Abstract
Please cite this paper as: Shanks et al. (2012) Epidemiological isolation causing variable mortality in Island populations during the 1918–1920 influenza pandemic. Influenza and Other Respiratory Viruses 6(6), 417–423.
Background During the 1918 pandemic period, influenza‐related mortality increased worldwide; however, mortality rates varied widely across locations and demographic subgroups. Islands are isolated epidemiological situations that may elucidate why influenza pandemic mortality rates were so variable in apparently similar populations.
Objectives Our objectives were to determine and compare the patterns of pandemic influenza mortality on islands.
Methods We reviewed historical records of mortality associated with the 1918–1920 influenza pandemic in various military and civilian groups on islands.
Results and Conclusions Mortality differed more than 50‐fold during pandemic‐related epidemics on Pacific islands [range: 0·4% (Hawaii) to 22% (Samoa)], and on some islands, mortality sharply varied among demographic subgroups of island residents such as Saipan: Chamorros [12%] and Caroline Islanders [0·4%]. Among soldiers from island populations who had completed initial military training, influenza‐related mortality rates were generally low, for example, Puerto Rico (0·7%) and French Polynesia (0·13%). The findings suggest that among island residents, those who had been exposed to multiple, antigenically diverse respiratory pathogens prior to infection with the 1918 pandemic strain (e.g., less isolated) experienced lower mortality. The continuous circulation of antigenically diverse influenza viruses and other respiratory infectious agents makes widespread high mortality during future influenza pandemics unlikely.
Keywords: 1918, mortality, Pacific islands, pandemic influenza
Introduction
The influenza pandemic of 1918–1920 was the most lethal natural event in recorded human history. Within a few months, the influenza A/H1N1 pandemic spread worldwide and killed 40–80 million people. 1 , 2 During the pandemic, influenza‐related mortality increased in all affected populations; however, it markedly varied across locations and among demographic subgroups at specific locations. For example, during the pandemic, previously healthy young adults had relatively high mortality risk; the unprecedented w‐shaped relationship between mortality and age remains unexplained. 3 Also, during the pandemic, influenza‐related mortality rates were extraordinarily high in some island and other isolated populations (e.g., Arctic). 4 This has led researchers to believe that mortality was because of a lack of cross‐protective immunity provided by prior influenza virus strains. The analysis of isolated communities, and specifically demographic subgroups within those communities, can help to understand the outcome of infection. Mortality differed by more than 10‐fold across apparently similar island populations and more than 30‐fold across demographic subgroups of specific island populations. 5
The 1918 influenza A/H1N1 virus was efficiently transmitted but not inherently or inevitably lethal. Most individuals who were infected with the pandemic strain had unremarkable clinical courses with complete recovery. Nearly all pandemic‐related deaths were because of post‐influenza bacterial pneumonias rather than direct pathologic effects of the virus. 6 In the absence of antivirals, antibiotics, mechanical ventilation, and intensive medical care, significant differences in mortality among various island populations were unlikely related to differences in medical care. Also, because most epidemics on isolated islands were caused by point source introduction of the pandemic strain (e.g., via the arrival of a single ship), mortality differences across affected islands were unlikely due to viral strain mutation.
It is thought that nearly all humans were immunologically susceptible to infection with the pandemic strain in 1918. The wide ranges of mortality across islands and across subgroups on the same islands suggest that the number and nature of prior respiratory infections (with influenza viruses and other respiratory infectious agents) may have significantly influenced clinical expressions and outcomes of infections with the pandemic strain. We hypothesize that exposures to few, and a limited diversity of, respiratory infectious agents prior to the 1918–1920 influenza pandemic enabled extremely high mortality in isolated communities, particularly islands, during the 1918–1920 influenza pandemic. 7 For this report, we examined the influenza mortality records from several island communities, both civilian and military, to assess the relationships between prior influenza and other respiratory infectious disease experiences and mortality during pandemic‐related epidemics in 1918–1920.
