormal abdominal imaging (p = 0.61), need for MCS (44.4% vs 22.2%, p = 0.23) or time from liver biopsy to HTx (p = 0.36). In a univariable logistic regression analysis, MELD-XI score and liver risk score at evaluation, as well as pre-operative MELD-XI score and liver risk score, were found to predict death <1 year post-HTx. Multivariable logistic regression analysis revealed that only the liver risk score at time of HTx evaluation and MELD-XI score immediately pre-HTx were independent predictors of death within 1 year after HTx (Table 4).
Table 3.
| A Baseline Characteristics of All Transplanted Patients (n = 36) With ≥1 Year Follow-up | |||
|---|---|---|---|
| Recipients surviving ≥1 year post-HTx (n = 27) |
Recipients dying < 1 year post-HTx (n = 9) |
p-value | |
| Age at HTx (years) | 55.1 ± 11.7 | 56.6 ± 9.0 | 0.73 |
| Gender [number of males (%)] | 25 (92.6%) | 8 (88.9%) | 1.0 |
| Race (n, %) | 0.94 | ||
| White | 14 (53.9%) | 6 (66.7%) | |
| African American | 6 (23.1%) | 1 (11.1%) | |
| Hispanic | 2 (7.7%) | 1 (11.1%) | |
| Other | 5 (18.5%) | 1 (11.1%) | |
| Etiology of heart failure (n, %) | 0.13 | ||
| Dilated cardiomyopathy | 9 (33.3%) | 0 (0%) | |
| Ischemic | 7 (25.9%) | 4 (44.4%) | |
| Other | 11 (40.7%) | 5 (55.6%) | |
| Laboratory data (at evaluation) | |||
| Alkaline phosphatase | 101.7 ± 60.4 | 146.8 ± 107.8 | 0.261 |
| GFR (ml/min/1.73 m2) | 147.4 ± 112.3 | 101.2 ± 38.4 | 0.07 |
| Platelets (×103/µl) | 182.8 ± 59.5 | 158.7 ± 78.9 | 0.34 |
| TP (mg/dl) | 6.8 ± 1.3 | 6.5 ± 0.6 | 0.38 |
| ALT (U/L) | 30.7 ± 25.3 | 20.2 ± 11.8 | 0.11 |
| AST (U/L) | 32.9 ± 26.7 | 22.9 ± 9.1 | 0.10 |
| Albumin (mg/dl) | 3.8 ± 0.7 | 3.6 ± 0.5 | 0.31 |
| Na (mEq/liter) | 136.3 ± 3.7 | 132.1 ± 4.3 | 0.01 |
| Creatinine (mg/dl) | 1.63 ± 1.49 | 1.74 ± 0.67 | 0.82 |
| Total bilirubin (mg/dl) | 1.25 ± 0.66 | 2.59 ± 1.58 | 0.036 |
| modMELD | 7.97 ± 2.28 | 10.16 ± 1.58 | 0.01 |
| MELD-XI | 14.53 ± 40.8 | 21.63 ± 6.43 | 0.0004 |
| HepBsAg/HepBcAb/HCVAb | 11 (42.3%) | 1 (11.1%) | 0.01 |
| Evaluation liver risk score | 31.0 ± 20.37 | 65.2 ± 28.58 | 0.0004 |
| B Fibrosis, Liver and Central Cardiac Hemodynamics and Cardiac Imaging of Patients Undergoing HTx Dichotomized by Survival to 1 Year | |||
|---|---|---|---|
| Recipients surviving ≥1 year post-HTx (n = 27) |
Recipients dying < 1 year post-HTx (n = 9) |
p-value |
|
| Liver imaging abnormality (n, %) | 0.61 | ||
| Cirrhosis/nodular | 8 (29.6%) | 2 (22.2%) | |
| Echogenic | 9 (33.3%) | 6 (66.7%) | |
| Fatty | 2 (7.4%) | 0 (0%) | |
| Amiodarone | 1 (3.7%) | 0 (0%) | |
| Liver imaging normal | 7 (25.9%) | 1 (11.1%) | |
| Fibrosis on evaluation of liver biopsy (0–4) | 0.15 | ||
| 0–2 | 26 (96.3%) | 7 (77.8%) | |
| > 2–4 | 1 (3.7%) | 2 (22.2%) | |
| Liver hemodynamics | |||
| Liver wedge pressure (mm Hg) | 20.2 ± 7.1 | 21.5 ± 8.2 | 0.72 |
