Abstract
Please cite this paper as: Belshe et al. (2010). Efficacy of live attenuated influenza vaccine in children 6 months to 17 years of age. Influenza and Other Respiratory Viruses 4(3), 141–145.
Background It has been suggested that live attenuated influenza vaccine (LAIV) may be less effective in older individuals because of prior wild‐type influenza infections. LAIV is currently approved in the United States, South Korea and Hong Kong for individuals 2–49 years of age.
Objective To examine data from previously published pediatric studies to determine the efficacy of LAIV in various age groups.
Methods Four studies in which the subject age range exceeded 36 months were identified: one 2‐year study comparing LAIV with placebo and three 1‐year studies comparing LAIV with trivalent inactivated influenza vaccine (TIV). Efficacy against any strain regardless of antigenic similarity to vaccine was analyzed by age; age groups were based on the study design and sample size. A logistic regression model was used to assess whether age, as a continuous variable, was an effect modifier on LAIV efficacy.
Results The efficacy of LAIV did not vary with age in children aged 15–84 months compared with placebo or in children aged 6 months to 17 years compared with TIV.
Conclusions The available data from prospective, randomized studies in children does not support the concept that prior repeated exposure to influenza, either through wild‐type infection or vaccination with live, attenuated or inactivated vaccines, reduces the efficacy of LAIV compared with placebo or TIV. The decreased immunologic responses to LAIV reported in older individuals or those with pre‐existing immunity do not appear to translate into reduced protection from influenza in children.
Keywords: Influenza, live attenuated influenza vaccine, trivalent‐inactivated influenza vaccine
Introduction
Multiple prospective, randomized, controlled studies have described the efficacy of live attenuated influenza vaccine (LAIV) in children and adolescents compared with placebo and with trivalent inactivated influenza vaccine (TIV). 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 LAIV provided levels of efficacy against influenza illness that, in the three comparative studies conducted, were significantly higher than those provided by TIV. 1 , 3 , 7 In contrast, comparative studies in adults ≥18 years of age have demonstrated variable results; studies have demonstrated comparable protection of the two vaccines, 12 , 13 , 14 , 15 , 16 greater protection with TIV, 17 , 18 , 19 and, in some cases, greater protection with LAIV. 19 For studies that have demonstrated greater protection with TIV in adults, authors have speculated that LAIV may be less effective in older individuals because of prior infections with similar wild‐type influenza strains. 15 , 17 , 18 , 19
Of the previously conducted studies of LAIV efficacy, only two analyzed efficacy by age group, and neither suggested a reduction in effectiveness of LAIV in children with increasing age. 1 , 3 The purpose of the present analysis is to more fully explore the efficacy of LAIV by age group based on data from previously conducted studies.
Studies comparing the safety of LAIV and TIV have demonstrated comparable safety in individuals 2 years of age and older. In one study, 3 among children 6–23 months of age, an increased rate of wheezing through 6 weeks following vaccination was associated with LAIV (5·9% LAIV versus 3·8% TIV); however, no increase was among children 24–59 months of age. LAIV is currently approved for use in the United States, South Korea and Hong Kong for the active immunization of eligible individuals 2–49 years of age against influenza.
Materials and methods
From a review of all available studies, only four LAIV efficacy studies in which the subject age range exceeded 36 months were identified for this post hoc analysis (Table 1): one 2‐year study of efficacy comparing LAIV to placebo and three 1‐year studies comparing LAIV with TIV [the studies were conducted by Aviron, Inc., Mountain View, CA, USA (now MedImmune) and Wyeth Research, Pearl River, NY, USA]. 1 , 3 , 4 , 5 , 7 In all studies, efficacy against any strain regardless of antigenic similarity to vaccine was analyzed. Age groups for analysis were defined based on the study design and sample size. The placebo‐controlled study (study 1 4 , 5 ) allowed meaningful analysis of smaller subgroups, and 12‐month cohorts were used. In the TIV‐controlled studies (studies 2 3 , 3 1 and 4 7 ), larger age cohorts were used as needed based on the study sample size. The large size of study 2 permitted 12‐month cohort analysis, but studies 3 and 4 were smaller, and children above and below the midpoint of the enrollment age range were analyzed. Where available (study 1), additional data on pre‐vaccination antibody levels were also analyzed by age group to better understand pre‐existing anti‐influenza immunity in the study population. Baseline pre‐vaccination serum antibody levels were evaluated in a small cohort of children (n = 203); subjects with a hemagglutinin inhibition titer ≤4 were considered seronegative, whereas those with a hemagglutinin inhibition titer of ≥8 were considered seropositive.
