Abstract
Please cite this paper as: Jeremy Sueker et al. (2010) Influenza and respiratory disease surveillance: the US military’s global laboratory‐based network. Influenza and Other Respiratory Viruses 4(3), 155–161.
The US Department of Defense influenza surveillance system now spans nearly 500 sites in 75 countries, including active duty US military and dependent populations as well as host‐country civilian and military personnel. This system represents a major part of the US Government’s contributions to the World Health Organization’s Global Influenza Surveillance Network and addresses Presidential Directive NSTC‐7 to expand global surveillance, training, research and response to emerging infectious disease threats. Since 2006, the system has expanded significantly in response to rising pandemic influenza concerns. The expanded system has played a critical role in the detection and monitoring of ongoing H5N1 outbreaks worldwide as well as in the initial detection of, and response to, the current (H1N1) 2009 influenza pandemic. This article describes the system, details its contributions and the critical gaps that it is filling, and discusses future plans.
Keywords: Influenza, military, surveillance
Background information
The US Department of Defense (DoD) invests considerable resources in global efforts to detect, analyze and prevent the spread of influenza and other respiratory infections within host‐country and DoD beneficiary populations. Physical crowding, rapid mass movement, physical stress and immunological naïveté render military populations especially susceptible to outbreaks of respiratory illness. 1 , 2 , 3 , 4 , 5 , 6 , 7 This is especially true of basic recruit and trainee groups in whom rates of acute respiratory infections have been traditionally higher than in older, seasoned troops. 8 Simultaneously, the DoD seeks to limit the potential for spreading influenza, given the role of militaries in 1918 and other notable pandemics. 9
The DoD Global Emerging Infections Surveillance and Response System (DoD‐GEIS), a division of the Armed Forces Health Surveillance Center (AFHSC) since early 2008, centralized DoD influenza and other respiratory disease surveillance efforts in 1998 and subsequently expanded worldwide surveillance and response initiatives starting in 2006. 4 , 10 , 11 The goal of the DoD influenza surveillance system is to identify and characterize antigenically diverse strains that threaten military beneficiary populations and global public health, and to work in collaboration with the US Centers for Disease Control and Prevention (CDC), the Food and Drug Administration (FDA), the World Health Organization (WHO), and other relevant organizations to develop vaccines and other effective responses. The system is built around a network of hub and satellite laboratories, with many joint ventures with host countries.
In January 2005, the US Congress passed H.R. 1815, Sec. 748: Pandemic Avian Flu Preparedness, providing DoD the opportunity to expand and strengthen its system. 12 The role of AFHSC/GEIS was further delineated in national and DoD influenza strategy documents. 13 , 14 , 15 With the third year of expanded operations concluded (2006–2008), and with the onset of the first pandemic of the 21st century due to a novel (H1N1) 2009 virus, this study endeavors to supplement previously published system description and achievements in 1998–2005, 11 and outline subsequent added system value, summarizes its expansion and growth, and outlines areas for further development. This study will not only focus on laboratory‐based surveillance, the backbone of AFHSC/GEIS influenza surveillance efforts, but also highlight complementary training and preparedness initiatives.
The system characteristics and functions
All DoD surveillance sites track influenza‐like illness (ILI) or febrile respiratory illness (FRI) and adhere to a standard case definition for enrolling participants and obtaining samples: oral temperature ≥38°C and a cough, sore throat or difficulty in breathing for ≤72 h. Because many participants are seen in a clinical setting, the system is multilayered, working to provide accurate and timely results to stakeholders while rapidly identifying the epidemiologically relevant characteristics of the virus and feeding this information, and sometimes the original specimen or isolate, into the WHO’s Global Influenza Surveillance Network (GISN) via the US CDC or other WHO Collaborating Centers.
