Abstract
Please cite this paper as: Vodeiko and Weir (2011). Determination of H5N1 vaccine potency using reference antisera from heterologous strains of influenza. Influenza and Other Respiratory Viruses 6(3), 176–187.
Background Standardization of inactivated influenza vaccines by hemagglutinin (HA) content is performed by the single radial immunodiffusion (SRID) method. Regulatory agencies prepare, calibrate, and distribute SRID reagent standards necessary for testing of seasonal influenza vaccines, and a similar process is used to produce potency reagents for candidate pandemic influenza vaccines that are manufactured for emergency stockpiles.
Objectives Because of the concerns in generating a timely strain‐specific potency antiserum for an emerging pandemic virus, we evaluated the feasibility of using heterologous potency reference antiserum as a replacement for a strain‐specific (homologous) antiserum in the SRID potency assay for stockpiled H5N1 vaccines.
Results The results indicate that a heterologous H5N1 antiserum can be used to determine the accurate potency of inactivated H5N1 influenza vaccines. Additionally, when H5N1 vaccine was subjected to an accelerated stability protocol, both homologous and heterologous antisera provided similar measurements of vaccine potency decline. Limitations to the heterologous antiserum approach to potency determination were shown by the inability of antiserum to recent seasonal H1N1 viruses to work in an SRID assay with the 2009 pandemic H1N1 A/California/07/2009 antigen.
Conclusions The data demonstrate the feasibility of using heterologous antiserum for potency determination of at least some candidate vaccines in case of a shortage or delay of homologous antiserum. Further, the results suggest the prudence of stockpiling a broad library of potency reagents including many strains of influenza viruses with pandemic potential to provide an added measure of assurance that reagent production would not be a bottleneck to vaccine production during a pandemic.
Keywords: Pandemic influenza, single radial immunodiffusion assay, vaccine potency
Introduction
Since 1977, standardization of the hemagglutinin (HA) content of inactivated influenza vaccines has been performed by the single radial immunodiffusion (SRID) method. 1 , 2 , 3 , 4 , 5 , 6 This assay is not technically demanding and can be standardized through the use of strain‐specific reagents provided by regulatory and other public health agencies. As a result of these considerations, and the fact that the vaccine potency as determined by SRID correlates with vaccine immunogenicity 7 , 8 , 9 , 10 which correlates with clinical benefit, 11 the SRID assay has been adopted and implemented worldwide by manufacturers and regulatory agencies to determine the potency of inactivated influenza vaccines. Although several alternative methods for HA quantification including techniques based on HPLC, 12 , 13 mass spectrometry, 14 and surface plasmon resonance (SPR) 15 have recently been described, none has yet been demonstrated to be suitable as a viable replacement assay, and the SRID technique remains as the standard method for testing and control of inactivated influenza vaccines.
The SRID assay is an agarose gel‐based assay which measures the diffusion and immunoprecipitation of the vaccine or reference antigen HA with a strain‐specific polyclonal antiserum. The antiserum is traditionally made in sheep using HA which has been cleaved from the virus with bromelain and purified before being used as an immunogen. The amount of antigen present is proportional to the area of the precipitin zone and is quantified by comparing a reference antigen of known HA content. Reference antigen standards are calibrated and distributed by the World Health Organization (WHO) Essential Regulatory Laboratories (ERL) that include the Center for Biologics Evaluation and Research at the U.S. Food and Drug Administration. When the antiserum used in the assay is derived from a variant virus strain within the same HA subtype (e.g., H1, H3, H5), precipitin zones in the SRID assay are often produced with a lower intensity than zones produced using the homologous antiserum. 2 , 3 In fact, when the SRID assay was developed for potency determination of influenza vaccines, the use of antiserum to a heterologous strain was not generally considered. It has been standard procedure for regulatory agencies and manufacturers to use a matched set of reference antigen and strain‐specific antiserum for each new component to be included in seasonal inactivated influenza vaccines.
Nevertheless, it was remarked some time ago that the constantly changing strains of virus incorporated in the vaccine required a major commitment of time and resources for the production of strain‐specific reagents. 6 Fortunately, the preparation of strain‐specific potency reagents, including the preparation of new antisera, rarely results in a delay in seasonal vaccine production and release. Preparedness for the emergence of a pandemic influenza virus, however, presents additional considerations for vaccine development and production, including that of reagent preparation. Several years ago, recommendations were made that libraries of candidate vaccine strains and their accompanying potency reagents should be developed as one means of preparing for a potential pandemic influenza virus. 16 Indeed, in the United States, significant effort has been made to produce stockpiles of influenza vaccines against viruses with pandemic potential, 17 , 18 , 19 including H5N1 viruses of antigenically distinct clades. The goal of this effort is to provide an immediate supply of “pre‐pandemic” vaccine until a new vaccine exactly matching the emerging pandemic vaccine becomes available. 20 To be useful during an emergency, sufficient supplies of potency reagents would need to be available for the stockpiled candidate vaccines. In addition, however, because of the time constraints and the inherent uncertainty in generating strain‐specific potency antiserum for a newly emerging pandemic virus, it would be extremely important to know whether existing heterologous potency antiserum was capable of substituting for a homologous antiserum that could only be made after the pandemic virus was identified. If so, stockpiled potency antiserum would provide an added measure of assurance that reagent production would not be a bottleneck to vaccine production and distribution.
Here, we describe an investigation into the use of cross‐reactive antibodies (heterologous antiserum) as a suitable replacement for a strain‐specific (homologous) antiserum in an SRID potency assay for H5N1 candidate vaccines representing three different virus clades. We find that for the three stockpiled H5N1 vaccines, conditions can be established under which the potency values in the presence of homologous and heterologous antisera are statistically indistinguishable from each other. The results demonstrate the feasibility of using heterologous antiserum for potency determination of at least some candidate pandemic vaccines in the event of a shortage or delay in availability of the homologous antiserum. The results suggest that producing stockpiles of potency antiserum to additional strains of potential pandemic viruses would increase the likelihood of having a workable antiserum reagent available in the event of another pandemic.