Table 1.
Study characteristics
| Study | Influenza season | Age at enrollment | n | Control | Geographic location |
|---|---|---|---|---|---|
| 1, year 1, Belshe | 1996–1997 | 15–71 months | 1602 | Placebo | United States |
| 1, year 2, Belshe | 1997–1998 | 27–83 months | 1358 | Placebo | United States |
| 2, Belshe | 2004–2005 | 6–59 months | 7852 | TIV | Global |
| 3, Ashkenazi | 2002–2003 | 6–71 months | 2085 | TIV | Europe/Israel |
| 4, Fleming | 2002–2003 | 6–17 years | 2202 | TIV | Europe/Israel |
LAIV, live attenuated influenza vaccine; TIV, trivalent inactivated influenza vaccine.
Study 1 was a 2‐year, multicenter, prospective, double‐blind trial conducted in the United States during the 1996–1998 influenza seasons in previously unvaccinated children 15–71 months of age. 4 , 5 The efficacy of LAIV was analyzed for children 15–23 months, 24–35 months, 36–47 months, 48–59 months and ≥60 months of age. Study 2 was a multinational, double‐blind, head‐to‐head trial conducted during the 2004–2005 influenza season in children 6–59 months of age. 3 Efficacy of LAIV compared with TIV was analyzed for children 6–23 months, 24–35 months, 36–47 months and 48–59 months of age. Studies 3 and 4 were randomized, open‐label studies conducted in Europe and Israel during the 2002–2003 influenza season of two doses of vaccine in children 6–71 months of age with recurrent respiratory tract infections and one dose of vaccine in children and adolescents 6–17 years of age with medically stable asthma, respectively. 1 , 7 Efficacy of LAIV compared with TIV was analyzed for children aged 6–35 and 36–71 months for study 3 and aged 6–11 and 12–17 years of age for study 4. All studies contacted subjects weekly throughout the influenza season and calculated efficacy based on the incidence of culture‐confirmed influenza illness.
In addition to the categorical age group analysis, a logistic regression model for each study was used to assess whether age, as a continuous variable, was an effect modifier on LAIV efficacy. The model used culture‐confirmed influenza illness as the dependent variable. Independent variables included treatment group, age as a continuous variable and the interaction between age and treatment. The interaction term was tested for significance to assess the impact of age on LAIV efficacy.
Results
In study 1, efficacy against all strains regardless of match to strains contained in the vaccine in year 1 ranged from 85 to 100% across the age cohorts analyzed, and there was no decrease with increasing age (Table 2). Similarly, after revaccination in year 2, efficacy did not vary by age, ranging from 84 to 92%. In the subset of subjects tested, baseline seropositivity increased with increasing age (Figure 1). In the youngest age group, 73 to 87% of subjects were seronegative to the vaccine strains, whereas in the oldest age group only 33 to 58% were seronegative. Baseline geometric mean titers also generally increased with increasing age (Figure 2); 8·9%, 12·5%, 11·4%, 18·9% and 18·5% of subjects aged 15–23, 24–35, 36–47, 48–59 and ≥60 months, respectively, had a baseline antibody titer >1:40.
Table 2.
