Abstract
Please cite this paper as: Papic et al. (2011) Liver involvement during influenza infection: perspective on the 2009 influenza pandemic. Influenza and Other Respiratory Viruses 6(3), e2–e5.
Elevation of liver transaminase levels is a frequent observation during systemic infections. The aim of our study was to investigate liver damage during pandemic 2009 influenza A/H1N1 infection in comparison with seasonal influenza. Serum levels of aspartate aminotransferase, alanine aminotransferase, and gamma‐glutamyl transpeptidase (GGT) were significantly higher in patients with pandemic influenza compared to seasonal influenza, which was strongly correlated with hypoxia. Moreover, a positive correlation between C‐reactive protein and serum GGT, alkaline phosphatase, and lactate dehydrogenase was noticed. Our findings support the hypothesis that the pandemic 2009 influenza A/H1N1 is an illness with a significant immune response to infection leading to hepatocellular injury.
Keywords: Alanine aminotransferase, H1N1, hypoxia, influenza, liver, systemic inflammatory response
Introduction
On June 11, 2009, the outbreak of a new strain of influenza A virus subtype H1N1 was officially declared by the World Health Organization to be the first influenza pandemic of the 21st century. 1 Reports of pandemic 2009 influenza A/H1N1 described a virus that is more pathogenic than seasonal influenza and that caused severe infection and complications in relatively young, otherwise healthy individuals. 1 , 2
The occurrence of liver involvement during influenza infection is intriguing owing to the virus characteristic of only infecting the epithelial cells of the respiratory tract. 3 Although the liver may not be the primary target organ of viral infection, it can be collaterally damaged. 3 , 4 Hepatic changes may be a consequence of the immune response to viral antigens with a close topographic association between the presence of viral antigens and the associated inflammatory infiltrates in liver. 3 , 5
In the novel pandemic 2009 influenza A/H1N1, it is still unclear how significant the liver damage is. The aim of our study was to compare liver injury during influenza A/H1N1 (2009) infection with seasonal influenza.
Patients and methods
A retrospective study that included cases of pandemic influenza during the outbreak of pandemic 2009 influenza A/H1N1 from June 1, 2009, until April 1, 2010, and seasonal influenza during 2008 and 2009 seasonal epidemics, was conducted at the University Hospital for Infectious Diseases Zagreb. Patient records were extracted and used for collection of clinical and laboratory data. Only cases with positive RT‐PCR and/or direct immunoflorescence of sputum or nasal swab were included in study.
Of the 294 cases reviewed, we excluded a total of 110 patients: 72 who were under 15 years of age, 11 with underlying liver or bile duct diseases, eight with medical conditions that could influence liver function tests (e.g., chemotherapy and hepatotoxic drugs), and 19 patients with positive blood cultures. The patients were classified into two groups: pandemic and seasonal.
We analyzed the clinical data and the results of routine biochemical and liver function tests performed on the first day of hospitalization before commencing antiviral therapy (oseltamivir).
Statistical analysis was performed using Prism (version 5.0) statistical software (GraphPad Software, San Diego, CA, USA). The demographic, clinical, and laboratory data were evaluated and presented descriptively. Fisher’s exact test and Mann–Whitney U test were used to compare the two groups, as appropriate. Pearson’s correlation coefficient was applied to measure the degree of association between the parametric variables, and Spearman’s rho for nonparametric variables. P < 0·05 values were considered significant.
Results
Patients’ characteristic
The pandemic group consisted of 97 patients: 54 (56·25%) men and 43 (43·75%) women, mean age 46·79 ± 15·69 years. The seasonal group included 86 patients: 52 (60·45%) men and 34 (39·55%) women, mean age 47·06 ± 22·55 years. In the seasonal group, all patients had influenza A, but only some of the strains were subtyped (of those 68·60% had A/H1N1 and 31·40% H3N2 subtype). There was no significant difference in sex and age between the two groups (P = 0·5504 and P = 0·9377, respectively).
