Abstract
Please cite this paper as: Fujimoto et al. (2012) Induction and maintenance of anti‐influenza antigen‐specific nasal secretory IgA levels and serum IgG levels after influenza infection in adults. Influenza and Other Respiratory Viruses 6(6), 396–403.
Objectives To determine the induction and changes in anti‐influenza virus secretory IgA (s‐IgA) levels in nasal washes and serum IgG levels in patients with influenza.
Methods The study recruited 16 patients with influenza aged 35·6 ± 9·6 years in 2007/2008 and 2008/2009 seasons. Nasal washes and serum were obtained throughout the first year. Anti‐viral s‐IgA levels and neutralization activities in nasal washes, and serum anti‐viral IgG levels and hemagglutination inhibition (HI) titers were measured.
Results Anti‐viral(H1N1) s‐IgA to total IgA ratio and neutralizing antibody titer were low in nasal washes of all patients, whereas serum levels of anti‐viral IgG and HI titers varied widely at day 1·4 ± 1·0 postinfection. Both nasal s‐IgA and serum IgG levels later increased significantly, reaching peak levels at day 9·6 ± 3·3 postinfection. The induced nasal s‐IgA then returned toward the initial levels within 300 days, although the levels at day 143 ± 70 were 3·03‐fold of the initial. Individual serum IgG levels also returned toward the initial levels within 300 days, although the mean levels remained high probably because of re‐infection in a subgroup of patients. Although influenza A (H3N2) was a minor epidemic subtype in both flu seasons, a significant rise in nasal anti‐viral (H3N2) s‐IgA levels and a slightly increase in serum IgG levels were noted.
Conclusion Low levels of nasal anti‐viral s‐IgA and neutralizing antibody were noted compared with a wide range of serum anti‐viral IgG and HI titers at the onset of infection. Elevated s‐IgA and IgG returned toward the initial levels within 300 days of infection with minor exceptions.
Keywords: Influenza virus, mucosal and systemic immunity, secretory nasal IgA, serum IgG
Introduction
Influenza, one of the most common infectious respiratory diseases, is associated with considerable morbidity and mortality in infants and aged individuals. 1 , 2 The first line of defense against respiratory pathogens is mucosal immunity, particularly nasopharyngeal mucosal immunity, which constitutes a major component of the immunological humoral‐ and cell‐mediated responses in the upper and lower airways. 3 All currently available influenza vaccines, with the exception of the cold‐adapted live flu vaccine, are administered intramuscularly or subcutaneously, induce a predominantly IgG‐mediated protection in the systemic immune compartment, but result in inadequate induction of anti‐viral secretory IgA (s‐IgA) antibodies with a wide cross‐protection activity at the airway mucosa. 4 , 5
It has been reported in many of mucosal vaccination experiments in animals, such as nasal influenza A virus (IAV) vaccination, that anti‐viral mucosal s‐IgA in nasal washes and/or bronchoalveolar fluid is the primary defense compound against IAV infection and that anti‐viral s‐IgA levels in the airway determine infection sensitivity of animals. 6 , 7 , 8 , 9 However, there is little clinical data on s‐IgA in nasal washes and bronchoalveolar fluid, and several questions remain unanswered: (i) What is the time course of mucosal s‐IgA induction in nasal washes of patients after IAV infection? and Are there differences in anti‐IAV s‐IgA levels among infants, children, adults, and the elderly populations? (ii) How long do the induced anti‐viral s‐IgA levels in nasal washes and serum IgG levels persist after IAV infection? (iii) What are the levels of anti‐IAV‐specific s‐IgA in nasal washes of human that can provide adequate protection against IAV infection? As a first step in answering these questions, we reported recently a new method for sampling nasal washes (nasopharyngeal aspiration) suitable for such studies. 10
The aim of this study was to define the time course of anti‐IAV‐specific s‐IgA induction as well as serum IgG induction over a period of approximately 1 year after seasonal IAV infection in adults. The results indicated that all patients tested with influenza had low levels of anti‐IAV s‐IgA and neutralizing antibody in nasal washes at the initial onset of illness, despite the wide‐range distribution of anti‐IAV IgG levels and hemagglutination inhibition (HI) titers in serum. The induced both s‐IgA in nasal washes and IgG in serum returned toward the initial levels during the 300‐day postinfection period, with minor exceptions.