The system can be roughly divided into host‐country population surveillance and global DoD beneficiary population – the armed forces and their families – surveillance. Host‐country surveillance is coordinated by the DoD’s five overseas medical research laboratories (Table 1) as well as by the US Army Public Health Command (Provisional) [USAPHC (Prov)] and its satellite activities in San Antonio, Texas, Public Health Region‐South (PHR‐South), in Landstuhl, Germany (PHR‐Europe), and in Camp Zama, Japan (PHR‐Japan). Each laboratory coordinates surveillance, and complementary training and infrastructure enhancement, in a cluster of regional countries. Efforts in each country are governed by bilateral agreements with a host‐government agency, often the Ministry of Health or Ministry of Defense. These partnerships focus on enhancing the domestic host‐country capacity to conduct national influenza surveillance and contribute unique respiratory samples to the global system.
Table 1.
Department of Defense overseas medical research laboratories
| Abbreviation | Title | Location |
|---|---|---|
| NMRCD | US Naval Medical Research Center Detachment | Lima, Peru |
| NAMRU‐2 | US Naval Medical Research Unit No. 2 | Jakarta, Indonesia |
| NAMRU‐3 | US Naval Medical Research Unit No. 3 | Cairo, Egypt |
| USAMRU‐K | US Army Medical Research Unit – Kenya | Nairobi, Kenya |
| AFRIMS | Armed Forces Research Institute of the Medical Sciences | Bangkok, Thailand |
Of the involved DoD overseas laboratories, the US Army Medical Research Unit – Kenya (USAMRU‐K, in Nairobi), the Armed Forces Research Institute of the Medical Sciences (AFRIMS, in Bangkok, Thailand) and USAPHC (Prov) satellite activities all utilize the US Air Force School of Aerospace Medicine (USAFSAM), in Brooks City Base, Texas, as a reference laboratory, with samples of interest joining the pool from DoD beneficiary populations. In contrast, the US Naval Medical Research Center Detachment (NMRCD, in Lima, Peru), the US Naval Medical Research Unit No. 2 (NAMRU‐2, in Jakarta, Indonesia) and the US Naval Medical Research Unit No. 3 (NAMRU‐3, in Cairo, Egypt, a WHO H5 Regional Reference Laboratory for influenza) interact directly with the CDC and WHO. All laboratories and activities provide the US CDC, the WHO and GenBank, with isolates or actual specimens as well as necessary sequencing information upon request. Following initial characterization, a subset of samples or influenza isolates is sent on to the CDC at its request for further characterization and antigenic analysis, and for possible inclusion in an annual panel of global reference viruses.
Surveillance within DoD beneficiary populations is coordinated through USAFSAM and the Department of Respiratory Disease Research at the US Naval Health Research Center (NHRC) in San Diego, California. Samples, accompanied by an epidemiologic questionnaire and the results of an influenza rapid test when available, are tested for a battery of likely respiratory pathogens (including influenza, parainfluenza, adenovirus, respiratory syncitial virus, rhinovirus and human metapneumovirus).
In addition to samples from the overseas laboratories, USAFSAM also receives routine samples from a network of sentinel sites at DoD healthcare facilities and tracks the active duty and dependent population outpatient visits through weekly review of 11 ILI‐related International Classification of Diseases‐9 codes in the military’s Standard Ambulatory Data Record system. Moreover, any US Air Force medical facility experiencing elevated ILI rates (>2 standard deviations of expected) is engaged and encouraged to submit samples as part of the surveillance system for the duration of this increased activity.
NHRC conducts population‐based FRI surveillance among trainees at the eight largest domestic recruit training facilities for the four US Armed Services and the Coast Guard. Recruits constitute a high‐risk population, combining immunological naïveté, high degree of physical and mental exertion, and crowding, and often receive their first influenza vaccine at the commencement of training. 1 , 2 , 7 , 8 Surveillance is also carried out on at least 20 US Navy ships from three fleets based in Norfolk, VA, San Diego, CA, and Yokosuka, Japan. Deployments often expose sailors to far‐reaching influenza and other respiratory viruses across the world, especially at ports‐of‐call.
AFHSC/GEIS also supports ILI surveillance at six civilian clinics straddling the California‐Mexico border as part of the Border Infectious Diseases Surveillance project, a joint NHRC and CDC engagement. A specimen from the second recognized US case of the novel (H1N1) 2009 pandemic influenza virus was obtained in this surveillance study. 16 , 17 In addition to the US Mexico border‐monitoring effort, the AFHSC/GEIS also supports three DoD research laboratories in monitoring influenza among US Foreign Service personnel and their dependents at over 80 US Embassies worldwide.