Materials and methods
SRID reagents
Reference antigens for three H5N1 influenza vaccine candidates (A/Vietnam/1203/2004, A/Indonesia/5/2005, and A/bhg/QL/A1/2005) have been produced by the Center for Biologics Evaluation and Research in collaboration with licensed vaccine manufacturers. Reference antigen for A/Anhui/1/2005 was prepared by the National Institute of Biological Controls and Standards (United Kingdom) in collaboration with a licensed vaccine manufacturer. Each reference antigen was prepared from an H5N1 reverse genetics‐modified, recombinant influenza virus vaccine candidate 21 generated by members of the World Health Organization Collaborating Centers for Influenza. All reference antigens were assigned an HA potency value (in μg/ml) through a collaborative calibration performed by the four WHO Essential Regulatory Laboratories [National Institutes of Biological Standards and Control (NIBSC), UK; Center for Biologics Evaluation and Research (CBER) of the Food and Drug Administration, USA; National Institute of Infectious Diseases (NIID), Japan; and Therapeutic Goods Administration (TGA), Australia].
Sheep immunization was performed in accordance with an approved animal protocol at CBER (A/Indonesia/5/2005 – S‐7854) or under contract to CBER (A/Vietnam/1203/2004 – S‐APS1; A/bhg/QL/1A/2005 – S‐112). Antisera were evaluated by SRID, calibrated, and lyophilized.
SRID method
The SRID assay developed by Wood and colleagues 4 for potency testing of inactivated influenza virus vaccines was recommended by the World Health Organization in 1978. 22 , 23 The assay is based on the diffusion of detergent‐disrupted virus preparations into an agarose gel containing specific anti‐HA serum. The SRID potency assay used at CBER is essential as described previously. 5 , 6 , 24 Minor modifications have been made in the agarose gel preparation. Briefly, a 1% solution of low‐melting agarose in phosphate‐buffered saline (PBS), pH 7·2, is melted and allowed to equilibrate to ∼50–55°C before addition of the appropriate amount of specific HA antiserum. After mixing well, the gel is poured in level plastic plates. The initial dilutions of vaccines and reference antigen (∼30–40 μg/ml) are prepared in deionized water to produce comparable zone sizes and then treated with 1% Zwittergent 3–14 for 30 minutes at room temperature. Additional dilutions are made in PBS, pH 7·2, and inoculated into 4‐mm wells punched into the agarose gel. Plates are incubated in a sealed, moist chamber at room temperature for a minimum of 18 hours before further processing. After 18 hours, gels are removed from the plastic plate in water and floated onto a piece of gel bond, covered with wet filter paper, and dried onto the gel bond. When completely dried, the filter paper is removed, and the gel is stained with 0·3% Coomassie Brilliant Blue for 10 minutes, destained, and dried.
Zone diameters are measured in two directions at right angles to the nearest 0·1 mm using an ImmuLab image scanner. Vaccine potency is computed by the parallel line bioassay method using reference and test vaccine dose–response curves (log antigen dilution versus log zone diameter). Statistical parameters for determining test validity are based upon correlation coefficient (r) and equality of slopes (t) between test and reference antigens. Average and standard deviation (SD) are calculated from at least four independent tests.
Hemagglutination inhibition assay
The hemagglutination inhibition assay (HAI) was performed in 96‐well plates (U‐bottom) by standard methods essentially as described previously 25 using 0·5% chicken red blood cells suspended in PBS (pH 7·2).
Influenza vaccines
Influenza H5N1 inactivated monovalent vaccines (A/Vietnam/1203/2004, A/Indonesia/05/2005 vaccine, and A/bar‐headed goose/QL/1A/2005) were produced and supplied by various licensed influenza vaccine manufacturers supported by the Biomedical Advanced Research and Development Authority, Department of Health and Human Services.
Results
Demonstration of cross‐reactivity
The three available H5N1 vaccine potency reference antisera (A/Vietnam/1203/2004, A/Indonesia/5/2005, and A/bar‐headed goose/QL/1A/2005) were evaluated for cross‐reactivity by hemagglutination inhibition (HI) assay (Table 1) and SRID assay (Figure 1). In the HI assay, each of the three hyperimmune sheep antisera made by immunization with purified bromelain‐cleaved virus hemagglutinin (HA) exhibited substantial cross‐reactivity with the H5N1 viruses from other clades and even with an H1N1 control virus (Table 1). In contrast, post‐infection ferret antisera demonstrated more noticeable differences between the H5N1 viruses of different clades and an H1N1 control virus. Although the results suggested the possibility that the sheep polyclonal antiserum contained antibodies that were cross‐reactive to the heterologous H5N1 HAs, there could be other explanations for the cross‐reactivity in the HI assay including non‐specific serum inhibitors in the sheep polyclonal antiserum. Nevertheless, cross‐reactivity of the three sheep polyclonal antisera was further evaluated in the SRID assay. Similar to the results of the HI analysis, potency antiserum from each H5N1 clade virus demonstrated cross‐reactivity with both homologous and heterologous H5N1 reference antigens by SRID (Figure 1). For each H5N1 antigen and vaccine samples manufactured from the same strain, clear visible precipitin rings were produced in the presence of the homologous reference antisera (Figure 1, left panels). Precipitin rings were also produced for each reference antigen and related vaccine samples in the presence of heterologous reference antiserum, although the quality of some of the precipitin rings was different in comparison with the homologous antiserum (Figure 1, center and right panels). For example, some of the rings appeared faint, but nevertheless these rings could be read and measured by the image scanner. In some gels, an interior double zone was observed (e.g., Figure 1B, right panel). Such double zones are occasionally observed in SRID assays with homologous antiserum. This phenomenon may be possibly due to precipitation of proteins other than HA as the antigen contains a mixture of proteins and the antiserum contains antibodies to more than HA. Regardless, the results taken together suggested that potency antiserum from a heterologous H5N1 virus strain might be suitable for potency analysis if the appropriate conditions for use in a potency assay could be established.
Table 1.
