Abstract
Please cite this paper as: Peebles et al. (2010) Influenza‐associated mortality among children – United States: 2007–2008. Influenza and Other Respiratory Viruses 5(1), 25–31.
Background Since October 2004, pediatric influenza‐associated deaths have been a nationally notifiable condition. To further investigate the bacterial organisms that may have contributed to death, we systematically collected information about bacterial cultures collected at non‐sterile sites and about the timing of Staphylococcus aureus specimen collection relative to hospital admission.
Methods We performed a retrospective, descriptive study of all reported influenza‐associated pediatric deaths in 2007–2008 influenza season in the United States.
Results During the 2007–2008 influenza season, 88 influenza‐associated pediatric deaths were reported. The median age was 5 (range 29 days – 17 years); 48% were <5 years of age. The median time from symptom onset to death was 4 days (range 0–64 days). S. aureus was identified at a sterile site or at a non‐sterile site in 20 (35%) of the 57 children with specimens collected from these sites; in 17 (85%) of these children, specimens yielding S. aureus were obtained within three days of inpatient admission. These 17 children were older (10 versus 4 years, median; P < 0·05) and less likely to have a high‐risk medical condition (P < 0·05) than children with cultures from the designated sites that did not grow S. aureus.
Conclusions S. aureus continues to be the most common bacteria isolated from children with influenza‐associated mortality. S. aureus isolates were associated with older age and lack of high‐risk medical conditions. Healthcare providers should consider influenza co‐infections with S. aureus when empirically treating children with influenza and severe respiratory illness.
Keywords: Influenza, mortality rates, Staphlyococcus aureus
Background
Seasonal influenza causes an estimated annual average of 226 000 hospitalizations and 36 000 deaths in the United States. The highest rates of influenza‐associated hospitalizations and death occur among the elderly, young children, and persons with certain high‐risk medical conditions. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 In October 2004, pediatric influenza‐associated deaths became a nationally notifiable condition to the U.S. National Notifiable Diseases Surveillance System, following reports of death in children associated with influenza in the 2003–2004 season. 9 , 10 , 11 During the 2006–2007 influenza season, an increase in influenza‐related pediatric deaths associated with Staphylococcus aureus infection was first reported. 12 This prompted collection of additional data on bacterial cultures from children with influenza‐related deaths during the 2007–2008 influenza season, including the culture results from any bacterial culture from a normally sterile site or specified non‐sterile site, and information on timing of specimen collection relative to hospital admission. Previously, information regarding cultures with negative results was not requested, and the timing for specimen collection relative to hospital admission was not collected. Here, we describe the reports of children with influenza‐associated mortality and bacterial co‐infection from the 2007–2008 influenza season.
Methods
A case was defined as the death of a US resident aged <18 from October 1, 2007–September 30, 2008 with laboratory evidence of an influenza virus type A or B infection. A positive laboratory test for influenza type A or B could occur before or after death and may be determined by any of the following methods: rapid influenza diagnostic test, viral isolation, enzyme immunoassay, fluorescent antibody staining, immunohistochemical staining of tissue samples, or reverse transcription polymerase chain reaction. Specimens for bacterial and viral culture were collected as part of routine clinical care and the postmortem examination, when applicable. CDC requested that respiratory specimens and postmortem lung specimens were sent to CDC laboratories if available. Influenza virus isolates, other viral isolates, and bacterial isolates were characterized at local, hospital, state, or CDC laboratories. Genotyping of available S. aureus isolates was performed at CDC by pulsed‐field gel electrophoresis (PFGE) using SmaI‐digested DNA. 13 Gel patterns were analyzed as previously described. 14
State or local health departments completed a standardized reporting form for each case of influenza‐associated pediatric mortality and transmitted the information to CDC via a web‐based interface hosted on CDC’s Secure Data Network. Through the standardized reporting form, information was collected on patient demographics, influenza laboratory test results, date and location of death, pre‐existing conditions, complications during the acute illness (including radiologically confirmed pneumonia), influenza vaccination history, and results of bacterial cultures obtained from normally sterile (blood, pleural fluid, chest tube fluid, or cerebral spinal fluid) and specified non‐sterile (endotracheal tube aspirates, tracheal aspirates, and bronchial washes) sites. Information was not collected on bacterial cultures obtained from nares or sputum, as positive cultures from these sites are more likely to represent colonization rather than infection. The reporting form includes a notes section for reporting additional information and for clarification. Information regarding bacterial isolation from postmortem lung biopsies and fungal co‐infections was not directly solicited; however, this information could be reported in a notes section of the reporting form. For our analysis, bacterial isolation from postmortem lung biopsies were included only if the specimen was collected on the calendar day of death or the calendar day following death.