Active‐duty US service members, who are highly immunized and whose dependents are well‐documented, also provide a valuable population for studies of annual influenza vaccine effectiveness (VE). NHRC conducts population‐based VE studies at its eight recruit training FRI surveillance locations 18 , 19 and USAFSAM derives its VE estimates based on a field‐expedient method involving a cohort from the immediate family members of active‐duty personnel and influenza‐positive index cases in the US Air Force. Additionally, population‐based, active duty component, non‐recruit military service members are routinely included in periodic assessments conducted by AFHSC epidemiology staff looking at vaccine‐specific VE estimates to better inform military vaccination policy. 20 , 21
Annual results from studies of global strain diversity and subtype‐specific VE are presented to the FDA’s Vaccine and Related Biologic Products Advisory Committee for influenza vaccine strain selection and also assist the DoD in evaluating the effectiveness of its annual influenza vaccination efforts.
Expansions and enhancements in 2006–2009
The AFHSC/GEIS influenza surveillance system expanded significantly between 2006 and 2008. The expansion focused on enhancing pandemic preparedness, both within the DoD and globally via support of host‐country detection and reporting capabilities required by the revised International Health Regulations 2005 (IHR 2005). 22 The enhancement strategy identified a set of priorities, including: (i) expanding the geographic breadth and diversity of ILI and FRI surveillance; (ii) increasing the number of ILI and FRI samples; (iii) enhancing the diagnostic capabilities of DoD laboratories, with a focus on high‐throughput H5 screening and increased biosecurity; (iv) enhancing the surveillance and laboratory capabilities of host‐country collaborators, through training and infrastructure enhancement; (v) investing in novel surveillance and diagnostic techniques and studies; and (vi) enhancing the preparedness of DoD combatant and regional medical commands through trainings and exercises.
Between 2005 and 2008, the network tripled the number of countries routinely submitting specimens [Table 2]. This included an expansion in South America from 2 countries to 10, as well as significant expansions in Africa, the Middle East and Asia. 23 Many of these represent the only sites where influenza isolates were submitted to the GISN from these countries, and have significantly improved host‐country compliance with the revised reporting requirements of the IHR 2005. 22
Table 2.
Selected indicators of Department of Defense influenza surveillance program growth, all sites, 2005–2008
| Fiscal year* | ILI samples processed | Influenza positive** | Influenza A | Influenza B | Original samples and isolates to CDC*** | Number of countries hosting ILI surveillance |
|---|---|---|---|---|---|---|
| 2005 | 11 940 | 2571 | 1057 | 858 | 550 | 24 |
| 2006 | 17 062 | 3382 | 2352 | 1015 | 444 | 29 |
| 2007 | 24 439 | 4976 | 3489 | 1278 | 148 | 56 |
| 2008 | 26 216 | 6089 | 3752 | 1750 | 522 | 72 |
| % Change, 2008 versus 2005 | 220 | 237 | 355 | 204 | 95 | 300 |
ILI, influenza‐like illness.
*DoD fiscal year: October 1 through September 30.
**By PCR test, culture or both.
***Influenza isolates or original samples requested by the WHO Collaborating Centre at the CDC, Atlanta, GA, USA, following initial characterization.
Sources of data: Internal reports by partners, March–April 2009, and Fiscal Year 2008 Annual Report, April 2009.
The number of ILI specimens tested in the laboratory for influenza also more than doubled during the 2005–2008 period in question. This growth reflects expansion in the number of countries, the number of sites within each country and the capacity of the principle partner laboratories. In addition, further enhancements in the year 2009 have resulted in influenza surveillance being implemented at 491 sites in 75 countries (Figure 1). The actual number of isolates submitted to the CDC declined slightly during the period in question. However, this likely represents improved efficiency, as the characterization capabilities at partner laboratories have grown, and most partners have the capability to submit full or partial hemagglutinin sequence information to CDC along with specimen typing and subtyping information. This enables CDC to request a smaller, better targeted subset of isolates and original samples. Additionally, challenges by the Indonesian government to the WHO’s practice of influenza virus sharing hindered work within Indonesia after January 2007.