Hemagglutination inhibition assay comparison between sheep potency reference antisera and post‐infection ferret antisera
| Antigen* | Potency reference antisera (sheep hyperimmune) | Post‐infection antisera (ferret) | ||||||
|---|---|---|---|---|---|---|---|---|
| A/Viet | A/Indo | A/bhg | Control Sheep† | A/Viet | A/Indo | A/bhg | Control Ferret‡ | |
| A/Viet virus (Clade 1) | 40 | 160 | 80 | 10 | 40 | 20 | 10 | 10 |
| A/Viet Ref. antigen (Clade 1) | 160 | 320 | 320 | 80 | 40 | 20 | 10 | 10 |
| A/Indo virus (Clade 2·1) | 40 | 640 | 160 | 10 | 10 | 640 | 10 | 10 |
| A/Indo Ref. antigen (Clade 2·1) | 160 | 2560 | 320 | 160 | 10 | 640 | 10 | 10 |
| A/bhg virus (Clade 2·2) | 80 | 320 | 640 | 10 | 10 | 160 | 160 | 10 |
| A/bhg Ref. antigen (Clade 2·2) | 40 | 320 | 320 | 40 | 10 | 160 | 160 | 10 |
| Control virus§ | 40 | 160 | 160 | 2560 | 10 | 10 | 10 | 320 |
| Control Ref. antigen§ | 80 | 640 | 640 | 2560 | 10 | 10 | 10 | 160 |
*Assay antigen was either (1) infectious virus or (2) inactivated virus (reference antigen). Homologous titers are indicated in bold.
†A/California/07/2009.
‡New Caledonia/20/1999.
§Control virus and reference antigen were matched to the corresponding control sheep antiserum (A/California/07/2009) and ferret antiserum (New Caledonia/20/1999).
Figure 1.
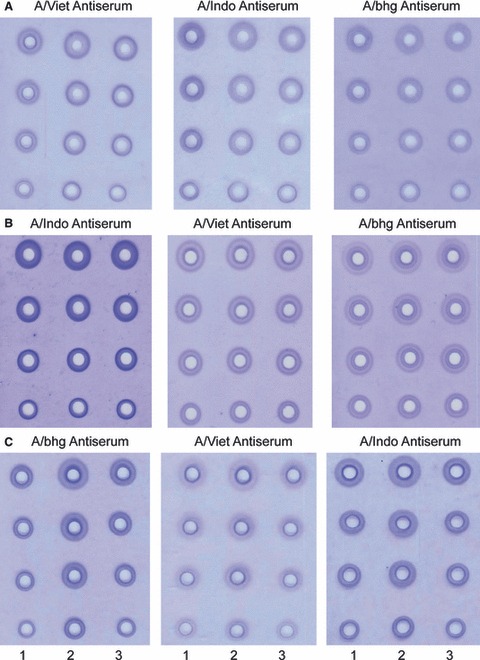
H5N1 single radial immunodiffusion (SRID) precipitin zones in the presence of homologous and heterologous potency antisera. SRID gels were run with reference antigen, and two samples of strain matched vaccine of A/Vietnam/1203/2004 (A), A/Indonesia/5/2005 (B), or Abhg/QL/1A/2005 (C) in the presence of homologous (left panels) or heterologous potency antiserum (center and right panels). Column 1 – dilutions of reference antigen. Column 2 and 3 – dilutions of vaccine.
Titration of the antigen standard in homologous and heterologous potency reference antisera
To determine whether a viable SRID assay could be established using heterologous H5N1 antisera, it was first necessary to determine whether there were workable ranges of reference antigen concentrations that could be found for each available heterologous potency reference antiserum. As the recommended concentration of each of the three potency reference antisera for use with its homologous and matching reference antigen is similar (7–10 μl/ml of gel), we chose to use a constant serum concentration equal to 10 μl/ml of gel for all assays and then determine whether a suitable dilution of the reference antigen could be found which would produce a precipitin ring with a diameter close to 8 mm and thus allow for a standard antigen curve to be setup over the optimal diameter range of ∼8·5 to ∼5·5 mm. An example of a preliminary titration for each reference antigen in the presence of homologous and heterologous potency reference antisera is shown in Table 2. Each of the four available reference antigens (A/Vietnam/1203/2004, A/Indonesia/5/2005, A/bhg/QL/1A/2005, and A/Anhui/1/2005) has an assigned potency value determined by a collaborative calibration effort of four WHO Essential Regulatory Laboratories and was used as the starting point for a series of dilutions from 1:2 to 1:10. SRID assays were performed for each series of antigen dilutions in the presence of the three available potency reference antisera: A/Vietnam/1203/2004 (S‐APS), A/Indonesia/5/2005 (S‐7854), and A/bhg/QL/1A/2005 (S‐112). For each of the ten series of SRID assays of antigen/antisera combinations, a dilution of reference antigen could be found which produced the desired precipitin ring diameter of approximately 8·0–8·5 mm (bold numbers). This dilution represents the optimal starting concentration of reference antigen that can be used to prepare a standard curve for the SRID assay using any of the three potency antisera, but the identified range of reference antigen concentrations suitable for use in the SRID assay in the presence of heterologous antisera is different from that used in homologous antiserum. For example, in the presence of 10 μl/ml of A/Indonesia/5/2005 antiserum, the initial concentration of antigen standard would be A/Vietnam – 38 μg/ml (1:4 dilution of reference antigen); A/Indonesia/5/2005 – 86 μg/ml (undiluted reference antigen); A/bhg/QL/1A/2005 – 69 μg/ml (undiluted reference antigen); and A/Anhui/1/2005 – 45 μg/ml (1:2 dilution of reference antigen). Nevertheless, as shown in Figure 2, the size of the precipitin ring was linear over the identified reference antigen concentration range for each of the four reference antigens in the presence of its corresponding homologous potency reference antisera and two heterologous potency reference antisera. Thus, the results indicated that H5N1 potency reference antisera was functional in an SRID assay with a heterologous H5N1 reference antigen and suggested that an assay with such an antigen/antiserum combination could be used to determine the potency of inactivated H5N1 influenza vaccines.
Table 2.