Efficacy of LAIV versus placebo by age and strain (study 1, years 1 and 2)
| Age, months (n)* | All strains** Efficacy, % (95% CI) | H3N2** | B** | ||||
|---|---|---|---|---|---|---|---|
| Attack rate, % | Efficacy, % (95% CI) | Attack rate, % | Efficacy, % (95% CI) | ||||
| LAIV | Placebo | LAIV | Placebo | ||||
| Year 1 | |||||||
| 15–23 (272) | 85 (58–95) | 1·2 | 11·1 | 90 (59–97) | 1·2 | 4·0 | 71 (−32 to 94) |
| 24–35 (359) | 96 (86–99) | 0·9 | 15·7 | 94 (79–99) | 0 | 9·0 | 100 (81–100) |
| 36–47 (327) | 87 (67–95) | 1·3 | 11·5 | 89 (63–97) | 0·9 | 5·2 | 83 (27–96) |
| 48–59 (332) | 100 (90–100) | 0 | 11·1 | 100 (85–100) | 0 | 7·4 | 100 (77–100) |
| ≥60 (312) | 91 (70–97) | 0 | 8·4 | 100 (79–100) | 1·4 | 8·4 | 84 (44–95) |
| 2Year 2 | |||||||
| 27–35 (246) | 84 (35–96) | 1·3 | 8·0 | 84 (35–96) | 0 | 0 | NA |
| 36–47 (294) | 85 (57–94) | 2·2 | 13·9 | 85 (57–95) | 0 | 0 | NA |
| 48–59 (274) | 92 (69–98) | 1·0 | 11·7 | 91 (65–98) | 0 | 1·3 | 100 (−50 to 100) |
| >60 (544) | 87 (71–94) | 1·9 | 14·2 | 87 (71–94) | 0 | 0 | NA |
LAIV, live attenuated influenza vaccine; NA, not applicable.
*Age strata in initial year of vaccination.
**Regardless of antigenic match to vaccine. Influenza A/H1N1 did not circulate during the study and was not isolated.
Figure 1.
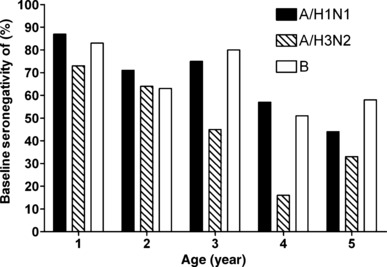
Baseline seronegativity by age and strain (study 1, year 1; n = 203).
Figure 2.
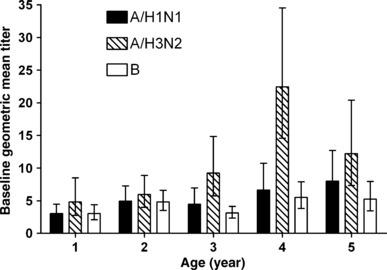
Baseline geometric mean titers (95% CI) by age and strain (study 1, year 1; n = 203).
In the first of the three TIV‐controlled studies, study 2, the relative efficacy of LAIV compared with TIV against all strains regardless of match ranged from 42 to 57% and was similar regardless of age (Table 3). Studies 3 and 4 similarly showed no decline in efficacy with increasing age (Table 4). In study 3, a trend showing higher relative efficacy of LAIV compared with TIV was seen in subjects 36–71 months of age compared with those 6–35 months of age, but this trend was because of the differential incidence of A/H3N2 versus A/H1N1 and B in the two age strata; analyzed by strain, the relative efficacy in each age group was similar. In all studies, efficacy against individual strains should be interpreted with caution because of the low number of influenza cases in some comparisons.
Table 3.
Relative efficacy of LAIV versus TIV by age and strain (study 2)
| Age, months (n) | All strains* Relative efficacy, % (95% CI) | H1N1* | H3N2* | B* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Attack rate, % | Relative efficacy, % (95% CI) | Attack rate, % | Relative efficacy, % (95% CI) | Attack rate, % | Relative efficacy, % (95% CI) | |||||
| LAIV | TIV | LAIV | TIV | LAIV | TIV | |||||
| 6–23 (3686) | 56 (40–68) | 0·1 | 0·3 | 67 (−56 to 95) | 0·7 | 4·1 | 83 (70–91) | 2·3 | 2·7 | 15 (−29 to 43) |
| 24–35 (2612) | 57 (40–69) | 0·1 | 0·3 | 78 (−79 to 99) | 1·0 | 5·6 | 82 (68–90) | 2·8 | 3·0 | 10 (−42 to 43) |
| 36–47 (846) | 42 (5–66) | 0 | 2·3 | 100 (63–100) | 1·7 | 3·4 | 48 (−29 to 81) | 4·1 | 4·8 | 12 (−69 to 55) |
| 48–59 (708) | 56 (25–75) | 0 | 2·0 | 100 (47–100) | 1·1 | 4·0 | 76 (22–95) | 5·0 | 7·5 | 25 (−37 to 60) |
LAIV, live attenuated influenza vaccine; TIV, trivalent inactivated influenza vaccine.