In the pandemic group, levels of liver enzymes were more often above normal range than in the seasonal group: aspartate aminotransferase (AST) (35·78% versus 18·60%, P = 0·01245), alanine aminotransferase (ALT) (26·31% versus 7·36%, P = 0·0016), gamma‐glutamyl transpeptidase (GGT) (36·84% versus 16·47%, P = 0·0025), and lactate dehydrogenase (LDH) (50·00% versus 33·33%, P = 0·0387, Fisher’s exact test). Most of the patients had mild hepatic lesions, representing a pattern of hepatocellular injury. In patients with liver enzyme abnormalities, the transaminase level was elevated 2·5‐fold and normalized rapidly over several days (Table 1).
Table 1.
Hematological and biochemical parameters
| Laboratory reference | Pandemic 2009 A influenza (H1N1) Mean (IQR) | Seasonal influenza (2007–2009) Mean (IQR) | P‐value* | |
|---|---|---|---|---|
| C‐reactive protein (mg/l) | <5 | 76·18 (22·20–98·95) | 56 (12·53–81·58) | 0·0122 |
| Leukocytes (×109/l) | 3·4–9·7 | 8·1 (5·275–9·825) | 8·24 (5·3–10·03) | 0·5911 |
| Thrombocytes (×109/l) | 158–424 | 184·09 (143–217) | 197 (148·5–227·0) | 0·0884 |
| Fibrinogen (g/l) | 1·8–3·5 | 6·00 (4·79–7·11) | 5·31 (3·25–6·08) | 0·0876 |
| d‐dimer (mg/l) | <0·35 | 0·58 (0·185–0·365) | 0·38 (0·155–1·203) | 0·7436 |
| Total bilirubin (μmol/l) | 3–20 | 18·04 (6·9–16·70) | 12·54 (6·30–14·75) | 0·1780 |
| AST (U/l) | 8–38 | 48·66 (20–59) | 31·91 (20·75–35·25) | 0·0172 |
| ALT (U/l) | 10–48 | 45·89 (18–47) | 27·33 (16–31) | 0·0017 |
| GGT (U/l) | Men: 11–55 Women: 9–35 | 71·79 (19–67) | 34·52 (15·50–38) | 0·0055 |
| ALP (U/l) | Men: 60–142 Women: 50–153 | 84·23 (55–90·25) | 77·08 (52–85·75) | 0·4314 |
| LDH (U/l) | <241 | 316·82 (202–360) | 250·22 (177·5–265·5) | 0·0096 |
IQR, interquartile range; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma‐glutamyl transpeptidase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase.
*Mann–Whitney U test. P < 0.05 values are significant
Relationship between peripheral oxygen saturation (SpO2), CRP, and liver injury
Hypoxemia (oxygen saturation <95%), measured by pulse oxymeter at the time of patient admission to hospital, was observed in 47·25% (43/91) of pandemic, but only in 18·6% (16/70) of the seasonal group (P < 0·0001, Fisher’s exact test). Peripheral oxygen saturation range was 84–100%. Sixteen patients had oxygen saturation ≤90% (17·58%) in the pandemic and 6 (8·57%) in the seasonal group. To identify the relationship between liver enzymes levels, C‐reactive protein (CRP), and LDH with SpO2, correlation–regression analysis was performed (Figure 1A, B).
Figure 1.
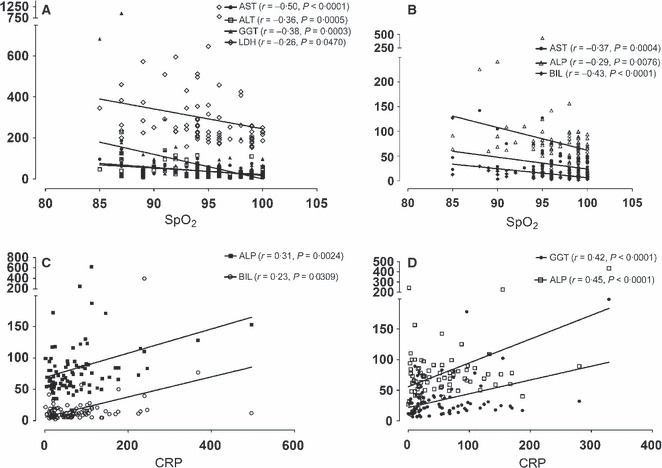
(A, B) Correlation between liver enzymes and peripheral oxygen saturation in pandemic 2009 influenza A/H1N1 and seasonal influenza, respectively. (C, D) Correlation between C‐reactive protein and liver enzymes in pandemic 2009 influenza A (H1N1) and seasonal influenza, respectively. Pearson’s r value and P‐value were presented. P < 0·05 is considered significant. AST, Aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma‐glutamyl transpeptidase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; BIL, total bilirubin; CRP, C‐reactive protein; SpO2, pulse oximeter oxygen saturation.