Single radial immunodiffusion (SRID) zone size of a dilution series of four H5N1 reference antigens in the presence of a constant concentration of three potency antisera
| Potency antiserum | SRID zone size (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1:2 | 1:3 | 1:4 | 1:5 | 1:6 | 1:7 | 1:8 | 1:9 | 1:10 | ||
| Dilutions of A/Vietnam Antigen ‐152 μg/ml | ||||||||||
| S‐APS Viet 10 μl/ml | 10·1 | 9·0 | 8·4 | 7·9 | 7·7 | 7·3 | 7·2 | 6·9 | 6·7 | 6·7 |
| S‐7854 Ind 10 μl/ml | 12·2 | 10·2 | 9·2 | 8·6 | 8·2 | 7·9 | 7·6 | 7·3 | 7·2 | 6·8 |
| S‐112 bhg 10 μl/ml | 15·2 | 13·4 | 12·0 | 11·3 | 10·4 | 9·7 | 9·3 | 8·9 | 8·6 | 7·9 |
| Dilutions of A/Indonesia Antigen – 86 μg/ml | ||||||||||
| S‐APS Viet 10 μl/ml | 8·4 | 7·4 | 7·1 | 6·6 | 6·5 | 6·5 | 6·1 | 6·1 | 5·9 | 5·8 |
| S‐7854 Ind 10 μl/ml | 8·4 | 7·2 | 6·9 | 6·8 | 6·7 | 6·6 | 6·5 | 6·4 | 6·4 | 6·4 |
| S‐112 bhg 10 μl/ml | 12·4 | 11·1 | 10·4 | 9·5 | 9·3 | 8·4 | 8·2 | 7·8 | 7·7 | 7·2 |
| Dilutions of A/bhg Antigen – 69 μg/ml | ||||||||||
| S‐APS Viet 10 μl/ml | 8·1 | 7·3 | 6·9 | 6·5 | 6·3 | 6·0 | 5·8 | 5·6 | 5·8 | 5·7 |
| S‐7854 Ind 10 μl/ml | 8·0 | 7 | 6·6 | 6·4 | 6·3 | 6·2 | 6·2 | 6·2 | 6·1 | 6·1 |
| S‐112 bhg 10 μl/ml | 9·7 | 7·9 | 7·2 | 6·9 | 6·6 | 6·4 | 6·2 | 6·2 | 6·2 | 6·1 |
| Dilutions of A/Anhui Antigen – 99 μg/ml | ||||||||||
| S‐APS Viet 10 μl/ml | 9 | 8·4 | 7·7 | 7·7 | 7·2 | 6·9 | 6·9 | 6·8 | 6·7 | |
| S‐7854 Ind 10 μl/ml | 9·8 | 8·2 | 7·7 | 7·1 | 7 | 6·7 | 6·6 | 6·5 | 6·5 | 6·4 |
| S‐112 bhg 10 μl/ml | 14·4 | 12·7 | 11·3 | 10·8 | 9·8 | 9·3 | 8·7 | 8·3 | 8·1 | 7·4 |
Figure 2.
Single radial immunodiffusion (SRID) analysis of reference antigens in the presence of homologous and heterologous potency antisera. Dilutions of four H5N1 potency reference antigens were analyzed by SRID using potency antiserum for A/Vietnam/1203/2004 (A), A/Indonesia/5/2005 (B), or A/bhg/QL/1A/2005 (C). Precipitin rings were measured in two directions to the nearest 0·1 mm for the determination of diameter.
Comparison of vaccine potency values in the presence of homologous and heterologous antisera
The preliminary titration of antigen standard in the presence of homologous and heterologous antisera described above allowed us to move to the next step of evaluating the potency of actual H5N1 vaccine samples. Although there have been a limited number of H5N1 vaccines produced by licensed vaccine manufacturers, we were able to obtain samples of vaccine lots manufactured for three different H5N1 strains – 2 lots of A/Vietnam/1203/2004, 1 lot of A/Indonesia/5/2005, and 1 lot of A/bhg/QL/1A/2005. For each of these vaccine samples, we measured the potency using the corresponding reference antigen in the presence of homologous antiserum and two heterologous antisera. As described in the preliminary studies, we limited the serum concentration to a single concentration of 10 μl/ml, and every assay was repeated in at least four independent experiments. Each agarose gel plate included one or more vaccine samples and the reference antigen corresponding to the vaccine virus strain at an optimal starting concentration as described in the previous section. In addition to the reference antigen that was used to construct the standard curve for the assay, a second sample of reference antigen at a different starting dilution was included as an additional unknown sample in comparison with the assigned reference antigen and the % difference between the two values calculated. Overall, the results exhibited within‐assay variability typical of the SRID method, with the coefficient of variation (CV) <15% in almost all assays. In two cases, the assay CV was >15%, but one of these used homologous antiserum and the other heterologous antiserum (Table 3– A/Vietnam vaccine 2, using A/Vietnam antiserum and A/bhg antiserum, respectively).
Table 3.
Potency of A/Vietnam/1203/04 reference antigen and vaccines using homologous (S‐APS1) and two heterologous (S‐7854 and S112) sera
| Antiserum* | Reference antigen #50 152 μg/ml Starting Concentration | Potency of A/Vietnam vaccines and reference antigen | |||
|---|---|---|---|---|---|
| A/Viet S‐APS1 | 51 μg/ml – 1:3 dilution | A/Viet Vaccine 1 | A/Viet Vaccine 2 | #50 A/Viet Ref Antigen† | Replicate/assigned (%)‡ |
| Average (n = 4) | 123·6 | 43·1 | 154 | 101 | |
| SD§ | 16 | 7 | 8 | ||
| A/Indo S‐7854 | 38 μg/ml – 1:4 dilution | A/Viet Vaccine 1 | A/Viet Vaccine 2 | #50 Viet | |
| Average (n = 4) | 122·4 | 40·9 | 146 | 96 | |
| SD | 10·4 | 4·6 | 10 | ||
| A/bhg S‐112 | 19 μg/ml – 1:8 dilution | A/Viet Vaccine 1 | A/Viet Vaccine 2 | #50 Viet | |
| Average (n = 4) | 126·9 | 38·8 | 142 | 93 | |
| SD | 12·1 | 9·3 | 17 | ||
*Potency antiserum used at 10 μl/ml per single radial immunodiffusion gel.
†Replicate sample of A/Vietnam reference antigen run and calculated as unknown and compared to assigned potency value of 152 μg/ml.
‡Value of reference antigen determined versus assigned HA concentration.
§SD – standard deviation.