Materials and methods
Patient population
A retrospective study of 16 adult patients (six men and 10 women; 24–60‐year old; mean age: 35·6 ± 9·6 years) was conducted. They suffered from IAV infection during the 2007/2008 and 2008/2009 flu seasons. They were diagnosed with influenza by clinical signs and symptoms and the rapid diagnosis Espline Influenza A&B‐N kit (Fujirebio Inc., Tokyo, Japan) on admission. Analysis of the most common features of influenza in this group showed fever (defined as body temperature ≥38°C) in 56% of the patients, pharyngitis in 81%, cough in 75%, nasal discharge in 100%, headache in 69%, and general malaise in 50% of the patients. Informed consent was obtained for enrolment in the study and for the use of stored nasopharyngeal aspirates and sera for quantitative analysis of antibodies. The study was approved by the Committee for Medical Ethics of Tokushima University Hospital. Eleven patients were infected in the 2007/2008 flu season and five were in the 2008/2009 flu season. In both flu seasons, IAV H1N1 subtype was prevalent in Japan. 11 , 12 Treatment was initiated within 48 h of the onset of fever and included 5‐day course of oseltamivir in 12 patients and zanamivir in four.
Sample collection
The nasal washes were collected from patients by using the nasal spray and aspiration method described recently. 10 Briefly, the nasal washes were collected by aspiration from both nostrils over 1‐min period from each side through a silicon tube (1·7 mm in diameter, MD‐33105; Akita‐Sumitomo Bake Co., Akita, Japan) and then trapped in a centrifuge tube connected to an evacuator (EP‐1500; Bluecross Co., Saitama, Japan). Finally, the silicon tube interior was rinsed with 1·0 ml of saline, and the collected lavage fluid was immediately cooled on ice, sonicated for 1 min, and centrifuged at 700 g for 10 min at 4°C. The supernatants were then stored at −30°C until use. Serum samples were simultaneously collected from each patient and stored at −30°C until use.
Nasal washes and serum were again collected from the same patients 2–4 times within the first year after the original infection. The obtained samples were divided into four time periods (Table 1): group 1 (samples collected within 3 days after the onset of illness, mean 1·4 ± 1·0 days, ±SD), group 2 (samples collected between 4 and 21 days after the onset of illness, mean, 9·6 ± 3·3 days), group 3 (samples collected at between 22 and 300 days after the onset of illness, mean, 143 ± 70 days), and group 4 (samples collected more than 300 days after the onset of illness, mean, 368 ± 25 days).
Table 1.
Changes in s‐IgA levels against viral antigens and neutralization activity in nasal washes and in serum levels of IgG antibodies and HI titers of IAV‐infected patients during follow‐up
| Days after onset of illness | Within 3 days (<3 days) | 4–21 days (<21 days) | 22–300 days (<300 days) | >300 days |
|---|---|---|---|---|
| Nasal lavage fluid (U/μg total IgA × 100) | ||||
| s‐IgA against A/H1N1 | 5·9 ± 2·9 | 31·4 ± 32·0* | 17·9 ± 10·4* | 8·6 ± 4·3 |
| s‐IgA against A/H3N2 | 4·3 ± 1·3 | 20·9 ± 19·0* | 21·0 ± 11·3* | 10·4 ± 4·9 |
| Neutralization activity against A/H1N1 (titer) | 6·0 ± 10·8 | 40·3 ± 47·6** | 27·9 ± 4·1 | 6·0 ± 2·8 |
| No. of samples | 8 | 16 | 9 | 4 |
| Sampling after onset of illness (days) | 1·4 ± 1·0 | 9·6 ± 3·3 | 143 ± 70 | 368 ± 25 |
| Serum (U/ml) | ||||
| IgG against A/H1N1 | 420 ± 324 | 858 ± 366* | 837 ± 375** | 1019 ± 628 |
| IgG against A/H3N2 | 493 ± 456 | 847 ± 412 | 794 ± 353 | 657 ± 211 |
| HI titer against A/H1N1 | 44 ± 32 | 89 ± 155 | 34 ± 19 | 47 ± 31 |
| HI titer against A/H3N2 | 41 ± 49 | 37 ± 35 | 40 ± 16 | 53 ± 23 |
| No. of samples | 8 | 16 | 10 | 3 |
| Sampling after onset of illness (days) | 1·4 ± 1·0 | 9·6 ± 3·3 | 146 ± 66 | 370 ± 31 |
Values are mean ± SD.