*Regardless of antigenic match to vaccine.
Table 4.
Relative efficacy of LAIV versus TIV age and strain (studies 3 and 4)
| Study | Age (n) | All strains* Relative efficacy, % (95% CI) | H1N1* | H3N2* | B* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Attack rate, % | Relative efficacy, % (95% CI) | Attack rate, % | Relative efficacy, % (95% CI) | Attack rate, % | Relative efficacy, % (95% CI) | ||||||
| LAIV | TIV | LAIV | TIV | LAIV | TIV | ||||||
| 3 | 6–35 months (952) | 31 (−30 to 64) | 0 | 1·1 | 100 (1–100) | 2·6 | 1·8 | −47 (−309 to 44) | 1·2 | 2·7 | 55 (−30 to 86) |
| 3–6 years (1133) | 70 (38–87) | 0 | 0·9 | 100 (−16 to 100) | 0·9 | 0·7 | −33 (−568 to 72) | 1·1 | 4·5 | 76 (39–92) | |
| 4** | 6–11 years (1376) | 31 (−8 to 57) | 0 | 0·6 | 100 (−62 to 100) | 1·5 | 1·0 | −53 (−373 to 48) | 3·8 | 5·9 | 36 (−7 to 63) |
| 12–17 years (835) | 30 (−43 to 66) | 0 | 0·5 | 100 (−369 to 100) | 1·6 | 1·5 | −3 (−270 to 70) | 2·3 | 3·3 | 32 (−67 to 73) | |
LAIV, live attenuated influenza vaccine; TIV, trivalent inactivated influenza vaccine.
*Regardless of antigenic match to vaccine.
**Conducted in children with stable, medically treated asthma, a population for whom there is a warning/precaution against the use of LAIV.
The results of the logistic regression model were consistent with those seen with the age group analysis. Age was not significantly correlated with vaccine efficacy in study 1 year 1, study 1 year 2, study 2, or study 4 (P = 0·55, 0·99, 0·93, 0·17, respectively). For study 3, vaccine efficacy increased slightly with age (P = 0·03); however, strain‐specific efficacy was not correlated for any of the strains (P = 0·99, 0·57 and 0·11 for A/H1N1, A/H3N2 and B, respectively).
Discussion
The data presented here in children and adolescents spanning a range of 17·5 years does not support the hypothesis that the efficacy of LAIV declines with increasing age. In study 1, as would be expected, a greater proportion of previously unvaccinated older children had pre‐existing serum antibody to the vaccine strains, reflecting increased previous natural infection with antigenically related viruses. Nevertheless, vaccine efficacy was equally high for older children and younger children, and thus there was no suggestion that increasing levels of pre‐existing anti‐influenza immunity influenced LAIV efficacy. Data presented here and elsewhere 2 also suggest that LAIV efficacy does not decline in those previously vaccinated with LAIV; in fact, a trend toward increased efficacy with revaccination has been reported in a meta‐analysis of four 2‐year studies in children younger than 7 years of age. 2 Prior vaccination with TIV has also not been shown to impact LAIV efficacy relative to TIV; specifically, in study 2, the relative efficacy of LAIV compared with TIV against all strains regardless of antigenic match was similar in children aged 6–59 months who were previously vaccinated with TIV (51%) and those previously unvaccinated (57%). 20 Accordingly, the available data from these prospective, randomized studies in children does not support the hypothesis that prior repeated exposure to influenza, either through wild‐type infection or vaccination with live, attenuated or inactivated vaccines, may reduce the efficacy of LAIV compared with placebo or TIV. This conclusion is also supported by the observation that LAIV retained its effectiveness in a consecutive multiseason open‐label study of 6569 children in which 50% of those in the study had received 1 or more doses of LAIV in the preceding 3 years of the study. 21
The finding that the protection provided by LAIV does not decline with age, prior vaccination or pre‐existing anti‐influenza antibody is somewhat counter‐intuitive given that immunologic responses to LAIV do decline in those with greater prior immunity to influenza. In children, serum antibody responses and shedding of LAIV vaccine strains have both been shown to decrease with increasing age, increasing baseline serum antibody levels and prior vaccination with LAIV. 4 , 22 , 23 , 24 Although achieving an HAI titer of 1:40 is generally considered a correlate of approximately 50% protection for inactivated influenza vaccines, 25 , 26 , 27 there is no established correlate of protection for LAIV. As a result, predicting the precise level of protection provided in specific subpopulations is not possible; however, the available data suggest that the above‐referenced age‐based differences in immunologic responses do not translate into reduced protection from influenza.