Dates of hospital admission and specimen collection were obtained for all patients from whom S. aureus was isolated from one of the designated sites. We focused our analyses on those patients from whom S. aureus was isolated from a specimen collected within three days of hospital admission, as we were attempting to identify patients in whom a bacterial co‐infection may have contributed to their severe presentation (as opposed to having been acquired during hospitalization).
Data were stored in SQL 2005 and Microsoft Access 2003 (Redmond, WA, USA) and analyzed using SAS 9.1 (SAS Institute, Cary, NC, USA). A chi‐square test was used to evaluate differences between proportions. A Wilcoxon two‐sample test was used to evaluate differences between medians. All comparisons were 2 sided, and a P‐value <0·05 was considered significant.
Results
During the 2007–2008 influenza season, a total of 88 influenza‐associated pediatric deaths were reported to CDC. Of the 88 children, 47 (53%) were boys and the median age at death was 5. Forty‐two (47%) of the children were <5 years of age, and 14 (16%) were <1 year of age (Figure 1). Most were white (Table 1).
Figure 1.
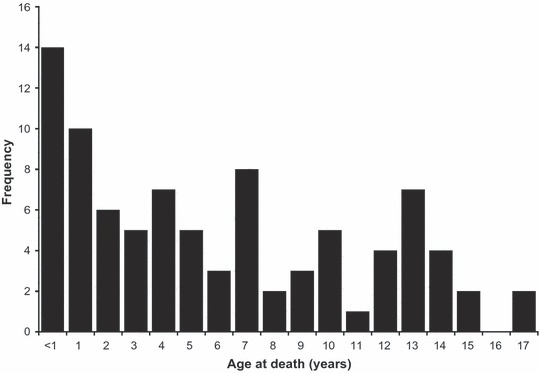
Frequency of age at death (years) among reported influenza‐associated pediatric deaths: USA October 1, 2007–September 30, 2008.
Table 1.
Race/ethnicity, location of death, and influenza type among children with influenza‐associated mortality – United States: 2007–2008
| Characteristic | N = 88 |
|---|---|
| Race/ethnicity, n (%) | |
| White | 46 (52) |
| Black | 14 (16) |
| Asian | 2 (2) |
| Hispanic | 20 (23) |
| American indian/Alaska native | 1 (1) |
| Unknown | 5 (6) |
| Location of death, n (%) | |
| Emergency department | 19 (22) |
| Intensive care unit | 47 (53) |
| Inpatient ward | 5 (6) |
| Operating room | 1 (1) |
| Outside the hospital | 16 (18) |
| Influenza Type, n/N (%) | |
| A | 54/84 (64) |
| H1 | 6/23 (26) |
| H3 | 17/23 (74) |
| B | 30/84 (36) |
| A and B co‐infected | 2/88 (2) |
| A/B undistinguished | 2/88 (2) |
The median time from onset of symptoms to death was four days, with 40% dying within three days and 72% dying within seven days of symptom onset. About half of the children died in the intensive care unit, and 40% died in the emergency department or outside the hospital (Table 1). Ventilation status was known for 50 children who died in the hospital (excluding emergency department) of which 48 (96%) required mechanical ventilation.