Methods
Data sources consisted of administrative, military, medical, and historical records, including official public health reports, mortality registries, commissioned reviews, and publications in medical journals during and following the 1918–1920 influenza pandemic period. Some of the reports and original data were drawn from unpublished sources that are not archived in readily searchable systems such as PubMED; thus, a systematic search strategy was not used to identify the useful and relevant data sources. Lists of references and bibliographies were reviewed to identify the reviews, registries, and reports published in English or French that recorded prospectively collected data from island populations that documented pandemic‐related mortality and numbers and characteristics of island residents. On most islands, the numbers of island residents who died during influenza pandemic‐related epidemics were specifically reported; it was not always possible, however, to determine the underlying causes of the deaths, for example, “influenza” versus “all respiratory deaths (including pneumonia and influenza).” Because of the massive social, military, and political disruptions during the First World War, the numbers of island residents during the pandemic period are necessarily estimates. To enable comparisons across islands and demographic subgroups, all mortality rates are presented as percentages.
Results
During pandemic‐related influenza epidemics from 1918 to 1920, pneumonia/influenza‐related mortality rates on various islands were estimated as Australia (0·4%), Hawaii (0·4%), New Zealand (0·7%), Fiji (5%), Mauritius (5·5%), Tonga (6·4%), Guam (6·6%), French Polynesia (16%), Nauru (18%), and Samoa (22%). 5 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 Thus during the period, respiratory illness–related mortality across Pacific islands varied by more than 50‐fold (range, 0·4–22%). On Fiji mortality was 5% among residents overall, but varied in relation to race‐ethnicity – from 1·4% among Europeans to 5·7% among Fijians (Table 1). 13 The colonial government of the time interpreted these figures through a racial lens seeing them as an indication of the relative fitness of different races in a social Darwinian sense. This is an untenable position today. It seems much more likely that epidemiological isolation was the differentiating factor between those with the highest mortality rates (e.g., Fijians) who had lived the greater part of their lives on small Pacific islands and the lower mortality immigrants from both India and Europe who had been exposed to a much larger number and wider range of respiratory pathogens, especially in childhood prior to immigration.
Table 1.
Variable civilian mortality in various island populations during the 1918–1920 influenza pandemic grouped by island and ethnicity
| Group and location | Percent mortality from total population | Reference |
|---|---|---|
| Europeans on Fiji | 1·4 of 4800 | 39 |
| Half Europeans on Fiji | 2·8 of 2700 | 39 |
| Indians on Fiji | 4·2 of 61 000 | 39 |
| Fijians on Fiji | 5·7 of 91 000 | 39 |
| Others on Fiji | 6·9 of 4200 | 39 |
| Caucasians on Hawaii | 0·3 of 55 000 | 17 |
| Chinese on Hawaii | 0·3 of 24 000 | 17 |
| Japanese on Hawaii | 0·4 of 109 000 | 17 |
| Filipinos on Hawaii | 0·7 of 21 000 | 17 |
| Hawaiians on Hawaii | 1·1 of 24 000 | 17 |
| Samoans on Western Samoa | 22 of 38 000 | 13 |
| Samoans on American Samoa | 0·0 | 13 |
| Polynesians on French Polynesia | 16·3 of 15 300 | 16 |
| New Caledonians | 0·0 | 35 |
| Nauru indigenous population | 18 of 1174 | 31 |
| Pacific migrant labor on Nauru | 39 of 251 | 31 |
| Chinese migrant labor on Nauru | 1 of 599 | 31 |
| Caroline Islanders on Saipan | 0·4 of 1500 | 12 |
| Chamorros on Saipan | 12 of 1500 | 12 |
| Chamorros on Guam | 6·6 of 13 000 | 13 |
Using published and archival material, we were able to investigate the implication of isolation on responses to the 1918 influenza pandemic more closely. For example, although mortality was relatively low on Hawaii compared with other Pacific islands, it was higher among Hawaiian (1·1%) than Caucasian and Chinese (0·3%) island residents (Table 1). 17 In 1918, the crew/passengers of a US Army transport ship were infected with influenza in the Philippines and carried it to the isolated island of Guam. During the ensuing epidemic, many resident Chamorros, but only one US sailor, died. 13 , 18 In 1964, another pandemic‐related epidemic affected the still very isolated western Caroline Islands. During the epidemic, there were many deaths, including among the adults, especially on the most isolated atolls (e.g., mortality %, by island: Ulithi, 1%; Woleai, 3%; Ifaluk, 6·5%). During 1964 pandemic‐related epidemics on Saipan and Guam, there was no significant influenza‐related mortality. 11 Prior infection of the latter in 1918 and thereafter protected them against mortality from the 1964 pandemic strain.