| Liver free (mm Hg) | 15.7 ± 6.6 | 16.7 ± 8.0 | 0.77 |
| Trans-liver gradient (mm Hg) | 8.8 ± 4.4 | 12.8 ± 7.2 | 0.06 |
| Echocardiogram | |||
| LA (mm) | 50.8 ± 10.8 | 53.2 ± 12.7 | 0.60 |
| LVEDD (mm) | 63.1 ± 12.6 | 66.0 ± 7.9 | 0.56 |
| LVEF (%) | 19.1 ± 8.5 | 31.1 ± 15.8 | 0.06 |
| Severe RV dysfunction (n, %) | 13 (50.0%) | 3 (33.2%) | 0.70 |
| Severe TR (n, %) | 8 (29.6%) | 1 (11.1%) | 0.40 |
| Hemodynamics | |||
| RA (mm Hg) | 13.2 ± 6.2 | 11.3 ± 7.7 | 0.49 |
| Mean PAP (mm Hg) | 32.4 ± 12.0 | 35.1 ± 10.4 | 0.57 |
| PCWP (mm Hg) | 22.3 ± 7.8 | 22.4 ± 9.8 | 0.98 |
| PVR (Wood units) | 3.2 ± 2.0 | 4.2 ± 2.2 | 0.25 |
| TPG (mPA–PCWP) (mm Hg) | 8.8 ± 4.4 | 12.8 ± 7.2 | 0.06 |
| RA/PAWP | 0.62 ± 0.30 | 0.52 ± 0.37 | 0.48 |
| PA SatO2 (%) | 56.1 ± 11.1 | 52.3 ± 10.3 | 0.40 |
| Pre-operative liver risk score | 26.8 ± 13.7 | 54.6 ± 25.1 | 0.01 |
| Duration liver biopsy to HTx (days) | 277 ± 462 | 137 ± 126 | 0.16 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GFR, glomerular filtration rate; HCVAb, hepatitis C virus antibody; HepBcAb, hepatitis B core antibody; HepBsAg, hepatitis B surface antigen; HTx, heart transplantation; LVEDD, left ventricular end-diastolic volume; MELD-XI, Model of End-stage Liver Dysfunction eXcluding International normalized ratio; modMELD, modified Model of End-stage Liver Dysfunction; TP, total protein.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GFR, glomerular filtration rate; HTx, heart transplantation; LA, left atrium; LVEDD, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; MELD-XI, modMELD, PA, pulmonary artery; PAP, pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PCWP, pulmonary capillary wedge pressure; RA, right atrium; SatO2, oxygen saturation; TPG, transpulmonary gradient; TR, tricuspid regurgitation.
Table 4.
Uni- and Multivariable Regression Analysis of Predictors of Death < 1 Year Post-HTx
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Evaluation MELD-XI | 1.32 | 1.08–1.61 | 0.01 | |||
| Evaluation albumin | 0.54 | 0.17–1.72 | 0.30 | |||
| Evaluation liver risk score | 1.06 | 1.02–1.1 | 0.01 | 1.08 | 1.01–1.15 | 0.02 |
| Abnormal liver sonogram (fibrosis or cirrhosis) | 2.80 | 0.29–26.6 | 0.37 | |||
| Fibrosis on liver biopsy | ||||||
| Fibrosis 0–2 | Reference | |||||
| Fibrosis > 2–4 | 7.43 | 0.59–94.3 | 0.12 | |||
| VAD pre-HTx | 2.80 | 0.57–13.8 | 0.21 | |||
| Pre-operative MELD–XI | 1.87 | 1.16–3.03 | 0.01 | 2.22 | 1.04–4.75 | 0.04 |
| Pre-operative albumin | 0.37 | 0.12–1.18 | 0.09 | |||
| Pre-operative liver risk score | 1.10 | 1.03–1.19 | 0.01 | |||
| Improved MELD-XI (evaluation to HTx) | 0.90 | 0.79–1.03 | 0.13 | |||
CI, confidence interval; HTx, heart transplantation; MELD-XI, Model of End-stage Liver Dysfunction eXcluding International normalized ratio; OR, odds ratio; VAD, ventricular assist device.