Information on pre‐existing conditions was known for 81/88 of the children; 39 (48%) had an Advisory Committee on Immunization Practices (ACIP)‐defined high‐risk medical condition. Of these, twenty children had one high‐risk condition, ten had two high‐risk conditions, five had three high‐risk conditions, and four had four high‐risk conditions. The high‐risk medical conditions reported were asthma (n = 15), moderate to severe developmental delay (n = 13), seizure disorder (n = 11), cardiac disease (n = 9), neuromuscular disease (n = 9), chronic pulmonary disease (n = 6), immunosuppressive condition (n = 3), cystic fibrosis (n = 2), metabolic disorder (n = 2), and renal disease (n = 1).
Fifty‐six (66%) of 85 children were recommended for vaccination by 2007–2008 ACIP criteria, and of these 48 had a known vaccination status for the 2007–2008 influenza season. Eleven (23%) of 48 recommended children received at least one dose of influenza vaccine in the 2007–2008 season at least 14 days prior to illness onset. Of these 11 children, six were completely vaccinated, one was partially vaccinated, and complete vaccination status could not be determined in four. One child who received the vaccine in the 2007–2008 season did not meet an age‐related or ACIP‐defined high‐risk criteria for vaccination.
Data on complications were known for 79 (90%) of the children. The complications most commonly reported were pneumonia (n = 34), acute respiratory distress syndrome (n = 25), sepsis (n = 15), seizures (n = 15), shock (n = 13), encephalopathy or encephalitis (n = 6), and bronchiolitis (n = 5).
Of the 57 children who had specimens for bacterial culture collected from one of the specified sterile or non‐sterile sites and had known results, 29 (51%) had positive bacterial cultures. S. aureus was the most commonly identified organism, identified in 20 (69%) of the 29 children with any positive culture. In 17 (85%) of these 20 children, specimens yielding S. aureus were collected within three days of inpatient admission (Table 2).
Table 2.
Children with bacteria isolated from specified sites among children with influenza‐associated mortality – United States: 2007–2008
| Among 53 children with specimens collected from sterile sites* n, (%) | Among 29 children with specimens collected from non‐sterile sites** n, (%) | Among 57 children with specimens collected from sterile sites* or non‐sterile sites** n, (%) | Among 10 children with positive postmortem lung specimen results*** n (negative results not obtained) | |
|---|---|---|---|---|
| ≥1 Organism identified | 16 (30) | 17 (59) | 29 (51) | 10 |
| Staphylococcus aureus | 11 (21) | 13 (45) | 20 (35) | 4 |
| Methicillin‐resistant Staphlyococcus aureus | 9 (17) | 5 (17) | 12 (21) | 2 |
| Methicillin‐susceptible Staphlyococcus aureus | 2 (4) | 7 (24) | 7 (12) | 1 |
| Susceptibility unknown | 0 | 1 (3) | 1 (2) | 1 |
| Other Gram‐positive bacterium | ||||
| Streptococcus pyogenes | 3 (6) | 1 (3) | 4 (7) | 2 |
| Streptococcus pneumoniae | 1 (2) | 0 | 1 (2) | 4 |
| Enterococcus | 2 (4) | 0 | 2 (4) | 0 |
| Streptococcus bovis | 1 (2) | 0 | 1 (2) | 0 |
| Beta hemolytic streptococcus | 0 | 0 | 0 | 0 |
| Gram‐negative bacterium | ||||
| Pseudomonas aeruginosa | 1 (2) | 1 (3) | 2 (4) | 0 |
| Moraxella catarrhalis | 0 | 2 (7) | 2 (4) | 0 |
| Stenotrophomones | 0 | 1 (3) | 1 (2) | 0 |
| Haemophilus influenzae (not‐typed) | 0 | 0 | 0 | 1 |
| Klebsiella oxytoca | 0 | 0 | 0 | 1 |
| Klebsiella pneumoniae | 0 | 0 | 0 | 1 |
*Blood culture, pleural fluid, chest tube fluid, or cerebral spinal fluid.
**Endotracheal tube aspirate, tracheal aspirate, or bronchial wash.
***Specimen collected on the day of death or the day after death.
Because specimens from non‐sterile sites may represent colonization or contamination, we evaluated the relationship between non‐sterile site cultures and radiologically confirmed pneumonia. Of the 29 children with cultures collected from non‐sterile sites, 13 of the 17 (76%) children with ≥1 isolate identified, and 10 of the 13 (77%) children with S. aureus identified had radiologically confirmed pneumonia. Overall, 25 of 29 (86%) children who had ≥1 isolate identified from one of the specified sterile or non‐sterile sites had radiologically confirmed pneumonia.