Table 3 shows the potency assay results for one lot of A/Vietnam/1203/2004 vaccine, one sample of a vaccine concentrate (an intermediate product in vaccine manufacturing), and a sample of A/Vietnam/1203/2004 reference antigen #50 determined in homologous and two heterologous H5N1 antisera. The results show that the reference antigen potency values in the presence of homologous and heterologous antisera were very similar to each other and to the assigned potency value of 152 μg/ml. Further, the potency values determined for each vaccine sample in all three antisera were similar, with the values obtained using heterologous antiserum differing from the values obtained using homologous A/Vietnam/1203/2004 antiserum by <10%.
4, 5 show the potency assay results for A/bhg/QL/1A/2005 and A/Indonesia/5/2005 vaccines and reference antigen, respectively. Similar to the results obtained in the analysis of A/Vietnam/1203/2004 vaccine, the potency values determined for A/bhg/QL/1A/2005 and A/Indonesia/5/2005 vaccines in heterologous antisera differed little from the value obtained using homologous antiserum (maximum difference of 3% for the A/Indonesia/5/2005 vaccine using A/bhg/QL/1A/2005 antiserum, Table 5). Potency values determined for each reference antigen using heterologous antisera also correlated well with the potency value obtained using homologous antiserum (maximum difference of 16% for the A/bhg/QL/1A/2005 reference antigen using A/Indonesia/5/2005 antiserum, Table 4). Taken together, the results obtained from SRID analysis of three different H5N1 vaccine strains demonstrate the feasibility of using heterologous H5N1 antisera for SRID potency assay of inactivated H5N1 vaccines.
Table 4.
Potency of A/bhg/Ql/1A/05 reference antigen and vaccine using homologous (S‐112) and two heterologous (S‐APS1 and S7854) sera
| Antiserum* | Reference antigen #63 69 μg/ml Starting concentration | Potency of A/bhg vaccine and reference antigen | ||
|---|---|---|---|---|
| A/bhg S‐112 | 69 μg/ml – undiluted | A/bhg Vaccine | #63 A/bhg Ref Antigen† | Replicate/assigned (%)‡ |
| Average (n = 16) | 119·4 | 77·6 | 113 | |
| SD§ | 4·4 | 4·2 | ||
| A/Viet S‐APS1 | 69 μg/ml – undiluted | A/bhg Vaccine | #63 A/bhg | |
| Average (n = 16) | 119·7 | 73·0 | 106 | |
| SD | 3·8 | 4·5 | ||
| A/Indo S‐7854 | 69 μg/ml – undiluted | A/bhg Vaccine | #63 A/bhg | |
| Average (n = 16) | 118·5 | 64·8 | 94 | |
| SD | 5·2 | 4·8 | ||
*Potency antiserum used at 10 μl/ml per single radial immunodiffusion gel.
†Replicate sample of A/bhg reference antigen run and calculated as unknown and compared to assigned potency value of 69 μg/ml.
‡Value of reference antigen determined versus assigned HA concentration.
§SD – standard deviation.
Table 5.
Potency of A/Indonesia/5/05 reference antigen and vaccine using homologous (S‐7854) and two heterologous (S‐APS1 and S112) sera
| Antiserum* | Reference antigen #59 86 μg/ml Starting concentration | Potency of A/Indo vaccine and reference antigen | ||
|---|---|---|---|---|
| A/Indo S‐7854 | 57 μg/ml – 1:1·5 dilution | A/Indo Vaccine | #59 A/Indo Ref Antigen† | Replicate/assigned (%)‡ |
| Average (n = 4) | 716 | 80 | 93 | |
| SD§ | 31 | 5 | ||
| A/Viet S‐APS1 | 86 μg/ml – undiluted | A/Indo Vaccine | #59 A/Indo | |
| Average (n = 8) | 713 | 87 | 101 | |
| SD | 48 | 7 | ||
| A/bhg S‐112 | 21·5 μg/ml – 1:4 dilution | A/Indo Vaccine | #59 A/Indo | |
| Average (n = 8) | 739 | 92 | 107 | |
| SD | 49 | 4 | ||
*Potency antiserum used at 10 μl/ml per single radial immunodiffusion gel.
†Replicate sample of A/Indonesia reference antigen run and calculated as unknown and compared to assigned potency value of 86 μg/ml.
‡Value of reference antigen determined versus assigned HA concentration.
§SD – standard deviation.
Heterologous potency antisera is stability‐indicating
While the results described above demonstrated that calculation and adjustment of the standard antigen for use with heterologous antiserum resulted in a valid potency measurement of an H5N1 vaccine strain homologous with the standard antigen, a necessary attribute of a potency assay is the ability to detect and quantify subpotent vaccines. To evaluate this issue, we set up an accelerated stability assay with a lot of A/Indonesia/5/2005 vaccine. Vaccine samples from this lot were stored at 4 and 37°C and assayed for potency by SRID at monthly intervals for 4 months using the homologous A/Indonesia/5/2005 potency reference antiserum or the A/Vietnam/1203/2004 potency reference antiserum. As shown in Figure 3, there was a negligible loss of potency when vaccine was stored at the recommended 4°C. On the other hand, significant potency loss was observed over the 4‐month observation period when vaccine was held at 37°C, and the measured potency loss was similar regardless of whether homologous or heterologous antiserum was used in the SRID potency assay.
Figure 3.
Single radial immunodiffusion (SRID) potency analysis of H5N1 A/Indonesia/5/2005 vaccine in an accelerated stability study. A/Indonesia/5/2005 vaccine was stored at the recommended temperature (4–8°C) or at 37°C and analyzed by SRID at monthly intervals in the presence of homologous A/Indonesia/5/2005 potency antiserum or heterologous A/Vietnam/1203/2004 potency antiserum.