*P < 0·01, **P < 0·05 versus the values of within 3 days.
Enzyme‐linked immunosorbent assay (ELISA)
The concentrations of total IgA and anti‐IAV‐specific s‐IgA in nasal washes and those of anti‐IAV‐specific IgG in serum were measured by ELISA as described previously 7 , 13 with minor modifications. The concentrations of total IgA were measured using a human IgA kit (Bethyl Laboratories, Montgomery, TX, USA) according to the instructions supplied by the manufacturer. For measurement of anti‐IAV‐specific antibody, the prevalent IAV strains were selected as coating ELISA antigens. In the 2007/2008 flu season, IAV/Solomon Islands/3/2006(H1N1)‐like subtype was identified in 88% of Japanese cases, 11 while in the 2008/2009 flu season, IAV/Brisbane/59/2007(H1N1)‐like subtype was identified in 64% of Japanese cases. 12 Although the IAV/H3N2 subtype was a minor subtype in both flu seasons, most epidemic strains were IAV/Hiroshima/52/2005(H3N2)‐like subtype in the 2007/2008 season and IAV/Uruguay/716/2007(H3N2)‐like subtype in the 2008/2009 season. 11 , 12 Therefore, for analysis of anti‐IAV‐specific s‐IgA and IgG in patients of the 2007/2008 flu season, A/Solomon Islands/3/2006(H1N1) and A/Hiroshima/52/2005(H3N2) split‐product HA vaccine antigens were used for coating in ELISA, while A/Brisbane/59/2007(H1N1) and A/Uruguay/716/2007(H3N2) split‐product HA vaccine antigens were used for ELISA analysis of samples in the 2008/2009 flu season.
For quantification of anti‐IAV‐specific s‐IgA in nasal washes, 96‐MicroWell Plates (Nalge Nunc International, Tokyo, Japan) were coated with the IAV vaccine antigens (0·1 μg/well) described previously, incubated overnight in phosphate‐buffered saline (PBS) at 4°C, and then blocked with 1% BSA in 50 mm Tris–HCl (pH 8·0) containing 0·14 m NaCl and 0·05% Tween‐20 (TTBS) for 1 h at room temperature. The nasal wash diluted with TTBS was added to each well and incubated for 2 h at room temperature. The plates were washed five times with TTBS, incubated with goat anti‐human IgA conjugated with horseradish peroxidase (Bethyl Laboratories) for 1 h at room temperature, and incubated again with a TMB Microwell Peroxidase Substrate System (KPL Inc., Gaithersburg, MD, USA) according to the instructions provided by the manufacturer. The produced chromogen was measured at an absorbance of 450 nm using a SpectraMax Plus384 autoreader (Molecular Devices Corp., Sunnyvale, CA, USA). Multiple samples taken from each patient were simultaneously evaluated in the same plate. Fluctuations in absorbance among plates were adjusted by control analyte. As the affinity‐purified human anti‐IAV‐specific s‐IgA standard for each IAV subtype is not commercially available, the anti‐IAV‐specific IgA concentrations in the nasal washes were determined from the standard regression curves with human IgA of known concentration in a human IgA quantitation kit (Bethyl Laboratories). The relative value of anti‐IAV‐specific s‐IgA was expressed as units (U); 1 U of anti‐IAV‐specific s‐IgA was determined from the regression curve as the point corresponding to 1 μg of human IgA detected in the assay system, as described previously. 13
As the concentration of nasal wash samples varies widely between individuals depending on the aspiration efficiency, history of nasal diseases, and patient age, the concentration of anti‐IAV‐specific s‐IgA (U/ml) was normalized by the total IgA (μg/ml) concentration in the same sample of nasal washes. The values of normalized anti‐IAV‐specific s‐IgA were highly reproducible in comparison with the values of anti‐IAV‐specific s‐IgA normalized by the amount of protein in nasal washes, particularly in the chronic nasal disease patients. Therefore, the serial changes in the nasal concentration of anti‐IAV‐specific s‐IgA throughout the study period were expressed relative to that of anti‐viral IAV‐specific s‐IgA to total IgA = [anti‐IAV‐specific s‐IgA levels (U/ml)/total IgA (μg/ml) × 100].