Post-operative complications after HTx in our cohort were frequent and included 14% with acute graft dysfunction requiring mechanical support, 19% with bleeding requiring reoperation, 22% needing prolonged intubation (48 hours after surgery), 19% with sternal wound infection, 6% with cerebrovascular accidents and 19% dying in-hospital. Recipients who died <1 year post-HTx more frequently had acute graft dysfunction requiring mechanical support (p < 0.01) and longer ventilation times (p = 0.01) and required more reoperations secondary to bleeding (p = 0.05) (Table 5). Of the 16 patients listed but not transplanted, 1-year survival after liver biopsy was only 50% (8 of 16).
Table 5.
Post-operative Outcomes of HTx Patients
| Recipients surviving > 1 year post-HTx (n = 27) |
Recipients dying < 1 year post-HTx (n = 9) |
p-value | |
|---|---|---|---|
| Graft dysfunction requiring MCS | 1 (3.7%) | 4 (44.4%) | 0.009 |
| Ventilator ≥48 h post-HTx | 3 (11.1%) | 5 (55.6%) | 0.013 |
| Creatinine > 2.5 mg/dl ≥48 h post-HTx | 7 (25.9%) | 5 (55.6%) | 0.125 |
| Reoperation for bleeding | 3 (11.1%) | 4 (44.4%) | 0.049 |
| Sternal wound infection | 6 (22.2%) | 1 (11.1%) | 0.652 |
HTx, heart transplantation; MCS, mechanical circulatory support.
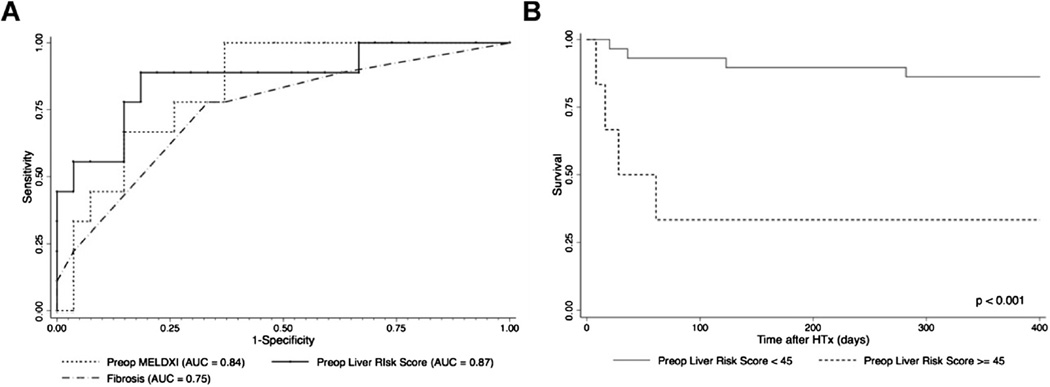
In the ROC analysis, with the cohort of 36 patients transplanted, a mean pre-operative liver risk score of <45 predicted higher 1-year survival post-transplant with a sensitivity of 88.9% and specificity of 78.9% (Figure 3A). According to Kaplan–Meier survival analysis, 1-year survival was 95.5% in patients with a score of <45, but only 42.9% in those with a score of ≥45 (Figure 3B). The numbers were too small to determine reliably whether fibrosis from cardiac congestion portended a worse prognosis compared with other forms of fibrosis.