Seasonal potency antiserum and the pandemic H1N1 vaccine
The most likely use of a heterologous antiserum for potency determination would be in an emergency setting such as the emergence of an H5N1 virus that differed from virus strains for which candidate vaccines and potency reagents had been prepared and stockpiled. Although challenging, the production of strain‐specific potency antiserum rarely delays the production of seasonal influenza vaccines. Nevertheless, it was of interest to see whether heterologous antiserum could work for seasonal vaccine potency determination. Indeed, in a series of preliminary SRID assays using several recent H3N2 potency antisera and homologous and heterologous H3N2 reference antigens, precipitin rings could be obtained in the typical range expected for a workable SRID potency assay (data not shown). Similarly, antiserum produced against the two most recent seasonal H1N1 strains, A/Solomon Islands/03/2006 and A/Brisbane/59/2007, produced precipitin rings with reference antigens for both of these vaccine strains (Figure 4, middle and right panels). As for the initial H5N1 studies, the quality of the precipitin rings differed in homologous versus heterologous antiserum and some of the observed zones in these particular gels were relatively faint. Although the assay conditions for these gels were not optimized, it seems likely that conditions could be established for successful assay setup with heterologous antiserum by manipulating antiserum/antigen concentrations and adjustment of the automated reader for fainter zones. In contrast, neither seasonal H1N1 potency reference antiserum was capable of producing precipitin rings when used with the reference antigen for the 2009 pandemic H1N1 A/California/07/2009, and antiserum to A/California/07/2009 did not cross‐react with either seasonal reference antigen in the SRID assay (Figure 4). Thus, although our data demonstrate the feasibility of using heterologous H5N1 antisera for SRID potency assay of the inactivated H5N1 vaccines that we tested, the data with seasonal and pandemic H1N1 reagents indicate that there are clearly limitations to this approach for more divergent virus strains.
Figure 4.
H1N1 seasonal and 2009 pandemic single radial immunodiffusion (SRID) precipitin zones in the presence of homologous and heterologous potency antisera. SRID gels were run with H1N1 reference antigens A/California/07/2009, A/Solomon Islands/03/2006, and A/Brisbane/59/2007 in the presence of homologous and heterologous potency antisera.
Discussion
The SRID assay has been the accepted method used to assess the potency of inactivated influenza vaccines for more than 30 years. It is a relatively simple assay from a technical point of view, measuring the diffusion and precipitation of the influenza HA in an agarose gel containing HA‐specific polyclonal antiserum. Quantification of the amount of HA present in the vaccine sample is based on comparison of an antigen standard with an assigned HA value. Both the antigen standard and the antiserum used in the assay are supplied by one of the WHO Essential Regulatory Laboratories. Thus, although the assay is simple and fairly easy to standardize among various laboratories, including vaccine manufacturers and regulatory agencies, it does require continuous production of new potency reagents as the recommended composition of the vaccine changes to match the predominant circulating strains of virus. The reference antigen is typically a preparation of inactivated whole virus, the HA of which matches that of the vaccine as closely as possible. Because of the large quantities of reagents needed to support worldwide vaccine manufacture, the reference antigen is usually produced at a commercial scale by a vaccine manufacturer and donated to one of the various regulatory agencies. The HA antigen content of the reference antigen is calibrated through a collaborative laboratory effort of the WHO ERLs. The antiserum to be used with the reference antigen in the SRID assay is typically prepared in sheep by immunization with virus HA which has been cleaved from the virus with the enzyme bromelain and purified. These potency reagents are then distributed by regulatory agencies to support vaccine manufacture.
Although the production and distribution of potency reagents is always challenging owing to the nature of the tight timelines for vaccine production, availability of reagents rarely impacts the availability of seasonal influenza vaccine. Nevertheless, concerns about the urgency of producing a new vaccine in the event of a pandemic virus emergence have led public health agencies to examine all aspects of vaccine development and production in an effort to condense the time required to make a vaccine available and to ensure that back‐up procedures are available should unforeseen circumstances be encountered. We recently described an alternative approach to producing potency antiserum that did not require the availability of the pandemic virus nor the purification of bromelain‐cleaved virus HA. 24 That work demonstrated the feasibility of using recombinant DNA expression techniques to generate potency reagents and provides a viable back‐up option for addressing this particular bottleneck in inactivated vaccine production. Here, we describe another approach that can be considered as an alternative to generating a new potency antiserum – the use of an antiserum made to a related virus strain. This approach could be a viable option for a rapid vaccine response to a pandemic virus if sufficient supplies of a workable antiserum were available.
The developers of the SRID method as an influenza potency assay did not recommend non‐homologous antiserum for use in potency testing. On the contrary, the importance of serum strain specificity was emphasized, and it was recommended that the potency antiserum be evaluated for specificity by using a variety of techniques such as HI, neuraminidase inhibition, and immuno double diffusion assays as well as SRID. 5 Nevertheless, the fact that potency antiserum is a polyclonal hyperimmune serum suggested that cross‐reactive antibodies would be present, and that by adjusting the antigen dose and antiserum concentration, conditions could be established for successful precipitation of the antigen in the SRID assay.
In initial investigations, we explored the cross‐reactivity between a number of H1, H3 and H5 reference antisera and the corresponding available reference antigens and found that acceptable precipitin zones in the SRID assay could be produced in the presence of heterologous antisera. To further investigate the practicality of using heterologous antisera as an alternative in the SRID assay, we focused on H5N1 influenza virus reagents that are currently available from the Center for Biologics Evaluation and Research. The three H5N1 viruses (A/Vietnam/1203/2004, A/Indonesia/5/2005, and A/bhg/QL/A1/2005) for which reference antigen and antiserum were available belong to different genetic clades (clades 1, 2·1, and 2·2, respectively) as defined by the WHO H5N1 virus evolution working group. 26 A fourth virus (A/Anhui/1/2005) for which limited quantities of reference antigen were available belongs to clade 2·3·4. Although of different clades, these four H5N1 virus HAs demonstrate substantial levels of antigenic homology, sharing more than a half of the antigenic sites discovered by mapping with H5 monoclonal antibodies. 27 , 28 , 29 , 30
For each H5N1 reference antigen, a dilution series could be found which resulted in a range of HA concentrations producing precipitin rings with diameters from ∼8·5 to ∼5·5 mm in the SRID test in the presence of either 10 μl/ml homologous or heterologous antisera, although the workable concentration range of the standard antigen differed in homologous versus heterologous antiserum. For example, while a starting concentration of 35 μg/ml of A/bhg/QL/1A/2005 was suitable for precipitation with 10 μl/ml of homologous A/bhg/QL/1A/2005 antiserum, a starting concentration of 69 μg/ml of reference antigen was necessary for use with 10 μl/ml of either A/Vietnam/1203/2004 or A/Indonesia/5/2005 antiserum. Most likely, the different antigen‐antiserum balances reflect the differences in strain‐specific and cross‐reactive antibodies in each potency antiserum. This fact is important for choosing the right reference antigen and vaccine dilution for SRID testing in the presence of heterologous antisera. In this type of assay setup, the reference antigen and the vaccine samples belong to the same virus strain, but the antiserum produced to a close virus relative can be used as long as the antigen–antibody combination produces readable precipitin rings in the optimal range of the assay. For the H5N1 potency reagents, we found that the dose–response of the heterologous antigen‐antiserum assay was linear as for the homologous antigen‐antiserum assay, and similar potency results were obtained when vaccine samples were assayed by both assays. Thus, we propose that such an assay setup can be used for potency testing if deemed necessary.