Figure 1.
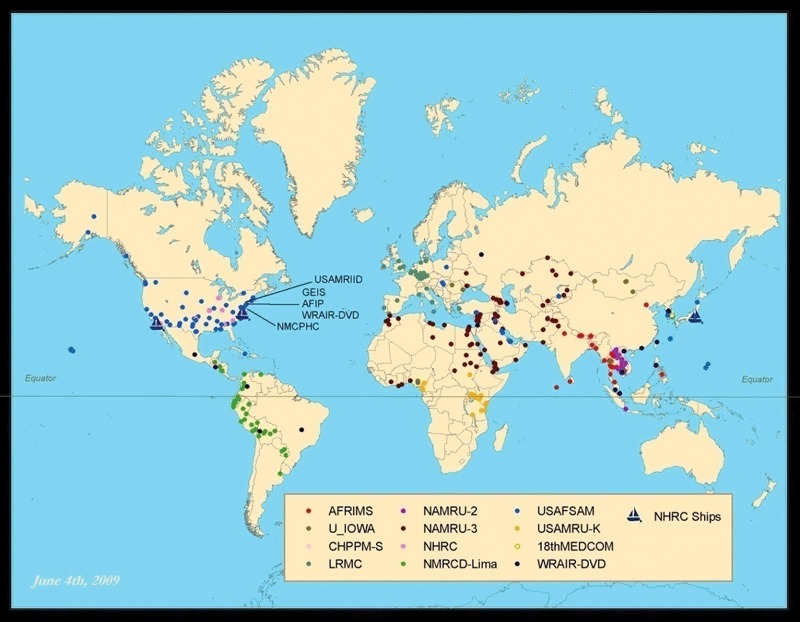
Department of Defense Global Emerging Infections Surveillance and Response System global influenza surveillance presence worldwide, as of June 2009.
Because of the geographically unique areas represented by the DoD surveillance system, the isolates and original specimens that it submits are often valuable in assisting the FDA and the WHO to construct their recommendations for influenza vaccine composition. Table 3 describes influenza virus strains identified by the DoD network, which have been included as seed strains in, or have directly influenced the composition of, several northern and southern hemisphere influenza vaccines in the past decade. Of note, three wild‐type isolates of the novel (H1N1) 2009 influenza viruses isolated by NHRC and USAFSAM investigators are currently in use as vaccine strains in support of large‐scale production of H1N1‐based pandemic vaccines (strains A/California/04/2009, A/California/07/2009 and A/Texas/05/2009). 24 , 25 , 26
Table 3.
Recent significant Department of Defense influenza isolate contributions to seasonal and pandemic influenza vaccine constitution
| Isolation year | Vaccine strain | Virus | Detection population (institution) | Contribution |
|---|---|---|---|---|
| 1999 | A/Moscow/10/99 (H3N2)‐like | A/Panama/2007 | Panama (USAFSAM) | Seed strain, northern and southern hemispheres: 2000–2004 |
| 1999 | A/New Caledonia‐like | A/Peru/1521/99 | Peru (NMRCD‐Lima) | Reference strain: 2000–2007 |
| 2006 | A/Wisconsin/67/2005 (H3N2)‐like | A/Nepal/921/2006 | Nepal (AFRIMS, WARUN) | Reference strain: 2007 |
| 2007 | A/South Dakota/4344/Ellsworth | A/South Dakota/6/07 | South Dakota (USAFSAM) | Seed strain (LAIV): 2008–2009 |
| 2009 | A/California/7/2009 (HlNl)v‐like | A/California/7/2009 A/California/4/2009 A/Texas/5/2009 | California (NHRC) California (NHRC) Texas (USAFSAM) | Seed strain: A/HlNlv 2009–2010 |
AFHSC/GEIS has also invested heavily in increasing the biosecurity and diagnostic capabilities of network laboratories. As more regional network laboratories are upgraded to biosafety level 3 (BSL‐3) status, potential novel influenza strains can be diagnosed locally in a more timely fashion than under the previous, highly centralized model, further supporting IHR 2005 reporting compliance. Since 2006, AFHSC/GEIS has funded the upgrade of six network laboratories to BSL‐3 status, to bring the network total to nine. Three upgrades are complete, and the remaining three are on‐track to be operational by the conclusion of 2010. Additionally, AFHSC/GEIS has supported the construction or renovation of BSL‐2 laboratories in more than 13 countries. At these laboratories, AFHSC/GEIS has funded the expanded use of PCR‐based screening platforms (including the FDA‐approved Human Influenza Virus Real‐Time RT‐PCR Detection and Characterization Panel developed by the CDC) in order to identify highly pathogenic strains prior to growing them in culture. As a consequence, all laboratories have increased their specimen testing capacity in the event they are forced to operate 24/7 during a pandemic.