The ELISA procedure was also used to determine serum anti‐IAV‐specific IgG levels. As there was no significant variability in serum protein concentrations, the row values of anti‐IAV‐specific IgG concentrations (U/ml) were used in the time course analysis.
Assay of hemagglutination inhibition and neutralizing activity
To measure hemagglutination inhibition (HI) activities, serum samples were treated overnight with receptor‐destroying enzyme (Denka Seiken Co., Tokyo, Japan) at 37°C to eliminate non‐specific HI factors, and the assay was conducted according to the protocol for HI testing established by the World Health Organization. 8 For neutralizing activities of nasal washes and serum, IAV/Solomon Islands/3/2006(H1N1) at 200 PFU and IAV/Brisbane/59/2007(H1N1) at 200 PFU, which were provided by the National Institute of Infectious Diseases of Japan, were incubated with 100 μl of diluted samples at room temperature for 30 min. Then the virus titers in the serially diluted neutralization mixtures were measured by the plaque assay, as described previously. 7 , 14
Statistical analysis
Values were expressed as mean ± SD. Differences between groups were examined for statistical significance using the Student’s t‐test or Welch’s t‐test. A P‐value <0·05 was considered statistically significant.
Results
Low levels of anti‐IAV(H1N1)‐specific s‐IgA and neutralizing antibody in nasal washes and wide variation in serum levels of anti‐IAV(H1N1)‐specific IgG and HI titers at the onset of illness
In the 2007/2008 and 2008/2009 winter flu seasons, the IAV H1N1 subtype was the most prevalent in Japan. We analyzed the ratio of anti‐IAV(H1N1)‐specific s‐IgA to total IgA and neutralizing antibody in nasal washes as a major local defense antibody at the site of virus entry, serum HI titer, and serum levels of anti‐IAV(H1N1)‐specific IgG, a major systemic defense antibody in adult IAV‐infected patients at 1·4 ± 1·0 days (onset of illness). The ratio of anti‐IAV(H1N1)‐specific s‐IgA in nasal washes was low in all samples tested (5·9 ± 2·9, anti‐IAV(H1N1) s‐IgA U/μg of total IgA × 100), with an upper limit of 12·0. In addition, the titer levels of neutralizing antibody in nasal washes were also low at 6·0 ± 10·8. However, serum levels of anti‐IAV(H1N1)‐specific IgG and HI titers varied from very low to high (Table 1 and 1, 2); in fact, the HI titers of five of eight IAV‐infected patients were relatively high (≥40) at the onset of illness.
Figure 1.
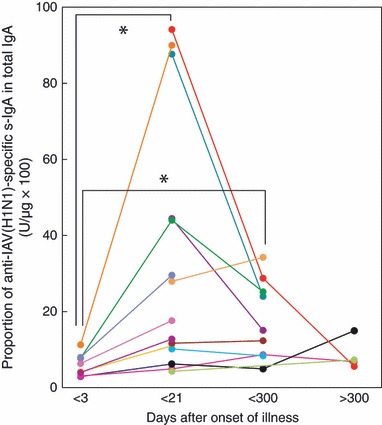
Kinetics of induction of anti‐IAV(H1N1)‐specific s‐IgA in nasal washes after IAV infection examined over a period of about 1 year. Data and lines represent the serial changes in anti‐IAV(H1N1)‐specific s‐IgA/total IgA ratio (U/μg × 100) of individual patients with different color symbols. *P < 0·01.
Figure 2.
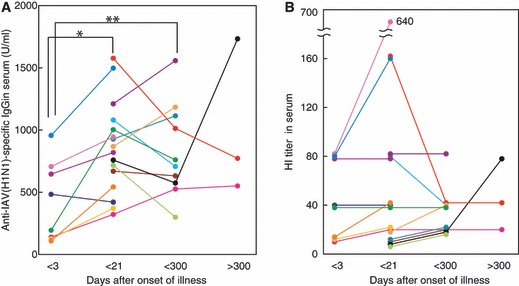
Kinetics of induction of anti‐IAV(H1N1)‐specific IgG (A) and HI titers (B) in serum after IAV infection examined over a period of about 1 year. Data and lines represent the serial changes in anti‐HA(H1N1)‐specific IgG (U/ml) of individual patients with different color symbols. *P < 0·01, **P < 0·05.