It revealed that AST, ALT, GGT, and LDH were negatively correlated with SpO2 in the pandemic group. Similarly, a negative correlation was observed in the seasonal group between AST, alkaline phosphatase (ALP), and bilirubin with SpO2.
Our study demonstrates a positive correlation between CRP and serum GGT, ALP, and LDH in seasonal and serum bilirubin and ALP in the pandemic group, as presented in Figure 1C, D.
Correlation between duration of hospitalization and liver involvement
In the pandemic group, duration of hospitalization was strongly correlated with age (r = 0·4292, P < 0·0001), CRP (r = 0·2840, P = 0·0064), bilirubin (r = 0·2421, P = 0·0208), GGT (r = 0·3133, P = 0·0025), ALP (r = 0·3924, P = 0·0009), and LDH (r = 0·2825, P = 0·0067) levels. In the seasonal group, duration of hospitalization was strongly correlated with age (r = 0·3220, P = 0·0025), CRP (r = 0·3235, P = 0·0024), bilirubin (r = 0·2767, P = 0·0108), and AST (r = 0·3730, P = 0·0004), ALT (r = 0·2604, P = 0·0154), GGT (r = 0·2745, P = 0·0110), and ALP (r = 0·2617, P = 0·0190) levels.
Discussion
Liver damage does not typically occur in seasonal influenza. To the best of our knowledge, this is the first report comparing liver involvement in seasonal influenza A (H1N1 and H3N2) and pandemic 2009 influenza A/H1N1.
Elevation of liver transaminase levels is a frequent observation during systemic infections. 3 , 4 , 6 However, the incidence of liver injury in influenza has not been established and the pathogenesis of liver involvement is still not well understood. Liver damage has been shown in animal models, revealing that virus replication is not needed to produce hepatocellular injury. 7 Production of cytokines (e.g., TNF‐alpha, IL‐6, IL‐8, IL‐10, and interferon alpha, interferon beta, and interferon gamma) is responsible for the hepatic oxidative stress leading to the hepatocyte injury. 7 , 8 Polakos et al. 5 introduced a so called collateral damage model and revealed that liver lesions occur because of the systemic immune response (SIRS) where, in the absence of viral antigens, the hepatocytes are damaged by viral‐specific CD8+ T cells.
Our study offers some interesting findings of liver involvement during influenza infection. First, liver damage was more intensive with pandemic 2009 A/H1N1 than during recent seasonal influenza A. According to our study, serum levels of AST, ALT, and GGT were significantly higher in the pandemic group.
Second, abnormalities in serum liver enzymes are strongly correlated with hypoxemia. Because hypoxemia (especially when severe) was more frequent in the pandemic group, the greater liver damage is presumably related to hypoxemia. However, no characteristic findings of ischemic hepatitis were found in any of our patients. All of the patients had mild, transient hepatic lesions.
Third, a positive correlation between CRP and serum liver enzymes was noted. Previous studies have found associations between GGT and ALP with CRP or other inflammatory parameters (e.g., leukocytes and fibrinogen). 9 , 10 , 11 CRP is a nonspecific biomarker of acute inflammation and is produced primarily in the liver. Our study revealed significantly higher levels of CRP in the pandemic group. Considering new increasing evidence 9 , 10 , 11 showing that liver enzymes are associated with oxidative stress and inflammation, we can presume that pandemic influenza promotes a more intense SIRS than seasonal influenza. SIRS finally produces perfusion disturbances of the tissue which contributes to hepatocellular damage and hypertransaminemia. 6 , 12 Bermejo‐Martin et al. 12 observed higher levels of pro‐inflammatory cytokines in pandemic 2009 influenza A/H1N1, and some of these can cause liver injury. 8 Exposure to influenza strains that circulated 50–60 years ago might provide cross‐protection against the influenza A/H1N1 (2009) virus. 13 Because the mean age of our study population is under 50 years, the possible role of the low preexisting immunity against pandemic 2009 influenza A/H1N1, which may affect the strength of the immunological response, cannot be excluded.