The only prospective, randomized study demonstrating a statistically significant reduction in efficacy with LAIV relative to TIV in adults was an open‐label study conducted on university campuses in Michigan; 17 this finding should be explored in additional studies in other settings. It is possible that variations in study endpoint and population, and season‐to‐season variability may contribute to the conflicting results regarding the relative efficacy of LAIV and TIV in adults. 14 , 19 , 28 In the more robust pediatric data, there is evidence to suggest that LAIV efficacy does not decline with increasing age. More research on influenza vaccination is needed to describe the efficacy of LAIV and TIV in older adolescents and adults. A large comparative study with a sample size in the range of 24 000 subjects may be required to answer such a question.
Acknowledgement
This study was sponsored by MedImmune, LLC, Gaithersburg, MD.
Conflict of Interest
Chris Ambrose, Tingting Yi, and Seth Toback are currently employees of MedImmune, LLC and as such receives a salary from the company.
References
- 1. Ashkenazi S, Vertruyen A, Aristegui J et al. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J 2006; 25:870–879. [DOI] [PubMed] [Google Scholar]
- 2. Rhorer J, Ambrose CS, Dickinson S et al. Efficacy of live attenuated influenza vaccine in children: a meta‐analysis of nine randomized clinical trials. Vaccine 2009; 27:1101–1110. [DOI] [PubMed] [Google Scholar]
- 3. Belshe RB, Edwards KM, Vesikari T et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med 2007; 356:685–696. [DOI] [PubMed] [Google Scholar]
- 4. Belshe RB, Gruber WC, Mendelman PM et al. Efficacy of vaccination with live attenuated, cold‐adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J Pediatr 2000; 136:168–175. [DOI] [PubMed] [Google Scholar]
- 5. Belshe RB, Mendelman PM, Treanor J et al. The efficacy of live attenuated, cold‐adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med 1998; 338:1405–1412. [DOI] [PubMed] [Google Scholar]
- 6. Bracco H, Farhat CK, Tregnaghi MW et al. Efficacy and safety of one and two doses of live attenuated influenza vaccine in vaccine‐naive children. Pediatr Infect Dis J 2009; 28:365–371. [DOI] [PubMed] [Google Scholar]
- 7. Fleming DM, Crovari P, Wahn U et al. Comparison of the efficacy and safety of live attenuated cold‐adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J 2006; 25:860–869. [DOI] [PubMed] [Google Scholar]
- 8. Forrest BD, Pride MW, Dunning AJ et al. Correlation of cellular immune responses with protection against culture‐confirmed influenza virus in young children. Clin Vaccine Immunol 2008; 15:1042–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lum LCS, Forrest BD, The LAIV Concomitant Vaccine Study Group . Influenza vaccine concurrently administered with a combination measles, mumps, and rubella vaccine to young children. Vaccine 2009. DOI:10.1016/j.vaccine.2009.11.054. [DOI] [PubMed]
- 10. Tam JS, Capeding MR, Lum LC et al. Efficacy and safety of a live attenuated, cold‐adapted influenza vaccine, trivalent against culture‐confirmed influenza in young children in Asia. Pediatr Infect Dis J 2007; 26:619–628. [DOI] [PubMed] [Google Scholar]
- 11. Vesikari T, Fleming DM, Aristegui JF et al. Safety, efficacy, and effectiveness of cold‐adapted influenza vaccine‐trivalent against community‐acquired, culture‐confirmed influenza in young children attending day care. Pediatrics 2006; 118:2298–2312. [DOI] [PubMed] [Google Scholar]
- 12. Hawksworth AW, Russell KL, Faix DJ et al. Effectiveness of the 2006‐2007 influenza vaccine: data from US military basic training centers [Abstract P725]. 6th Meeting of the Options for Control of Influenza Diseases, 17–23 June 2007, Toronto, ON, Canada.