Kinetics of induction of nasal anti‐IAV(H1N1)‐specific s‐IgA and serum IgG after IAV infection
Influenza A virus infection resulted in significant induction of nasal anti‐IAV(H1N1)‐specific s‐IgA and neutralizing antibody titers, and serum anti‐IAV‐specific IgG and HI titers after infection, reaching peak levels between days 4 and 21 (Table 1 and 1, 2). These results are almost identical to those reported in the kinetics studies on antibody production in mice after induction of nasal IAV infection 15 and also in children vaccinated with live attenuated IAV. 16 Serum HI titers in all except three patients increased after infection, particularly in patients with HI titers ≥80 at the onset of illness. However, a mild or no increase in HI titer after infection was observed in patients with HI titers of <80 at the onset of illness (Figure 2B). Subsequently, the increased nasal s‐IgA level in each patient returned toward the respective initial level, although the mean levels of nasal s‐IgA and neutralizing antibody titers were still 3·03‐ and 4·65‐fold higher than the initial values, respectively, within the 300‐day after the onset of illness (Table 1). At 368 ± 25 days postinfection, nasal s‐IgA levels of three of four patients were within the initial levels, but the s‐IgA of one patient had increased (Figure 1).
The high levels of serum anti‐IAV(H1N1)‐specific IgG and HI titer in each patient (who showed peak levels during 4 and 21 days after infection) returned toward the initial levels during the follow‐up period (<300 days postinfection) (Figures 2A and 2B). However, serum HI titers of five of 10 patients and those of two of three patients were ≥40 between 22 and 300 days and after 300 days postinfection, respectively. These high values from the patients increased the mean HI titers of anti‐IAV(H1N1) throughout the follow‐up period (Figure 2B and Table 1).
Serial changes in nasal anti‐IAV(H3N2) s‐IgA and serum IgG after infection
Although IAV(H3N2) was a minor epidemic subtype in both the 2007/2008 and 2008/2009 winter flu seasons in Japan, the kinetics of anti‐IAV(H3N2)‐specific s‐IgA induction in nasal washes and serum IgG induction were similar to those of nasal anti‐IAV(H1N1)‐specific s‐IgA and serum IgG, although the changes in the levels were relatively mild in anti‐IAV(H3N2) s‐IgA/IgG than those in anti‐IAV(H1N1) s‐IgA/IgG (3, 4). While changes in serum anti‐IAV(H3N2) IgG levels were observed during the follow‐up period, almost no changes in the levels of HI titer were observed, with several minor exceptions (Figure 4B). Thus, the mean serum HI titers against IAV(H3N2) did not change during the period (Table 1).
Figure 3.
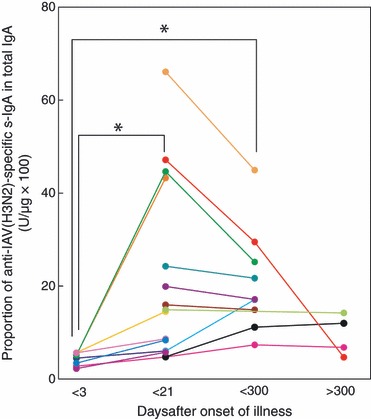
Kinetics of induction of anti‐IAV(H3N2)‐specific s‐IgA in nasal washes after IAV infection examined over a period of about 1 year. Data and lines represent the serial changes in anti‐HA(H3N2)‐specific s‐IgA/total IgA ratio (U/μg × 100) of individual patients with different color symbols. *P < 0·01.
Figure 4.
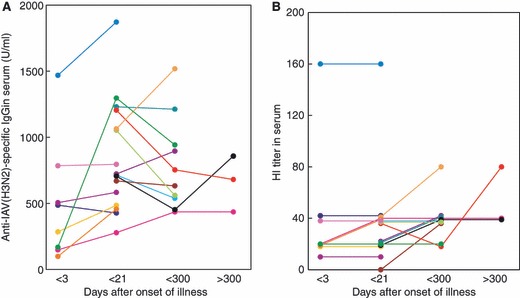
Kinetics of induction of anti‐IAV(H3N2)‐specific IgG (A) and HI titers (B) in serum after IAV infection examined over a period of about 1 year. Data and lines represent the serial changes in serum anti‐HA(H3N2)‐specific IgG (U/ml) levels of individual patients with different color symbols.