One concern about the use of heterologous potency antiserum in the SRID assay was whether the assay would remain stability‐indicating, i.e., capable of accurately measuring subpotent vaccine. This might be the situation if the majority of cross‐reactive antibodies in the heterologous antiserum that were responsible for precipitation in the SRID were not conformation‐specific antibodies. Reassuringly, in our study in which an A/Indonesia/5/2005 vaccine was subjected to an accelerated stability protocol over the course of 4 months, both homologous and heterologous antisera provided similar measurements of vaccine potency decline. Although additional studies might be needed for other heterologous antigen‐antiserum combinations, the results again strongly suggested the feasibility of using heterologous potency antiserum if the appropriate antigen‐antiserum concentration conditions can be established.
Strategies to prepare for and confront pandemic influenza have included the development of multiple interventions such as antiviral drugs, personal protective equipment, and vaccines, 31 , 32 and considerable effort has been expended in the United States to produce stockpiles of influenza vaccines against viruses with pandemic potential. 17 , 18 , 19 Having sufficient supplies of potency reagents for these vaccines is not only necessary for use of these pre‐pandemic vaccines, but as shown here, stockpiles of potency antiserum can also potentially serve as the potency antiserum for the new vaccine that is produced in response to a pandemic, assuming there is sufficient cross‐reactivity. This suggests that libraries of stockpiled potency reagents should be expanded to increase the likelihood of having cross‐reactive SRID antiserum available in the event of a pandemic. 16 Clearly, however, there are limitations to this heterologous antiserum approach as shown by the inability of antiserum to recent seasonal H1N1 viruses to work in an SRID assay with the 2009 pandemic H1N1 A/California/07/2009 antigen. This is probably due to the extensive antigenic variation between the A/California/07/2008‐like viruses and the seasonal H1N1 viruses that were in circulation before the pandemic. Nevertheless, we would argue that this suggests the wisdom of stockpiling an even broader library of potency reagents to include many strains of influenza viruses with pandemic potential including H2, H7, and H9 viruses. As recently noted, H2N2 that is genetically similar to the 1957 virus continues to circulate in swine, a situation not unlike that which produced the 2009 H1N1 pandemic. 33
Of course, stockpiling of potency reagents such as antiserum assumes that the SRID will remain the standard potency assay for inactivated vaccines for the foreseeable future. As noted earlier, several newer technologies are under investigation as potential influenza potency assays. 12 , 13 , 14 , 15 At the present time, though, none of the newer techniques has been shown to be as practical as the SRID for standardized testing nor has any been shown to measure a biological function of the HA antigen that can be correlated with clinical benefit. In addition, some of these techniques may still require a specific potency antiserum to mirror the specific binding of antigen and antibodies measured in the SRID. 15 In summary, our results support the generation of an extensive library of potency reagents for influenza strains with pandemic potential, particularly antiserum, as an essential aspect of pandemic influenza preparedness.
Acknowledgements
We thank Zhiping Ye and Maryna Eichelberger (CBER/FDA) for critical reading of the manuscript. This work was supported in part by the Biomedical Advanced Research and Development Authority (BARDA), Department of Health and Human Services.
References
- 1. Schild GC, Henry‐Aymard M, Pereira HG. A quantitative, single‐radial‐diffusion test for immunological studies with influenza virus. J Gen Virol 1972; 16:231–236. [DOI] [PubMed] [Google Scholar]
- 2. Schild GC, Smith JW, Cretescu L, Newman RW, Wood JM. Strain‐specificity of antibody to haemagglutinin following inactivated A/port chalmers/1/73 vaccine in man: evidence for a paradoxical strain‐specific antibody response. Dev Biol Stand 1977; 39:273–281. [PubMed] [Google Scholar]
- 3. Mostow SR, Schild GC, Dowdle WR, Wood RJ. Application of the single radial diffusion test for assay of antibody to influenza type A viruses. J Clin Microbiol 1975; 2:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wood JM, Schild GC, Newman RW, Seagroatt V. An improved single‐radial‐immunodiffusion technique for the assay of influenza haemagglutinin antigen: application for potency determinations of inactivated whole virus and subunit vaccines. J Biol Stand 1977; 5:237–247. [DOI] [PubMed] [Google Scholar]
- 5. Williams MS, Mayner RE, Daniel NJ et al. New developments in the measurement of the hemagglutinin content of influenza virus vaccines by single‐radial‐immunodiffusion. J Biol Stand 1980; 8:289–296. [DOI] [PubMed] [Google Scholar]
- 6. Williams MS. Single‐radial‐immunodiffusion as an in vitro potency assay for human inactivated viral vaccines. Vet Microbiol 1993; 4:253–262. [DOI] [PubMed] [Google Scholar]
- 7. Cate TR, Couch RB, Parker D, Baxter B. Reactogenicity, immunogenicity, and antibody persistence in adults given inactivated influenza virus vaccines – 1978. Rev Infect Dis 1983; 5:737–747. [DOI] [PubMed] [Google Scholar]
- 8. La Montagne JR, Noble GR, Quinnan GV et al. Summary of clinical trials of inactivated influenza vaccine – 1978. Rev Infect Dis 1983; 5:723–736. [DOI] [PubMed] [Google Scholar]