The benefit of exposure to diverse respiratory pathogens and/or environmental antigens is also observed when residents of isolated island communities were recruited into the US, French, and UK armed forces. 18 , 19 , 20 The influenza‐related mortality experiences of islanders in military service are informative to the extent that they varied from the experiences of their counterparts who remained on their home islands. As expected, US soldiers serving in the Philippines, Hawaii, and Puerto Rico experienced lower influenza‐related mortality rates compared with their locally recruited counterparts. 20 (Table 2) Of interest though, mortality rates were generally lower among recruited Philippino, Hawaiian, and Puerto Rican soldiers than their civilian counterparts on the respective islands – particularly if the soldiers were “seasoned” (e.g., had completed recruitment training and were not new in their current assignments). 20 , 21 The differences in the mortality risk from influenza among the individuals of the same ethnicity suggest that epidemiological factors – in addition to host (e.g., genetic) factors – were significant determinants of risk of lethal outcomes after influenza illnesses.
Table 2.
Influenza mortality rates in US, French, and UK military units either operating in or coming from island areas during 1918–1919 pandemic
| Group and location | Percent mortality from total population | Reference |
|---|---|---|
| US soldiers in Philippines | 0·14 of 7381 | 20 |
| Philippino soldiers in Philippines | 1·02 of 5982 | 20 |
| US soldiers in Hawaii | 0·09 of 5631 | 20 |
| Hawaiian soldiers in Hawaii | 0·55 of 1627 | 20 |
| US soldiers in Panama | 0·13 of 8286 | 20 |
| Puerto Rican soldiers in Panama | 0·0 of 3500 | 20 |
| Puerto Rican soldiers in Puerto Rico | 0·70 of 12 600 | 20 |
| French Polynesian soldiers in France | 0·13 of 1057 | 22 |
| New Caledonian soldiers in France | 0·18 of 2113 | 22 |
| New Zealand soldiers in Europe | 0·8 of 30 000 | 10 |
| New Zealand soldiers on Samoa | 1·8 of 600 | 10 |
| New Zealand soldiers as recruits in NZ | 2·3 of 12 000 | 10 |
| New Zealand Maori soldiers in Europe | 2·8 of 800 | 10 |
| New Zealand soldiers on troopship | 6·9 of 1200 | 30 |
This is supported by the data acquired for Polynesian and Melanesian soldiers recruited into the French Army who experienced relatively low influenza‐related mortality while working in labor battalions in France; there was a nearly 50‐fold difference in mortality between them and their civilian counterparts in French Polynesia. 22 (1, 2) The distinguishing epidemiological factor in these examples is military service in Europe which would have certainly exposed the soldiers to a high level of respiratory infections prior to 1918.
The experiences of New Zealand and Australia (that were relatively isolated at this time) differed in regard to the timing (separated by 4 months) and severity of their respective influenza epidemics. Despite considerable historic and cultural similarities, influenza‐related mortality was twice as high among the residents of New Zealand as Australia, and on New Zealand, mortality rates were much higher among the Polynesian Maori (4·2%) than among the Caucasian immigrants (0·6%). 14 In the fall of 1918, large numbers of soldiers from Australia and New Zealand were engaged in the First World War in Europe. During the fall–winter influenza epidemic period, Caucasian soldiers from Australia and New Zealand had similar influenza‐related mortality rates (approximately 0·7%); however, mortality in the New Zealand Army’s Maori battalion was approximately four times higher — nearly the same as among Maori still in New Zealand. 10 , 23 , 24 (Table 2) The higher susceptibility of the Maori population may reflect their relatively higher isolation prior to joining the military.