Figure 3.
Evaluation of the liver risk score. (A) AUC curve statistic for comparison of pre-operative MELD-XI, pre-operative liver risk score and degree of fibrosis on liver biopsy specimens. (B) One-year survival of patients after HTx based on pre-operative liver risk scores dichotomized by 45.
Mortality after HTx listing and after MCS
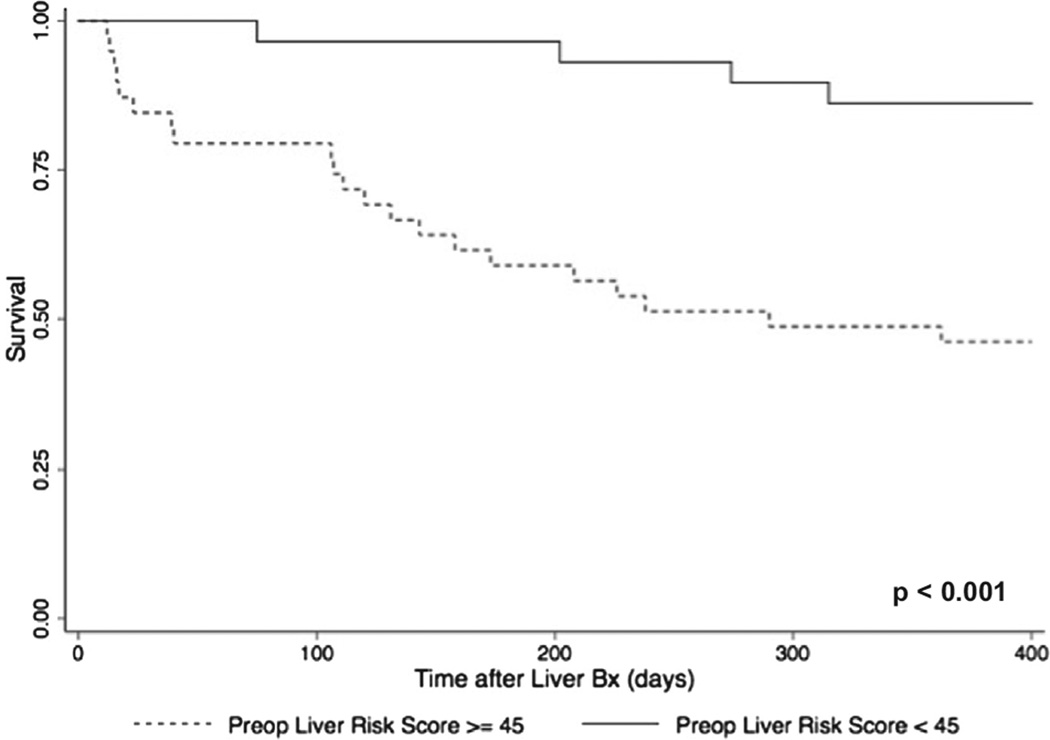
Based on a cut-off of the liver risk score of 45 as an indicator of high risk, we evaluated whether this cut-point could yield information regarding 1-year survival after liver biopsy in the 52 patients listed for HTx. One-year survival after listing was 86.7% (standard error [SE] = 0.07) in risk, but 45.5% (SE = 0.11) in high-risk (p < 0.001) patients. In a Cox proportional hazards regression analysis, patients with a liver risk score of ≥45 at evaluation for HTx had a higher risk of dying compared with patients having a score of <45 (HR 3.94, 95% CI 1.77–8.79, p < 0.001), even after the adjustment of pre-operative albumin level (HR 4.35, 95% CI 1.48–12.8, p = 0.001) (Figure 4). For the 14 patients who underwent VAD therapy as bridge to transplant (LVAD or BiVAD/TAH), 1-year survival after VAD surgery was 85.7% (SE = 0.13) in low-risk patients compared with 57.1% (SE = 0.18) in high-risk patients (p = 0.24).