Ten children had ≥1 bacterial isolate identified from a postmortem lung specimen. The same species of bacteria was also isolated at one of the specified culture sites for three of these children. S. aureus and S. pneumoniae were the most common bacteria identified, each reported in four children (Table 2).
Among the 23 children from whom S. aureus was isolated from one of the specified sterile or non‐sterile sites or a postmortem lung specimen, 12 (52%) had a methicillin‐resistant Staphlyococcus aureus (MRSA) isolate recovered, 9 (39%) had a methicillin‐susceptible Staphlyococcus aureus (MSSA) isolate recovered, and 2 (9%) had a S. aureus isolate with an unknown methicillin susceptibility recovered. Of the 12 MRSA isolates, six were available for genotyping, five were identified as PFGE type USA300, and one was PFGE type USA600. Four of the 5 USA‐300 isolates and the USA‐600 isolate were collected within three days of hospital admission. Two of the 9 MSSA isolates were available for genotyping and were identified as PFGE types USA200 and USA800; the USA‐200 isolate was collected within three days of admission while the USA‐800 isolate was not.
Viral co‐infections were uncommonly reported (Table 3). The most common viral co‐infections with influenza were adenovirus and respiratory syncytial virus (RSV). Fungal isolates were reported in three children. Candida parapsilosi and Candida krusei were isolated from blood cultures in a 13‐year‐old child. This child also had hemophagocytic syndrome that was believed to be secondary to influenza virus infection. Two children had Candida albicans isolates recovered: one by blood culture and the other by endotracheal tube fluid culture. The time periods from illness onset to death in the three children with fungal isolates were 23, 17, and 21 days, respectively.
Table 3.
Viral co‐infections among children with influenza‐associated mortality – United States: 2007–2008
| Viral co‐infections | N = 10 children |
|---|---|
| Adenovirus (type 3; C; unspecified) | 3 |
| Respiratory syncytial virus | 3 |
| Human metapneumovirus | 1 |
| Enterovirus | 1 |
| Herpes simplex virus type 1 | 1 |
| Rhinovirus | 1 |
| Echovirus | 1 |
| Total viral co‐infections | 11 |
The 17 children who had S. aureus isolated at a sterile or non‐sterile site within three days of hospital admission were significantly older, were significantly less likely to have one or more high‐risk medical condition, had significantly more time from symptom onset until death, were more likely to have radiologically confirmed pneumonia, were more likely to be infected with influenza B virus, and were less likely to be recommended for vaccination by 2007–2008 ACIP criteria than the 37 children who had cultures from the specified sites that did not grow S. aureus (Table 4).
Table 4.
Factors associated with isolation of Staphylococcus aureus within three days of hospital admission* among children with influenza‐associated mortality who had bacterial culture results from specified sites – United States: 2007–2008
| Characteristic | S. aureus isolate obtained within 3d of admission* (N = 17) | No laboratory evidence of S. aureus* (N = 37) | P‐value |
|---|---|---|---|
| Age, median (range) year | 10 (0–15) | 4 (0–17) | <0·01 |
| Median days from onset to death (range) | 8 (1–43) | 4 (0–52) | <0·05 |
| Radiologically confirmed pneumonia n/N (%) | 12/16 (75) | 15/36 (42) | <0·05 |
| ≥1 ACIP‐defined high‐risk medical condition** n/N (%) | 4/16 (25) | 20/32 (63) | <0·05 |
| Influenza A infection n/N (%) | 7/16 (44) | 27/37 (73) | <0·05 |
| Influenza B infection n/N (%) | 9/16 (56) | 10/37 (27) | <0·05 |
| Recommended for vaccination by 2007–2008 ACIP criteria n/N (%) | 5/16 (31) | 26/32 (81) | <0·01 |
ACIP, Advisory Committee on Immunization Practices.