Discussion
Our study clearly showed that anti‐viral s‐IgA levels and neutralizing antibody titers in the nasal washes were low at the onset of infection, irrespective of the levels of anti‐viral IgG and HI titers in the serum. In particular, serum HI titers in over half of IAV‐infected patients were ≥40 at the time of infection, a critical value for evaluation of the efficacy of influenza vaccine 17 and a value reported to be associated with protection from influenza illness in up to 50% of patients in human challenge studies. 18 These results suggest that nasal s‐IgA levels associate with the infectivity of IAV. In the present study, anti‐viral s‐IgA levels in nasal washes were expressed relative to the total IgA [anti‐IAV‐specific s‐IgA (U/ml)/total s‐IgA (μg/ml) × 100], because the concentration of nasal washes varied among the patients. The mean ratio at the onset of illness was 5·9 ± 2·9 (U/μg × 100), and the maximal value in the group was 12·0. These anti‐viral s‐IgA levels were almost consistent to those reported previously in 10 pediatric IAV‐infected patients within 48 h of the onset of fever. 13
It has been reported that mucosal s‐IgA is primarily involved in cross‐protection of the mucosal surface against variant IAV infection, and the mechanism of broad‐spectrum cross‐protection could be explained by the wide‐range cross‐reactivity of s‐IgA. 19 , 20 , 21 , 22 , 23 We recently found cross‐neutralization activity for nasal fluids of minipigs after intranasal vaccination of IAV antigen. 8 In the present study, we found patients with increased levels of s‐IgA induction against both H1N1 and H3N2 subtypes, although IAV H1N1 was the predominant subtype in the 2007/2008 and 2008/2009 flu seasons in Japan. The results could probably be explained by the following (although we did not identify the subtype of the infecting IAV strain in the clinical samples): (i) The induced s‐IgA in nasal washes has cross‐reactivity between H1N1 subtype and H3N2 subtype in a manner similar to the reported cross‐reactivity in nasal fluids of animals after intranasal vaccination. 8 (ii) Some of the epitopes of H1N1 subtype resemble those of H3N2 subtype and induce heterosubtype immunity by stimulation of pre‐existing immunological s‐IgA memory. 24 (iii) As we used a split‐inactivated viral preparation as an antigen for ELISA assay, it is possible that the induced s‐IgA is the antibody against common viral proteins other than hemagglutinin and neuraminidase.
To our knowledge, there are only a limited number of reports on the serial changes in nasal immune responses in patients with influenza. Nasal vaccination has been reported in clinical studies using virosome‐based influenza vaccine, 20 a Proteosome™‐trivalent inactivated influenza vaccine 21 (ID Biomedical Corporation of Québec, laval, QC, Canada) and a live attenuated IAV vaccine. 16 Furthermore, only little information is available on the serial changes in s‐IgA concentration of patients after IAV infection. 25 To determine the kinetics of mucosal and systemic antibody induction in response to the IAV(H1N1) subtype infection in 2007/2008 and 2008/2009 flu seasons, we analyzed the levels of anti‐IAV(H1N1)‐specific s‐IgA in nasal washes and those of IgG in serum of patients over a period of about 1 year.
The induced levels of nasal s‐IgA and serum IgG antibodies at day 9·6 ± 3·3 after infection were significantly higher than those at day 1·4 ± 1·0 postinfection. It is possible to explain that the levels at day 1·4 ± 1·0 represent pre‐infection levels, because nasal s‐IgA level is known to increase rapidly 4 days after nasal virosome‐based influenza vaccination 20 and 1 week after treatment of patients with IAV(H1N1) cold‐adapted reassortant virus. 16 Further follow‐up analysis of individual patients showed a decrease in nasal anti‐IAV‐specific s‐IgA levels before 300 postinfection days, and the average levels of anti‐IAV‐specific s‐IgA and neutralizing activity at day 143 ± 70 were about 57% and 69% of the peak level, respectively. The levels subsequently returned to the pre‐infection levels after about 1 year (368 ± 25 day). The results are in general agreement with those of Shvartsman and Grigorieva 25 who reported persistently high titers of antibodies against IAV in nasal secretions for 4–8 months in mild cases of infection and for more than 8 months in severe cases complicated with pneumonia. Induced serum anti‐IAV(H1N1)‐specific IgG levels were also decreased in a large number of patients, although slowly, during 1‐year follow‐up period, but the mean values in Table 1 were 0·98‐ and 1·2‐fold of the peak levels recorded at days 146 ± 66 and 370 ± 25, respectively. These findings were the reflection of data of a small group of patients who showed an increase in serum IgG levels, HI titers, and nasal s‐IgA levels during the follow‐up period, probably due to IAV re‐infection. Although we tried to detect the viral re‐infection by the rapid diagnosis Influenza A&B‐N kit after the initial series of experiments for about 1 year, viral antigen was not detected probably because of long‐term storage of nasal washes after sampling. The results of the present study on the kinetics of antibody induction by natural IAV infection (Table 1) are roughly consistent with the data of live influenza vaccination. 26 , 27 In these studies, both nasal s‐IgA and serum IgG levels reached maximum values at 1 month, then gradually decreased to pre‐immunization levels in 6 months.