The effects of isolation on responses to infection are not restricted to the 1918 pandemic influenza strain. In 1918, the densely populated highlands of New Guinea were unknown to the outside world. Because of the rigid quarantine of Australia, it is likely that the 1918 influenza pandemic did not affect New Guinea. In 1964, influenza outbreaks in isolated New Guinea highland areas produced high mortality, particularly among the adult males. 25 Furthermore, through 1996, isolated highland groups in the Indonesia province of Papua experienced high mortality during epidemics caused by influenza. 26
During the 1918–1920 pandemic period, the highest influenza‐related mortality anywhere in the world was reported from Samoa. 4 , 13 , 27 In the 19th century, the Samoan islands were partitioned into Western and American Samoa; the two geopolitical entities had ethnically identical populations. During the influenza pandemic period, more than one‐fifth of all residents of Western Samoa, but no residents of American Samoa, died. The survival of American Samoans reflected the effectiveness of a maritime quarantine that was instituted by the military governor in Pago Pago (Table 1). 18 , 28 Quarantines of isolated islands can delay but not permanently prevent the introductions of pandemic influenza virus strains. In 1926, a less virulent form of influenza arrived in American Samoa; during the epidemic, 25% of the residents became ill but <0·1% died in sharp contrast to the experience on Western Samoa in 1918. 29
During the First World War, a New Zealand battalion of mostly Caucasian soldiers felt to be unfit for duty in Europe occupied Samoa. When influenza arrived in Samoa, mortality was approximately 10 times higher among Samoan residents than among the New Zealand soldiers who were stationed there. Many New Zealand soldiers on Samoa had close contact with the island residents, for example, during disaster relief and burial duties. Of note, mortality rates were similar among the New Zealand soldiers on Samoa and those in recruit camps in New Zealand; however, mortality was much higher among the New Zealand soldiers in recruit camps than among the civilians in communities adjacent to the camps. 10 , 14 , 24 (Table 2) When a group of New Zealand soldiers bound for Europe encountered influenza at a West African port, 7% died on board the ship or shortly after landing in the UK. 30 The experiences of New Zealand soldiers overall suggest that their mortality risk during the 1918 pandemic period was not exclusively determined by their ethnic backgrounds but was related to prior respiratory infection experiences (e.g., isolation of their home communities) as well as the epidemiological contexts in which their influenza infections occurred.
During the pandemic period, there were large mortality differences in relation to the ethnicities of industrial workers on small Pacific islands. For example, Pacific Islander (from Marshall and Caroline Islands) and Chinese laborers were employed on the phosphate island of Nauru. When pandemic influenza arrived in 1920, the mortality rate was very high among the Pacific Islanders, intermediate among the native Nauruans, and very low among the Chinese workers. 31 (Table 1) The mortality experience on Nauru in relation to ethnicity in 1920 was also reflected during a less lethal influenza epidemic in 1948 on Ocean Island, another phosphate island with contract mining workers where only the Ellice Islanders (1·7%) and no Chinese or Europeans died from influenza. 32 Racial/ethnic group is thus very unlikely to be a key mortality determinant but probably is a surrogate for number and range of previous respiratory infections prior to arrival on Nauru or Ocean Island.
In some ways, the example of Saipan can be seen as the exception that proves the rule. Distinct ethnic groups on the same island had very different mortality rates possibly due to the differences in their isolation during the previous pandemic in the 1890s. In 1919, a Japanese ship introduced pandemic influenza to Saipan in the Northern Mariana Islands. The Chamorro and Caroline Islander residents of the island lived in close proximity to each other in adjacent communities. During the pandemic‐related epidemic on the island, there was more than a 30‐fold difference in mortality among the Chamorros and Caroline Islanders. 12 (Table 1) Saipan and Guam are separated by 120 miles. In 1919, Chamorro people lived on both islands (many had migrated to Saipan from Guam early in the 20th century). Caroline Islanders had migrated to Saipan in the 19th century following their displacement from Truk. 33 , 34 The influenza pandemic of 1890 likely spared the relatively isolated Saipan which at the time had no regular maritime contact with Guam. We were unable to find any record of influenza‐like infection in this isolated population before 1919, but surmise that the influenza epidemic of 1919 was likely the first exposure to influenza for most Caroline Islanders on Saipan. In contrast, many Chamorros on Saipan had probably been exposed to influenza (prior to 1900) while living on Guam. During the 1919 epidemic on Saipan, mortality was relatively low among the Caroline Islanders (possibly during first lifetime infections with influenza virus) but very high among the Chamorros (likely during second ever infections with influenza), suggesting that previous exposure to a particular respiratory pathogen is not always beneficial.