Figure 4.
One-year survival after liver biopsy. Kaplan–Meier survival analysis for all patients after liver biopsy based on high- vs low-risk liver risk scores.
Discussion
Previous work from our group and others has demonstrated that increased MELD and MELD-XI scores portend poor outcomes in patients with advanced HF, after MCS and post-HTx.7,10 As these scores may reflect dynamic parameters of liver function, they respond and fluctuate to HF therapies, mechanical support and following HTx. Liver fibrosis of any etiology may indicate irreversible hepatic damage and, therefore, many HTx centers use non-invasive liver imaging such as abdominal sonography and computed tomography, or liver biopsy and invasive hemodynamic assessment for the characterization of patients with preexisting and potentially irreversible liver dysfunction. The clinical assumption underlying this strategy is that impaired liver hemodynamics and abnormal biopsy findings may identify a high-risk post-operative course related to profound bleeding, vasodilatory shock and increased risk for infection and subsequent death. Although a high MELD score can identify a high-risk HTx candidate, liver biopsy provides specific information regarding structural liver impairment, even under optimal hemodynamic conditions.
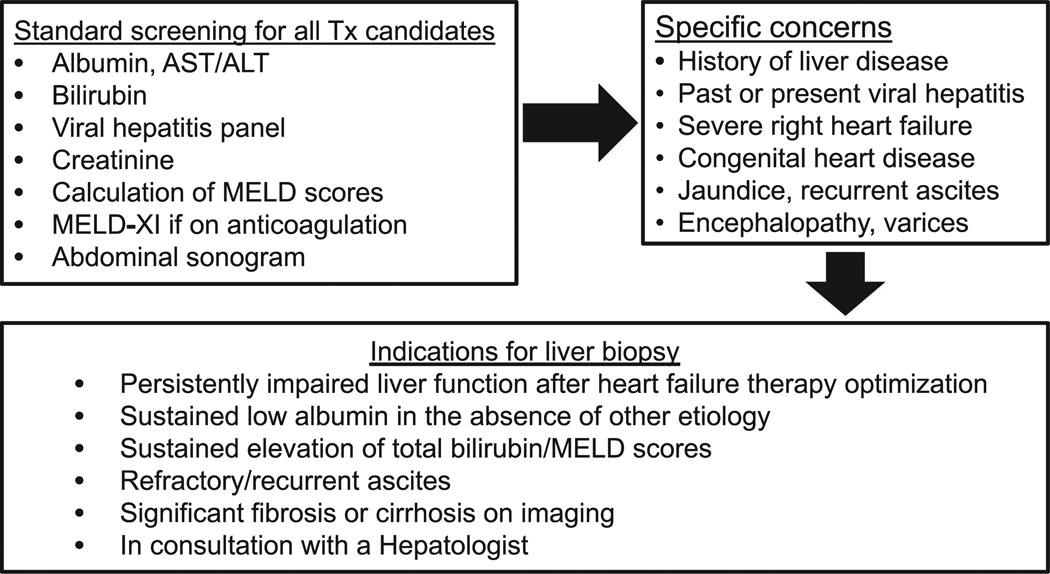
Our study is the largest series of HTx candidates with presumed advanced liver disease who underwent liver biopsy as part of their HTx evaluation with 1-year follow-up post-HTx. We have defined a novel liver risk score as the combination of MELD-XI and degree of fibrosis on liver biopsy in patients with suspected liver dysfunction who are potential candidates for HTx. We showed that a liver risk score of ≥45 at the time of HTx evaluation can identify patients carrying a high 1-year mortality risk before and after HTx, regardless of the use of MCS as bridging therapy. A flow diagram of the algorithm and general indications for liver biopsy at our institution has been proposed, as shown in Figure 5.
Figure 5.
Suggested diagnostic algorithm in patients evaluated for HTx with suspected liver disease.