We have recently reported attenuation of inducible respiratory immunoresponses by oseltamivir treatment in mice infected with IAV 9 and non‐significant increases in anti‐IAV‐specific s‐IgA in nasal washes of pediatric IAV‐infected patients treated with oseltamivir for 5 days. 13 These results are considered to be because of the suppression of viral replication and viral antigen production by the anti‐viral agent. However, adult patients in the present study (age, 35·6 ± 9·6 years), who were treated with oseltamivir or zanamivir for 5 days within 48 h after onset of illness, showed significant increase in s‐IgA in nasal washes and IgG in serum at day 9·6 ± 3·3. These findings suggest that despite the antiviral treatment and probably the low production of viral antigen doses in mucosal loci, nasal s‐IgA antibodies increase after stimulation of pre‐existing immunological s‐IgA memory in adult patients, but not in naïve children aged 5·9 ± 3·3 years reported previously. 13
In summary, our results showed low nasal levels of anti‐IAV‐specific s‐IgA and neutralizing antibody in all tested patients who were infected with IAV at the onset of illness, although highly variable serum levels of anti‐viral IgG and HI titers were observed. The induced nasal s‐IgA levels and neutralizing antibody titers returned toward the initial levels during the 300‐day postinfection period, and the mean levels of anti‐IAV s‐IgA and neutralizing antibody titer were 3·03‐ and 4·65‐fold of the initial levels, respectively, at postinfection day 143 ± 70. Induced serum IgG levels in the majority of patients slowly returned toward the initial levels during the 300‐day postinfection period, although some patients were found to have high anti‐viral IgG levels and HI titers in serum probably due to IAV re‐infection. Taken together, the findings presented in this study provide important information on s‐IgA levels with regard to the sensitivity to IAV infection as well as data that could be potentially useful for evaluation of intranasal IAV vaccination.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This study was supported by a Grant‐in‐Aid (21249061), the Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, and Science and Technology of Japan.
References
- 1. Lipatov AS, Govorkova EA, Webby RJ et al. Influenza: emergence and control. J Virol 2004; 78:8951–8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim HW, Brandt CD, Arrobio JO, Murphy B, Chanock RM, Parrott RH. Influenza A and B virus infection in infants and young children during the years 1957‐1976. Am J Epidemiol 1979; 109:464–479. [DOI] [PubMed] [Google Scholar]
- 3. Holmgren J, Czerkinsky C. Mucosal immunity and vaccine. Nat Med 2005; 11:S45–S53. [DOI] [PubMed] [Google Scholar]
- 4. Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild type virus. J Clin Microbiol 1986; 24:157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tamura SI, Asanuma H, Ito Y et al. Super cross‐protective effect of nasal vaccination to subcutaneous inoculation with influenza hemagglutinin vaccine. Eur J Immunol 1992; 22:477–481. [DOI] [PubMed] [Google Scholar]
- 6. Tamura S, Kurata T. Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn J Infect Dis 2004; 57:236–247. [PubMed] [Google Scholar]
- 7. Mizuno D, Ide‐Kurihara M, Ichinomiya T, Kubo I, Kido H. Modified pulmonary surfactant is a potent adjuvant that stimulates the mucosal IgA production in response to the influenza virus antigen. J Immunol 2006; 176:1122–1130. [DOI] [PubMed] [Google Scholar]
- 8. Nishino M, Mizuno D, Kimoto T et al. Influenza vaccine with Surfacten, a modified pulmonary surfactant, induces systemic and mucosal immune responses without side effects in minipigs. Vaccine 2009; 27:5620–5627. [DOI] [PubMed] [Google Scholar]