Pandemic influenza was introduced to French Polynesia when a ship from California landed in Tahiti. During the ensuing epidemic, >16% of the total population died; however, mortality varied across the islands from 11·9% on Maketea to 17·8% on Tahiti. 16 New Caledonia, another French colony in the Pacific, escaped influenza entirely in 1918–1920; protection from the pandemic was attributed to the strict quarantine of ships leaving from Australia. When influenza did arrive in New Caledonia in July 1921, a large proportion of the population was infected, but there were very few deaths as on American Samoa in 1926. 35
Discussion
In this review of experiences during the 1918–1920 pandemic period, influenza–pneumonia‐related mortality varied by more than 50‐fold during pandemic‐related epidemics on various Pacific islands; mortality seemed correlated with the island’s degree of contact with outside epidemiological sources as marked by the number of steamships arriving in the major island ports. In the early 20th century, islands were connected to the outside world by trans‐oceanic steamships (which travelled at <14 knots); obviously, the interconnectedness of island communities has enormously changed since then. The extreme globalization that occurred in the last century has enabled continuous circulation of antigenically diverse influenza viruses and other respiratory microbial agents; hence, the epidemiological conditions that set the stage for the extraordinarily lethal 1918–1920 pandemic are unlikely to recur. 7
Other aspects such as socioeconomic status, along with living and environmental conditions, may have also played a major role in population‐level mortality. In 1918, the most isolated island populations may have had more virulent clinical expressions of influenza because their lung microenvironments were not well “conditioned” by prior inflammatory events. (Figure 1) Epidemiological and animal model data demonstrate that infection with a respiratory pathogen alters subsequent immune responses to unrelated respiratory pathogens; the effect is long lasting and does not depend on T and B lymphocytes. The alteration may arise from airway remodeling of damaged tissues, alteration or redistribution of lymphatics and vascular tissues, modification of epithelial cells, and/or resetting of the activity of innate immunity. 36 Islanders with a narrow range of exposures to respiratory infections prior to 1918 would have experienced the 1918 influenza virus with lungs qualitatively different from those who had had multiple respiratory infections prior to 1918 (e.g., military service veterans).
Figure 1.
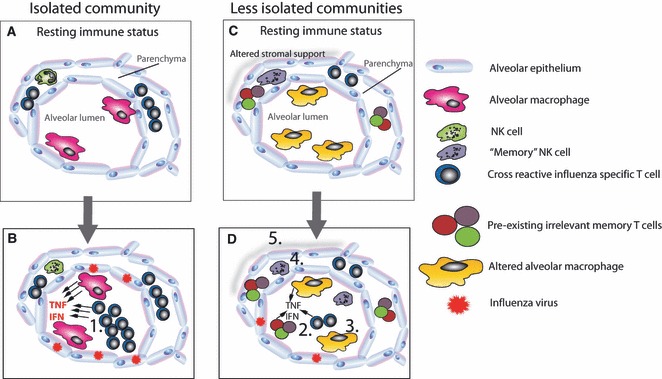
The impact of infection exposure on the development of pathology during the 1918 influenza pandemic. An isolated community will have a less infection‐experienced lung microenvironment. If cross‐reactive T cells are present, they may be over‐represented (A). In the absence of cross‐reactive antibody, influenza will replicate unchecked causing prolonged innate immunity (B). Should specific T cells be present, they expand exponentially because other infections have not adjusted their memory clone size. These too contribute to the inflammatory microenvironment. In an infection‐experienced host (C), the lung at rest is very different. No one T‐cell population is over‐represented, innate “memory” NK cells are present, and airway macrophages are blunted in their responsiveness. During a subsequent influenza infection (D), the whittled down cross‐reactive and bystander T cells enter the lung (2) but are exposed to a reduced inflammatory microenvironment because airway macrophages are less responsive (3) and “memory” NK cells have already reduced the viral load (4). Furthermore, lung remodeling (5) by prior infection has made the lung more resilient.
Repeated infections with antigenically diverse respiratory agents continuously alter and further partition the memory T‐cell pool; as a result, no single specificity is over‐represented among the memory T cells. Over‐representation of a memory T‐cell clone may not benefit the host when re‐exposed to the cognate T‐cell epitope. For example, when CD8+ T cells lyse virally infected cells, they produce abundant quantities of pro‐inflammatory cytokines with characteristic clinical manifestations, for example, cachexia, fever, vascular leakage, and respiratory failure. Successive infections may alter the relative proportions of each memory T‐cell clone in an attrition process. There is limited space to accommodate memory T cells, and diverse respiratory infections progressively decrease pre‐existing T‐cell populations. A richly diverse T‐cell repertoire, as in persons with many previous respiratory infections, could lead to a smaller clonal burst size with less immunopathologic effects.