The AFHSC/GEIS influenza surveillance system has also been at the vanguard of Highly Pathogenic Avian Influenza H5N1 diagnostic support in resource‐limited settings, including the laboratory diagnosis of 184 cases of influenza A/(H5N1) in six countries. In 2008, the AFHSC/GEIS network was involved in the diagnosis of eight human H5N1 cases, which represents 18% of the global total for the calendar year, and as of mid‐December 2009, 38 cases (37 in Egypt, 1 in Cambodia) out of 51 (75%) (Ref. 27; P. Blair, personal communication, 15 December 2009).
Furthermore, AFHSC/GEIS projects in host‐countries have funded laboratory equipment, supplies and training to increase the surveillance and diagnostic capabilities of these collaborative endeavors. All contributing laboratories and clinical surveillance sites are provided with the equipment needed to obtain, store and ship quality respiratory specimens. Training programs have focused on laboratory diagnostic and isolation techniques, outbreak responses, and institution review board and human use protocols.
AFHSC/GEIS has also invested in a number of novel surveillance approaches. Partner organizations are carrying out community and household‐based cohort studies to examine the transmission of influenza from birds, pigs and horses to humans in high‐exposure settings in Southeast Asia (Thailand and Cambodia), Central Asia (Mongolia), West Africa (Nigeria) and Eastern Europe (Romania). In South America, AFHSC/GEIS has also supported the expansion of ALERTA, a real‐time electronic disease reporting system in four countries that is optimized for resource‐limited settings. 28 , 29 AFHSC/GEIS has established the capability through partners to perform routine serologic studies of influenza exposure and seroconversion utilizing the DoD’s serum repository – the largest in the world. 30 AFHSC/GEIS is also working to reduce the DoD’s reliance on influenza rapid tests in austere settings through a joint venture with the CDC and FDA to add an influenza A/H5 and novel A/H1‐specific real‐time PCR assay to a ruggedized field‐deployable JBAIDS (Joint Biological Agent Identification and Diagnostic System) package already in use at over 300 US military clinics and hospitals worldwide. Furthermore, future expansion and inclusion is being sought to add the CDC influenza panA, panB, A/H1, A/H3 and A/H7 assays.
AFHSC/GEIS has additionally sponsored regional training seminars for DoD pandemic planners and public health professionals around the world to accelerate the implementation of DoD‐wide and international pandemic preparedness efforts. Since 2006, AFHSC/GEIS has supported over 46 training seminars, reaching over 2900 public health practitioners from 53 countries.
In the fall of 2007, the Institute of Medicine (IOM) published a comprehensive review of the first year of activities funded by HR 1815. 31 The committee commended the AFHSC/GEIS network for its expansion efforts undertaken. The full report suggested improving domestic and international coordination of surveillance efforts and improving the bi‐directional communication with field surveillance sites. To that end, AFHSC/GEIS has established closer working relations with other domestic agencies, most notably the CDC, Department of Health and Human Services (DHHS), and Department of State through meetings that align missions and surveillance efforts. The AFHSC/GEIS program is also actively seeking to improve coordination with the WHO and other international agencies to enable its network to most effectively contribute to global influenza surveillance efforts.