*Identified at a normally sterile site (blood, pleural fluid, chest tube fluid, cerebral spinal fluid) or specified non‐sterile site (endotracheal tube aspirate, tracheal aspirate, bronchial wash).
**Children receiving long‐term aspirin therapy who might be at risk for experiencing Reye syndrome after influenza virus infection or those with chronic pulmonary (including asthma), cardiovascular (except hypertension), renal, hepatic, hematologic, or metabolic disorders (including diabetes mellitus). Children with immunosuppression or any condition (e.g., cognitive dysfunction, spinal cord injuries, seizure disorders, other neuromuscular disorders) that can compromise respiratory function or the handling of respiratory secretions or that can increase the risk for aspiration.
To further evaluate those children with a likely S. aureus co‐infection (as opposed to colonization), we analyzed data from the 14 children who had S. aureus isolates identified within three days of hospital admission from a sterile sites or specified non‐sterile sites and had a radiologically confirmed pneumonia. As mentioned earlier, we compared them with the 37 children who had cultures from the specified sites that did not grow S. aureus. The findings were consistent with those mentioned earlier with the exception of the association with influenza B virus.
Discussion
This report describes the 88 reports of influenza‐associated deaths in children from the 2007–2008 influenza season and provides more detailed information about isolation of potentially pathogenic bacteria from specified sites in these children. Among children for whom results of bacterial cultures from sterile sites or specified non‐sterile sites were available, almost half had positive bacterial cultures. S. aureus was identified in 69% of these children, the majority (85%) of whom had specimens yielding S. aureus obtained within three days of inpatient admission, indicating that they may have been admitted with a bacterial co‐infection or complication of influenza. As reported previously, young age and high‐risk medical conditions were common among influenza‐associated pediatric deaths. 12 , 15 Healthcare providers should suspect influenza S. aureus co‐infection among children with severe respiratory illness and a suspected or confirmed influenza infection, and treat with appropriate antimicrobial and antiviral agents.
An increase in the number of influenza‐associated pediatric deaths in association with S. aureus co‐infection was first recognized during the 2006–2007 influenza season, and S. aureus remained the most commonly identified bacteria among these children in the 2007–2008 season. Comparisons with data from previous years can only be made from sterile sites because information about isolation of bacteria from non‐sterile sites was not collected. One (2%) child with a sterile‐site S. aureus isolate was identified among 47 influenza‐associated pediatric deaths in the 2004–2005 influenza season, compared with 2/46 (4%) in 2005–2006 and 18/72 (25%) in 2006–2007. 12 In the 2007–2008 season, S. aureus was isolated from sterile‐site cultures in 11 (12·5%) of 88 children. Comparisons between these numbers should be made with caution because the proportion of children from whom bacterial cultures were obtained likely varied each year.
Those children from whom S. aureus was isolated within three days of admission from sterile or specified non‐sterile specimens were more likely to be older and were less likely to have a high‐risk pre‐existing condition than those children with cultures of the specified sites that did not grow S. aureus. The older median age among children with positive S. aureus cultures could be related to higher S. aureus nasal carriage rates among older children. In 2003–2004, a nationally representative carriage survey determined that children aged 5–11 and 11–19 were significantly more likely to be colonized with S. aureus than children aged 1–5. 16 Whereas frequencies of S. aureus nasal carriage have not increased significantly, MRSA nasal carriage has increased. 16 As MRSA nasal carriage increases, there is a potential for increasing co‐infection with MRSA and influenza virus, resulting in severe morbidity and mortality in children.
While one cannot consider pathogens isolated from specified non‐sterile sites (endotracheal tubes, tracheal aspirates, bronchial washes) to be definitively causative pathogens in pneumonia, we believe that there is some value in evaluating specimens from these sites. Given the challenges of pneumonia diagnostics, these specimens, while not from sterile sites, but from the lower respiratory tract, can provide insights into the causative pathogens of pneumonia and the epidemiology of children with co‐infection.