A critical issue that arises from our study is the clinical need for more consideration of combined heart–liver Tx in patients with advanced HF and severe liver disease.24 Data from the United Network for Organ Sharing (UNOS) database show that 112 combined heart–liver Txs were performed between 2003 and 2011 (based on OPTN data as of May 1, 2013). Between 2003 and 2007, the 1- and 5-year survival rates were 91.2% and 85.1%, respectively. For the period 2008–2011, 1-year survival decreased to 81.8%. Thus, combined heart–liver Tx is a viable option, but it remains difficult to determine uniformly which patients have this indication.
Current UNOS policies define a combined organ allocation priority in patients listed for heart–liver Tx.25 In an analysis of the UNOS data set focusing on combined heart–liver Tx between 1987 and 2004, only 29.6% of patients listed survived to Tx.26 That study showed lower MELD scores (15.2 vs 28.8) in waitlist survivors who reached Tx, suggesting that there is likely an optimal time-point at which a patient is not too sick to survive to dual-organ Tx. Advocacy for MELD “exception points” on the basis of advanced HF and a resulting need for dual-organ transplantation may be the only viable strategy to optimize success in transplanting this unique population.26
Limitations of our study include potential selection bias of patients who underwent liver biopsy, the small number of patients, and the lack of prospective validation. Our study represents a retrospective assessment of the practice and findings of a single institution with all the associated limitations of a single-center study, but it is the largest series thus far and may serve as a foundation for other centers to report their practices and results. A future goal would be to identify non-invasive parameters, such as more detailed liver imaging as well as novel biomarkers of liver function and fibrosis, that may replace biopsy as well as provide a tool for serial assessment for ongoing risk stratification while awaiting Tx.
In conclusion, liver disease in the context of HF is an increasingly common clinical problem with implications for the management of these patients both before and after HTx. We propose a method of incorporating liver biopsy findings into the assessment of HTx candidates that can offer unique predictive information regarding post-HTx outcomes. Our approach defines risk prediction that may assist in triaging patients who may be too sick to undergo isolated HTx. Further work will be required to clarify whether these patients may benefit from combined heart–liver Tx.
Supplementary Material
Acknowledgments
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government. This work was supported by a grant from the National Center for Advancing Translational Sciences, National Institutes of Health (NIH) (UL1 TR000040 to M.F.); the National Heart, Lung, and Blood Institute, NIH (HL095742, HL101272 and HL114813 to P.C.S.); the Irving Institute of Clinical and Translational Research at Columbia University (UL1 RR024156); and the Health Resources and Services Administration (Contract No. 234-2005-370011C).
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
Supplementary data associated with this article can be found in the online version at www.jhltonline.org.
References
- 1.Heart Failure Society of America. 2010 Comprehensive heart failure practice guidelines. J Card Fail. 2010;16:e1–e180. doi: 10.1016/j.cardfail.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Costanzo MR, Dipchand A, Starling R, et al. The International Society for Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–956. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 3.Schulze PC, Jiang J, Yang J, et al. Preoperative assessment of high risk candidates to predict survival after heart transplantation. Circ Heart Fail. 2013;6:527–534. doi: 10.1161/CIRCHEARTFAILURE.112.000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slaughter MS. Long-term continuous flow left ventricular assist device support and end-organ function: prospects for destination therapy. J Cardiothorac Surg. 2010;25:490–494. doi: 10.1111/j.1540-8191.2010.01075.x. [DOI] [PubMed] [Google Scholar]