Although duration of hospitalization was limited owing to hospital capacities during the influenza outbreak, there was still evident a relationship between the duration of hospitalization and the results of the liver enzyme tests.
There are limited data of liver abnormalities during chronic conditions associated with both hypoxia and inflammation. Liver damage was connected to chronic obstructive pulmonary disease, which is strengthening our presumption of hypoxia and SIRS as the causative agents. 14 , 15 Whether liver enzymes provide clinical information additional to CRP requires further studies.
The main limitation of this study is the retrospective nature of the collected data. In hospitalized patients, clinical attention is on the acute infection of the respiratory tract, so the clinical importance of liver involvement remained unclear. As previously mentioned, this is the first report comparing liver involvement in seasonal and pandemic influenza.
These findings support the concept of the pandemic influenza A/H1N1 (2009) as an illness with significant immune response that in combination with moderate hypoxia caused by influenza respiratory illness may lead to hepatocellular injury and finally result in different clinical and laboratory features than in previous seasonal epidemics.
Acknowledgements
None.
Potential conflicts of interest
None.
Funding
N. Papic was supported by an ESCMID Training Fellowship program and Croatian Science Foundation Doctoral Fellowship Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bautista E, Chotpitayasunondh T, Gao Z et al. Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 2010; 362:1708–1719. [DOI] [PubMed] [Google Scholar]
- 2. Chowell G, Bertozzi SM, Colchero MA et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 2009; 361:674–679. [DOI] [PubMed] [Google Scholar]
- 3. Adams DH, Hubscher SG. Systemic viral infections and collateral damage in the liver. Am J Pathol 2006; 168:1057–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shimizu Y. Liver in systemic disease. World J Gastroenterol 2008; 14:4111–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polakos NK, Cornejo JC, Murray DA et al. Kupffer cell‐dependent hepatitis occurs during influenza infection. Am J Pathol 2006; 168:1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carrillo‐Esper R, Pérez‐Bustos E, Ornelas‐Arroyo S, Albores‐Saavedra J, Uribe M. Liver involvement in severe human influenza a H1N1. Ann Hepatol 2010; 9:107–111. [PubMed] [Google Scholar]
- 7. Fislova T, Gocnik M, Sladkova T et al. Multiorgan distribution of human influenza A virus strains observed in a mouse model. Arch Virol 2009; 154:409–419. [DOI] [PubMed] [Google Scholar]
- 8. Tiegs G. Cellular and cytokine‐mediated mechanisms of inflammation and its modulation in immune‐mediated liver injury. Z Gastroenterol 2007; 45:63–70. [DOI] [PubMed] [Google Scholar]
- 9. Bo S, Gambino R, Durazzo M et al. Associations between gamma‐glutamyl transferase, metabolic abnormalities and inflammation in healthy subjects from a population‐based cohort: a possible implication for oxidative stress. World J Gastroenterol 2005; 11:7109–7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee DH, Jacobs DR Jr. Association between serum gammaglutamyltransferase and C‐reactive protein. Atherosclerosis 2005; 178:327–330. [DOI] [PubMed] [Google Scholar]
- 11. Kerner A, Avizohar O, Sella R et al. Association between elevated liver enzymes and C‐reactive protein: possible hepatic contribution to systemic inflammation in the metabolic syndrome. Arterioscler Thromb Vasc Biol 2005; 25:193–197. [DOI] [PubMed] [Google Scholar]
- 12. Bermejo‐Martin JF, Ortiz de Lejarazu R, Pumarola T et al. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care 2009; 13:R201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hancock K, Veguilla V, Lu X et al. Cross‐reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009; 361:1945–1952. [DOI] [PubMed] [Google Scholar]
- 14. Henrion J, Minette P, Colin L, Schapira M, Delannoy A, Heller FR. Hypoxic hepatitis caused by acute exacerbation of chronic respiratory failure: a case‐controlled, hemodynamic study of 17 consecutive cases . Hepatology 1999; 29:427–433. [DOI] [PubMed] [Google Scholar]
- 15. Minakata Y, Sugiura H, Yamagata T et al. Prevalence of COPD in primary care clinics: correlation with non‐respiratory diseases . Intern Med 2008; 47:77–82. [DOI] [PubMed] [Google Scholar]