- 13. Hawksworth AW, Russell KL, Strickler JK, Gaydos JC, Malone JL, Ryan MAK. Effectiveness of the 2004–2005 influenza vaccine: data from US military basic training centers [Abstract 1012]. 43rd Annual Meeting of IDSA, 6–9 October, 2005, San Francisco, CA.
- 14. Treanor JJ, Kotloff K, Betts RF et al. Evaluation of trivalent, live, cold‐adapted (CAIV‐T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild‐type influenza A (H1N1), A (H3N2), and B viruses. Vaccine 1999; 18:899–906. [DOI] [PubMed] [Google Scholar]
- 15. Ohmit SE, Victor JC, Rotthoff JR et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med 2006; 355:2513–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strickler JK, Hawksworth AW, Myers C, Irvine M, Ryan MA, Russell KL. Influenza vaccine effectiveness among US military basic trainees, 2005‐06 season. Emerg Infect Dis 2007; 13:617–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Monto AS, Ohmit SE, Petrie JG et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med 2009; 361:1260–1267. [DOI] [PubMed] [Google Scholar]
- 18. Wang Z, Tobler S, Roayaei J, Eick A. Live attenuated or inactivated influenza vaccines and medical encounters for respiratory illnesses among US military personnel. JAMA 2009; 301:945–953. [DOI] [PubMed] [Google Scholar]
- 19. Eick AA, Wang Z, Hughes H, Ford SM, Tobler SK. Comparison of the trivalent live attenuated vs. inactivated influenza vaccines among U.S. military service members. Vaccine 2009; 27:3568–3575. [DOI] [PubMed] [Google Scholar]
- 20. Data on File. Gaithersburg, MD: MedImmune, Inc, 2006. [Google Scholar]
- 21. Piedra PA, Gaglani MJ, Kozinetz CA et al. Trivalent live attenuated intranasal influenza vaccine administered during the 2003‐2004 influenza type A (H3N2) outbreak provided immediate, direct, and indirect protection in children. Pediatrics 2007; 120:e553–e564. [DOI] [PubMed] [Google Scholar]
- 22. Belshe RB, Gruber WC, Mendelman PM et al. Correlates of immune protection induced by live, attenuated, cold‐adapted, trivalent, intranasal influenza virus vaccine. J Infect Dis 2000; 181:1133–1137. [DOI] [PubMed] [Google Scholar]
- 23. Block SL, Reisinger KS, Hultquist M, Walker RE. Comparative immunogenicities of frozen and refrigerated formulations of live attenuated influenza vaccine in healthy subjects. Antimicrob Agents Chemother 2007; 51:4001–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Block SL, Yogev R, Hayden FG, Ambrose CS, Zeng W, Walker RE. Shedding and immunogenicity of live attenuated influenza vaccine virus in subjects 5–49 years of age. Vaccine 2008; 26:4940–4946. [DOI] [PubMed] [Google Scholar]
- 25. Hobson D, Curry RL, Beare AS, Ward‐Gardner A. The role of serum haemagglutination‐inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972; 70:767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull 1979; 35:69–75. [DOI] [PubMed] [Google Scholar]
- 27. Potter CW, Jennings R, Nicholson K, Tyrrell DA, Dickinson KG. Immunity to attenuated influenza virus WRL 105 infection induced by heterologous, inactivated influenza A virus vaccines. J Hyg (Lond) 1977; 79:321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nichol KL, Mendelman PM, Mallon KP et al. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA 1999; 282:137–144. [DOI] [PubMed] [Google Scholar]