- 5.Kamdar F, Boyle A, Liao K, et al. Effects of centrifugal, axial and pulsatile left ventricular assist device support on end-organ function in heart failure patients. J Heart Lung Transplant. 2009;28:352–359. doi: 10.1016/j.healun.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Kamdar F, John R, Eckman P, et al. Post-cardiac transplant survival in the current era in patients receiving continuous-flow left ventricular assist devices. J Thorac Cardiovasc Surg. 2013;145:575–581. doi: 10.1016/j.jtcvs.2012.09.095. [DOI] [PubMed] [Google Scholar]
- 7.Choksi A, Cheema F, Shahzad K, et al. Hepatic dysfunction and survival after heart transplantation: application of the MELD scoring system for outcome prediction. J Heart Lung Transplant. 2012;31:591–600. doi: 10.1016/j.healun.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelow JM, Desai AS, Hochberg CP, et al. Clinical predictors of hepatic fibrosis in chronic advanced heart failure. Circ Heart Fail. 2010;3:59–64. doi: 10.1161/CIRCHEARTFAILURE.109.872556. [DOI] [PubMed] [Google Scholar]
- 9.Samsky MD, Patel CB, DeWald TA, et al. Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol. 2013;61:2397–2405. doi: 10.1016/j.jacc.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 10.Martin PY, Gines P, Schrier RW. Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N Engl J Med. 1998;339:533–541. doi: 10.1056/NEJM199808203390807. [DOI] [PubMed] [Google Scholar]
- 11.Kato TS, Stevens GR, Jiang J, et al. Risk stratification of ambulatory patients with advanced heart failure undergoing evaluation for heart transplantation. J Heart Lung Transplant. 2013;32:333–340. doi: 10.1016/j.healun.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim MS, Kato TS, Farr M, et al. Hepatic dysfunction in ambulatory patients with heart failure—application of the MELD Sscoring system for outcome prediction. J Am Coll Cardiol. 2013;61:2253–2261. doi: 10.1016/j.jacc.2012.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Kato TS, Shulman B, et al. Liver dysfunction as a predictor of outcomes in patients with advanced heart failure requiring ventricular assist device support: use of the Model of End-stage Liver Disease (MELD) and MELD eXcluding INR (MELD-XI) scoring system. J Heart Lung Transplant. 2012;31:601–661. doi: 10.1016/j.healun.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews JC, Pagani FD, Haft JW, et al. Model for End-stage Liver Disease score predicts left ventricular assist device operative transfusion requirements, morbidity and mortality. Circulation. 2010;121:214–220. doi: 10.1161/CIRCULATIONAHA.108.838656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 16.Heuman DM, Mihas AA, Habib A, et al. MELD-XI: a rational approach to “sickest first” liver transplantation in cirrhotic patients requiring anticoagulation therapy. Liver Transplant. 2007;13:30–37. doi: 10.1002/lt.20906. [DOI] [PubMed] [Google Scholar]
- 17.Kalambokis G, Manousou P, Vibhakorn S, et al. Transjugular liver biopsy—indications, adequacy, quality of specimens and complications—a systematic review. J Hepatol. 2007;47:284–294. doi: 10.1016/j.jhep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Lefkowitch JH, Scheuer PJ. Scheuer’s liver biopsy interpretation. 8th. Philadelphia: Saunders; 2010. [Google Scholar]
- 19.Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007;47:598–607. doi: 10.1016/j.jhep.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Batts KP, Ludwig J. Chronic hepatitis: an update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1412. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Kleiner DE, Brunt EM, van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 22.Wanless IR, Huang W-Y. MacSween’s pathology of the liver. 6th. Edinburgh: Churchill Livingstone Elsevier; 2012. Vascular disorders; pp. 611–612. [Google Scholar]
- 23.Scientific Registry of Transplant Recipients. [Accessed January 15, 2015]; http://www.SRTR.org/ [Google Scholar]
- 24.Cannon RM, Hughes MG, Jones CM, et al. A review of the United States experience with combined heart–liver transplantation. Transplant Int. 2012;25:1223–1228. doi: 10.1111/j.1432-2277.2012.01551.x. [DOI] [PubMed] [Google Scholar]
- 25.Organ Procurement and Transplantation Network. Policy 3.6. [Accessed January 2015]; http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_8.pdf/
- 26.Porrett PM, Desai SS, Timmins KJ, et al. Combined orthotopic heart and liver transplantation: the need for exception status listing. Liver Transplant. 2004;10:1539–1544. doi: 10.1002/lt.20279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.