- 9. Quinnan GV, Schooley R, Dolin R, Ennis FA, Gross P, Gwaltney JM. Serologic responses and systemic reactions in adults after vaccination with monovalent A/USSR/77 and trivalent A/USSR/77, A/Texas/77, B/Hong Kong/72 influenza vaccines. Rev Infect Dis 1983; 5:748–757. [DOI] [PubMed] [Google Scholar]
- 10. Wright PF, Cherry JD, Foy HM et al. Antigenicity and reactogenicity of influenza A/USSR/77 virus vaccine in children‐‐a multicentered evaluation of dosage and safety. Rev Infect Dis 1983; 5:758–764. [DOI] [PubMed] [Google Scholar]
- 11. Hobson D, Curry RL, Beare AS, Ward‐Gardner A. The role of serum haemagglutination‐inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972; 70:767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kapteyn JC, Porre AM, de Rond EJ et al. HPLC‐based quantification of haemagglutinin in the production of egg‐ and MDCK cell‐derived influenza virus seasonal and pandemic vaccines. Vaccine 2009; 27:1468–1477. [DOI] [PubMed] [Google Scholar]
- 13. Garcia‐Canas V, Lorbetskie B, Bertrand D, Cyr TD, Girard M. Selective and quantitative detection of influenza virus proteins in commercial vaccines using two‐dimensional high‐performance liquid chromatography and fluorescence detection. Anal Chem 2007; 79:3164–3172. [DOI] [PubMed] [Google Scholar]
- 14. Williams TL, Luna L, Guo Z et al. Quantification of influenza virus hemagglutinins in complex mixtures using isotope dilution tandem mass spectrometry. Vaccine 2008; 26:2510–2520. [DOI] [PubMed] [Google Scholar]
- 15. Nilsson CE, Abbas S, Bennemo M, Larsson A, Hamalainen MD, Frostell‐Karlsson A. A novel assay for influenza virus quantification using surface plasmon resonance. Vaccine 2010; 28:759–766. [DOI] [PubMed] [Google Scholar]
- 16. Wood JM. Developing vaccines against pandemic influenza. Philos Trans R Soc Lond B Biol Sci 2001; 356:1953–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. US Department of Health and Human Services . HHS Pandemic Influenza Plan. Washington, DC, 2005. Available at http://www.hhs.gov/pandemicflu/plan/pdf/HHSPandemicInfluenzaPlan.pdf (Accessed 19 April 2011).
- 18. US Department of Health and Human Services . HHS Pandemic Planning Update, a Report from Secretary Michael O. Leavitt. Washington DC, 2006. Available at http://www.flu.gov/professional/pdf/panflu20060313.pdf (Accessed 19 April 2011).
- 19. US Homeland Security Council . National Strategy for Pandemic Influenza. Washington DC, 2005. Available at http://permanent.access.gpo.gov/lps64971/nspi.pdf (Accessed 19 April 2011).
- 20. US Homeland Security Council . Implementation Plan for the National Strategy for Pandemic Influenza. Washington DC, 2006. Available at http://www.flu.gov/professional/federal/pandemic‐influenza‐implementation.pdf (Accessed 19 April 2011).
- 21. Webby RJ, Perez DR, Coleman JS et al. Responsiveness to a pandemic alert: use of reverse genetics for rapid development of influenza vaccines. Lancet 2004; 363:1099–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. WHO . WHO Expert Committee on Biological Standardization, Thirtieth report. Geneva: World Health Organization, 1979, Annex 3 (WHO Technical Report Series, No. 638). [PubMed] [Google Scholar]
- 23. WHO . Recommendations for production and quality control of inactivated influenza vaccines In: WHO Expert Committee on Biological Standardization. Fifty‐fourth report. Geneva: World Health Organization, 2005, Annex 3 (WHO Technical Report Series, No. 927). [Google Scholar]
- 24. Schmeisser F, Vodeiko GM, Lugovtsev VY, Stout RR, Weir JP. An alternative method for preparation of pandemic influenza strain‐specific antibody for vaccine potency determination. Vaccine 2010; 28:2442–2449. [DOI] [PubMed] [Google Scholar]
- 25. Palmer DF, Coleman MT, Dowdle WR, Schild GC. Advanced Laboratory Techniques for Influenza Diagnosis. Washington, D.C.: U.S. Department of Health Education and Welfare, 1975. [Google Scholar]
- 26. WHO . Continuing Progress Towards a Unified Nomenclature System for the Highly Pathogenic H5N1 Avian Influenza Viruses. Geneva: World health Organization, 2009. Available at http://www.who.int/csr/disease/avian_influenza/guidelines/nomenclature/en/index.html (Accessed 22 August 2011). [Google Scholar]
- 27. Rudneva IA, Kushch AA, Masalova OV et al. Antigenic epitopes in the hemagglutinin of Qinghai‐type influenza H5N1 virus. Viral Immunol 2010; 23:181–187. [DOI] [PubMed] [Google Scholar]
- 28. Wu WL, Chen Y, Wang P et al. Antigenic profile of avian H5N1 viruses in Asia from 2002 to 2007. J Virol 2008; 82:1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaverin NV, Rudneva IA, Ilyushina NA et al. Structure of antigenic sites on the haemagglutinin molecule of H5 avian influenza virus and phenotypic variation of escape mutants. J Gen Virol 2002; 83:2497–2505. [DOI] [PubMed] [Google Scholar]
- 30. Kaverin NV, Rudneva IA, Govorkova EA et al. Epitope mapping of the hemagglutinin molecule of a highly pathogenic H5N1 influenza virus by using monoclonal antibodies. J Virol 2007; 81:12911–12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schuchat A, Bell BP, Redd SC. The science behind preparing and responding to pandemic influenza: the lessons and limits of science. Clin Infect Dis 2011; 52(Suppl 1):S8–S12. [DOI] [PubMed] [Google Scholar]
- 32. Jennings LC, Monto AS, Chan PK, Szucs TD, Nicholson KG. Stockpiling prepandemic influenza vaccines: a new cornerstone of pandemic preparedness plans. Lancet Infect Dis 2008; 8:650–658. [DOI] [PubMed] [Google Scholar]
- 33. Nabel GJ, Wei CJ, Ledgerwood JE. Vaccinate for the next H2N2 pandemic now. Nature 2011; 471:157–158. [DOI] [PubMed] [Google Scholar]