Few viral co‐infections were reported. The frequency of reported viral co‐infections is consistent with reports from the 2003–2004 influenza season, when adenovirus and RSV infections were also the most commonly reported viral co‐infections. 15 Information regarding viral co‐infections, including the site of virus isolation, was limited, and not systematically collected in all children.
The proportions of influenza types identified in these children were similar to the proportions circulating in the United States during the 2007–2008 season. Nationally, 71% and 29% of viruses typed were influenza A and influenza B, respectively, in the 2007–2008 season. 17 By comparison, among the 84 children with known virus type information that were infected with only one influenza type, 64% and 36% were infected with influenza A and B, respectively.
Vaccination remains the best way to prevent influenza and its complications. During the 2007–2008 influenza season, the ACIP recommended annual influenza vaccination for all children aged 6–59 months and children aged 5–18 years with an ACIP‐defined high‐risk condition. 18 In February 2010, the ACIP recommended universal influenza vaccination. 19 Vaccination is now recommended for all persons ≥6 months, regardless of age or high‐risk medical conditions. This new recommendation could make a strong impact on pediatric influenza‐associated deaths including those with S. aureus co‐infection, because many of the deaths in the 2007–2008 season occurred among children who were older or previously healthy, and not recommended for vaccination.
These data are subject to some limitations. First, reports of influenza‐associated pediatric mortality are made passively by healthcare providers, and some deaths meeting the case definition were likely not reported. Testing for influenza is at the discretion of the physician, and some children were likely never tested for influenza. Additionally, the influenza rapid antigen test, a common diagnostic method for influenza has a relatively low sensitivity 20 , 21 , 22 , 23 , 24 that could lead to undiagnosed cases. Second, the children were not systematically tested for bacterial, viral, or fungal pathogens, or radiologically confirmed pneumonia, and thus our results are limited by the testing that was performed as part of routine clinical care and postmortem analysis. The children labeled as “no lab evidence of S. aureus” had culture(s) collected at ≥1 specified sterile or non‐sterile site that did not grow S. aureus; however, it is possible that these children had a S. aureus isolate present at a site that was not cultured. Third, some bacterial cultures obtained from non‐sterile sites could represent colonization rather than co‐infections. However, we carefully selected only respiratory specimens that were collected in the trachea or bronchi to maximize identifying infecting versus colonizing bacterial isolates. Fourth, not all bacterial cultures were obtained within three days of hospital admission, and therefore we may have misclassified some patients who were admitted with bacterial co‐infections.
Influenza‐associated mortality among children is a rare event; however, healthcare providers should be mindful of the severe outcomes associated with influenza in children, especially those with high‐risk medical condition. Critically ill children should be promptly treated with anti‐viral medications, unless contraindicated, upon influenza diagnosis or suspicion. Because isolation of S. aureus potentially representing co‐infection was identified in a substantial proportion (19%) of children with influenza‐associated mortality within three days of hospital admission, healthcare providers should consider administering antimicrobial agents active against locally circulating strains of S. aureus when empirically treating children with influenza‐like illness and severe respiratory illness.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Acknowledgements
We thank Lenee Blanton and Krista Kniss for technical assistance with the Influenza Associated Pediatric Mortality Database. We also thank Greg Fosheim for characterizing S. aureus isolates, and Carrie Reed and Christine Dao for statistical assistance.