Conclusions and future directions
The DoD global influenza surveillance system strives to be a valuable asset to the DoD and the global public health community. Its specimen catchment area includes regions noted for their regular contribution to global strain circulation, such as Southeast Asia and regions from which strain circulation information is limited, including South America, Africa and the Middle East. 32 Since the system’s initial expansion in 1998, samples from the DoD network have contributed significantly to the annual and pandemic vaccine composition, three times being selected as seed viruses. The detection of the first four cases of a pandemic (H1N1) 2009 influenza virus in the USA has led to an additional contribution in pandemic vaccine development. 16 , 17 , 24 , 25 , 26
As a contributor to the WHO’s GISN, the DoD system is unparalleled in scope. A key feature of this surveillance system is that it is laboratory‐based (e.g. providing pathogen‐specific laboratory testing and confirmatory data) and encompasses a large network of military and civilian health facilities, research laboratories, and field locations that accrue specimens from nearly 500 participating sites in 75 countries around the world. This extensive network is positioned to detect the emergence of significant mutations in the pandemic (H1N1) 2009 influenza virus as they emerge. With the responsibility of conducting surveillance in many regions across the world, this network must also grapple with difficult but important issues. Paramount among these is the issue regarding the ownership of viruses sent to the WHO at the request of the Indonesian Ministry of Health and the development and sharing of vaccines from these viruses. 33 As a WHO partner in this emerging debate regarding domestic sovereignty of viruses, the DoD is committed to a constructive resolution that balances domestic rights with global public health needs.
Recent congressional supplemental funding has enabled a significant expansion in capability, geographic coverage and in the commencement of new initiatives. The IOM review 31 recognized the AFHSC/GEIS program for contributing to the development of laboratory and communications infrastructure within partner countries as well as for building DoD and host‐country laboratory and human resource capacity that resulted in expanded information about avian influenza and acute respiratory diseases. Major new undertakings have included the expansion of host‐country civilian and military laboratory diagnostic and training capacity, the propagation of multi‐pathogen screening technologies, development of new, high‐throughput, diagnostic detection platforms, and significant expansion into austere, low‐resource settings previously under‐represented by global surveillance efforts.
Disclaimer
The opinions and assertions contained herein are solely those of the authors and do not reflect the official policy or position of the US Department of Defense (DoD) or of its subordinate services (Army, Navy or Air Force) medical authorities.
Financial support
This study was supported by the US Military’s Defense Health Program funding of the DoD Global Emerging Surveillance and Response System (DoD‐GEIS) and the Armed Forces Health Surveillance Center (AFHSC).
Additional members of the DoD Influenza Working Group
US Naval Health Research Center (NHRC): Anthony Hawksworth, CDR Dennis Faix, Dr. Christopher Meyers; US Air Force Shool of Aerospace Medicine (USAFSAM): Col William Courtney, Dr. Elizabeth Macias, Major Thomas Gibbons; US Naval Medical Research Unit No. 3 (NAMRU‐3): Dr. Moustafa Mansour, CAPT Kenneth Earhart; US Naval Medical Research Center Detachment (NMRCD): LCDR Tadeusz Kochel, Ms. Gloria Chauca, Dr. Alberto Laguna‐Torres; Armed Forces Medical Research Institute of the Medical Sciences (AFRIMS): Col Rodney Coldren, Maj Richard Jarman LTC Robert Gibbons; US Army Medical Research Unit‐Kenya (USAMRU‐K): Dr. Wallace Bulimo, Dr. Karen Saylors; US Naval Medical Research Unit No. 2 (NAMRU‐2): LCDR Gary Brice, CDR Timothy Burgess, CDR Shannon Putnam; US Naval and Environmental Preventive Medicine Unit No. 2 (NEPMU‐2): CDR Danny Shiau, CDR Eric Kasowski, LCDR Timothy Styles: Pacific Air Forces/US Pacific Command (PACAF/PACOM): Major Wesley Palmer; US Army Public Health Command, Public Health Region‐Europe (PHR‐Europe): LTC John Maza, LCDR Michael Cooper; Landstuhl Regional Medical Center (LRMC): MAJ Thomas Palys; US Army Public Health Command, Public Health Region‐South (PHR ?South): Ms. Angela Owens.