The human immune system evolved when most people lived in small family/tribal groups in the context of social isolation. Relatively recent social, political, economic, and cultural changes increased the mixing of human populations with different infectious disease experience on both the individual and population levels. Thus, it is not surprising that there is a period of epidemiological adjustment once an isolated area becomes connected to new pools of infectious disease pathogens and human populations. For example, the linking of dense urban populations by trade routes enabled continuous chains of transmission – and great epidemics – of measles and smallpox. Industrialization and warfare accelerated and enhanced the process.
During the 2009 A/H1N1 pandemic, mortality was relatively high among the individuals who were pregnant, obese, or otherwise immunocompromised (e.g., chemotherapy); 37 however, the magnitudes, natures, and distributions of mortality during the 2009 and 1918 pandemics differed enormously – even though both pandemics were caused by antigenically similar A/H1N1 influenza viruses. 38 The influenza pandemic of 1918 remains a unique event with several unexplained clinical and epidemiologic characteristics; the findings of this and other historical reviews suggest that the extreme mortality associated with the 1918 pandemic is unlikely to recur. More detailed and comprehensive understanding of the pathophysiologic mechanisms (e.g., immunopathogenicity, virus‐bacterial interactions) that enabled the extreme mortality of the 1918 pandemic would inform preparations for and responses to future pandemics.
Conflicts of interest
No author claims any conflict of interest.
Funding
The Global Emerging Infectious Disease and Response System (GEIS), which is part of the Armed Forces Health Surveillance Center of the US Department of Defence, provided funding for this project.
Disclaimer
The opinions expressed are those of the authors and do not necessarily reflect those of the Australian Defence Force or the US Department of Defense.
Contributors
GDS conceived the epidemiological study, gathered the data, assisted in the analysis, and wrote the first draft of the manuscript. TH and JB assisted in the analysis, interpretation and writing of the manuscript.
Acknowledgements
The authors thank Jeff Taubenberger for providing ideas crucial to our thinking and the many medical librarians who helped us locate historical information.
References
- 1. Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis 2006; 12:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murray CJ, Lopez AD, Chin B, Feehan D, Hill KH. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918–20 pandemic: a quantitative analysis. Lancet 2006; 368:2211–2218. [DOI] [PubMed] [Google Scholar]
- 3. Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis 2007; 195:1018–1028. [DOI] [PubMed] [Google Scholar]
- 4. Crosby A. America’s Forgotten Pandemic: The Influenza of 1918. Cambridge: Cambridge University Press, 1998. [Google Scholar]
- 5. Cliff A, Haggett P, Smallman‐Raynor M. Island Epidemics. Oxford: Oxford University Press, 1990. [Google Scholar]
- 6. Brundage JF, Shanks G. Deaths from bacterial pneumonia during the 1918–19 influenza pandemic. Emerg Infect Dis 2008; 14:1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shanks G, Brundage JF. The lethal consequences of pathogenic immune responses among young adults during the 1918 influenza pandemic. Emerg Infect Dis 2012; http://wwwnc.cdc.gov/eid/pages/ahead‐of‐print.htm . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McArthur N. Island Populations of the Pacific. Canberra: Australian National University Press, 1968. [Google Scholar]
- 9. Brown P, Gajdusek DC, Morris JA. Virus of the 1918 influenza pandemic era: new evidence about its antigenic character. Science 1969; 166:117–119. [DOI] [PubMed] [Google Scholar]
- 10. Government Printer . New Zealand Expeditionary Force Roll of Honour. Wellington: Government Printer, 1924. [Google Scholar]
- 11. Brown P, Gajdusek DC, Morris JA. Epidemic A2 influenza in isolated Pacific island populations without pre‐epidemic antibody to influenza virus types A and B, and the discovery of other still unexposed populations. Am J Epidemiol 1966; 83:176–188. [DOI] [PubMed] [Google Scholar]
- 12. Crampton HE. On the differential effects of the influenza epidemic among native peoples of the Pacific islands. Science 1922; 55:90–92. [DOI] [PubMed] [Google Scholar]
- 13. Jordan E. Epidemic Influenza: A Survey. Chicago: American Medical Association, 1923. [Google Scholar]
- 14. Rice GW. Black November: The 1918 Influenza Pandemic in New Zealand, 2nd edn Christchurch: Canterbury University Press, 2005. [Google Scholar]
- 15. Scragg R. Historical epidemiology in Papua New Guinea. P N G Med J 1977; 20:102–109. [PubMed] [Google Scholar]
- 16. Allard M. L’epidemie d’influenza de 1918–1919 dans les colonies francaises de L’Oceanie. AMedPhC 1922; 20:66–72. [Google Scholar]
- 17. Schmitt R, Nordyke E. Influenza deaths in Hawaii, 1918–1920. Hawaii J Hist 1999; 33:101–117. [Google Scholar]
- 18. Navy Department . Report of the US Navy Surgeon General. Washington DC: Navy Department, 1919. [Google Scholar]
- 19. The War Office . Statistics of the Military Effort of the British Empire during the Great War. London: The War Office, 1922. [Google Scholar]
- 20. Report of the Surgeon General Vol 1 part 2. War Department Annual Reports, 1919. Washington, 1920:1466–1489.