References
- 1. Thompson WW, Shay DK, Weintraub E et al. Influenza‐associated hospitalizations in the United States. JAMA 2004; 292:1333–1340. [DOI] [PubMed] [Google Scholar]
- 2. Thompson WW, Shay DK, Weintraub E et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–186. [DOI] [PubMed] [Google Scholar]
- 3. Barker WH, Mullooly JP. Impact of epidemic type A influenza in a defined adult population. Am J Epidemiol 1980; 112:798–811. [DOI] [PubMed] [Google Scholar]
- 4. Mullooly JP, Bridges CB, Thompson WW et al. Influenza‐ and RSV‐associated hospitalizations among adults. Vaccine 2007; 25:846–855. [DOI] [PubMed] [Google Scholar]
- 5. O’Brien MA, Uyeki TM, Shay DK et al. Incidence of outpatient visits and hospitalizations related to influenza in infants and young children. Pediatrics 2004; 113:585–593. [DOI] [PubMed] [Google Scholar]
- 6. Keren R, Zaoutis TE, Bridges CB et al. Neurological and neuromuscular disease as a risk factor for respiratory failure in children hospitalized with influenza infection. JAMA 2005; 294:2188–2194. [DOI] [PubMed] [Google Scholar]
- 7. Neuzil KM, Wright PF, Mitchel EF Jr, Griffin MR. The burden of influenza illness in children with asthma and other chronic medical conditions. J Pediatr 2000; 137:856–864. [DOI] [PubMed] [Google Scholar]
- 8. Fiore AE, Shay DK, Broder K et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep 2008; 57(RR‐1):1–60. [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention (CDC) . Severe morbidity and mortality associated with influenza in children and young adults–Michigan, 2003. MMWR Morb Mortal Wkly Rep 2003; 52:837–840. [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention (CDC) . Update: influenza‐associated deaths reported among children aged <18 years‐United States, 2003–2004 influenza season. MMWR Morb Mortal Wkly Rep 2004; 52:1286–1288. [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention (CDC) . Update: influenza‐associated deaths reported among children aged <18 years–United States, 2003–04 influenza season. MMWR Morb Mortal Wkly Rep 2004; 52:1254–1255. [PubMed] [Google Scholar]
- 12. Finelli L, Fiore A, Dhara R et al. Influenza‐associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics 2008; 122:805–811. [DOI] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention (CDC) . Oxacillin resistant Staphylococcus aureus on PulseNet (OPN): Laboratory Protocol for Molecular Typing of S. aureus by Pulsedfield Gel Electrophoresis (PFGE) cited. Available at http://www.cdc.gov/ncidod/dhqp/pdf/ar/ar_mras_PFGE_s_aureus.pdf (accessed 02 July 2010).
- 14. McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed‐field gel electrophoresis typing of oxacillin‐resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol 2003; 41:5113–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhat N, Wright JG, Broder KR et al. Influenza‐associated deaths among children in the United States, 2003–2004. N Engl J Med 2005; 353:2559–2567. [DOI] [PubMed] [Google Scholar]
- 16. Gorwitz RJ, Kruszon‐Moran D, McAllister SK et al. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J Infect Dis 2008; 197:1226–1234. [DOI] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention (CDC) . Influenza activity–United States and worldwide, 2007–2008 season. MMWR Morb Mortal Wkly Rep 2008; 57:692–697. [PubMed] [Google Scholar]
- 18. Fiore AE, Shay DK, Haber P et al. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56(RR‐6):1–54. [PubMed] [Google Scholar]
- 19. Fiore AE, Uyeki TM, Broder K et al. Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59(RR‐8):1–62. [PubMed] [Google Scholar]
- 20. Ghebremedhin B, Engelmann I, Konig W, Konig B. Comparison of the performance of the rapid antigen detection actim Influenza A&B test and RT‐PCR in different respiratory specimens. J Med Microbiol 2009; 58(Pt 3):365–370. [DOI] [PubMed] [Google Scholar]
- 21. Grijalva CG, Poehling KA, Edwards KM et al. Accuracy and interpretation of rapid influenza tests in children. Pediatrics 2007; 119:e6–e11. [DOI] [PubMed] [Google Scholar]
- 22. Landry ML, Cohen S, Ferguson D. Real‐time PCR compared to Binax NOW and cytospin‐immunofluorescence for detection of influenza in hospitalized patients. J Clin Virol 2008; 43:148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pregliasco F, Puzelli S, Mensi C et al. Influenza virological surveillance in children: the use of the QuickVue rapid diagnostic test. J Med Virol 2004; 73:269–273. [DOI] [PubMed] [Google Scholar]
- 24. Uyeki TM, Prasad R, Vukotich C et al. Low sensitivity of rapid diagnostic test for influenza. Clin Infect Dis 2009; 48:e89–e92. [DOI] [PubMed] [Google Scholar]