References
- 1. Russell KL, Broderick MP, Franklin SE, et al. Transmission dynamics and prospective environmental sampling of adenovirus in a military recruit setting. J Infect Dis 2006; 194:877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gray GC, Callahan JD, Hawksworth AW, Fisher CA, Gaydos JC. Respiratory diseases among U.S. military personnel: countering emerging threats. Emerg Infect Dis 1999; 5:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gray GC, Goswami PR, Malasig MD, et al. Adult adenovirus infections: loss of orphaned vaccines precipitates military respiratory disease epidemics. For the Adenovirus Surveillance Group. Clin Infect Dis 2000; 31:663–670. [DOI] [PubMed] [Google Scholar]
- 4. Canas LC, Lohman K, Pavlin JA, et al. The Department of Defense laboratory‐based global influenza surveillance system. Mil Med 2000; 165(7 Suppl. 2):52–56. [PubMed] [Google Scholar]
- 5. Williams RJ, Cox NJ, Regnery HL, et al. Meeting the challenge of emerging pathogens: the role of the United States Air Force in global influenza surveillance. Mil Med 1997; 162:82–86. [PubMed] [Google Scholar]
- 6. Gaydos JC, Top FH Jr, Hodder RA, Russell PK. Swine influenza a outbreak, Fort Dix, New Jersey, 1976. Emerg Infect Dis 2006; 12:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Russell KL, Hawksworth AW, Ryan MA, et al. Vaccine‐preventable adenoviral respiratory illness in US military recruits, 1999–2004. Vaccine 2006; 24:2835–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Russell KL. Respiratory infections in military recruits; in Lenhart M, Lounsbury D, North RJ. (eds): Textbooks of Military Medicine: Recruit Medicine. Washington, DC: Borden Institute, Walter Reed Army Medical Center, 2006; 227–253. [Google Scholar]
- 9. Barry JM. The Great Influenza: The Epic Story of the Deadliest Plague in History. New York: Viking Penguin, 2004. [Google Scholar]
- 10. Kelley PW. A commentary on the military role in global influenza surveillance. Am J Prev Med 2009; 37:260–261. [DOI] [PubMed] [Google Scholar]
- 11. Owens AB, Canas LC, Russell KL, et al. Department of Defense Global Laboratory‐Based Influenza Surveillance: 1998–2005. Am J Prev Med 2009; 37:235–241. [DOI] [PubMed] [Google Scholar]
- 12. US Congress (ed.). H.R. 1815, Section 748: Pandemic Avian Flu Preparedness, 2005.
- 13. Department of Homeland Security . National Strategy for Pandemic Influenza: Implementation Plan. Homeland Security Council, The White House: Washington, DC, 2006. [Google Scholar]
- 14. Department of Homeland Security . National Strategy for Pandemic Influenza: Implementation Plan One Year Summary. Homeland Security Council, The White House: Washington, DC, 2007. [Google Scholar]
- 15. Office of the Assistant Secretary of Defense for Homeland Defense . Department of Defense Implementation Plan for Pandemic Influenza. Department of Defense: Washington, DC, 2006. [Google Scholar]
- 16. US Centers for Disease Control and Prevention (CDC) Update: swine influenza A (H1N1) infections – California and Texas, April 2009. Morb Mortal Wkly Rep 2009; 58:435–437. [PubMed] [Google Scholar]
- 17. CDC . Swine influenza A (H1N1) infection in two children – Southern California, March–April 2009. Morb Mortal Wkly Rep 2009; 58:400–402. [PubMed] [Google Scholar]
- 18. Russell KL, Ryan MA, Hawksworth A, Freed NE, Irvine M, Daum LT, NHRC Respiratory Disease Surveillance Team . Effectiveness of the 2003–2004 influenza vaccine among U.S. military basic trainees: a year of suboptimal match between vaccine and circulating strain. Vaccine 2005; 23:1981–1985. [DOI] [PubMed] [Google Scholar]