- 9. Takahashi E, Kataoka K, Fujii K et al. Attenuation of inducible respiratory immune responses by oseltamivir treatment in mice infected with influenza A virus. Microbes Infect 2010; 12:778–783. [DOI] [PubMed] [Google Scholar]
- 10. Fujimoto C, Kido H, Sawabuchi T et al. Aspiration of nasal IgA secretion in normal subjects by nasal spray and aspiration. Auris Nasus Larynx 2009; 36:300–304. [DOI] [PubMed] [Google Scholar]
- 11. Infectious Disease Surveillance Center, National Institute of Infectious Diseases of Japan . 2007/08 influenza season, Japan. Infect Agents Surveill Rep 2008; 29: 297–299. Available at http://idsc.nih.go.jp/iasr/29/345/tpc345.html (Accessed 3 August 2010). [Google Scholar]
- 12. Infectious Disease Surveillance Center, National Institute of Infectious Diseases of Japan . 2008/09 influenza season, Japan. Infect Agents Surveill Rep 2009; 30: 285–286. Available at http://idsc.nih.go.jp/iasr/30/357/tpc357.html (Accessed 3 August 2010). [Google Scholar]
- 13. Sawabuchi T, Suzuki S, Iwase K et al. Boost of mucosal secretory immunoglobulin A response by clarithromycin in paediatric influenza. Respirology 2009; 14:1173–1179. [DOI] [PubMed] [Google Scholar]
- 14. Dulbecco R. Production of plaques in monolayer tissue cultures by single particles of an animal virus. Proc Natl Acad Sci USA 1952; 38:747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tamura S, Iwasaki T, Thompson AH et al. Antibody‐forming cells in the nasal‐associated lymphoid tissue during primary influenza virus infection. J Gen Virol 1998; 79:291–299. [DOI] [PubMed] [Google Scholar]
- 16. Murphy BR, Nelson DL, Wright PF, Tierney EL, Phelan MA, Chanock RM. Secretory and systemic immunological response in children infected with live attenuated influenza A virus vaccines. Infect Immun 1982; 36:1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goodeve A, Potter CW, Clark A, Jennings R, Scild GC, Yetts R. A graded dose study of inactivated surface antigen influenza B vaccine in volunteers. J Hyg (Lond) 1983; 90:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Couch RB, Kasel JA. Immunity to influenza in man. Annu Rev Microbiol 1983; 37:529–549. [DOI] [PubMed] [Google Scholar]
- 19. Renegar KB, Small PA Jr, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol 2004; 173:1978–1986. [DOI] [PubMed] [Google Scholar]
- 20. Durrer P, Glück U, Spyr C et al. Mucosal antibody response induced with a nasal virosome‐based influenza vaccine. Vaccine 2003; 21:4328–4334. [DOI] [PubMed] [Google Scholar]
- 21. Langley JM, Halperin SA, McNeil S et al. Safety and immunogenicity of a ProteosomeTM‐trivalent inactivated influenza vaccine, given nasally to healthy adults. Vaccine 2006; 24:1601–1608. [DOI] [PubMed] [Google Scholar]
- 22. Murphy BR, Clements ML. The systemic and mucosal immune response of humans to influenza A virus. Curr Top Microbiol Immunol 1989; 146:107–116. [DOI] [PubMed] [Google Scholar]
- 23. Liew FY, Russell SM, Appleyard G, Brand CM, Beale J. Cross‐protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur J Immunol 1984; 14:350–356. [DOI] [PubMed] [Google Scholar]
- 24. Tamura S, Tanimoto T, Kurata T. Mechanisms of broad cross‐protection provided by influenza virus infection and their application to vaccines. Jpn J Infect Dis 2005; 58:195–207. [PubMed] [Google Scholar]
- 25. Shvartsman YS, Grigorieva SK. Secretory immunity in influenza. J Infect Dis 1979; 140:837–843. [DOI] [PubMed] [Google Scholar]
- 26. Clements ML, Murphy BR. Development and persistence of local and systemic antibody responses in adults given live attenuated or inactivated influenza A virus vaccine. J Clin Microbiol 1986; 23:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beyer WE, Palache AM, de Jong JC, Osterhaus AD. Cold‐adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta‐analysis. Vaccine 2002; 20:1340–1353. [DOI] [PubMed] [Google Scholar]