- 21. Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis 2006; 6:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Memoire des hommes: morts pour la France: Ministere de la Defense, 2011.
- 23. Shanks G, MacKenzie A, McLaughlin R et al. Mortality risk factors during the 1918–19 influenza pandemic in the Australian army. J Infect Dis 2010; 201:1880–1889. [DOI] [PubMed] [Google Scholar]
- 24. Commonwealth War Graves Commission . Debt of honour register. http://www.cwgc.org/debt_of_honour.asp: Commonwealth War Graves Commission, 2011. [Google Scholar]
- 25. Barnes R. Epidemiology of 1964–1965 influenza outbreak in the Sepik district. P N G Med J 1966; 9:127–132. [Google Scholar]
- 26. Corwin AL, Simanjuntak CH, Ingkokusumo G et al. Impact of epidemic influenza A‐like acute respiratory illness in a remote jungle highland population in Irian Jaya, Indonesia. Clin Infect Dis 1998; 26:880–888. [DOI] [PubMed] [Google Scholar]
- 27. Grey F. Notes on epidemic broncho‐pneumonia (Spanish influenza) in Samoa. Med J Aust 1919; 1:359–361. [Google Scholar]
- 28. Govt of New Zealand . Report of the Samoa Epidemic Commission. Wellington: Govt of New Zealand, 1919. [Google Scholar]
- 29. Larsen A. Some medical observations in the Pacific Islands and Dutch East Indies. Cal West Med 1934; 40:413–417. [PMC free article] [PubMed] [Google Scholar]
- 30. Summers J, Wilson N, Baker M, Shanks G. Mortality risk factors for pandemic influenza on New Zealand troop ship, 1918. Emerg Infect Dis 2010; 16:1931–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shlomowitz R. Differential mortality of Asians and Pacific Islanders in the Pacific labour trade. J Aust Popul Assoc 1990; 7:116–127. [DOI] [PubMed] [Google Scholar]
- 32. Isaacs A, Edney M, Donnelley M, Ingram M. Influenza in an isolated community: an epidemic on Ocean Island. Lancet 1950; 1:64–66. [DOI] [PubMed] [Google Scholar]
- 33. US Navy . Civil Affairs Handbook: Mandated Marianas Islands. OPNAV P22‐8. Washington DC: US Navy, 1944. [Google Scholar]
- 34. Joseph A, Murray V, Bender L. Chamorros and Carolinians of Saipan. Cambridge: Harvard University Press, 1951. [Google Scholar]
- 35. Peltier F. L’epidemie d’influenza qui a sevi en Nouvelle Caledonie en 1921. Bulletin de l’Office International d’Hygiene Publique 1922; 14:676–685. [Google Scholar]
- 36. Wissinger E, Goulding J, Hussell T. Immune homeostasis in the respiratory tract and its impact on heterologous infection. Semin Immunol 2009; 21:147–155. [DOI] [PubMed] [Google Scholar]
- 37. Nguyen‐Van‐Tam J, Openshaw P, Hashim A et al. Risk factors for hospitalization and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May–Sep 2009). Thorax 2010; 65:645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wei C, Boyington J, Dai K et al. Cross‐neutralization of the 1918 and 2009 influenza viruses: role of glycans in viral evolution and vaccine design. Sci Transl Med 2010; 2:24ra21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. UK Ministry of Health . Report on the Pandemic of Influenza 1918–19. Reports on Public Health and Medical Subjects. London: UK Ministry of Health, 1920. [Google Scholar]