- 19. Strickler JK, Hawksworth AW, Myers C, Irvine M, , Ryan MA, Russell KL. Influenza vaccine effectiveness among US military basic trainees, 2005–06 season. Emerg Infect Dis 2007; 13:617–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eick AA, Wang Z, Hughes H, Ford SM, Tobler SK. Comparison of the trivalent live attenuated vs. inactivated influenza vaccines among U.S. military service members. Vaccine 2009; 27:3568–3575. [DOI] [PubMed] [Google Scholar]
- 21. Wang Z, Tobler S, Roayaei J, Eick A. Live attenuated or inactivated influenza vaccines and medical encounters for respiratory illnesses among US military personnel. JAMA 2009; 301:945–953. [DOI] [PubMed] [Google Scholar]
- 22. World Health Organization (WHO) International Health Regulations, 2nd ed. World Health Organization, Press: Geneva, Switzerland, 2008. [Google Scholar]
- 23. Armed Forces Health Surveillance Center (AFHSC) . DoD Global Emerging Infections Surveillance and Response System: Fiscal Year 2008 Annual Report. 2009. [cited; 8 September 2009]. Available at: http://www.geis.fhp.osd.mil/GEIS/aboutGEIS/annualreports/GEISAR08.pdf
- 24. US Food and Drug Administration (FDA) . Transcript for the Vaccines and Related Biological Products Advisory Committee, in FDA‐CBER Vaccines and Related Biological Products Advisory Committee. Gaithersburg, MD: CASET Associates, Ltd, 2009; p. 1–244. [Google Scholar]
- 25. World Health Organization (WHO) . Characteristics of the emergent influenza A (H1N1) viruses and recommendations for vaccine development. 2009. [cited 11 August 2009; 1–6]. Available at: http://www.who.int/csr/resources/publications/swineflu/H1N1Vaccinevirusrecommendation26May2009.pdf (Accessed 26 May 2009).
- 26. World Health Organization (WHO) . Table: summary of available candidate vaccine viruses for development of pandemic (H1N1) 2009 virus vacccines. 2009. [cited 11 August 2009]. Available at: http://www.who.int/csr/disease/swineflu/guidance/vaccines/candidates/en/index.html (Accessed 31 July 2009).
- 27. WHO . Table: cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. Available at: http://www.who.int/csr/disease/avian_influenza/country/ (Accessed 17 December 2009).
- 28. Johnson PR, Blazes DL. Using cell phone technology for infectious disease surveillance in low‐resource environments: a case study from Peru; in Institute of Medicine (ed.): Global Issues in Infectious Disease Surveillance and Detection. Washington, DC: The National Academies Press, 2007; 136–153. [Google Scholar]
- 29. Soto G, Araujo‐Castillo RV, Neyra J, et al. Challenges in the implementation of an electronic surveillance system in a resource‐limited setting: ALERTA in Peru. BMC Proc 2008; 2(Suppl. 3):S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rubertone MV, Brundage JF. The Defense Medical Surveillance System and the Department of Defense serum repository: glimpses of the future of public health surveillance. Am J Public Health 2002; 92:1900–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Institute of Medicine (US) . Committee for the Assessment of DoD‐GEIS Influenza Surveillance and Response Programs Review of the DoD‐GEIS Influenza Programs: Strengthening Global Surveillance and Response. Washington, DC: National Academies Press, 2008; xxii, 226. [Google Scholar]
- 32. Russell CA, Jones TC, Barr IG, et al. The global circulation of seasonal influenza A (H3N2) viruses. Science 2008; 320:340–346. [DOI] [PubMed] [Google Scholar]
- 33. Sedyaningsih ER, Isfandari S, Soendoro T, Supari SF. Towards mutual trust, transparency and equity in virus sharing mechanism: the avian influenza case of Indonesia. Ann Acad Med Singapore 2008; 37:482–488. [PubMed] [Google Scholar]


